Introduction
In nature, parasites are usually found sharing the host's resources with an array of other symbionts, including parasites, pathogens, mutualists and microbiota. These co-infections can result in complex interactions, with potentially important implications for the evolution and epidemiology of diseases, as well as for the fitness and wellbeing of the hosts (Haine et al. Reference Haine, Boucansaud and Rigaud2005; Mideo, Reference Mideo2009; Jones et al. Reference Jones, White and Boots2010). One case of particular interest is when co-infecting parasites have conflictive evolutionary interests, as is the case when horizontally transmitted parasites (HTPs) and vertically transmitted parasites (VTPs) co-occur in the same host (Ben-Ami et al. Reference Ben-Ami, Rigaud and Ebert2011; reviewed by Haine et al. Reference Haine, Boucansaud and Rigaud2005; Haine, Reference Haine2008; Jones et al. Reference Jones, White and Boots2010). VTPs and HTPs are both selected to minimize host mortality, as both benefit of the extended period over which transmission may occur. Conflicts are however expected to arise when it comes to the host's fecundity (Jones et al. Reference Jones, White and Boots2010). VTPs are transmitted from mother to offspring and are thus under strong selection to minimize the damage to the host (Bull et al. Reference Bull, Molineux and Rice1991; Lipsitch et al. Reference Lipsitch, Siller and Nowak1996). Instead, as vertical transmission is most efficient when host fecundity is high, VTP parasites may be expected to maximize their fitness by boosting the reproductive output of their hosts (Zug and Hammerstein, Reference Zug and Hammerstein2015; but see Baton et al. Reference Baton2013). By contrast, HTPs are typically transmitted between unrelated hosts and are therefore not directly concerned by an increase in host fecundity. Instead, they will be selected to castrate their hosts if the host's energy is redirected to maximize parasite transmission (Jaenike, Reference Jaenike1996; O'Keefe and Antonovics, Reference O'Keefe and Antonovics2002; Jones et al. Reference Jones, White and Boots2010). Recent theoretical work has shown that such conflicts of interest can have important consequences for the evolutionary trajectory of both types of parasites, as well as for the fitness of the host (Jones et al. Reference Jones, White and Boots2010).
The mosquito Culex pipiens is naturally infected by both parasite types: Wolbachia, a maternally inherited endosymbiotic bacterium whose prevalence in Cx. pipiens populations is close to 100% (Rasgon and Scott, Reference Rasgon and Scott2003; Duron et al. Reference Duron2005; Dumas et al. Reference Dumas2013), and the avian malaria parasite Plasmodium relictum SGS1 which is found infecting ca. 4% of field-collected Cx. pipiens mosquitoes in the South of France (Zélé et al. Reference Zélé2014b). Wolbachia spreads rapidly and efficiently in mosquito populations through cytoplasmic incompatibility (CI), a form of reproductive manipulation whereby infected females have a reproductive advantage over uninfected ones (Werren et al. Reference Werren, Baldo and Clark2008; Engelstadter and Hurst, Reference Engelstadter and Hurst2009). In most cases CI is sufficient by itself to ensure the spread of non-mutualist Wolbachia variants through the host populations (Zug and Hammerstein, Reference Zug and Hammerstein2015). However, both models and field observations have shown that selection may favour variants that also increase the relative fecundity of the infected females (Turelli, Reference Turelli1994). These ‘Jekyll and Hyde’ infections, so-called because Wolbachia acts both as a beneficial symbiont and a reproductive parasite (Jiggins and Hurst, Reference Jiggins and Hurst2011), are expected to be particularly efficient at invading and spreading across host populations (Zug and Hammerstein, Reference Zug and Hammerstein2015).
Plasmodium, on the other hand, is one of the HTPs that frequently expresses its virulence by partially castrating its vectors: several species of malaria parasites have been shown to significantly reduce the fecundity (number of eggs) and fertility (number of hatched larvae) of mosquitoes (reviewed in Hurd, Reference Hurd2003, Reference Hurd2009). The effects of P. relictum on Cx. pipiens fecundity are particularly acute, with highly infected mosquitoes laying up to 40% fewer eggs than their uninfected counterparts (Vézilier et al. Reference Vézilier2012). Although neither the mechanistic nor the putative adaptive nature of these fecundity reductions have been entirely resolved, the reproductive curtailment induced by Plasmodium has been widely assumed to be an adaptive strategy of the parasite aimed at increasing mosquito survival (Schwartz and Koella, Reference Schwartz and Koella2001; Ferguson et al. Reference Ferguson, Rivero and Read2003; Hurd, Reference Hurd2003).
A previous work in this system has shown the existence of significant interactions between Wolbachia and P. relictum in mosquitoes: Wolbachia increases the susceptibility of Cx. pipiens mosquitoes to P. relictum (Wolbachia-infected mosquitoes have higher parasite burdens, Zélé et al. Reference Zélé2014a) and Plasmodium-infected females suffer lower mortality rates if they are also infected with Wolbachia (Zélé et al. Reference Zélé2012) suggesting that Wolbachia has a protective effect against Plasmodium-induced mortality and that, contrary to what has been shown in other systems (Hoffmann et al. Reference Hoffmann, Ross and Rasic2015), Wolbachia increases mosquito tolerance to the infection.
Here, we explore the effects of Wolbachia–Plasmodium co-infections on mosquito fecundity. For this purpose we first carry out a two-way factorial experiment to determine the effect of Wolbachia and Plasmodium, singly or in co-infections, on the number of eggs laid by Cx. pipiens mosquitoes (Experiment 1). We make two specific predictions: (1) as a strictly VTP sharing a long co-evolutionary history with its host (Atyame et al. Reference Atyame2011), Wolbachia should have a positive effect on the fecundity of mosquitoes in the absence of a Plasmodium infection, and (2) in Plasmodium-infected mosquitoes the fecundity will be the net result of the balance between the Wolbachia-driven gains and the Plasmodium-driven losses, with the actual outcome depending on the relative strength of, and interactions between, these opposing physiological forces. In other words, we expect Wolbachia to (partially) protect mosquitoes against the Plasmodium-driven fecundity reductions.
To deepen our understanding of the protective effect of Wolbachia against Plasmodium-driven losses, we then carried out a second experiment where we aimed to establish whether there is a negative correlation between fecundity and Plasmodium infection intensity quantified as the number of oocysts in the midgut (Experiment 2). We call this our tolerance experiment because the slope of host fitness against infection intensity is also known as disease tolerance (Raberg et al. Reference Raberg, Sim and Read2007) and, to our knowledge, it has never been quantified for Plasmodium-infected mosquitoes. Whether Plasmodium has an all-or-nothing (infected/uninfected) effect on mosquitoes, or whether its effects depend on the intensity of the infection has been the subject of some recent discussion (Churcher et al. Reference Churcher2017). A previous work has shown that Plasmodium burden correlates negatively with mosquito longevity (Dawes et al. Reference Dawes2009). We therefore further predict that: (3) the degree of fecundity reduction in mosquitoes will be proportional to the intensity of the infection, and that: (4) Wolbachia may be able to increase the tolerance (i.e. reduce the slope of the fitness–burden relationship) of the mosquitoes to a Plasmodium infection. We discuss the potential mechanisms and implications underlying the conflicting effects of these two parasites on mosquito reproduction.
