INTRODUCTION
Trematodes of the family Cyclocoelidae Stossich, 1902 parasitize the nasal cavity, hypothalamus, orbit, oesophagus, trachea, air sacs, intestine, liver, kidneys and abdominal cavity of birds feeding on mollusks. Feizullaev (Reference Feizullaev1980) indicated that reports of Cyclocoelum spp. from the trachea and thoracic cavity should be considered as records of worms located at the boundary of trachea with bronchi. Two specimens of Cyclocoelum obscurum (Leidy, 1887) were reported from human lungs in a delta of the Volga river, Russia (Kurockin in Feizullaev, Reference Feizullaev1980), which suggests some limited zoonotic potential of cyclocoelids. For all central European members of this family, wetland-associated mollusks serve as intermediate hosts, and only Morishitium polonicum (Machalska, 1980) uses the xerothermic snails of the genus Helicella based on experimental infection data (Timon-David, Reference Timon-David1955, Reference Timon-David1957). Although family Cyclocoelidae was erected over a century ago, the status of many cyclocoelid species remains controversial or uncertain. Numerous keys to this family have previously been published (Kossack, Reference Kossack1911; Witenberg, Reference Witenberg1923, Reference Witenberg1926; Bashkirova, Reference Bashkirova1950; Dubois, Reference Dubois1959; Feizullaev, Reference Feizullaev1980; Kanev et al. Reference Kanev, Radev, Fried, Gibson, Jones and Bray2002; Dronen, Reference Dronen2007; Dronen and Tkach, Reference Dronen and Tkach2014; Dronen and Blend, Reference Dronen and Blend2015). Yamaguti (Reference Yamaguti1971) further divided this family into three subfamilies. According to Yamaguti, all of the genera analysed in this study, namely Cyclocoelum Brandes, 1892, Harrahium Witenberg, 1926, Hyptiasmus Kossack, 1911, Morishitium Witenberg, 1928, and Uvitellina Witenberg, 1923, belonged to the subfamily Cyclocoelinae Stossich, 1902, as characterized by an absent external seminal vesicle, funnel-shaped buccal cavity, simple (not diverticulate) caeca and cercariae encysted within the rediae in which they developed. Subsequently, Kanev et al. (Reference Kanev, Radev, Fried, Gibson, Jones and Bray2002) changed the classification of subfamilies within the Cyclocoelidae and used the position of the ovary relative to the testes as a major diagnostic character. The localization of the ovary between the testes was associated with Cyclocoelinae, whereas the pretesticular localization of the ovary characterized Haematotrephinae Dollfus, 1948 (which, among others, contains the genera Harrahium and Uvitellina analysed in this study) and the posttesticular localization of the ovary characterized Ophthalmophaginae Harrah, 1922. More recently, Dronen (Reference Dronen2007) changed the diagnostic characters of particular subfamilies. In his view, Cyclocoelinae possessed an intertesticular ovary, forming a triangle with the testes, whereas Haematotrephinae possessed the ovary ranging from pretesticular to opposite the anterior testis forming a triangle with the testes. Considering Haematotrephinae, the genus Harrahium was distinguished by a postpharyngeal position of the genital pore and vitelline fields that are not posteriorly confluent. A postpharyngeal genital pore and posteriorly confluent vitelline fields distinguish the genus Uvitellina. Dronen (Reference Dronen2007) also proposed the validity of three additional subfamilies not mentioned above. Among these subfamilies, Hyptiasminae Dollfus, 1948 (which, among others, contains the genera Hyptiasmus and Morishitium analysed in this study) was characterized by an intertesticular ovary and tandem to nearly tandem testes. The genus Hyptiasmus was characterized by a prepharyngeal genital pore and posteriorly confluent vitelline fields. The genus Morishitium was distinguished by a postpharyngeal genital pore and posteriorly non-contiguous vitelline fields (Dronen, Reference Dronen2007; Dronen and Blend, Reference Dronen and Blend2015). This classification system was retained in the most recent revision of the Cyclocoelidae by Dronen and Blend (Reference Dronen and Blend2015), and only Haematotrephus tringae (Brandes, 1892) was reclassified to the genus Harrahium Witenberg, 1926 within the same subfamily.
Surprisingly, only negligible phylogenetic data are available for cyclocoelids. Littlewood and Bray (Reference Littlewood and Bray2001) published the 18S rDNA sequence of Cyclocoelum mutabile (Zeder, 1800). Several 28S rDNA sequences are also available. These sequences include the 28S rDNA of Cyc. mutabile (Olson et al. Reference Olson, Cribb, Tkach, Bray and Littlewood2003), six isolates of Circumvitellatrema momota Dronen, Greiner, Ialeggio & Nolan, 2009 (Libert et al. Reference Libert, Jouet, Ferte, Lemberger and Keck2012) and a specimen of Typhlocoelum Stossich, 1902, which has not been identified to species (Tkach et al. Reference Tkach, Kudlai and Kostadinova2016). The 5·8S rDNA and ITS2 were analysed in four specimens of Cir. momota (Libert et al. Reference Libert, Jouet, Ferte, Lemberger and Keck2012). However, there are no molecular data verifying the taxonomic position of species and genera within the Cyclocoelidae.
In Central Europe, nine species within the Cyclocoelidae have been reported, including the type species of the genus Cyclocoelum, Cyc. mutabile (Zeder, 1800) (Vojtěchovská-Mayerová, Reference Vojtěchovská-Mayerová1952), Cyc. obscurum (Leidy, 1887) (Sitko et al. Reference Sitko, Faltýnková and Scholz2006), Haematotrephus lanceolatum (Wedl, 1858) (Macko, Reference Macko1960; so far, the species has not been found in the Czech Republic), Harrahium tringae (Brandes, 1892) (Sitko, Reference Sitko1969), Hyptiasmus arcuatus (Brandes, 1892 of Stossich, 1902) (Buša, Reference Buša1960; absent in the Czech Republic), Hyptiasmus oculeus Kossack, 1911 (Sitko et al. Reference Sitko, Faltýnková and Scholz2006), M. polonicum [Sitko et al. Reference Sitko, Faltýnková and Scholz2006, reported as Morishitium elongatum (Harrah, 1921)], Skrjabinocoelum petrowi Kurashvili, 1953 (Macko et al. Reference Macko, Špakulová, Vasilková and Oros2011; absent in the Czech Republic) and Uvitellina vanelli (Rudolphi, 1819) (Sitko et al. Reference Sitko, Faltýnková and Scholz2006).
