Introduction
Myxozoans are a group of highly specialized endoparasites, characterized by morphologically reduced spores, and complex life cycles that involve both vertebrate (predominantly fish) and invertebrate (annelids or bryozoans) hosts (Kent et al. Reference Kent, Andree, Bartholomew, El-Matbouli, Desser, Devlin, Feist, Hedrick, Hoffmann, Khattra, Hallett, Lester, Palenzeula, Siddall and Xiao2001; Lom and Dyková, Reference Lom and Dyková2006). With more than 2400 described species (Zhang, Reference Zhang2011) they have adapted to hosts in freshwater, marine and terrestrial habitats, and this adaptability may have been crucial for their persistence and diversity. There is strong morphological, molecular, phylogenetic and structural evidence for the relationships between myxozoans and free-living Cnidaria (Siddal et al. Reference Siddal, Martin, Bridge, Desser and Cone1995; Jiménez-Guri et al. Reference Jiménez-Guri, Philippe, Okamura and Holland2007; Holland et al. Reference Holland, Okamura, Hartikainen and Secombes2011; Nesnidal et al. Reference Nesnidal, Helmkampf, Bruchhaus, El-Matbouli and Hausdorf2013; Takeuchi et al. Reference Takeuchi, Sekizuka, Ogasawara, Yokoyama, Kamikawa, Nozaki, Konishi-Sugita, Ohnish and Kuroda2015); however, the mechanisms behind the transitions to parasitism, and subsequent radiation of these organisms remain obscure (Okamura et al. Reference Okamura, Gruhl, Bartholomew, Okamura, Gruhl and Bartholomew2015).
Ceratomyxa Thélohan, 1892, is the second largest myxozoan genus, with over 240 described species, most of which infect the gall bladders of marine teleosts (Eiras, Reference Eiras2006; Gunter et al., Reference Gunter, Whipps and Adlard2009; Gunter and Adlard, Reference Gunter and Adlard2010; Fiala et al. Reference Fiala, Hlavnicková, Kodádková, Freeman, Bartosová-Sojková and Atkinson2015). Ceratomyxa myxospores are distinctly elongated, crescent-shaped or arcuate, with two shell valves and two subspherical polar capsules located at the spore apex, adjacent to a straight suture line (Lom and Dyková, Reference Lom and Dyková2006). Three other genera have morphologically similar myxospores: Myxodavisia (Zhao, Zhou, Kent and Whipps, Reference Zhao, Zhou, Kent and Whipps2008) myxospores are distinguished from Ceratomyxa on the basis of having valve cell appendages (Zhao et al. Reference Zhao, Zhou, Kent and Whipps2008); Palliatus Shulman Kovaleva and Dubina, 1979 myxospores have a membranous veil (Lom and Dyková, Reference Lom and Dyková2006), and Meglitschia Kovaleva, 1988 myxospores differ from other Ceratomyxa spp. by being highly arcuate, with large and elongated polar capsules (Lom and Dyková, Reference Lom and Dyková2006). Available molecular data for Myxodavisia bulani (Fiala, Kodadkova, Freeman, Bartošova-Sojkova and Atkinson, Reference Fiala, Hlavnicková, Kodádková, Freeman, Bartosová-Sojková and Atkinson2015), and Palliatus indecorus (Shulman Kovaleva and Dubina, 1979), demonstrate that these taxa cluster in a basal position within the ‘Ceratomyxa’ clade of the marine lineage in ssrDNA-based phylogenetic studies (Fiala et al. Reference Fiala, Hlavnicková, Kodádková, Freeman, Bartosová-Sojková and Atkinson2015; Adriano and Okamura, Reference Adriano and Okamura2017; Zatti et al. Reference Zatti, Atkinson, Bartholomew, Maia and Adriano2017). Molecular data from additional taxa, particularly from type species, are needed to better assess the validity of maintaining these groups as four distinct genera (Fiala et al. Reference Fiala, Hlavnicková, Kodádková, Freeman, Bartosová-Sojková and Atkinson2015; Adriano and Okamura, Reference Adriano and Okamura2017).
Within the Ceratomyxa, only six species have been described from strictly freshwater environments, and five of these are from the Amazon basin: C. mylei (syn. Meglitschia mylei) (Azevedo, Ribeiro, Clemente, Casal, Lopes, Al-Quraishy and Matos, Reference Azevedo, Ribeiro, Clemente, Casal, Lopes, Matos, Al-Quraishy and Matos2011), C. microlepsis (Azevedo, Rocha, Casal, Sao Clemente, Matos, Al-Quraishy and Matos, Reference Azevedo, Rocha, Casal, São Clemente, Matos, Al-Quraishy and Matos2013), C. amazonensis (Mathews, Naldoni, Maia and Adriano, Reference Mathews, Naldoni, Maia and Adriano2016), C. vermiformis Adriano and Okamura, Reference Adriano and Okamura2017 and C. brasiliensis (Zatti, Atkinson, Bartholomew, Maia and Adriano, Reference Zatti, Atkinson, Bartholomew, Maia and Adriano2017). One of these Amazonian species, C. vermiformis, has elongated plasmodial stages that exhibit coordinated, undulatory locomotion, provided by plasmodial cytoskeleton elements, in the bile of its host (Adriano and Okamura, Reference Adriano and Okamura2017). Superficially similar worm-like movement has been observed in myxoworm stages of taxa in class Malacosporea, where the movement is supported by four sets of longitudinal muscles (Gruhl and Okamura, Reference Gruhl and Okamura2012).