Materials and methods
Mosquito and Plasmodium strains
We used two isogenic lines of Cx. p. quinquefasciatus that share the same nuclear genome, but differ in their Wolbachia infection. The first line, Slab (which, for simplicity reasons will be henceforth termed wSL), was collected in California in 1954 (Georghiou et al. Reference Georghiou, Metcalf and Gidden1966) and is naturally infected by the Wolbachia wPip(Sl) strain (Duron et al. Reference Duron, Fort and Weill2006a). The second line, w(-), was generated by an antibiotic (tetracycline hydrochloride) treatment of wSL larvae for three consecutive generations to eliminate the Wolbachia infection (as described in Duron et al. Reference Duron2006b). The w(-) line was initiated from over 10 000 wSL larvae. To eliminate the potential side-effects of the tetracycline, the w(-) line was reared for ca. 25 generations before the experiment took place under standard laboratory, tetracycline-free, conditions.
We used one lineage of P. relictum known as SGS1. This Plasmodium lineage has been thus far recorded in 29 bird species belonging to eight families of the Passeriformes in Eurasia and Africa (MalAvi database, Bensch et al. Reference Bensch, Hellgren and Perez-Tris2009) and in 4% of the Cx. pipiens mosquitoes collected in the Montpellier region (France, Zélé et al. Reference Zélé2014b). The strain used in the experiments was isolated from wild sparrows collected in 2009 in the region of Dijon (France) and passaged to canaries (Serinus canaria) through intraperitoneal injection. The strain has been since maintained in our animal house by carrying out regular passages between our stock canaries every ca. 3 weeks. A diagnostic PCR technique (Waldenstrom et al. Reference Waldenstrom2004) was carried out on all experimental canaries prior to the onset of the experiments to ensure that they were free from any previous Plasmodium infection. Each canary was tested five times to avoid false negatives.
Experiment 1
Newly hatched (L1) larvae from the two different mosquito lines were placed in plastic trays (34 cm × 23 cm × 7 cm) filled with 1 L of water (Eau de Source, Carrefour, France) at a constant density of 300 larvae per tray (n = 10 trays per line). The experiments took place under standard temperature (24 ± 2 °C), humidity (65 ± 5%) and photoperiod (12L:12D) conditions. Larvae were fed ad libitum on brewer's yeast on the first day and on ground Tetramin® fish flakes thereafter (2nd and 4th day: 200 mg; 6th day: 400 mg). Water and food were changed every two days. On day 7 post-hatching, each plastic tray was individually placed inside an ‘emergence cage’ (40 cm × 28 cm × 31 cm) and emerged adults were allowed to feed ad libitum on a 10% glucose water solution.
Experimental canaries (n = 10) were haphazardly allocated to one of two treatments: half of them were experimentally infected with our SGS1 Plasmodium lineage (‘experimental cages’; birds parasitaemia ranged 0.87–2.43%), the other half were left as uninfected controls (‘control cages’). Experimental infections took place by intraperitoneal injection of ca. 50–100 µL of blood from our infected canary stock. Parasitaemia was regularly monitored from the tenth day of infection onwards using thin blood smears as described by Valkiūnas (Reference Valkiūnas2005). Mosquito blood feeding took place 10 days after the infection, to coincide with the acute phase of the parasitaemia (Vézilier et al. Reference Vézilier2010). The parasitaemia and haematocrit (quantified as the Packed Cell Volume, PVC) of the bird were quantified immediately prior to the mosquito blood meal.
To explore the effect of Plasmodium and of Wolbachia on mosquito fecundity, groups of 75 adult Cx. pipiens females (6–8-day old) from each line (wSL and w(-)) were haphazardly chosen from the 20 emergence cages (10 per mosquito line) ca. 12 days after emergence and placed together to feed overnight inside an experimental cage (n = 5 infected cages, n = 5 control cages). To simplify the identification of the strains, 2 days before the blood meal the mosquitoes were marked using a small amount (1 µg/female) of either pink or blue fluorescent powder (RadGlo® JST) applied as a dust storm (Service, Reference Service1993). Preliminary trials have shown that at this concentration the dust has no effect on mosquito survival and fecundity or parasite burden (Vézilier et al. Reference Vézilier2012; Zélé et al. Reference Zélé2012), and is only detectable using a binocular microscope. The two colours were used in rotation to mark the two strains, so that the strain-colour code was switched from cage to cage.
On day 1 post-blood meal (pbm), all engorged females were placed individually in numbered plastic tubes (30 mL) covered with a mesh (haematin tubes). Food was provided in the form of a cotton pad soaked in a 10% glucose solution. Five days later (day 6 pbm), all mosquitoes were transferred to a new tube containing 4 mL of mineral water (Eau de Source Carrefour) to allow the females to lay their eggs (oviposition tubes). Haematin tubes were stored at 4 °C for subsequent quantification of the amount of haematin excreted at the bottom of each tube as an estimate of the blood meal size as described in (Vézilier et al. Reference Vézilier2010). To obtain an estimate of the infection success (the infection status of dead mosquitoes cannot be established), on day 7 pbm, fifteen blood-fed females from each of the infected cages were haphazardly sampled from the tubes, and killed with CO2. One wing was taken out and measured along its longest axis as an estimate of body size (this parameter is also been shown to positively correlate with female fecundity in Aedes mosquitoes; Briegel, Reference Briegel1990; Armbruster and Hutchinson, Reference Armbruster and Hutchinson2002), and their midguts were dissected in PBS (Standard Phosphate Buffered Saline) and examined under a microscope to assess oocyst prevalence and burden (Vézilier et al. Reference Vézilier2010).
The oviposition tubes were provided daily with a cotton pad soaked in mineral water placed on top of each tube. In these conditions, 90% of the females lay their eggs in a single day in the form of one or several rafts (Vézilier et al. Reference Vézilier2012). The oviposition tubes were checked daily for the presence of eggs. Egg rafts were photographed using a binocular microscope equipped with a numeric camera, after which they were put back in the insectary where they were checked daily until the emergence of the larvae. Larvae were killed by adding 5 mL of 100% ethanol to the tube, and tubes were stored at 4 °C for later counting. After oviposition, females were measured (wing length) and allocated to one of the two mosquito lines by examining their colour under a binocular microscope. Egg number was recorded by manually counting the number of eggs on the photographs using the Mesurim Pro freeware (Academie d'Amiens, France). Larvae were counted directly under the binocular microscope.
Experiment 2
To quantify fecundity and oocystaemia simultaneously, the procedure was identical to that of Experiment 1 save for a few differences. First, mosquito larvae were fed with 400 mg (instead of 200 mg) of ground Tetramin® fish flakes on the 4th day after hatching. Second, adult Cx. pipiens females from each line (wSL and w(-)) were placed to feed inside six experimental cages, each containing an infected bird (parasitaemia ranging from 1.55 to 28.9%; no uninfected birds were used in this experiment). Third, the number of females dissected per mosquito line and experimental cage was increased to 50, and dissections took place on days 8–10 pbm, after oviposition took place. Fourth, egg rafts were photographed for fecundity but not kept for fertility (larval hatching rate) assessment.
Statistical analysis
Analyses were carried out using the R statistical package (version 3.3.2). The different statistical models built to analyse the data are described in the Supplementary Materials Table S1). The general procedure for building the statistical models was as follows. Models were built by including mosquito lines [with, wSL, or without, w(-), Wolbachia], parasite treatment (exposed to an infected or a control bird), haematin excreted (a proxy for blood meal size) and mosquito wing size as fixed explanatory variables, and experimental cage as a random explanatory variable. In addition, as egg production has been shown to be a saturating function of haematin quantity (Vézilier et al. Reference Vézilier2010), the quadratic term haematin2 was added to the minimal model when it improved significantly the model fit.