In this study, we employed combined molecular and comparative morphological analysis to address the phylogenetics of central European Cyclocoelidae. Particularly, we examined whether there are any differences between Cyclocoelum and Hyptiasmus, and Cyclocoelum and Harrahium. The species of Cyclocoelum and Hyptiasmus share similar morphologies and overlapping spectra of the intermediate hosts (Sitko et al. Reference Sitko, Faltýnková and Scholz2006; Gibson et al. Reference Gibson, Bray and Harris2016). The life cycles of Harrahium are incompletely understood, but spectra of definitive hosts overlap with those of Cyclocoelum (Sitko et al. Reference Sitko, Faltýnková and Scholz2006; Gibson et al. Reference Gibson, Bray and Harris2016). In contrast to the three above-mentioned genera, the genus Morishitium differs according to its life cycle, which involves xerothermophilous snails of the genus Helicella (Sitko et al. Reference Sitko, Faltýnková and Scholz2006; Gibson et al. Reference Gibson, Bray and Harris2016). In this study, we also attempt overcoming the problems associated with the comparative analyses of the morphology of the Cyclocoelidae present in museum collections, which have been processed by multiple independent researchers, as only a single person acquired, fixed and measured the extensive collection of specimens analysed in this study. The members of Cyclocoelidae characteristically respond to the pressure applied during the preparation of slides by pronounced changes of their body shapes, body measures and organ locations, which are particularly important when comparing the data from different regions and different decades (data not shown). We conducted the first conclusive phylogenetic analyses of the taxonomic position of central European Cyclocoelidae based on two nuclear (ITS2, 18S rDNA) and two mitochondrial (CO1, ND1) DNA loci. We provided comparative measurements of the examined central European Cyclocoelidae, and addressed their host-specific prevalence and intensity of infection based on an extensive cohort of birds examined from 1962 to 2016.
MATERIAL AND METHODS
Sampling
We examined over 17 000 individuals of 240 bird species for the prevalence assessment of the cyclocoelids from 1962 to 2016 (det. & coll. J. Sitko). All examined birds were collected in the Czech Republic (48°39′N–50°59′N, 12°19′E–18°29′E), primarily in the eastern parts of the country. Note that this may be considered as a limitation of this study as species, which complete their life cycle in brackish waters and in Pannonian part of the Central Europe, are underrepresented in our samples. The list of the sampling sites positive for each respective helminth species is provided in Table S1. All helminths were obtained from birds submitted for deposition in the Comenius Museum in Přerov, Czech Republic. All birds were already dead from various causes when received by the museum. All the bird carcasses were processed immediately when received or were frozen down and examined and fixed within next 2 months.
For the phylogenetic analyses, we examined representative specimens of the Cyclocoelidae collected in the Czech Republic in the vicinity of Mikulov 48·78°N, 16·67°E, Tovačov 49·42°N, 17·30°E and Záhlinice 49·17°N, 17·28°E from Sep-2011 to Mar-2016, and fixed and stored in 96% ethanol for further analyses. The list of individuals examined is provided in Table 1.
Table 1. New sequences of members of Cyclocoelidae, collected from the Czech Republic, generated throughout the course of the present study. NCBI GenBank accession numbers are indicated
For the comparative morphological analysis, we stained representative specimens using Semichon's carmine, followed by dehydration through an alcohol series, and mounting in Canada balsam. For the analyses of egg length, we measured the longest egg present within each examined adult individual. The dimensions are shown as a range, indicated in μm (mean ± s.d.). All other data are shown as the means ± s.d. unless otherwise stated.
DNA extraction, amplification and sequencing
We washed the cyclocoelids stored in 96% ethanol twice for 15 min using 1 mL of buffer containing 10 mm Tris–HCl (pH 7·5) and 5 mm EDTA. Subsequently, we extracted the DNA using a NucleoSpin Tissue XS kit (Macherey Nagel, Düren, Germany) according to the manufacturer's instructions. We next amplified the DNA using the following polymerase chain reaction (PCR) mix: 10 mm Tris–HCl (pH 8·8), 50 mm KCl, 1·5 mm MgCl2, 0·1% Triton X-100, 0·2 mm dNTP (each), 1 µ m 5′ primer, 1 µ m 3′ primer, 0·5 U of Taq DNA polymerase (Top-Bio, Prague, Czech Republic) and 300 ng of extracted genomic DNA. The total reaction volume was 25 µL. The primers used targeted nuclear 18S rDNA and ITS2 loci, and mitochondrial CO1 and ND1 loci (Table 2). We performed the PCR using an Eppendorf Mastercycler Pro thermal cycler (Eppendorf, Hamburg, Germany) for 36 cycles with 15 s denaturation at 94 °C, 2-min annealing at 53–57 °C, followed by a 1–3-min extension at 72 °C. Cycling was initiated with a 2-min denaturation at 94 °C and terminated after a 5-min incubation at 72 °C. We subsequently purified the amplified DNA using USB Exo-SAP-IT (Affymetrix, Santa Clara, CA) and subjected it to bidirectional Sanger sequencing using an ABI 3130 DNA Analyser (Applied Biosystems, Foster City, CA). The resulting consensus DNA sequences were submitted to GenBank under accession numbers KU877883–KU877912 and KX097822–KX097823 (Table 1).