During a survey of Myxozoa of the Amazon basin in Brazil, we found a novel, freshwater Ceratomyxa species that parasitized the catfish Brachyplatystoma rousseauxii (Castelnau, 1855) (Siluriformes: Pimelodidae). Known as ‘dourada’ in Portuguese, this fish performs the longest strictly freshwater migration in the world, a journey of ~11 600 km (Barthem et al. Reference Barthem, Goulding, Leite, Cañas, Forsberg, Venticinque, Petry, Ribeiro, Chuctaya and Mercado2017). Dourada spawn in Andean tributaries of the headwaters of the Amazon (Barthem and Goulding, Reference Barthem and Goulding1997) and immature fish are transported downriver to the Amazon estuary region, where juveniles remain for 1·5–2 years. After this growth period, they then move upstream into the lower and middle Amazon, where they inhabit river channels and floodplains for another year (Batista and Alves-Gomes, Reference Batista and Alves-Gomes2006). Reproductive migration of this species back to the headwaters occurs June–November, when the river level starts to rise due to the rainy season (Batista and Alves-Gomes, Reference Batista and Alves-Gomes2006). Carvajal-Vallejos et al. (Reference Carvajal-Vallejos, Duponchelle, Desmarais, Cerqueira, Querouil, Nunez, Garcia and Renno2014) observed some evidence of genetically distinct dourada populations in the Amazon basin, which have specific spawning areas.
We sampled dourada from three widely separated rivers within the Amazon basin, and based on morphological, ssrDNA and internal transcribed spacer region (ITS-1) sequencing identified the same novel Ceratomyxa species in the gall bladder of fish from each locality. Lack of ITS-1 variation suggested that the parasite has a uniform population structure across broad spatial scales, a feature we suggest is evidence of panmixia in the parasite population as a consequence of the extensive migrations of the fish host.
Material and methods
Fish and parasite sampling
Thirty B. rousseauxii were collected by net from three localities (GPS coordinates given in Results section): fish were collected from the Tapajós River, Pará State, Brazil in October 2014 and March 2015 (average length 44 cm, range 34–59 cm, N = 17); from the Amazon River, Amapá State, Brazil, in October 2015 (length 52 cm, N = 1) and from the Solimões River, Amazonas State in December 2015 (average length 77 cm, range 33–103 cm, N = 12) (Fig. 1). The catches were authorized by the Brazilian Ministry of the Environment (SISBIO n° 44268-4), and the examination methodology was approved by the Ethics Committee on Animal Use, University of Campinas (CEUA/UNICAMP n° 3846). Fish were euthanized by overdose of benzocaine solution and necropsied. Gall bladders were removed and ruptured. Bile samples were examined by light microscope and those containing myxosporeans were subdivided and fixed in 10% neutral-buffered formalin for spore measurement (Lom and Arthur, Reference Lom and Arthur1989) and in 100% ethanol for DNA sequencing. Myxospores obtained from ten different plasmodia from two fish specimens (from Tapajós and Solimões rivers) were photographed using a Carl Zeiss Axio Imager A2 light microscope equipped with Axio Cam and AxioVision AxioVs 40V4.8.2 software, using differential interference contrast. Measurements of formalin-fixed myxospores followed the general guidelines of Lom and Arthur (Reference Lom and Arthur1989), and Gunter et al. (Reference Gunter, Whipps and Adlard2009) for Ceratomyxa spp., with modifications suggested by Adriano and Okamura (Reference Adriano and Okamura2017) for strongly arcuate myxospores. We use the more structurally accurate term ‘polar tubule’ instead of ‘polar filament’ (Ben-David et al. Reference Ben-David, Atkinson, Pollak, Yossifon, Shavit, Bartholomew and Lotan2016). Type myxospores were also air-dried onto glass slides, stained with Giemsa, and deposited in the Museum of Zoology ‘Adão José Cardoso’ University of Campinas (UNICAMP), São Paulo State, Brazil.
