Introduction
The free-living nematode Caenorhabditis elegans was introduced as a model organism more than half a century ago by Sidney Brenner (Brenner, Reference Brenner2002). Since then, this 1 mm free-living soil nematode has been extensively studied in the laboratory, and C. elegans research has contributed to the discovery and understanding of many biological processes relevant to other multicellular organisms. A milestone discovery was the elucidation of the origin and fate of each of the 959 somatic cells of this organism (Sulston and Horvitz, Reference Sulston and Horvitz1977). This allowed development from the perspective of the individual cell to be understood. Other landmarks were the reconstruction of the complete set of synaptic connections in its nervous system (White et al. Reference White, Southgate, Thomson and Brenner1986) and the discovery of apoptosis genes (Hedgecock et al. Reference Hedgecock, Sulston and Thomson1983). Equally important, C. elegans-driven research has allowed the development of new techniques and tools for broad applications. The introduction of GFP as an in vivo reporter brilliantly illustrates this concept (Chalfie et al. Reference Chalfie, Tu, Euskirchen, Ward and Prasher1994). Another key discovery made in C. elegans is gene silencing by RNA interference (RNAi) (Fire et al. Reference Fire, Xu, Montgomery, Kostas, Driver and Mello1998). A timeline of significant discoveries made in C. elegans can be found in (Corsi et al. Reference Corsi, Wightman and Chalfie2015). The success of C. elegans as a metazoan model lies in its biological simplicity combined with its easiness to grow, manipulate and perform genetics studies. Key attributes are its rapid life cycle (~3 days) (Fig. 1A), large progeny (~300 eggs per generation), low maintenance cost, long-term cryopreservation, transparency and its invariant cell number and development (Hall and Altun, Reference Hall and Altun2008). The intensive study of this organism has led to the accumulation of knowledge and to the development of a large repertoire of tools and resources over time. A list of high-quality C. elegans resources freely available is provided in Table 1. Importantly, since its inception the C. elegans research community promotes sharing of reagents and ideas.

Fig. 1. Caenorhabditis elegans is a nematode (A) Life cycle of C. elegans. The length of time the animal spends at certain stages at 22 °C is indicated in hours (h). Eggs of approximately 30 cells are laid outside at about 40 min post-fertilization. Embryonic development continues ex utero during 9 h. After hatching as L1 (558 cells) development, continues under favourable conditions, through four stages interrupted by moulting. Approximately 300 eggs are laid per generation per adult worm. Under adverse conditions (e.g. starvation, crowding, high temperature), C. elegans enters the diapause cycle (dark grey) and gives rise to dauer larva (an enduring larval form), which can survive for up to 4 months. There are two C. elegans sexes, a self-fertilizing hermaphrodite (XX, 959 somatic cells) and a male (XO, 1031 somatic cells). Males arise infrequently (0·1%) by spontaneous non-disjunction in the hermaphrodite germ line and at a higher frequency (up to 50%) through mating (hermaphrodites can also be induced to generate male progeny spontaneously at a higher rate by treatment at high temperature) (Hall and Altun, Reference Hall and Altun2008). (B) Simplified phylogeny of the Phylum Nematoda. In all groups, there are free-living and parasitic species. Traditional clades (I–V) are indicated. Caenorhabditis elegans belongs to clade V, which includes several vertebrate parasitic species such as Necator spp. and Haemonchus spp. The phylogeny is based on (Blaxter and Koutsovoulos, Reference Blaxter and Koutsovoulos2015), Enoplia is a now recognized as a basal lineage (Mark Blaxter, personal communication).
Table 1. Open-access informational and experimental resources

For helminth parasitologists, and particularly for those who work in parasitic nematodes, C. elegans provides additional advantages. In contrast to C. elegans, nematode parasites are inherently difficult to grow, maintain and manipulate, and only in rare cases their lifecycle can be maintained in the laboratory. Indeed, none of the medically relevant parasitic nematodes can be maintained through the entire lifecycle without passing through a host. Nematodes are monophyletic (Blaxter and Koutsovoulos, Reference Blaxter and Koutsovoulos2015) and have a remarkably similar anatomy, development plan and life cycle through four larval stages (Fig. 1B). It is worth mentioning that C. elegans resistant and dispersal larval stage, the dauer larval stage (Fig. 1A), closely resembles free-living L3 stage of parasitic nematodes (Crook, Reference Crook2014). The dauer stage is highly resistant to stress. It acts as a dispersal stage, either through its own locomotion or by hitchhiking on a larger invertebrate to resume development as L4 in a propitious environment (Felix and Braendle, Reference Felix and Braendle2010). It is thought that this resistant larval stage may have been the evolutionary precursor of the L3 infective stage, and important for the recurrent emergence of parasitism from free-living species in the nematode lineage (Crook, Reference Crook2014).
How this amenable free-living relative that naturally lives in the soil can serve as a powerful tool for many studies in which nematode parasitologists are interested will be reviewed in following sections.