Table 2. Primers used for the amplification and sequencing of mitochondrial and nuclear DNA loci in Cyclocoelidae

Alignments and phylogenetic analyses
Newly generated sequences, sequences obtained from NCBI GenBank as of 5-Mar-2016, and sequences of corresponding outgroups were aligned using ClustalW (gap opening penalty 7, gap extension penalty 2 for both pairwise and multiple alignments, DNA weight matrix IUB, transition weight 0·1). We manually corrected the alignments for any inconsistencies, trimmed the aligned sequences, and removed short-length sequences from the alignments; only trimmed sequences were utilized for further analyses. The trimmed ITS2 locus included partial 5·8S rDNA and partial (close to full length) ITS2 sequence, and it corresponded to nt. 3–565 (563 bp) of Cir. momota JQ886068 (Table S2). The trimmed 18S rDNA locus (partial LSU rRNA coding sequence) corresponded to nt. 142–726 (585 bp) of Cyc. mutabile AJ287494 (Table S3; species identification of this specimen was likely incorrect, cf. the analyses below). The trimmed CO1 locus (partial CO1 coding sequence) corresponded to nt. 49–351 (303 bp) of Philophthalmus gralli Mathis & Leger, 1910 JQ675731 (Table S4). The trimmed ND1 locus (partial ND1 coding sequence) corresponded to nt. 1–435 (435 bp) of Parafasciolopsis fasciolaemorpha Ejsmont, 1932 EF612500 (Table S5).
Maximum-likelihood fits of the 24 nucleotide substitution models were performed as described (Řezáč et al. Reference Řezáč, Gasparo, Král and Heneberg2014), with all sites used for the analyses, including gaps. For each model, we calculated the Bayesian information criterion, corrected Akaike information criterion and maximum-likelihood values. For the ITS2 locus, we analysed ten sequences with a total of 593 positions in the final dataset (Table S6). For the 18S rDNA locus, we analysed eight sequences with a total of 587 positions in the final dataset (Table S7). For the CO1 locus, we analysed nine sequences with a total of 306 positions in the final dataset (Table S8). For the ND1 locus, we analysed 11 sequences with a total of 435 positions in the final dataset (Table S9). We used best-fit models for the follow-up phylogenetic analyses. We employed a bootstrap procedure at 1000 replicates. We used the nearest-neighbour interchange as the maximum-likelihood heuristic method of choice to determine the tree inference when the initial tree was formed using a neighbour joining algorithm.
We used the maximum-likelihood method to estimate inter- and intrasite evolutionary divergence in the Cyclocoelidae. We calculated the number of base differences per site by averaging over all sequence pairs between groups (distance) ± s.e. and employed a bootstrap procedure at 1000 replicates. The models used to estimate inter- and intrasite evolutionary divergence based on the 18S rDNA and CO1 loci were identical to those used to construct the respective tree. However, to analyse the ITS2, we employed the Kimura 2-parameter model (Kimura, Reference Kimura1980) with the non-uniformity of evolutionary rates among sites modelled using a discrete Gamma distribution (+G) with five rate categories. To analyse the ND1 locus, we employed the Tamura–Nei model (Tamura and Nei, Reference Tamura and Nei1993) with the non-uniformity of evolutionary rates among sites modelled using a discrete Gamma distribution (+G) with five rate categories. The latter two models were selected based on the maximum-likelihood method as a replacement of the Hasegawa–Kishino–Yano model, which does not facilitate the calculation of inter- and intrasite evolutionary divergence based on the program used (Tamura et al. Reference Tamura, Peterson, Peterson, Stecher, Nei and Kumar2011).
To corroborate the data obtained from the maximum-likelihood analyses, we employed Bayesian inference. To infer the tree topologies using a Bayesian approach, we converted the ClustalW alignments generated in MEGA5 to the Nexus format in Mesquite 3·04, manually adjusted the accessory information, and performed the Bayesian analysis in MrBayes 3·2·5 using the mixed model of nucleotide substitution. The Bayesian analysis included four Monte Carlo Markov chains for 10 000 000 generations and trees sampled every 1000th generation, with the average standard deviation (s.d.) of split frequencies not exceeding 0·01. We discarded the first 25% of samples as burn-in. After discarding the burn-in samples, we used the remaining data to generate a 50% majority-consensus tree with the posterior probabilities of branches indicated. We visualized the resulting trees in FigTree 1·4·2. We obtained the following summary statistics for analyses performed: average s.d. of split frequencies 0·0009–0·0023, maximum s.d. of split frequencies 0·0029–0·0072, average potential scale reduction factor 1·000–1·003, and maximum potential scale reduction factor 1·000–1·007.
RESULTS
Central European Cyclocoelidae
During the extensive long-term examination of Czech birds, we confirmed the presence of seven cyclocoelids, namely Cyc. mutabile, Cyc. obscurum, Har. tringae, Hyp. arcuatus, Hyp. oculeus, M. polonicum and U. vanelli. Due to a strong decrease in the abundance of northern lapwing Vanellus vanellus, we did not collect any U. vanelli specimens during the recent decade, and thus this species was excluded from the phylogenetic analyses. Similarly, we identified Hyp. arcuatus in only a single host individual decades ago; thus, this species was also excluded from the phylogenetic analyses. The status of all other species was both morphologically and genetically examined. The species used for phylogenetic analyses were identified based on a morphological examination in their core hosts, i.e., in song thrush Turdus philomelos and common blackbird Turdus merula (M. polonicum), Eurasian coot Fulica atra (Cyc. mutabile and Hyp. oculeus), spotted redshank Tringa erythropus (Cyc. obscurum and Har. tringae) and common greenshank Tringa nebularia (Cyc. obscurum).