The response variables used in the analyses were either linearized using a Box-Cox transformation (Crawley, Reference Crawley2007) then analysed using a linear model with normal error distributions (fixed effects model: lm; mixed effects model: lme, package nlme), or analysed using a generalized linear mixed effect model (glmer, lme4 package) using the appropriate family of error distribution (e.g. binomial when the response variable was a proportion). Count (e.g. oocysts number) and proportion (e.g. hatching rate) data were often greatly over-dispersed. Several methods to deal with overdispersion are currently available such as using ‘quasi’ families in GLMs (Generalized Linear Models; Crawley, Reference Crawley2007), or explicitly modelling the source of extra-variation in the data (e.g., β-binomial or negative-binomial models). However, to our knowledge, it is not currently possible to account for quasi-, negative-binomial, or β-binomial distribution within the usual mixed model glmer procedure. For this reason, we used instead a mixed model glmmadmb procedure (glmmADMB package) from AD Model Builder (Fournier et al. Reference Fournier2012) with the appropriated error structure (Table S1).
Maximal models, including all higher-order interactions, were simplified by sequentially eliminating non-significant terms and interactions to establish a minimal model (Crawley, Reference Crawley2007). The significance of the explanatory variables was established using F-tests or a LRT (likelihood ratio test), which is approximately distributed as a χ 2 distribution (Bolker, Reference Bolker2008). The significant values given in the text are for the minimal model, while non-significant values correspond to those obtained before deletion of the variable from the model.
Ethical statement
Animal experiments were carried out in strict accordance with the ‘National Charter on the Ethics of Animal Experimentation’ of the French Government, and all efforts were made to minimize suffering. Experiments were approved by the Ethical Committee for Animal Experimentation established by the authors’ institution (CNRS) under the auspices of the French Ministry of Education and Research (permit number CEEA- LR-1051).
Results
Experiment 1
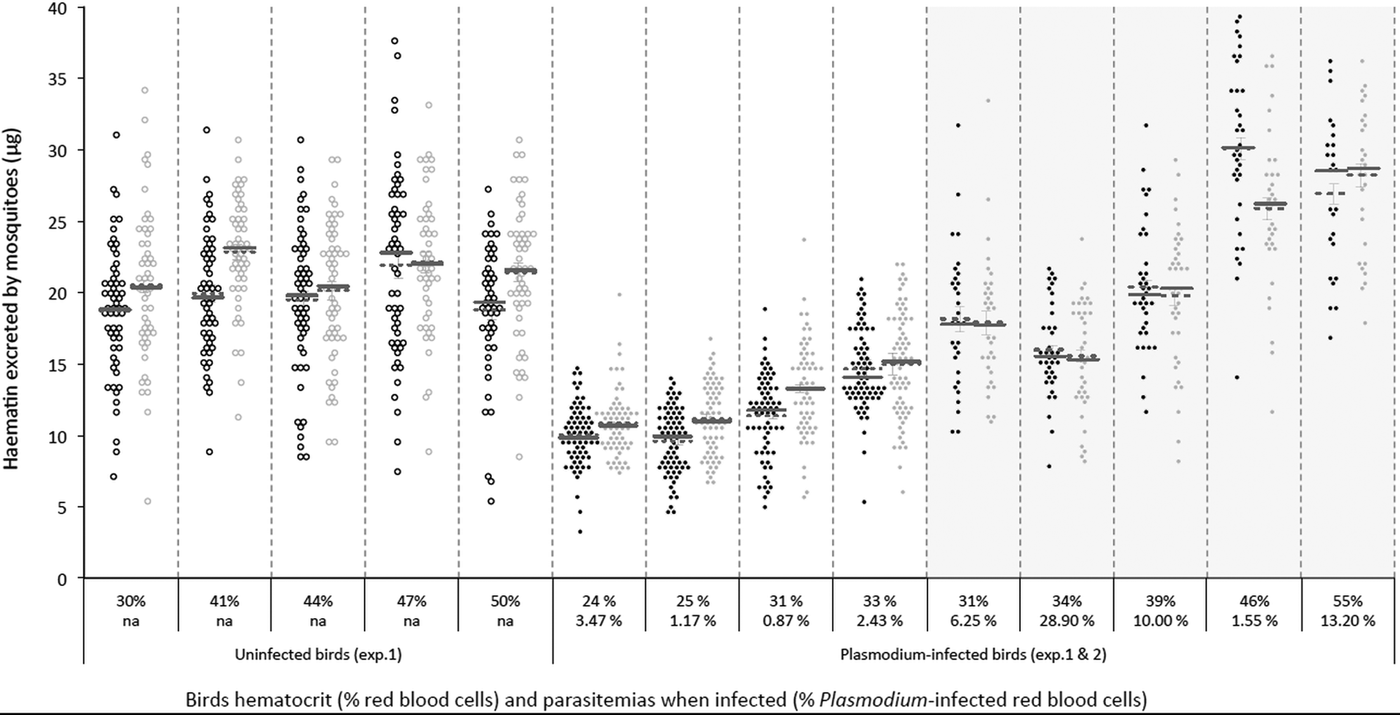
To explore the effect of Plasmodium and of Wolbachia on mosquito fecundity, groups of Wolbachia-infected (wSL) and uninfected (w(-)) adult Cx. pipiens females were fed on either Plasmodium-infected (n = 5) or uninfected birds (n = 5). Mosquitoes fed on four of the five infected birds showed a very high infection rate (% of infected mosquitoes in each of these cages ± s.e.: 61.5 ± 1.9, 88.0 ± 1.3, 89.3 ± 1.1 and 80.8 ± 1.5) whereas, for unknown reasons, in the fifth cage only 24.2 ± 1.3% of the mosquitoes became infected. For this reason, this cage was eliminated from further analyses. There was no significant difference in size between mosquitoes from the two different lines [adult wing size, mean ± s.e. 3.27 ± 0.01 and 3.29 ± 0.01 for w(-) and wSL, respectively; model 1, F 1,1087 = 2.97, P = 0.09, Supplementary Materials Fig. S1.A; for this and all other analyses see Supplementary Materials Table S1]. Overall, w(-) females excreted marginally (albeit significantly) less haematin than wSL ones (mean ± s.e., 15.47 ± 0.25 µg and 16.94 ± 0.26 µg, respectively, model 2, χ 21 = 7.31, P < 0.0001, Fig. S1.B). In both lines, the amount of excreted haematin depended on both the haematocrit of the bird and on the presence of Plasmodium (Plasmodium × haematocrit interaction, model 2, χ 21 = 21.13, P < 0.0001, Fig. 1; Fig. S2). There were, however, no significant differences between the two mosquito lines either in the prevalence (probability of containing at least one oocyst, model 3, χ 21 = 0.79, P = 0.37) or in the intensity (number of oocytsts; model 4, χ 21 = 0.02, P = 0.89) of the infection (Supplementary Materials Table S2).