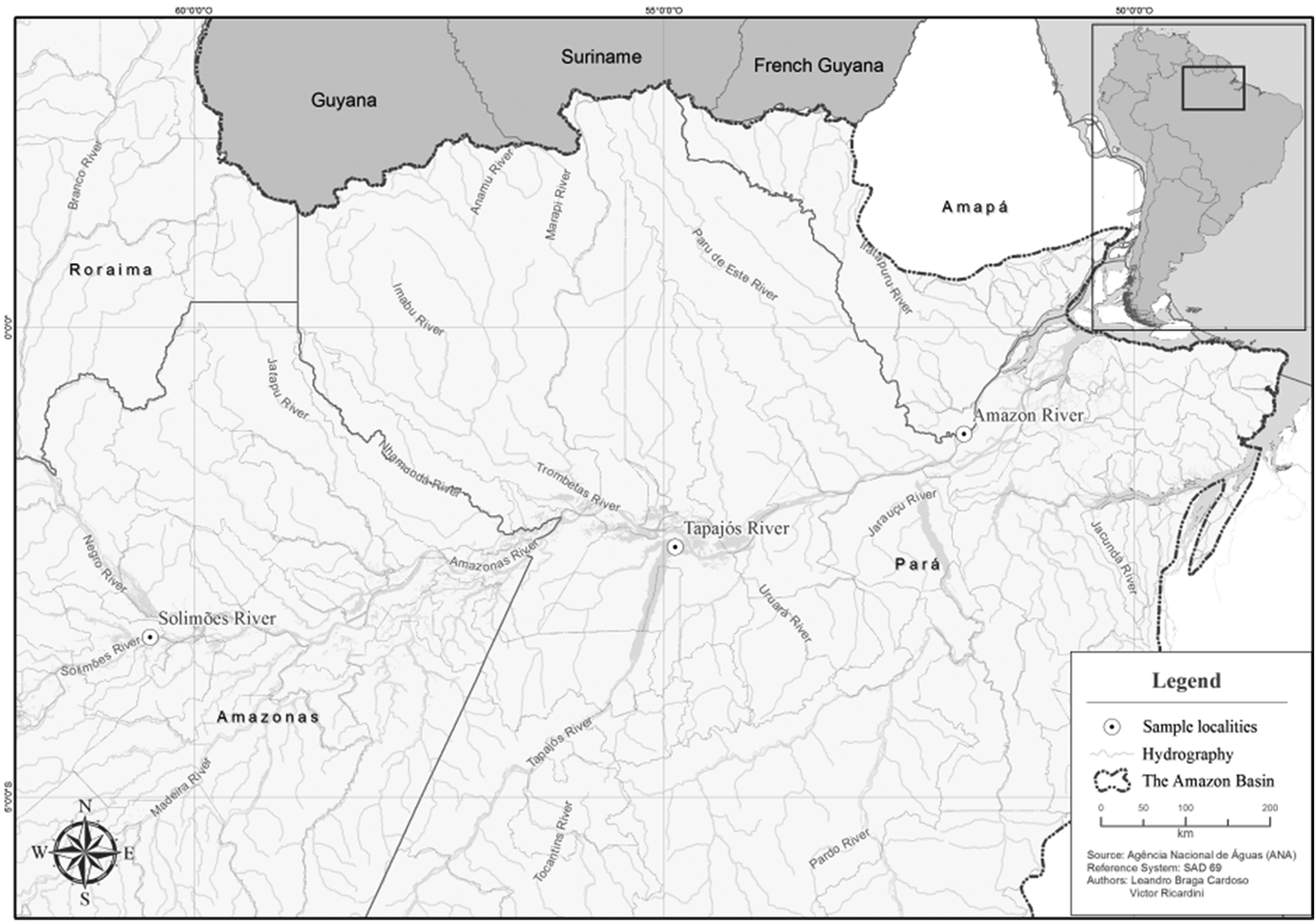
Fig. 1. Map of the myxozoan Ceratomyxa gracillima n. sp. collection localities in the Amazon basin: Solimões River (Amazonas State); Tapajós River (Pará State) and Amazon River (Amapá State).
Molecular studies
DNA was extracted from 50 µL of infected bile preserved in ethanol. The sample was pelleted at 15 700 G for 10 min and the ethanol removed. DNA was extracted from the pellet using a DNeasy® Blood & Tissue Kit (animal tissue protocol) (Qiagen Inc., Redwood City, California, USA).
The ssrDNA was amplified using a semi-nested PCR: first round amplification targeted the entire ssrDNA with universal primers 18E (CTGGTTGATTCTGCCAGT; Hillis and Dixon, Reference Hillis and Dixon1991) and 18R (CTACGCAAACCTTGTTACG; Whipps et al. Reference Whipps, Adlard, Bryant and Kent2003) (Fig. 2). PCR was conducted in 20 µL reaction volumes, comprising: 1 µL template DNA (10–50 ng μL−1), 0·25 µL GoTaq Flexi polymerase (Promega, San Luis Obispo, California, USA), 0·2 µL mm each dNTPs, 0·50 µL each primer (10 pL), 4 µL 5 × GoTaq Flexi clear buffer, 2·4 µL MgCl2 (1·5 mm), 0·50 µL BSA, 0·4 µL Rediload dye (Invitrogen, Carisbad, California, USA) and 10·05 µL ultrapure water. PCR was performed on a PTC-200 Thermocycler (MJ Research Inc., Watertown, Massachusetts, USA) with initial denaturation at 95 °C for 2 min, followed by 35 cycles of 94 °C for 30 s, 62 °C for 30 s (or 58 °C and 60 s with second-round primers) and 72 °C for 120 s, followed by a terminal extension at 72 °C for 7 min. Semi-nested second-round PCR generated overlapping fragments using primers 18E and ACT1R (AATTTCACCTCTCGCTGCCA; Hallett and Diamant, Reference Hallett and Diamant2001) and MXATK2f (ACGCTTGCGAAGYGTGCCTT, Zatti et al. Reference Zatti, Atkinson, Bartholomew, Maia and Adriano2017) with 18R (Fig. 2).

Fig. 2. Schematic representation of part of the ribosomal DNA of Ceratomyxa gracillima n. sp., which shows locations of gene regions and PCR primers.
For the ITS-1 amplification, the first round was performed with the novel primer MYXATK3f (CATTTGAGGGCGTTAGTACTTG) paired with NC13R (GCTGCGTTCTTCATCGAT, Gasser et al. Reference Gasser, Chilton, Hoste and Beveridge1993) followed by a second round with ALL1F (GCGGCTTAATTTGACTCAACACGGG, Hallett et al. Reference Hallett, Atkinson and El-Matbouli2002) and NC13R (Fig. 2). These primers produced fragments that extended from the ssrDNA through the ITS-1 and terminated in the 5·8S. The PCR protocol was the same as above, but with an annealing temperature of 60 °C. All amplicons were electrophoresed in 1·5% agarose gels with Tris-borate-EDTA buffer (0·045 m Tris-borate, 0·001 m EDTA, pH 8·0) stained with SYBRsafe (Invitrogen By Life Technologies, Maryland, USA) alongside a 1 kb Plus DNA Ladder (Invitrogen By Life Technologies, Maryland, USA), then analysed on a Compact Digimage System transilluminator (Major Science, Saratoga, California, USA). Products from PCRs that produced a single bright band were purified with QIAquick PCR Purification Kit (Qiagen) according to the manufacturer's instructions, then sequenced in both directions. Sequencing reactions were performed using a BigDye 102 Terminator v3.1 Cycle Sequencing kit (Applied Biosystems, California, USA) in an ABI 3730 DNA 103 Analyzer (Applied Biosystems) at the Oregon State University Center for Genome Research and Biocomputing.