Phylogenetic analyses of specimens isolated from birds
Maximum-likelihood analysis of nuclear (ITS2 and 18S rDNA) and mitochondrial (CO1 and ND1) DNA loci revealed that all of the analysed species represent distinct taxa supported well according to the molecular evidence; with all of the four DNA loci tested being species specific (Fig. 1). The analyses also included the only previously available two sequences of the loci tested, namely, 18S rDNA of an unknown cyclocoelid from Calidris canutus (Littlewood and Bray, Reference Littlewood and Bray2001) and ITS2 of Cir. momota from Momotus momota (Libert et al. Reference Libert, Jouet, Ferte, Lemberger and Keck2012). The ITS2 sequence of Cir. momota represented a distinct taxon that was supported well by molecular evidence. We corrected several obvious errors in the 18S rDNA sequence of the cyclocoelid from C. canutus and treated this specimen as Harrahium sp. instead of its original designation as Cyc. mutabile according to Littlewood and Bray (Reference Littlewood and Bray2001), as wading birds do not typically host Cyc. mutabile (cf. next subchapter), but rather host Harrahium spp., namely Cyc. obscurum (re-classified to the Harrahium below) and Har. tringae, assuming that the species identification was erroneous due to the large amount of information processed in the otherwise excellent book by Littlewood and Bray (Reference Littlewood and Bray2001). Consistent with this change, the corrected sequence of the cyclocoelid specimen from C. canutus segregated with 18S rDNA of Cyc. obscurum. However, the sequence was not as close to Har. obscurum comb. n. KU877905 as expected from sequences of the same species tested for relatively conserved 18S rDNA locus (Fig. 1A).

Fig. 1. Maximum-likelihood analysis of sequences of nuclear [18S rDNA (A) and ITS2 (B)] and mitochondrial DNA loci [CO1 (C) and ND1 (D)] of Cyclocoelidae. Bars indicate the number of substitutions per one nucleotide.
Although we only analysed one to three individuals of each species, both the hypervariable mitochondrial loci displayed intraspecific variability in at least one of the analysed species (CO1 in Cyc. obscurum and M. polonicum and ND1 in Cyc. mutabile, Cyc. obscurum and M. polonicum) (Table 3). All of the intraspecific variability reached ⩽0·010 base differences per site. The nuclear loci tested did not display any intraspecific variability (Table 3).
Table 3. Estimates of intra- and interspecific evolutionary divergences of Cyclocoelidae from the Czech Republic examined in this study, and specimens with DNA sequences available in the NCBI GenBank database. The estimates are based on sequences of 18S rDNA, ITS2, CO1 and ND1 DNA loci. Distance: The number of base differences per site generated by averaging over all sequence pairs between groups. As the outgroups, we used the sequences of non-cyclocoelid species with the highest similarity to the analysed specimens as revealed by NCBI Blast algorithm. Thus, sequences of different organisms were used for different DNA loci based on their public availability. The sequences of the following species were used: Fasciola gigantica AJ011942 (Fasciolidae, 18S rDNA), Isthmiophora hortensis AB189982 (Echinostomatidae, ITS2), Fasciola hepatica KF111622 (Fasciolidae, CO1), and Parafasciolopsis fasciolaemorpha EF612500 (Fasciolidae, ND1)

The genus Cyclocoelum appears to be paraphyletic. The DNA of the type species, Cyc. mutabile, shared the highest similarity with Cir. momota (as suggested by ITS2) and less similarity with Hyp. oculeus (as suggested by ITS2, 18S rDNA and ND1 loci). Particularly, the analysis of ITS2 provided sufficient resolution, justifying the existence of the three genera (Fig. 1, Table 3). However, other species of genus Cyclocoelum, Cyc. obscurum did not cluster with any of the three above species, and the analyses of multiple DNA loci have repeatedly showed that this species shares the highest DNA similarity with genus Harrahium, represented in our analyses by Har. tringae. The identical result was obtained by the maximum-likelihood analyses of ITS2, CO1 and ND1 loci (Fig. 1), and using the Bayesian approach (Figs S1–S4). In the analyses of 18S rDNA, the sequence of Har. tringae was absent, but, again, Cyc. obscurum clustered separately from Cyc. mutabile, Hyp. oculeus and also from M. polonicum. Thus, we suggest the re-classification of Cyc. obscurum as Har. obscurum (Leidy, 1887) Sitko and Heneberg, 2016 comb. n.
The above change also questions the validity of subfamilies within the Cyclocoelidae. Dronen (Reference Dronen2007) and Dronen and Blend (Reference Dronen and Blend2015) suggested that Cyclocoelinae include the genera Cyclocoelum and Circumvitellatrema Dronen, Greiner, Ialeggio & Nolan, 2009, whereas Hyptiasminae were suggested to include the genera Hyptiasmus and Morishitium, and Haematotrephinae were suggested to include the genera Harrahium and Uvitellina. However, the maximum-likelihood analysis of the four loci tested does not support the above division. Cyclocoelinae (represented by Cyc. mutabile and Cir. momota) form an ingroup of Hyptiasminae (represented by Hyp. oculeus and M. polonicum) according to the analysis of ITS2 and ND1 (Fig. 1B and D). Also the maximum-likelihood analyses of 18S rDNA and CO1 suggested that Cyclocoelinae and Hyptiasminae form a single clade within the family, but the bootstrap support of the particular nodes was not convincing to suggest such re-classification without examining more species. Due to the changes in the species spectrum included, the subfamily Cyclocoelinae Stossich, 1902 sensu lato includes species with an intertesticular ovary that forms a triangle with the testes or that is nearly in line with tandem, or nearly tandem testes.
There is also poor support for the position of genus Morishitium. This genus forms a long branch when subjected to maximum-likelihood analyses of any of the four DNA loci tested and is likely associated with its own subfamily. It appears it forms its own clade together with members of current Hyptiasminae, except genus Hyptiasmus, that is, the genera Allopyge Johnston, 1913, Prohyptiasmus Witenberg, 1923 and Morishitium. They would be characterized by an intertesticular ovary that is nearly in line with tandem, or nearly tandem testes and are expected to comprise members of the rejected subfamily. Again, the examination of more species within the above genera is needed to confirm the existence of such clade.