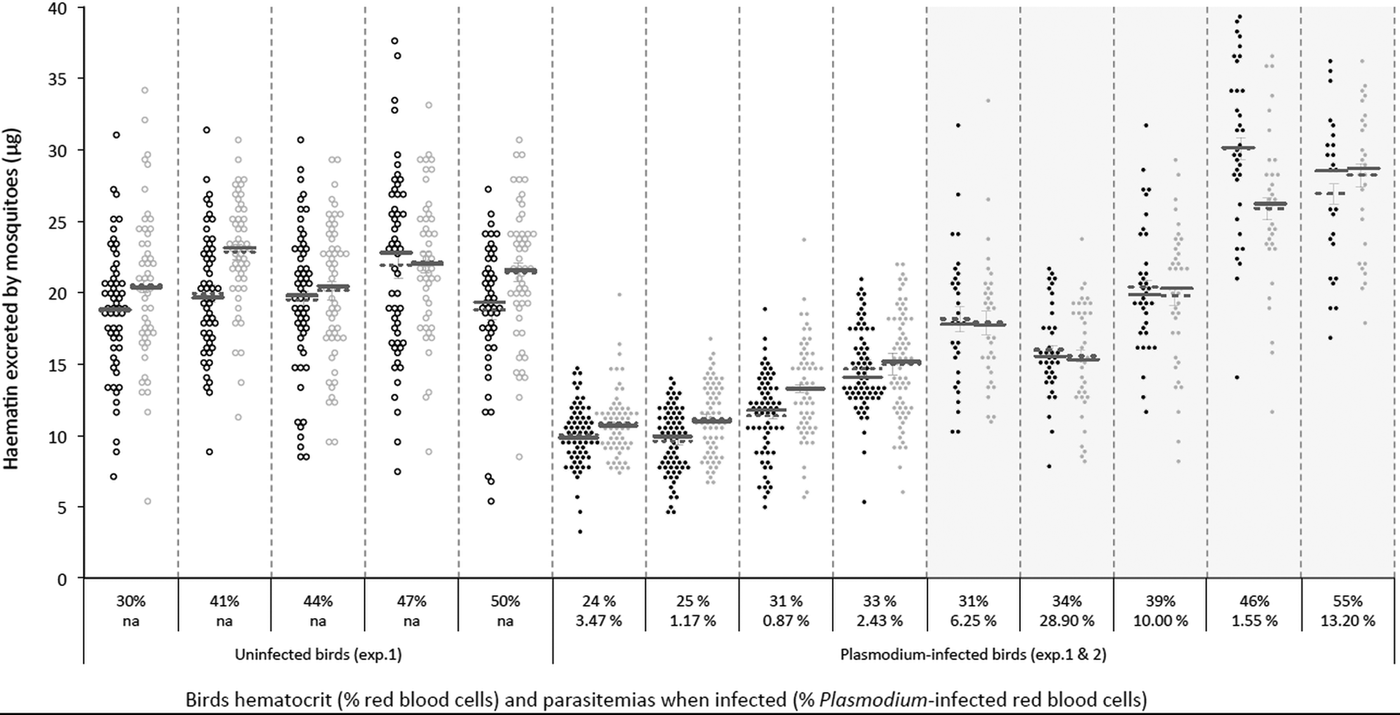
Fig. 1. Distribution plot of the amount of haematin excreted by both Cx. pipiens lines according to the haematocrit (quantified as % Packed Cell Volume; upper level x-axis labels) and the Plasmodium infection intensity (quantified as % red blood cells infected; second level x-axis labels) of the birds they fed on. Wolbachia-infected (wSL; grey circles) and uninfected (w(-); black circles) mosquitoes exposed (full circles) or not (empty circles) to Plasmodium infected blood in Experiment 1 (light background) and 2 (shaded background; this experiment only includes Plasmodium-infected females). Horizontal lines represent means (dotted line) and medians (solid lines).
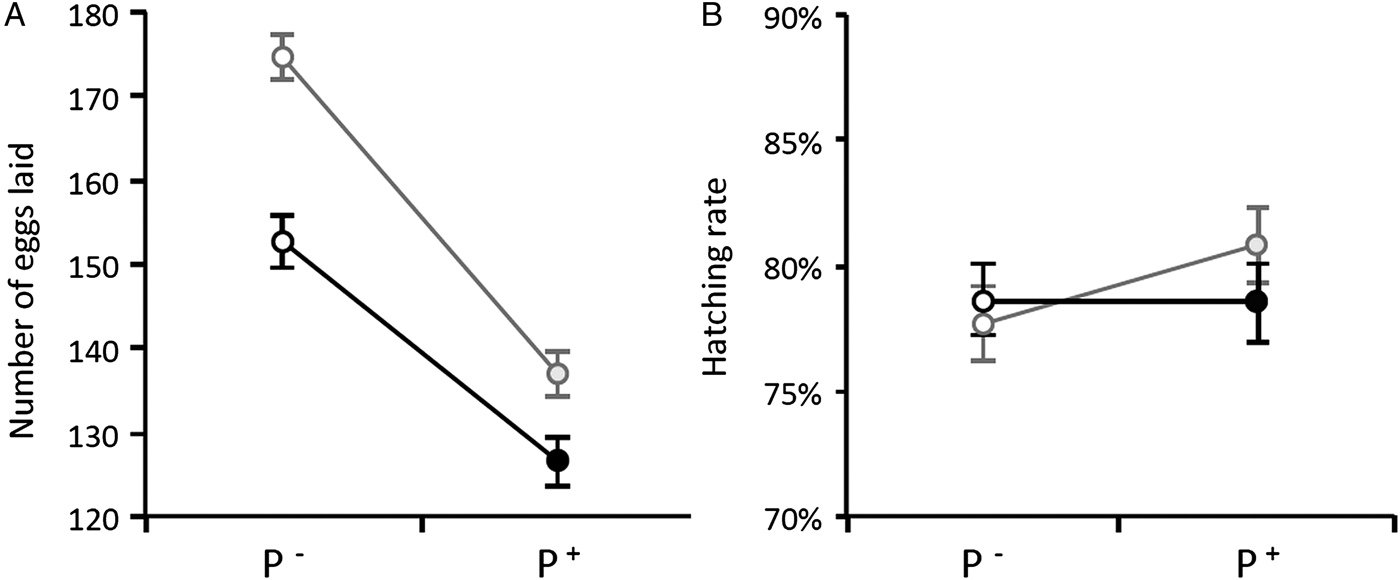
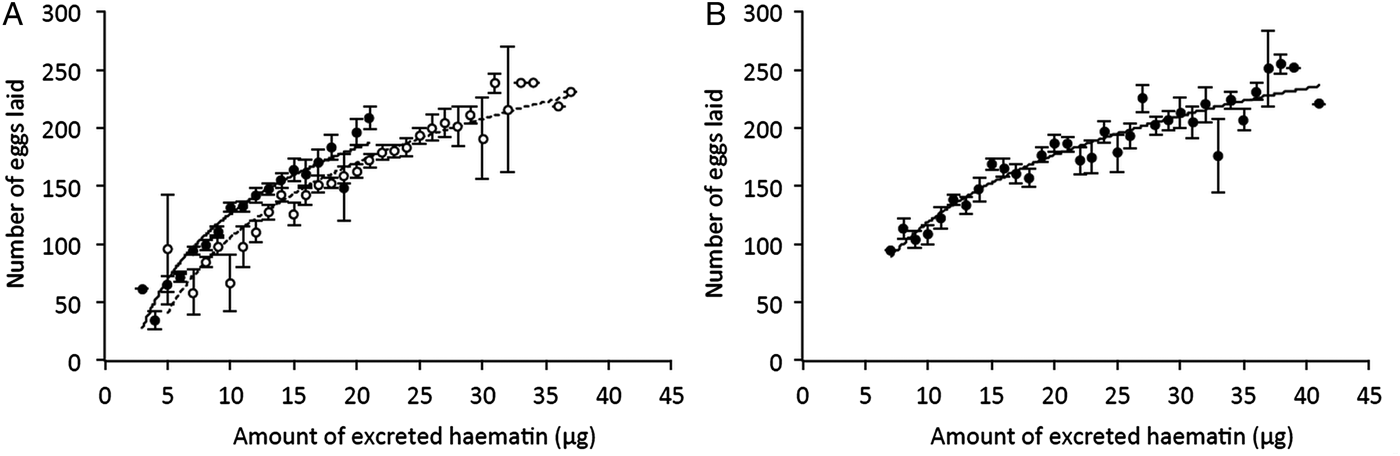
Once the females were transferred to the oviposition tube, it took them on average 3 days to lay their eggs. The number of eggs laid by females (henceforth fecundity) was strongly dependent on whether the females were infected or not by Wolbachia: egg rafts of wSL females contained on average 17 ± 3 eggs more than rafts from w(-) (model 5: χ 21 = 37.48, P < 0.0001). Fecundity was also strongly dependent on whether the females were infected or not by Plasmodium: egg rafts of Plasmodium-infected females contained on average 32 ± 3 fewer eggs than rafts from their uninfected counterparts (model 5, χ 21 = 7.76, P = 0.005, Table S2). There was, however, no significant interaction between the infection by both parasites: the Plasmodium-induced fecundity reduction was not buffered by the presence of Wolbachia (model 5, χ 21 = 2.32, P = 0.13, Fig. 2A). As expected, haematin, its quadratic term haematin2, and mosquito size were found to be strong predictors of the amount of eggs laid (model 6, haematin: χ 21 = 87.80, P < 0.0001, Fig. 3A; haematin2: χ 21 = 29.01, P < 0.0001; wing length: χ 21 = 10.08, P = 0.001). However, neither haematin, haematin2, nor mosquito size affect the significance of Wolbachia, Plasmodium or their interaction in the model (model 5 vs model 6).