Sequence assembly, alignment and phylogenetic analyses
Forward and reverse sequences were aligned by eye and assembled into consensus sequences using BioEdit (Hall, Reference Hall1999). Chromatograms were examined for polymorphic loci, indicated by coincident peaks. We used a visual threshold for recording secondary peaks only if they were at least 50% of the height of the primary peak. Consensus sequences were searched using BLASTn (Altschul et al. Reference Altschul, Madden, Schaffer, Zhang, Zhang, Miller and Lipman1997) with the GenBank database to identify the most closely related taxa.
Phylogenetic analyses were performed on an alignment of 81 ssrRNA sequences from related species, which included all available Ceratomyxa spp., P. indecorus and M. bulani, retrieved from the NCBI database (accession numbers are indicated in the phylogenetic tree). Sequences were aligned with ClustalW (Thompson et al. Reference Thompson, Gibson, Plewniak, Jeanmougin and Higgins1997) using default parameters. Gaps were treated as missing data. Phylogenetic trees were calculated using maximum likelihood (ML) and maximum parsimony (MP) methods. The optimum evolutionary model for the dataset was obtained by the Akaike information criterion using jModelTest 0.1.1 (Posada, Reference Posada2008), which identified GTR + I + G as the best-fit model. ML analysis was made using PhyML version 3.0 (Guindon and Gascuel, Reference Guindon and Gascuel2003) implemented via the web server (http://www.atgc-montpellier.fr/phyml/) (Guindon et al. Reference Guindon, Dufayard, Lefort, Anisimova, Hordijk and Gascuel2010). MP was computed in PAUP* v.4.0b10 (Swofford, Reference Swofford2002) employing a heuristic search with ten repetitions of random sequence addition, the ACCTRAN-option, with tree bisection and reconnection branch swapping. Basal myxosporean taxa Chloromyxum auratum (AY971521) and Chloromyxum clavatum (JQ793641) were used as outgroups. Bootstrap support was calculated using 1000 replicates for both methods. Trees were visualized using Figtree 1.3.1 (Rambaut, Reference Rambaut2008) and annotated in Adobe Photoshop (Adobe Systems Inc., California, USA). Sequence divergence among all Ceratomyxa from Amazon were estimated using p-distance and distance (base pairs differences) methods in MEGA 7.0 (Kumar et al. Reference Kumar, Stecher and Tamura2016) using default parameters.
Results
Morphological, molecular and host data supported description of a new Ceratomyxa species. We found the parasite in gall bladders of B. rousseauxii at each of the three sample locations: 82% (14/17) from the Tapajós River, 100% (1/1) from the Amazon River and 50% (6/12) from the Solimões River. The average prevalence was 70% (21/30). Parasite plasmodia were elongated, and exhibited undulatory, worm-like locomotion, swimming freely in the bile.
Morphological description and taxonomic summary
Phylum: Cnidaria Verrill, 1865
Unranked sub-phylum: Myxozoa Grassé, 1970
Class: Myxosporea Bütschli, 1881
Order: Bivalvulida Shulman, 1959
Family: Ceratomyxiidae Doflein, 1899
Genus: Ceratomyxa Thélohan, 1892
Ceratomyxa gracillima n. sp. (Figs 3–5).
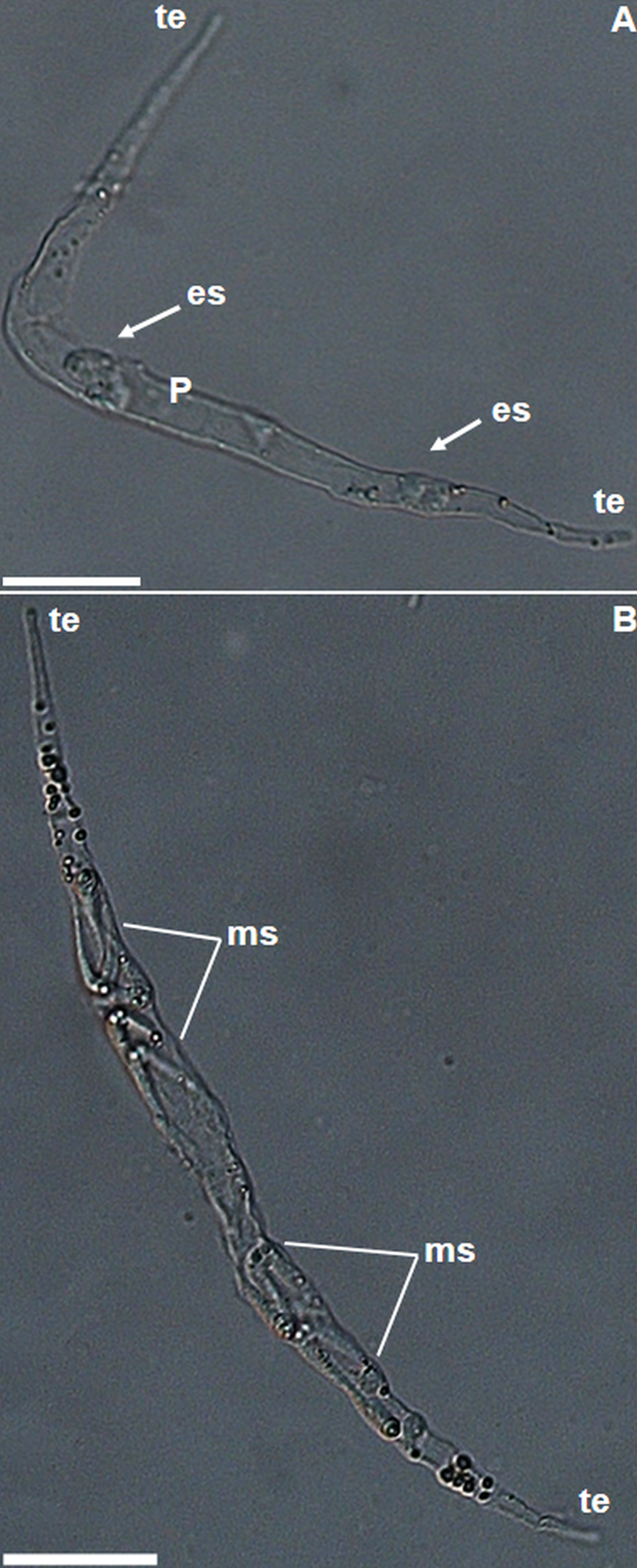