Subfamily Haematotrephinae newly includes Har. obscurum comb. n., which was re-classified from genus Cyclocoelum. In addition, the DNA specimen labeled as Cyc. mutabile and isolated from Calidris canutus by Littlewood and Bray (Reference Littlewood and Bray2001) clusters with this subfamily. Correct classification of this specimen to species is unclear. This specimen is clearly not Cyc. mutabile, but the maximum-likelihood analysis also rejected its classification as Har. obscurum comb. n., as this specimen is distant from the 18S rDNA locus of this species isolated from T. nebularia in this study (Fig. 1A). Reflecting the re-classification of Har. obscurum comb. n. to Haematotrephinae Dollfus, 1948 sensu Dronen and Blend (Reference Dronen and Blend2015), the key diagnostic character of this subfamily should be changed as follows: members of this subfamily have a pretesticular or intertesticular ovary that forms a triangle with the testes.
We further confirmed the phylogenetic classification of the Cyclocoelidae within Echinostomata according to the method of Olson et al. (Reference Olson, Cribb, Tkach, Bray and Littlewood2003). However, additional research is needed to settle the position of families within Echinostomata, as cyclocoelids share higher sequence similarity of the analysed loci with Echinostomatidae Looss, 1899 and Fasciolidae Railliet, 1895 than with Philophthalmidae Looss, 1899. However, as we analysed predominantly hypervariable loci, these suggestions should be confirmed by the analysis of loci showing intermediate to low variability.
We corroborated the data obtained with maximum-likelihood analysis by inferring the tree topologies using a Bayesian approach. The Bayesian approach confirmed all of the key conclusions of the maximum-likelihood analyses (Figs S1–S4).
Ecology of central European Cyclocoelidae
The host-specific prevalence (Table 4) and intensity of infection (Table 5) of the Cyclocoelidae were strongly family specific. The differences in prevalence in examined Czech birds suggest strict separation of ecological niches for Cyc. mutabile and Hyp. oculeus (Rallidae), Har. obscurum comb. n. and Har. tringae (Scolopacidae), Hyp. arcuatus (Anatidae), U. vanelli (Charadriidae) and M. polonicum (Turdidae, with occasional findings also in Sturnidae, Muscicapidae and Prunellidae). Harrahium obscurum comb. n. in Tringa erythropus and Tringa nebularia showed the highest prevalences (30 and 22%, respectively), with satellite hosts also represented by Tringa glareola, Tringa ochropus and Philomachus pugnax. Harrahium tringae showed a similarly high prevalence in its core host species, Tringa erythropus (prevalence 20%), and satellite hosts also were of similar spectrum as those of Har. obscurum comb. n., with Tringa glareola and Philomachus pugnax also confirmed as definitive hosts among the examined Czech birds. Other cyclocoelids were less prevalent, but some species specialized in more abundant hosts were relatively frequently identified. These species included Cyc. mutabile and Hyp. oculeus, which parasitized Fulica atra. Of these species, only Cyc. mutabile was identified in Gallinula chloropus, despite a relatively large number of common moorhens examined (n = 50). Hyptiasmus arcuatus was identified only once (in Anas clypeata), as its intermediate hosts are likely associated with salty or brackish waters, which were absent from the study area. We identified A. attenuatum in multiple turdids, with relatively low prevalence in Turdus merula, but at 4·6% prevalence in Turdus philomelos. Whether Turdus iliacus and Luscinia megarhynchos should be considered other core hosts remains unknown, as the number of examined individuals of these two species was low. In addition, we also identified M. polonicum in multiple other species, but we assumed that these results were incidental findings in satellite hosts. Moreover, we identified U. vanelli only in V. vanellus, with a moderate overall prevalence (5·2%; Table 4). After a population crash of the local northern lapwing populations, we examined only a limited number of lapwings in the last two decades, and no individuals examined recently were infected with U. vanelli.
Table 4. Host-specific prevalence of Cyclocoelidae in the Czech Republic from 1962 to 2016

Table 5. Intensity of infection by Cyclocoelidae in the Czech Republic from 1962 to 2016

Five of the seven cyclocoelid species caused infections of only low intensity. These species included Cyc. mutabile, Har. tringae, Hyp. arcuatus, Hyp. oculeus and U. vanelli. In contrast, the intensity of Har. obscurum comb. n. infection was high in its core host, Tringa erythropus, reaching 18·6 ± 17·0 individuals per infected bird, but this helminth occurred at low infection intensities in all satellite hosts and at moderate infection intensity also in Tringa nebularia. Thus, Tringa nebularia should also be considered a satellite rather than a core host of Har. obscurum comb. n., but the examination of more individuals is needed to provide a definitive answer. Another species with high intensities of infections was M. polonicum, displaying the highest intensities of infection in Turdus philomelos and Turdus merula (14·9 ± 36·1 and 28·6 ± 37·8, respectively), with a high intensity of infection also observed in incidental records in Turdus iliacus and Erithacus rubecula, but not in other satellite hosts (Table 5).
The records of Har. obscurum comb. n. in the ruff Philomachus pugnax, Har. tringae in wood sandpiper Tringa glareola, and M. polonicum in common starling Sturnus vulgaris, redwing Turdus iliacus, European robin Erithacus rubecula, common nightingale Luscinia megarhynchos and dunnock Prunella modularis are new host records (Tables 4 and 5). We corroborated the above-suggested spectrum of core Cyclocoelidae host–parasite interactions (except U. vanelli) using DNA sequencing as described above.