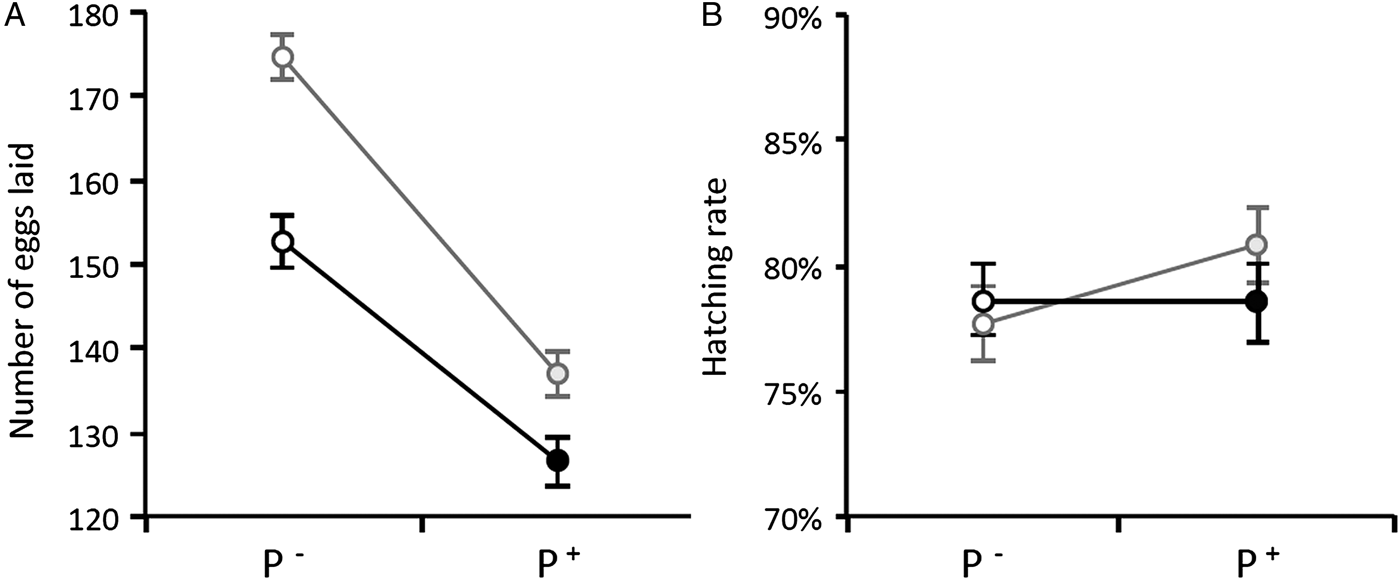
Fig. 2. Wolbachia and Plasmodium infection effects on Cx. pipiens fecundity (A) and egg hatching rate (B) in Experiment 1. Figures represent means ± s.e. Grey circles: wSL females, black circles: w(-) females. Empty circles: females fed on a control bird (P−), full circles: females fed on a Plasmodium-infected bird (P+). Only mosquitoes whose rafts were productive (i.e. from which at least one larva emerged) were included in the analysis.
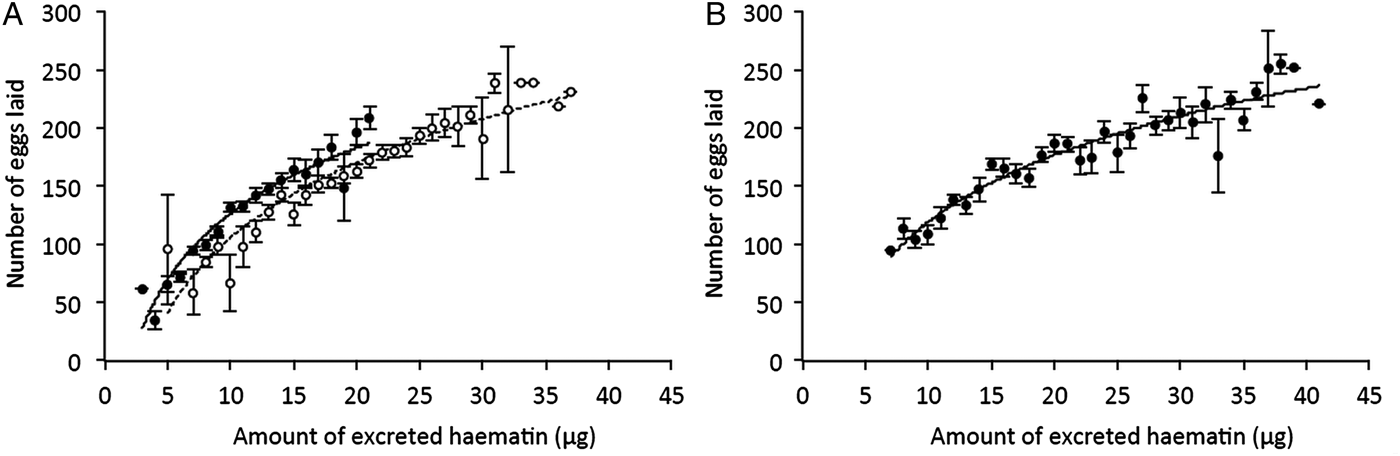
Fig. 3. Relationship between the amount of haematin excreted and the number of eggs laid by Cx. pipiens females in Experiments 1 (A) and 2 (B). Figures represent means ± s.e. number of eggs laid for each haematin value (haematin values were rounded up to the nearest integer) for Plasmodium-infected (full circles) and – uninfected (empty circles) mosquito females (i.e. independently of their Wolbachia infection status; Experiment 2 only includes Plasmodium-infected females). The fitted curves correspond to the logarithmic trend of the data plot.