Fig. 3. Light photomicrographs of plasmodia of Ceratomyxa gracillima n. sp. from the gallbladder of Brachyplatystoma rousseauxii. (A) Immature plasmodium. Note the tapered ends (te) at both poles, and early sporogonic stages (es) in the medial region of the plasmodium (P). (B) Mature plasmodium showing mature myxospores (ms). Bars: 20 µm.

Fig. 4. Light photomicrographs of Ceratomyxa gracillima n. sp. A and B: mature myxospores. Bars: 10 µm.

Fig. 5. Schematic drawing of Ceratomyxa gracillima n. sp. showing details of the principal measured components. SL, spore length; ST, spore thickness; VL, valves length; PA, posterior angle. Bar: 10 µm.
Vegetative stages
Motile, elongated plasmodia at different stages of development were found swimming freely in the bile of B. rousseauxii. Immature plasmodia contained few sporogonic stages in medial regions and no mature myxospores (Fig. 3A), and showed an average length of 112 µm (range 72–134 µm, N = 7); and average width of 8·9 (range 4·6–11·8 µm, N = 7). Mature plasmodia contained mature myxospores in their medial portion (Fig. 3B). Average plasmodia length 181 µm (range 173–246 µm, N = 10) and average width 11·4 µm (range 7·2–16·2 µm, N = 10).
Mature myxospores
Spores were strongly arcuate in side view, average total length 28 µm (range 23·3–35·3 µm, N = 33); average spore length of 4·4 µm (range 3·0–5·7 µm, N = 33); average spore thickness 7·0 µm (range 6·0–8·2 µm, N = 33). Two spherical, equal-sized polar capsules, diameter 1·9 µm (range 1·5–2·5 µm, N = 30), located anteriorly, adjacent to the straight suture. Each polar capsule containing a polar tubule with 2–3 turns around the longitudinal axis. Posterior angle averaged 36·6° (range 35°–40°, N = 10) (Figs 4A, B and 5).
Type host: Brachyplatystoma rousseauxii (Siluriformes: Pimelodidae)
Prevalence across the three sites: 21 of 30 (70%).
Type locality: Tapajós River, municipality of Santarém, Pará State, Brazil, (02°20′28″S, 54°52′56″W). Other localities: Amazon River, municipality of Vitoria do Jari, Amapá State (01°08′17″S, 51°48′31″W) and Solimões River, municipality of Manacapuru, Amazonas State (03°18′08″S, 60°28′00″W).
Site of infection: Lumen of gall bladder.
Type material: A glass slide with fixed, stained myxospores (syntype) was deposited in the Museum of Zoology ‘Adão José Cardoso’ University of Campinas (UNICAMP), São Paulo, Brazil (Zuec MYX 65). Concatenated ssrDNA, ITS-1 and partial 5·8S sequences were deposited in the NCBI GenBank database (accession numbers: KY934182 from the Tapajós River, KY934183 from the Solimões River and KY934184 from the Amazon River).
Etymology: The specific name gracillima (in English, gracile = slender, thin, slim) refers to the features of the plasmodia.
Molecular and phylogenetic analyses
We obtained partial rDNA sequences (18S-ITS-1-5.8S) for C. gracillima n. sp. from each of the three localities (1839 bp from the Tapajós River, 1855 bp from the Solimões River and 1849 bp from the Amazon River). Apart from differences in length, the sequences were identical. The BLASTn search revealed no other sequence with more than 94% similarity to C. gracillima n. sp. The ITS-1 region began approximately 1635 bp from the start of primer 18E and was about 165 bp long. At four positions – 1725, 1747, 1763 and 1809 bp – we observed ~50/50 mixtures of alleles as single nucleotide polymorphisms (SNPs) at the same positions in the C. gracillima n. sp. sequences from the three rivers.