Comparative morphology of central European Cyclocoelidae
As the cyclocoelid species discussed in this study were not previously subjected to comparative DNA analysis, and we complement here DNA analyses with measurements of key morphologic features of the analysed species as identified using a combined morphological and genetic approach (Tables 6 and 7) and provide representative photographs (Figs 2 and 3) of the species analysed. The body measurements were largely consistent with previously published data. Notably, we did not identify any adult individuals of M. polonicum with large eggs. As pointed out by Dronen and Blend (Reference Dronen and Blend2015), the description of M. polonicum by Machalska (Reference Machalska1980a ) suggested that specimens from Turdus philomelos have a maximum egg size 139 × 69 µm2, whereas the individuals obtained from Turdus merula had larger eggs (150 × 81 µm2). Dronen and Blend (Reference Dronen and Blend2015) suggested that this finding might indicate the presence of two Morishitium species in Polish turdids. However, in our extensive material examined in this study, we did not record any individuals of Morishitium with eggs longer than 145 µm in T. philomelos as well as in T. merula (Table 7), and the analyses of DNA (Fig. 1) suggested that both T. philomelos and T. merula host the mutually identical species of Morishitium (or that the long-egged species is absent in the study area, and its niche is used by the short-egged species).
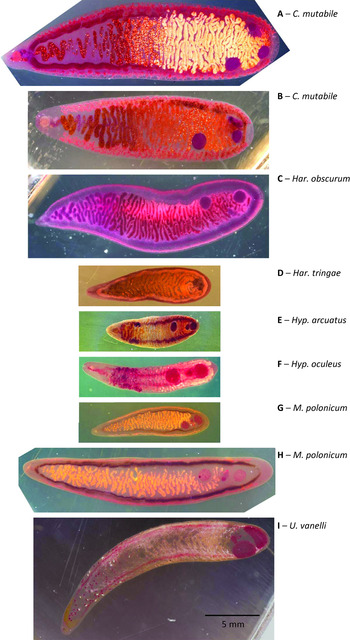
Fig. 2. Representative microphotographs of species of Cyclocoelidae analysed in this study, stained in Semichon's carmine. (A) Cyclocoelum mutabile, host Fulica atra, juvenile, air sac, sampling site and date: Klec, Czech Republic, 10-Nov-1968, specimen 1009–14. (B) Cyclocoelum mutabile, host Fulica atra, adult male, air sac, sampling site and date: Lomnice nad Lužnicí, Czech Republic, 20-Mar-1968, specimen 1911–62. (C) Cyclocoelum obscurum, host Tringa erythropus, adult male, air sac, sampling site and date: Lomnice nad Lužnicí, Czech Republic, 12-Sep-1967, specimen 1709–4. (D) Harrahium tringae, host Philomachus pugnax, adult female, air sac, sampling site and date: Záhlinice, Czech Republic, 23-May-1985, specimen 3548–18. (E) Hyptiasmus arcuatus, host Anas penelope, nasal cavity, sampling site and date: Záhlinice, Czech Republic, 15-Dec-2000, specimen 11, 121–20. (F) Hyptiasmus oculeus, host Fulica atra, adult female, nasal cavity, sampling site and date: Tovačov, Czech Republic, 12-Apr-1999, specimen 7438–255. (G) Morishitium polonicum, host Turdus merula, adult female, air sac, sampling site and date: Záhlinice, Czech Republic, 15-Mar-2003, specimen 9931–247. (H) Morishitium polonicum, host Turdus philomelos, adult male, air sac, sampling site and date: Záhlinice, Czech Republic, 27-Jul-2007, specimen 12079–170. (I) Uvitellina vanelli, host Vanellus vanellus, adult male, air sac, sampling site and date: Buk, Czech Republic, 31-Mar-1965, specimen 2877–44. Scale bar 5 mm applies to all the subfigures. Note that a pressure was applied when fixing the helminths in 1960s, which reproducibly causes relocation of some of their organs.

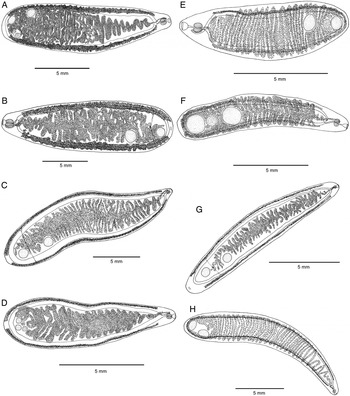
Fig. 3. Representative drawings of species of Cyclocoelidae analysed in this study. (A) Cyclocoelum mutabile (pressure-fixed). (B) Cyclocoelum mutabile (live specimens and specimens fixed with minimal coverslip pressure). (C) Cyclocoelum obscurum. (D) Harrahium tringae. (E) Hyptiasmus arcuatus. (F) Hyptiasmus oculeus. (G) Morishitium polonicum. (H) Uvitellina vanelli.
Table 6. Measurements of Cyclocoelidae based on the adult individuals collected in the Czech Republic from 1962 to 2016. Host species of the individuals described: Cyclocoelum mutabile – 30 specimens from Fulica atra; Harrahium obscurum comb. n. – 30 specimens from Tringa erythropus; and Harrahium tringae – two specimens from Philomachus pugnax, 4 specimens from Tringa erythropus and one specimen from Tringa glareola, and Uvitellina vanelli – eight specimens from Vanellus vanellus. The data are shown as a range (mean ± s.d.). Besides body measurements, the table also contains key features used for an identification of each species

a In Uvitellina vanelli, the oral sucker is absent. The measurements are provided for a distance from pharynx to the anterior body extremity and width of the oral fissure.
Table 7. Measurements of Cyclocoelidae based on the adult individuals collected in the Czech Republic from 1962 to 2016. Host species of the individuals described: Hyptiasmus arcuatus – 2 specimens from Anas clypeata; Hyptiasmus oculeus – 25 specimens from Fulica atra; Morishitium polonicum – 30 specimens from Turdus philomelos and separately analysed 30 specimens from Turdus merula. The data are shown as a range (mean ± s.d.). Besides body measurements, the table also contains key features used for an identification of each species

a In Hyptiasmus spp., the oral sucker is absent. The measurements are provided for a distance from pharynx to the anterior body extremity and width of the oral fissure.