On average, 97% of the egg rafts laid by Cx. pipiens females were viable (i.e. they showed non-null hatching rates; Table S2). This proportion was positively correlated with the number of eggs contained in the raft (model 7, χ 21 = 58.92, P < 0.0001) but was not influenced by either Plasmodium or Wolbachia (model 7, χ 21 = 0.02, P = 0.88 and χ 21 = 0.34, P = 0.56, respectively). The proportion of larvae hatched in each raft (hatching rate, Table S2) was strongly dependent on the number of eggs contained in the raft, but also on the amount of excreted haematin (model 8, χ 21 = 98.82, P < 0.0001, and χ 21 = 46.66, P < 0.0001, respectively). However, there was no effect of either Wolbachia, Plasmodium, or their interaction on hatching rate (model 8, χ 21 = 0.14, P = 0.71, χ 21 = 1.80, P = 0.18 and χ 21 = 1.68, P = 0.19, respectively; Fig. 2B).
Experiment 2
In this experiment, fecundity and oocystaemia were quantified simultaneously for wSL and w(-) adult Cx. pipiens females fed on six different Plasmodium-infected bird (no uninfected birds were used in this experiment). For unknown reasons, one of the birds resulted in extraordinary high number of oocysts in mosquitoes (up to 1825 oocysts; mean ± s.e. 487 ± 49) compared to all the other ones combined (mean ± s.e. 25 ± 2 oocysts). Therefore, we decided to exclude this bird from the analysis. Despite being kept under identical conditions, and in several rearing trays, w(-) mosquitoes were significantly larger than wSL mosquitoes (mean ± s.e. 3.66 ± 0.01 and 3.62 ± 0.01 for w(-) and wSL, respectively; model 9, F 1,306 = 5.04, P = 0.03; Fig. S1.A; Table S2). As was the case in Experiment 1, the amount of haematin excreted by the two mosquito lines depended on the haematocrit of the bird (model 10, χ 21 = 98.56, P < 0.0001, Fig. 1). In both strains, the number of eggs laid is strongly correlated with haematin (and its quadratic term haematin2, model 14, haematin: χ 21 = 26.42, P < 0.0001; haematin2: χ 21 = 10.48, P = 0.001, Fig. 3B) and mosquito size (χ 21 = 13.94, P = 0.0002). As a result, w(-) females laid a significantly higher number of eggs than wSL ones (mean ± s.e. 188 ± 4 and 166 ± 3 for w(-) and wSL, respectively; model 14, χ 21 = 14.40, P = 0.0001, Fig. 4).
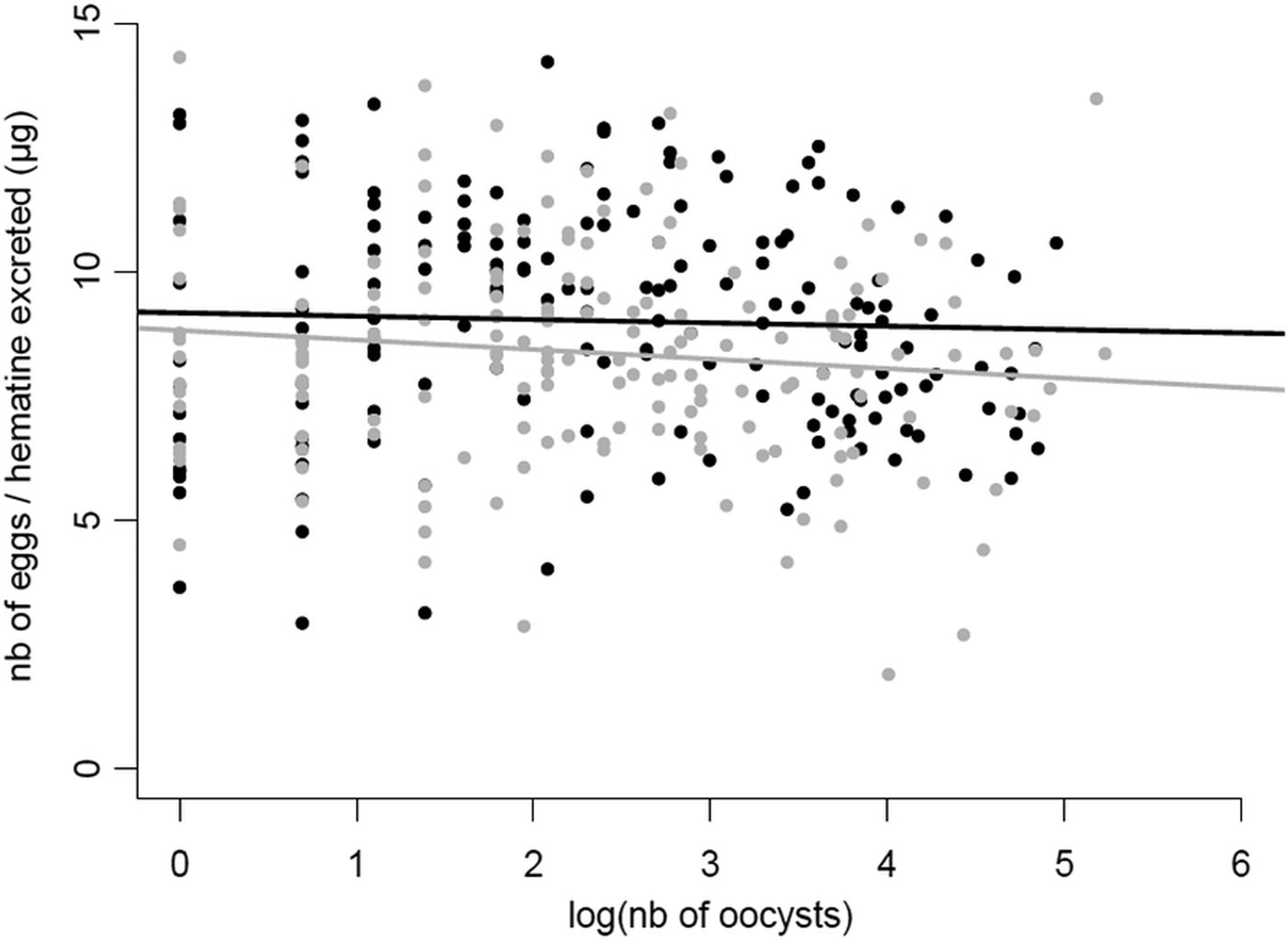
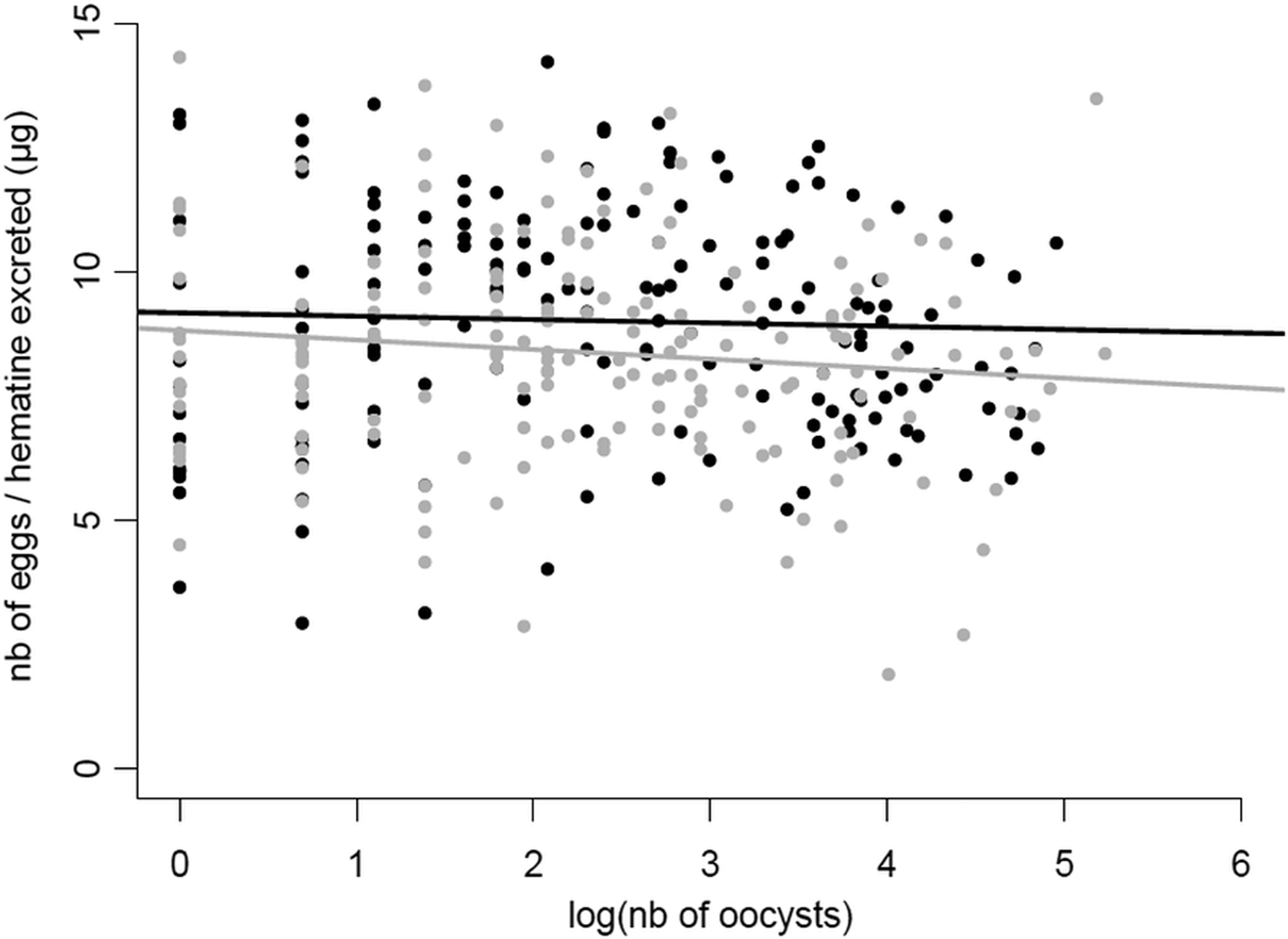
Fig. 4. Relationship between the number of oocysts in the midgut of Cx. pipiens females and the number of eggs they laid in Experiment 2. The number of eggs laid has been corrected by the amount haematin excreted (μg) for each mosquito female. The number of oocysts has been log transformed to improve the fit of the model. The fitted lines correspond to the linear trend of the data plots. Grey dots and line: wSL females, black dots and line: w(-) females.
Contrary to expectations, we found no negative relationship between mosquito fecundity and the intensity of the Plasmodium infection, quantified as the number of oocysts in the mosquito midgut (model 15, χ 21 = 0.90, P = 0.34), and no effect of Wolbachia on the slope of the fecundity-intensity regression (model 15, oocysts*Wolbachia interaction: χ 21 = 1.53, P = 0.22; Fig. 4). Similarly, no significant differences between the two mosquito lines were found either in the prevalence (probability of containing at least one oocyst, model 11, χ 21 = 0.04, P = 0.83) or in the intensity of infection (number of oocysts; model 12, χ 21 = 0.46, P = 0.50; Table S2; Fig. S2).
Discussion
Multiple infections can have many different outcomes on the host's life history traits. Most often than not, these co-infections have cumulative deleterious effects on the host, which are mediated by a higher parasite diversion of host resources or to cumulative damages to the hosts due to a greater overall parasite load (Morand, Reference Morand2011). However, in certain situations, co-infections may lead to lower virulence than single infections, most notably when parasites competitively suppress each other within the host (Balmer et al. Reference Balmer2009; Rodrigues et al. Reference Rodrigues2016). Co-infections involving HTPs and VTPs are also expected to have non-cumulative effects on the host because of the conflicting evolutionary interests