Phylogenetic analysis revealed two main clades, A and B (Fig. 6). The large clade A contains a subclade A1 of exclusively Ceratomyxa marine species, plus two small marine subclades. Subclade A2 comprised Ceratomyxa sp. (DQ377699), Ceratomyxa synaphobranchi (Fiala, Hlavnickova, Kodadkova, Freeman, Bartošova-Sojkova and Atkinson, Reference Fiala, Hlavnicková, Kodádková, Freeman, Bartosová-Sojková and Atkinson2015), and P. indecorus, all of which are parasites of deep water marine fish. Subclade A3 comprised parasites of sharks and cod.

Fig. 6. Consensus maximum likelihood phylogenetic tree based on ssrDNA sequences of Ceratomyxa gracillima n. sp., other Ceratomyxa spp. plus Palliatus indecorus and Myxodavisia bulani. Nodal supports are indicated for maximum likelihood (ML) and maximum parsimony (MP), respectively, with a bootstrap of 1000 replicates. Branches with bootstrap ⩽70% support are blank. GenBank accession numbers are shown after taxon names. Clade A: Marine Ceratomyxa spp., including P. indecorus. Clade B: Amazonian Ceratomyxa spp. plus C. leatherjacketi, C. tunisiensis and M. bulani.
Ceratomyxa gracillima n. sp. clustered in the smaller clade B with C. vermiformis, C. amazonensis and C. brasiliensis to form a subclade of species with exclusively freshwater Amazonian fish hosts. This subclade was sister to Ceratomyxa leatherjacketi (Fiala, Hlavnickova, Kodadkova, Freeman, Bartošova-Sojkova and Atkinson, Reference Fiala, Hlavnicková, Kodádková, Freeman, Bartosová-Sojková and Atkinson2015) and Ceratomyxa tunisiensis (Thabet, Mansoura, Omar and Zouaria, 2015). Together with M. bulani these isolates collectively formed an early-diverging clade within the marine myxosporean lineage (Fig. 6).
Discussion
We found a novel Ceratomyxa species, C. gracillima n. sp. in the bile of an economically important Amazonian catfish, B. rousseauxii. The parasite developed in the gall bladder and the myxospores are typical of Ceratomyxa spp., apart from being arcuate in frontal view and having extended valve cells characteristic of Meglitschia. There are, however, limited morphological characters to distinguish Meglitschia from Ceratomyxa, and a distinction of the two genera is not supported by molecular data (Zhao et al. Reference Zhao, Zhou, Kent and Whipps2008; Adriano and Okamura, Reference Adriano and Okamura2017). Accordingly, we compared the morphology of C. gracillima n. sp. primarily with other freshwater Ceratomyxa spp. and then, with other congeners from marine/brackish hosts, guided by the species checklist of Eiras (Reference Eiras2006) and other recent taxonomic studies (Gunter et al. Reference Gunter, Whipps and Adlard2009; Azevedo et al. Reference Azevedo, Ribeiro, Clemente, Casal, Lopes, Matos, Al-Quraishy and Matos2011; Azevedo et al. Reference Azevedo, Rocha, Casal, São Clemente, Matos, Al-Quraishy and Matos2013; Fiala et al. Reference Fiala, Hlavnicková, Kodádková, Freeman, Bartosová-Sojková and Atkinson2015; Mathews et al. Reference Mathews, Naldoni, Maia and Adriano2016; Adriano and Okamura, Reference Adriano and Okamura2017; Zatti et al. Reference Zatti, Atkinson, Bartholomew, Maia and Adriano2017).
The strongly arcuate myxospores of C. gracillima n. sp. resemble those of both C. vermiformis, a parasite of Colossoma macropomum, and C. mylei, a parasite of Myleus rubripinnis. Both of these hosts are serrasalmid fishes from the Amazon basin. Ceratomyxa gracillima n. sp. myxospores are longer, narrower and have smaller polar capsules with fewer turns of the polar tubules than those of C. vermiformis and C. mylei (Table 1). The elongate plasmodia of C. vermiformis have blunt ends and a gradient in maturation of developmental stages demonstrates that one of these blunt ends (the anterior end) serves as a centre of growth (a growth pole) (Adriano and Okamura, Reference Adriano and Okamura2017). The elongate plasmodia of C. gracillima n. sp. have thin, tapering ends (Fig. 3A, B), and we did not observe a distinct growth pole in this species (although early developmental stages were present in medial regions). Another Amazonian myxozoan, C. brasiliensis, has elongated plasmodia but these were not observed to be motile (Zatti et al. Reference Zatti, Atkinson, Bartholomew, Maia and Adriano2017). Ceratomyxa gracillima n. sp. was distinct from most Ceratomyxa species on the basis of its strongly arcuate spores and elongated valve cells. The myxospores of other ceratomyxids with arcuate spores (C. insolita, C. inconstans Jameson, 1929, and C. sphairophora Davis, 1917) differ in valve lengths and spore thickness relative to C. gracillima n. sp., C. vermiformis and C. mylei myxospores (Meglitsch, Reference Meglitsch1960; Eiras, Reference Eiras2006; Azevedo et al. Reference Azevedo, Ribeiro, Clemente, Casal, Lopes, Matos, Al-Quraishy and Matos2011; Adriano and Okamura, Reference Adriano and Okamura2017).