DISCUSSION
Cyclocoelidae are considered to be an unsettled taxonomic group. The identification of species of the family Cyclocoelidae is not trivial, morphologic differences among the Cyclocoelum, Harrahium and Hyptiasmus spp. are negligible (Dronen and Blend, Reference Dronen and Blend2015), their developmental cycles are highly similar (Sitko et al. Reference Sitko, Faltýnková and Scholz2006; Gibson et al. Reference Gibson, Bray and Harris2016), their diagnostic characters can be skewed by applying different strengths of pressure during the preparation of slides (cf. Fig. 3A and B), and mature eggs are also difficult to measure because rediae emerge immediately upon contact with water. All the hitherto suggested classification systems were based on morphological characters, as molecular phylogenetic analysis of the family Cyclocoelidae was never conducted, with the exception of positioning the Cyclocoelidae within the higher taxonomical units.
The analysis of the newly obtained DNA sequences of central European cyclocoelid species confirmed the classification of Cyc. mutabile, Har. obscurum comb. n., Har. tringae, Hyp. oculeus, M. polonicum and U. vanelli as valid species (Fig. 1). DNA analysis suggested a paraphyletic origin of genus Cyclocoelum. One clade was represented by the type species, Cyc. mutabile, whereas the other clade was represented by Cyc. obscurum. The specimens of Cyc. obscurum clustered with Har. tringae in all four DNA loci tested (Fig. 1); thus, we suggested the re-classification of Cyc. obscurum as Har. obscurum comb. n. The results of the DNA analysis thus provided data consistent with Bykhovskaya-Pavlovskaya (Reference Bykhovskaya-Pavlovskaya1949), who did not observe any differences between the genera Cyclocoelum and Harrahium at the morphological level. Here, we showed differences at the molecular level, but the boundary between the genera needs to be redrawn as soon as the DNA of more species is tested.
Harrahium obscurum comb. n. is a cosmopolitan species, distributed across Europe, Asia, North Africa and North and Central America, where it parasitizes various charadriiform birds. However, this species was originally described from the stomach of a fish. The type host report is considered to be an error, as there are no cyclocoelids that would parasitize the stomachs of fishes (Dronen and Blend, Reference Dronen and Blend2015). Morphologically, the most similar species is Cyclocoelum pseudomicrostomum Harrah, 1922. Harrahium obscurum comb. n. differs from C. pseudomicrostomum by having a rudimentary oral sucker, shorter cirrus sac (248–579 µm / 4–5% of body length compared with 1325 µm / 9% of body length in C. pseudomicrostomum) and larger eggs (138–162 × 70–94 µm compared with 102 × 51–66 µm) (Harrah, Reference Harrah1922; Dronen and Blend, Reference Dronen and Blend2015). Regarding the differential diagnosis from C. mutabile, Feizullaev (Reference Feizullaev1980) stated that the vitelline fields of C. mutabile are broad and not confluent, whereas the vitelline fields of Har. obscurum comb. n. are narrow but confluent, and that the genital pore is prepharyngeal in C. mutabile, but postpharyngeal in Har. obscurum comb. n. (Feizullaev, Reference Feizullaev1980).
The measurements of Har. obscurum comb. n. according to Dubois (Reference Dubois1959) largely matched the specimens examined in this study, with the following exceptions: the specimens examined herein were larger (13 000–28 000 µm compared with 6000–13 000 µm), with a larger oral sucker width (184–552 µm vs 115 µm) and with only a partially overlapping pharynx width (184–350 µm vs 115–264 µm), ovary width (343–596 µm vs 275–463 µm) and testes width (457–1341 µm vs 596 µm), but a nearly identical size of eggs (133–157 × 70–87 µm vs 138–162 × 70–94 µm) (Table 6; Dubois, Reference Dubois1959; Dronen and Blend, Reference Dronen and Blend2015). However, the measurements largely matched those by Feizullaev (Reference Feizullaev1980), who reported the following dimensions: body length 6000–30 500 µm, body width 3500–7500 µm, pharynx 120–460 × 120–350 µm, intestine length 250–1100 µm, testes 460–1300 × 300–1400 µm, ovary 250–900 × 270–300 µm, eggs 96–175 × 49–94 µm.
The situation with M. polonicum is also complicated. The only species of Morishitium reported previously from the Czech Republic was M. elongatum (Sitko et al. Reference Sitko, Faltýnková and Scholz2006), which was recently re-classified as Allopyge elongatum (Dronen and Blend, Reference Dronen and Blend2015). Sitko et al. (Reference Sitko, Faltýnková and Scholz2006) reported this species from Czech turdids and sturnids. The same species was reported from turdids in Poland (Rząd et al. Reference Rząd, Sitko, Wysocki and Stępniewski2011, Reference Rząd, Sitko, Sałamantin and Wysocki2014). However, A. elongatum is restricted to South and East Asia (China, India, Vietnam), and to the Americas, hosted by charadriiform, galliform and several passeriform (Corvidae, Mimidae, Muscicapidae, Passeridae) families but not by Turdidae or Sturnidae (Dronen and Blend, Reference Dronen and Blend2015). Body measurements and key diagnostic characters of A. elongatum differ from those found in our specimens (and also in specimens retrieved from Poland) (Machalska, Reference Machalska1980a ; Dronen and Blend, Reference Dronen and Blend2015). Importantly, it was Machalska (Reference Machalska1980a , Reference Machalska b ), who already described a new cyclocoelid from turdids in Poland. She termed it Cyclocoelum polonicum, and this species was later re-classified as M. polonicum (Dronen and Blend, Reference Dronen and Blend2015). Some authors considered Cyc. polonicum as a junior synonym of A. elongatum (Rząd et al. Reference Rząd, Sitko, Sałamantin and Wysocki2014), which caused the above-listed misidentifications. Here we thus propose that all the previous records of Morishitium (Allopyge) spp. from the Czech Republic and Poland should be considered as records of M. polonicum. Currently, there are four Morishitium spp. infecting the thrushes, namely Morishitium bivesiculatum (Prudhoe, 1944), Morishitium dollfusi (Timon–David, 1950), Morishitium petrowi (Oganesov, 1959) and M. polonicum. The available material does not allow us to test, whether all of them represent valid species but based on the body measurements, the species occurring in the Czech Republic and Poland matches clearly the measurements provided by Machalska (Reference Machalska1980a ). Characteristic features include an intertesticular ovary that forms nearly a straight line with the nearly tandem testes, a postpharyngeal genital pore and the vitelline fields, which are not confluent posteriorly. Rudimental oral sucker is present. Body measurements of the Czech specimens examined in this study were nearly identical with those reported by Machalska (Reference Machalska1980a ) from Poland: body length 8680–12 870 µm vs 7138–13 109 µm, oral sucker width 202–313 µm vs 208–323 µm, pharynx width 230–313 µm vs 208–323 µm, cirrus sac length 164–432 µm vs 219–474 µm, ovary width 202–377 µm vs 265, testes width 340–1118 µm vs 498 µm, size of eggs 81–139 × 58–90 µm vs 81–139 × 46–69 µm. Upper limits of body measurements of Czech M. polonicum from T. merula are based on helminths retrieved from birds affected by low intensity infection only, and are close to those reported for M. dollfusi in Eurasian magpie Pica pica, but the possible synonymy of M. polonicum and M. dollfusi can be revealed by the DNA analyses only.