associated with these two different transmission strategies. HTPs may be selected to castrate their hosts if this leads to increased transmission (Jaenike, Reference Jaenike1996; O'Keefe and Antonovics, Reference O'Keefe and Antonovics2002), while VTPs are strongly predicted to have a protective effect on host fecundity (Smith and Dunn, Reference Smith and Dunn1991; Ebert and Herre, Reference Ebert and Herre1996). Our results show that Plasmodium drastically decreases the fecundity of Cx. pipiens females by about 20% (Fig. 2A), and that this decrease is all-or-nothing effect which is independent of the parasite's burden (Fig. 4). Wolbachia, on the other hand increases fecundity by roughly 10%, irrespective of whether the mosquito is infected or not by Plasmodium (Fig. 2A) but does not alter the tolerance (quantified as the slope of the fecundity-burden relationship) of mosquitoes to a Plasmodium infection (Fig. 4). Overall, although Wolbachia-infected mosquitoes fare better than uninfected ones, Wolbachia does not confer a sufficiently higher reproductive boost to mosquitoes to compensate for the large reproductive losses inflicted by Plasmodium. Below, we discuss the potential mechanisms and implications underlying the conflicting effects of these two symbionts on mosquito reproduction.
Plasmodium effects on fecundity
Drastic reductions in mosquito fecundity are a common outcome of Plasmodium infections (reviewed in Hurd, Reference Hurd2003, Reference Hurd2009), and agree with previous results obtained in our laboratory with this same experimental system (Vézilier et al. Reference Vézilier2012). The reproductive curtailment induced by Plasmodium has been widely assumed to be an adaptive strategy of the parasite aimed at increasing mosquito survival (Schwartz and Koella, Reference Schwartz and Koella2001; Ferguson et al. Reference Ferguson, Rivero and Read2003; Hurd, Reference Hurd2003). This appealing hypothesis rests on the crucially important role of mosquito longevity for Plasmodium transmission (Vézilier et al. Reference Vézilier2012). To date, however, the issue of whether the Plasmodium-driven fecundity reduction is an adaptive strategy of the parasite aimed at increasing longevity or whether it is simply the result of a competition for nutrients between the parasite and the mosquito remains unresolved.
In Anopheles mosquitoes, Plasmodium seems to reduce fecundity through a combination of an impaired intake of yolk protein by the ovaries (Hogg et al. Reference Hogg, Carwardine and Hurd1997; Ahmed et al. Reference Ahmed2001), and an increase in egg resorption (Carwardine and Hurd, Reference Carwardine and Hurd1997; Hopwood et al. Reference Hopwood2001) mediated by follicular cell apoptosis (Ahmed et al. Reference Ahmed2001). Whether similar mechanisms operate in Plasmodium-infected Cx. pipiens mosquitoes remains to be established. Reduction in fecundity may also operate through a Plasmodium-driven reduction in the quantity or the quality of the blood meal, either because nutrients are scavenged by the parasite or as a host's response to the infection. Plasmodium infected hosts are strongly anaemic (Experiment 1, Fig. 1), and anaemia reduces the amount of protein available for egg production. As a result, mosquitoes having fed on a Plasmodium-infected bird excreted significantly less haematin than uninfected ones (Fig. S1.B). Comparison of uninfected and infected birds from Experiment 1 (Fig. 1), and a significant interaction between haematocrit and Plasmodium infection, however, suggest that haematin excretion is lower in Plasmodium infected mosquitoes despite similar haematocrit values, suggesting that, in addition to feeding on poor quality blood, mosquitoes feeding on infected birds may also have taken smaller blood meals (note that such comparison is not possible in Experiment 2 as all birds were infected). Finding a satisfactory method to quantify blood meal size that is independent of the amount of haemoglobin in the blood, the main drawback of haematin, would go a long way towards disentangling the effects of anaemia and clarifying the central role seemingly played by blood meal size in these interactions (Pigeault et al. Reference Pigeault2015).