Table 1. Comparison of morphometry of Ceratomyxa gracillima n. sp. with Amazonian Ceratomyxa spp. with arcuate myxospores
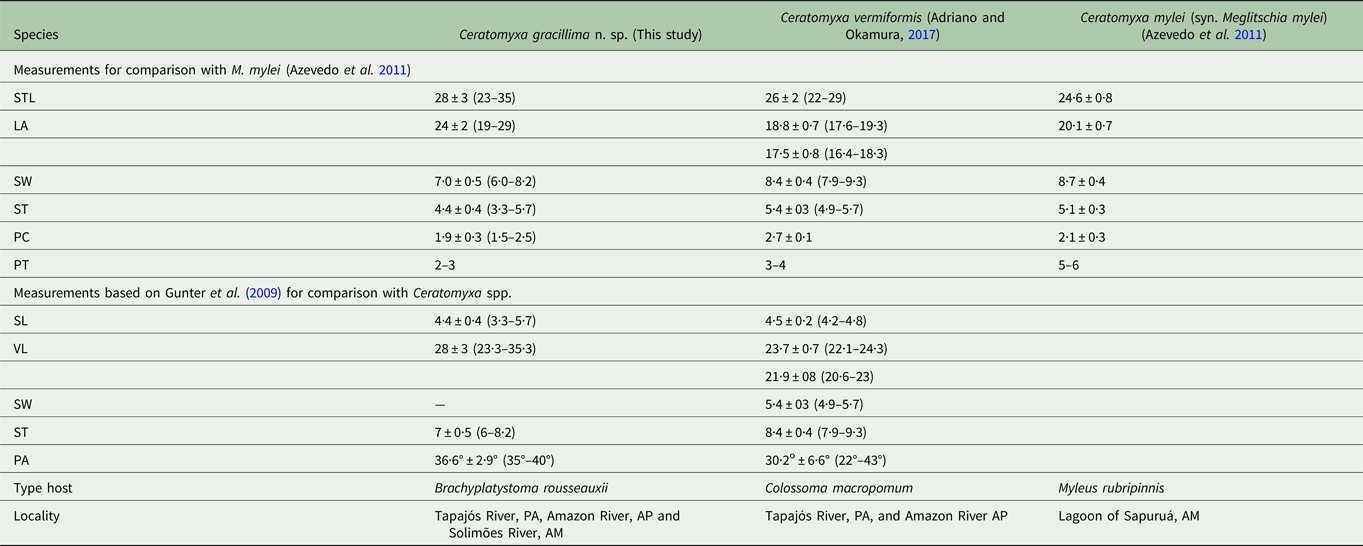
Dimensions are given in micrometres expressed as the mean ± standard deviation followed by the range in parentheses. STL, spore total length; LA, length of appendices; SW, spore width; ST, spore thickness; PC, polar capsule diameter; PT, polar tubule turns; SL, spore length; VL, valve length; PA, posterior angle; PA, Pará State; AP, Amapá State; AM, Amazonas State.
The ssrDNA sequence data that are available clearly distinguish C. gracillima n. sp. from its congeners. Thus, it was at least 5·2% different from its nearest relative, C. vermiformis (Table 2), but we note that there are no molecular data for the Amazonian species C. mylei for comparison. Levels of interspecific genetic variation amongst myxosporeans vary (Ferguson et al. Reference Ferguson, Atkinson, Whipps and Kent2008; Carriero et al. Reference Carriero, Adriano, Silva, Ceccarelli and Maia2013), and Ceratomyxa species have been shown to differ by 2% to more than 20% (Gunter et al. Reference Gunter, Whipps and Adlard2009). The interspecific differences between available ssrDNA sequences for Amazonian Ceratomyxa spp. range from 2·4% (C. brasiliensis vs C. amazonensis) to 6·9% (C. gracillima n. sp. vs C. amazonensis) (see Table 2).
Table 2. Pairwise identities of ssrDNA sequences from Ceratomyxa species described from Amazon basin fish hosts, adjusted for missing data. The upper triangular matrix shows the number of nucleotide differences; the lower triangular matrix shows the percentage nucleotide difference

Despite the lack of ssrDNA sequence data for one of the Amazonian ceratomyxids (C. mylei), we consider that the considerable morphological differences in plasmodia and spores described above and the exploitation of the different fish hosts provide evidence consistent with species-level differences and hence the designation of C. gracillima as a new species.
As ssrDNA is typically conserved within isolates of the same species, the neighbouring, more variable ITS-1 was explored to identify intraspecific differences (strains/genotypes) (Whipps and Kent, Reference Whipps and Kent2006; Atkinson and Bartholomew, Reference Atkinson and Bartholomew2010a, Reference Atkinson and Bartholomewb). Accordingly, we identified a series of polymorphic loci (SNPs) in the ITS-1 which were evident as two mixed peaks at the same four locations in all sequence chromatograms. The absence of variability in the sequences in this genome region was surprising and is evidence that the parasite population is genetically uniform for this highly variable region despite wide geographic separation of the collection localities. In a straight line, the Solimões River site is ~660 km from the Tapajós River site and around 1000 km from the Amazon River site, while the distance between Tapajós and the Amazon sites is ~370 km. The similarity of ITS-1 sequences amongst parasites collected from such widely separated localities leads us to hypothesize that this uniformity is evidence of high gene flow across broad spatial scales, and is evidence of panmixia. We consider that this mixing is a result of the fish host's exceptionally long-distance migration from tributaries in the foothills of the Andes to the coastal estuary of Brazil, and eventually back again (Barthem and Goulding, Reference Barthem and Goulding1997; Barthem et al. Reference Barthem, Goulding, Leite, Cañas, Forsberg, Venticinque, Petry, Ribeiro, Chuctaya and Mercado2017). Pathogens and parasites are potentially carried with fish along this migration route and their populations may therefore be mixed over great distances.