The combined morphological and genetic approach revealed that infections with the cyclocoelids are highly host family specific (Tables 4 and 5). Cyclocoelum mutabile typically parasitizes Rallidae, typically coots and gallinules. Both the Harrahium spp. analysed in this study parasitize waders of the family Scolopacidae, typically Tringa spp., as well as P. pugnax. We identified Hyp. arcuatus only in Anatidae, whereas Hyp. oculeus was only present in coots. Morishitium polonicum was most frequently identified in T. philomelos, but was also identified with a lower prevalence in numerous other passerine birds feeding at least occasionally on terrestric snails. Lastly, U. vanelli was specialized for lapwings only. Previous reports on the overlap of the host spectrum cannot be rejected, but need to be referred with caution. These include the findings of Cyc. mutabile in galliform, anseriform, pelecaniform and phoenicopteriform birds, Har. obscurum comb. n. in anseriform, galliform and coraciiform birds and in humans, Har. tringae in anseriform birds and Hyp. oculeus in charadriiform birds (Feizullaev, Reference Feizullaev1980). Such records require further confirmation using combined morphological and genetic methods.
Another question raised in this study is associated with the validity of subfamilies within the Cyclocoelidae. The particular subfamilies had a problematic status, as they were only supported by a small number of autapomorphies. Analysis of DNA sequences had low support for most of the nodes questioned, and included only a few species out of those known globally. Thus, we did not propose re-classifications of particular subfamilies despite several changes are likely in the future. One of the most striking issues is associated with Cyclocoelinae and Hyptiasminae. As regards Cyclocoelinae, Dronen and Blend (Reference Dronen and Blend2015) classified the species within this group as possessing an intertesticular ovary that formed a triangle with the testes. This subfamily included a single genus with a prepharyngeal genital pore (Cyclocoelum) and multiple genera with a postpharyngeal genital pore (Selfcoelum Dronen et al., 2006, Psophiatrema Dronen and Kinsella, 2009 and Circumvitellatrema). The analyses in the present study included only two species that were classified into genus Cyclocoelum (Cyc. mutabile and Cyc. obscurum, here re-classified as Har. obscurum comb. n.) and a previously sequenced specimen of Cir. momota. Regarding the subfamily Hyptiasminae Dollfus, 1948, Dronen and Blend (Reference Dronen and Blend2015) classified member species as having an intertesticular ovary that is nearly in line with the tandem or nearly tandem testes. Within the Hyptiasminae, two genera with a prepharyngeal genital pore (Hyptiasmus and Prohyptiasmus) and two genera with a postpharyngeal genital pore (Allopyge and Morishitium) have been recognized by Dronen and Blend (Reference Dronen and Blend2015). Among these species, the genetic analyses in the present study included the genera Hyptiasmus and Morishitium. The analyses collectively suggested that Cyclocoelinae form an ingroup of Hyptiasminae. Further analyses should address the possible rejection of Hyptiasminae Dollfus, 1948 sensu Dronen and Blend (Reference Dronen and Blend2015), reflecting the priority of Cyclocoelinae, and the re-classification of Hyptiasmus to Cyclocoelinae Stossich, 1902 sensu Dronen and Blend (Reference Dronen and Blend2015). Morishitium polonicum formed a long branch in analyses of all of the four DNA loci tested. The topology and branch characteristics suggest that Morishitium might represent a separate lineage warranting status in a separate subfamily in the Cyclocoelidae. The above is by the results of the analyses of all loci, except 18S rDNA, where however the resolution was low to reject the above (Fig. 1). Consequently, further analyses should address a possibility of a proposal for a new subfamily encompassing Allopyge, Morishitium and Prohyptiasmus, i.e., all members of the Hyptiasminae, except genus Hyptiasmus.
In conclusion, the combined molecular and comparative morphological analysis of central European Cyclocoelidae confirmed Cyclocoelidae as an unsettled taxonomic group. Particularly surprising was the re-classification of Cyc. obscurum to the genus Harrahium. Further research should address possible rejection of the subfamily Hyptiasminae, and erection of the subfamily containing all of the members of the Hyptiasminae, except Hyptiasmus. Despite the uncertainties regarding higher classification units, we confirmed that all of the morphologically identified species represent valid taxa, the existence of which is supported by both morphological and genetic evidence, and which display strict niche separation in terms of host specificity and selectivity at least within the examined central European cohort of birds. Specimens of non-European origin are required to shed more light on the classification of species and genera, which were not available to us.
SUPPLEMENTARY MATERIAL
The supplementary material for this article can be found at https://doi.org/10.1017/S0031182016001955.
ACKNOWLEDGEMENT
We thank Milan Řezáč (Crop Research Institute, Prague) for instrumentation support.
FINANCIAL SUPPORT
The study was supported by the project PRVOUK P31/2012 from the Charles University (to J. B. and P. H.).