Wolbachia effects on fecundity
Although Wolbachia is a VTP and therefore would benefit from increasing mosquito fecundity, the ability of this parasite to induce CI allows it to invade host populations even in the absence of a fitness advantage. Invasion has even been observed when Wolbachia exerts a negative effect on key host fitness components such as fecundity (Xi et al. Reference Xi, Khoo and Dobson2005; Hoffmann et al. Reference Hoffmann2011; Walker et al. Reference Walker2011; Nguyen et al. Reference Nguyen2015). Our results from Experiment 1, however, show a beneficial effect of Wolbachia on Cx. pipiens fecundity. The previous work on natural systems has shown either a beneficial effect (Dobson et al. Reference Dobson, Marsland and Rattanadechakul2002; Baton et al. Reference Baton2013) or no effect (Rasgon and Scott, Reference Rasgon and Scott2003) of Wolbachia infection status on mosquito fecundity. These results however contrast with the fecundity reductions found in artificial Wolbachia–mosquito combinations (Xi et al. Reference Xi, Khoo and Dobson2005; McMeniman et al. Reference McMeniman2009; Joshi et al. Reference Joshi2014; but see Fu et al. Reference Fu2010). Although the Wolbachia-induced fecundity boost persists when mosquitoes are infected with a partially castrating parasite such as Plasmodium, the increase is not sufficient to compensate for the drastic Plasmodium-driven losses (Fig. 2A).
These results could not be replicated in Experiment 2. In Experiment 2, mosquitoes were significantly larger than those in Experiment 1, and w(-) females were significantly larger than wSL females (Fig. S1.A). One potential explanation for these size differences is that they may have been the result of an unintentional increase in larval food dose on one of the feeding days (see above), although it is less clear to us why this would have given a size advantage to the Wolbachia-free line. Irrespective of the underlying mechanism, the result of this experiment is likely to have been biased by this unexplained size differences between the two mosquito lines, as size is positively correlated with both blood meal size and fecundity.
The mechanisms underlying this increase in fecundity are unknown. In Drosophila mauritiana flies, Wolbachia-infected females produce about four times more eggs than their non-infected counterparts through the combined action of an increased mitotic activity, and a decreased programmed cell death (apoptosis) in the fly's germarium (Fast et al. Reference Fast2011). Inhibition of apoptosis by Wolbachia was also found to be necessary for oogenesis completion in the wasp Asobara tabida (Bazzocchi et al. Reference Bazzocchi2007; Pannebakker et al. Reference Pannebakker2007). Most remarkably, Wolbachia have the genetic machinery to influence iron utilization of hosts (Brownlie et al. Reference Brownlie2009; Kremer et al. Reference Kremer2009). Indeed, in Wolbachia the expression level of iron metabolism genes is directly influenced by the presence of iron in the diet (Kremer et al. Reference Kremer2009) and this regulation can confer fecundity benefit for hosts reared on iron-restricted or -overloaded diets (Brownlie et al. Reference Brownlie2009). As iron is an abundant compound of vertebrate blood, Wolbachia may thus play a pivotal, albeit unrecognized, role as nutritional mutualist in mosquitoes. In this regard, a recent observation in Anopheline mosquitoes is worthy of interest: Wolbachia has the capacity to increase the rate at which eggs were laid (Shaw et al. Reference Shaw2016), suggesting that it accelerates blood meal digestion and/or egg maturation. Further work needs to be carried out in our system to establish the mechanism underlying the increase in fecundity observed in Wolbachia-infected mosquitoes.
Wolbachia effects on tolerance to Plasmodium
The slope of loss of fitness with parasite burden has been termed ‘tolerance’ and is an essential concept in disease ecology as it can have drastic consequences for both the evolution and epidemiology of diseases (Raberg et al. Reference Raberg, Sim and Read2007; Read et al. Reference Read, Graham and Raberg2008). Partial castration is the most common virulence phenotype in mosquitoes infected with Plasmodium, with up to 40% of eggs being lost to the infection (Vézilier et al. Reference Vézilier2012). However, to our knowledge, no study has investigated whether the loss of eggs in mosquitoes is a function of Plasmodium burden, although an early report comparing the fecundity of mosquitoes with low and high oocyst burdens showed no significant difference between the two groups (Hogg and Hurd, Reference Hogg and Hurd1995). Here we show, for the first time, that the intensity of the Plasmodium infection is not correlated with the degree of reproductive loss (Fig. 4) which suggests that resource competition may not be a major driver of the fecundity reduction. These results contrast with the correlation found between parasite density and mosquito survival rates, albeit in a non-natural mosquito–Plasmodium combination (Dawes et al. Reference Dawes2009).
While most studies are entirely focused on tolerance as a genetically determined trait, environmental factors such as resource availability, can also influence tolerance (Sternberg et al. Reference Sternberg2012). To our knowledge, the role of endosymbiotic bacteria as a factor in tolerance to disease in mosquito vectors has never been explicitly investigated. Here we show that, under our particular set of experimental conditions, Wolbachia does not have an effect on the slope of mosquito fecundity and Plasmodium burden. Further work needs to be done to establish whether mosquito tolerance to Plasmodium may be modulated by other environmental factors such as temperature (Murdock et al. Reference Murdock2014) or nutrient availability (Caragata et al. Reference Caragata2016).
Concluding remarks
This is the first study aiming to determine the outcome of the co-infection between Wolbachia and Plasmodium on mosquito fecundity. We show that, Wolbachia-infected mosquitoes fare better in terms of fecundity than their uninfected counterparts but that Wolbachia does not protect its host against the detrimental effect of Plasmodium on their fecundity. These and previous results showing that Wolbachia buffers the effects of Plasmodium on mosquito longevity (Zélé et al. Reference Zélé2012) support the hypothesis by which Wolbachia-carrying females are generally in better condition than those from which Wolbachia has been cleared, lending them more resilient against the negative effects of concomitant parasites. This so-called ‘Jeckyll and Hide’ strategy may contribute to explain why Wolbachia is near to or at fixation in worldwide populations of the C. pipiens complex (Duron et al. Reference Duron2005). Our results thus open up interesting question about the potential implications of Wolbachia on the transmission dynamic of malaria parasites and may have implications for the use of Wolbachia as a potential tool to combat malaria.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0031182017001330.
Acknowledgements
The authors would like to thank Gabriele Sorci for providing us with the parasite isolates, Mylène Weill's group for the mosquito strains, Antoine Nicot, Julien Vézilier and Romain Pigeault for their help in the experiment. We also thank Pascal Boutinaud and Nathalie Barougier for their help in maintaining the canaries in the animal house and Philippe Perret for his invaluable help in all bird matters. Radiant Color NV kindly provided the mosquito pigments.
Financial support
This project is funded by a grant from the Agence Nationale de la Recherche (grant number ANR 07SEST009 IRMAL) to A.R, and F.Z. was funded by a Ph.D. grant from the CNRS and the Languedoc-Roussillon Region.