The addition of C. gracillima n. sp. to ML and MP molecular phylogenetic analyses of Ceratomyxa species provides further support for a distinct Amazonian Ceratomyxa lineage (Mathews et al. Reference Mathews, Naldoni, Maia and Adriano2016; Adriano and Okamura, Reference Adriano and Okamura2017; Zatti et al. Reference Zatti, Atkinson, Bartholomew, Maia and Adriano2017). Our analysis revealed C. gracillima n. sp. as sister to the morphologically similar C. vermiformis and both are placed in a subclade that also contains C. brasiliensis and C. amazonensis. Those species, together with C. tunisiensis, C. leatherjacketi and M. bulani, in turn form a well-supported and apparently early-diverging ceratomyxid clade (clade B; Fig. 6). This apparent early-diverging position for the Amazonian freshwater lineage relative to the larger, fully marine Ceratomyxa clade may be a genetic signal of historical marine incursions into the Amazon basin (Zatti et al. Reference Zatti, Atkinson, Bartholomew, Maia and Adriano2017).
Although the Amazonian Ceratomyxa spp. studied so far have been reported only in hosts belonging to evolutionary lineages of freshwaters species (i.e. Serrasalmidae, Cichlidae and Pimelodidae) (Nelson et al. Reference Nelson, Grande and Wilson2016; Bloom and Lovejoy, Reference Bloom and Lovejoy2017), several vertebrate and invertebrate freshwater organisms from the Amazon region are closely related to extant marine lineages, including stingrays, croakers, sponges, mollusks, parasitic monogenoids (Boeger and Kritsky, Reference Boeger and Kritsky2003; Lovejoy et al. Reference Lovejoy, Albert and Crampton2006; Cooke et al. Reference Cooke, Chao and Beheregary2011; Bloom and Lovejoy, Reference Bloom and Lovejoy2017). Several hypotheses have been proposed to explain the origin of these marine-derived lineages, including opportunistic invasions via estuaries, vicariance events and marine incursions (Lovejoy et al. Reference Lovejoy, Albert and Crampton2006; Hoorn et al. Reference Hoorn, Wesselingh, Steege, Bermudez, Mora, Sevink, Sanmartin, Sanchez-Meseguer, Anderson, Figueiredo, Jaramillo, Riff, Negri, Hooghiemstra, Lundberg, Stadler, Sarkinen and Antonelli2010; Bloom and Lovejoy, Reference Bloom and Lovejoy2017). There is evidence, based on fossil records, geomorphological history, distributional data and phylogenetic results, that marine incursions into South America occurred between late Oligocene and early Miocene were involved in the adaptation of marine fish to the freshwater environment in the Amazon (Cooke et al. Reference Cooke, Chao and Beheregary2011; Bloom and Lovejoy, Reference Bloom and Lovejoy2017). Apart from South America, the occurrence of Ceratomyxa spp. in hosts inhabiting exclusively freshwater (or estuarine) environments is exceptional and restricted to Ceratomyxa hongtzensis Hsieh and Chen, 1984, Ceratomyxa anguillae Tuzet and Ormières,1957 and Ceratomyxa hungarica Molnár, 1992 (Eiras, Reference Eiras2006; Froese and Pauly, Reference Froese and Pauly2013). In South America, there is still no record of Ceratomyxa species infecting freshwater fish outside the Amazon basin (Eiras, Reference Eiras2006; Adriano and Oliveira, Reference Adriano and Oliveira2017). In this context, the contrast between the uncommon presence of Ceratomyxa spp. infecting freshwater hosts from other continents and watersheds and the recent finding of five species related to marine taxa parasitizing Amazonian freshwater fish (Azevedo et al. Reference Azevedo, Ribeiro, Clemente, Casal, Lopes, Matos, Al-Quraishy and Matos2011, Reference Azevedo, Rocha, Casal, São Clemente, Matos, Al-Quraishy and Matos2013; Mathews et al. Reference Mathews, Naldoni, Maia and Adriano2016; Adriano and Okamura, Reference Adriano and Okamura2017; Zatti et al. Reference Zatti, Atkinson, Bartholomew, Maia and Adriano2017) suggest that this freshwater diversity may have arisen from ancestral marine ceratomyxids that invaded the Amazon during marine incursions in the late Oligocene and early Miocene, and subsequently adapted to freshwater environments. Subsequent adoption of freshwater hosts possibly leads to the radiation of Amazonian ceratomyxids shown by phylogenetic analyses. If this hypothesis is valid, then dating of the transition of ancestral Ceratomyxa species, from marine to freshwater, could provide an indication of the rate of molecular evolution for myxozoans, which is potentially valuable and unique in the understanding of the evolution of these enigmatic parasites.
Acknowledgements
The authors thank Dr Lincoln Corrêa for the help with fieldwork; the fishermen of the community of Jari do Socorro, Pará State; Jari, Amapá State and Manacapuru, Amazônia State for the provision of the fish, and Prof Dr Regina Maura Bueno Franco from University of Campinas for providing some equipment used in this study. We also thank the anonymous reviewers for their comments that helped to improve this paper.
Financial support
Part of this research was conducted while SAZ was a visiting scholar at Oregon State University, USA, which was funded by the CAPES within the Ministry of Education, Brazil (grant n. BEX/6729/2015-00). SAZ was also supported by a Ph.D. scholarship provided by CAPES to UNICAMP. This study was also supported by the São Paulo Research Foundation – FAPESP (Procs. Nos. 2013/21374-6 and 2016/22047-7) to EAA. EAA received a research productivity grant from the Brazilian Fostering Agency CNPq (Proc. No. 301886/2016-4).