WormBase and WormBase parasite: key resources in an information ERA
There is a wealth of open access resources for those working in C. elegans and nematodes that provide information on a range of topics (Table 1). WormBase (http://www.wormbase.org) is a central repository for research data on the biology, genetics and genomics of C. elegans and other nematodes. WormBase integrates several online resources (see Table 1), such as Textpresso, an information extracting and processing package for the biological literature. WormBook, the online text companion to WormBase, is a comprehensive and open-access resource covering topics related to the biology of C. elegans and other nematodes. Indeed, several chapters dedicated to parasitic nematodes help to bridge the C. elegans and the parasitic nematode research communities. In this review, we will emphasize the genomic and gene information currently available through Wormbase and its recent relative WormBase ParaSite (Howe et al. Reference Howe, Bolt, Cain, Chan, Chen, Davis, Done, Down, Gao, Grove, Harris, Kishore, Lee, Lomax, Li, Muller, Nakamura, Nuin, Paulini, Raciti, Schindelman, Stanley, Tuli, Van Auken, Wang, Wang, Williams, Wright, Yook, Berriman, Kersey, Schedl, Stein and Sternberg2016, Reference Howe, Bolt, Shafie, Kersey and Berriman2017).
Caenorhabditis elegans was the first animal genome to be sequenced, curated and annotated and became the animal reference genome (Consortium, Reference Consortium1998). This contributed to accelerating genome sequencing and annotation of the human and other animal genomes, notably those of nematode species. Caenorhabditis elegans genome is manually revised and refined incorporating literature information and RNAseq data (mRNA and non-coding RNA) from experiments, and updated regularly by WormBase curators. RNA-seq is important for transcriptomic profiling and to identify novel transcripts and alternative splicing events. In turn, this provides accurate gene models and genome annotation for further studies. Importantly, WormBase contains information on resources for each gene (e.g. gene model, clones, mutant strains), functional information of genes and gene products (e.g. phenotypes), and its interface allows easy exploration of data. Recently, FPKM (fragment per kilobase of exon per million reads mapped) expression values of cDNA libraries across all stages of C. elegans life cycle have been included in Wormbase. This transcript profiling data includes, in addition to the classical stages an embryonic time series at 30- min intervals (Hashimshony et al. Reference Hashimshony, Feder, Levin, Hall and Yanai2015). This provides a correlation between developmental stages and their underlying molecular activity. For genes that have parasitic nematode orthologues this transcriptomic profiling may serve as a proxy and to generate and test-specific hypothesis.
Fortunately, the number of both free-living and parasitic worm genomes sequenced has sharply increased in the last years due to independent and combined efforts such as the 959 nematode genome initiative (http://www.nematodes.org/nematodegenomes/index.php/Main_Page). In response to the challenge of having good quality genomes annotated a new sub-portal from WormBase, WormBase ParaSite, has recently been launched as a collective effort (Howe et al. Reference Howe, Bolt, Cain, Chan, Chen, Davis, Done, Down, Gao, Grove, Harris, Kishore, Lee, Lomax, Li, Muller, Nakamura, Nuin, Paulini, Raciti, Schindelman, Stanley, Tuli, Van Auken, Wang, Wang, Williams, Wright, Yook, Berriman, Kersey, Schedl, Stein and Sternberg2016, Reference Howe, Bolt, Shafie, Kersey and Berriman2017). Both databases include three parasitic genomes (Brugia malayi, Onchocerca volvulus and Strongyloides ratti) for which physical maps, careful automatic and manual annotation are continuously being refined (Howe et al. Reference Howe, Bolt, Cain, Chan, Chen, Davis, Done, Down, Gao, Grove, Harris, Kishore, Lee, Lomax, Li, Muller, Nakamura, Nuin, Paulini, Raciti, Schindelman, Stanley, Tuli, Van Auken, Wang, Wang, Williams, Wright, Yook, Berriman, Kersey, Schedl, Stein and Sternberg2016). WormBase ParaSite is publicly updated three times a year and its release 7 (April 2017) includes 84 nematode species (101 genomes) as well as 30 platyhelminthes species (33 genomes). RNAseq expression data from experiments in eight helminth species has also been included in WormBase ParaSite, helping to improve gene models. The database allows comparative genomics to be performed easily and provides foundations for the application of modern functional genomics approaches to this group of pathogens. Importantly, the WormBase community forum has approximately 2000 members that discuss general nematode biology issues and Wormbase curators provide help to users.
Similar to WormBase, genes can be searched in WormBase ParaSite by a variety of criteria (including gene name, blast tools, text mining) through an amenable browser. For a given gene different information can be easily explored (e.g. transcript models, functional annotations, cross-references to other resources, genomic context). The gene page is the main entrance to explore helminth comparative genomics and infer the history of genes and gene families (Fig. 2A). The pipeline organizes worm and other reference species genes in homologous clusters, constructs protein multiple alignments for each cluster, and produces a gene tree which takes into account sequence-based phylogeny and the species phylogeny, which can be used to understand gene history by speciation and gene duplication (Fig. 2B). Advanced search tools and export data is available through the Biomart tool available on the site. This advanced searcher allows the generation of lists of genes of interest (e.g. phosphatases) and to retrieve specific information about them. Future plans for WormBase ParaSite include the identification of putative targets for anthelmintic drugs and linking gene products to the ChEMBL database of medicinal chemistry (Davies et al. Reference Davies, Nowotka, Papadatos, Dedman, Gaulton, Atkinson, Bellis and Overington2015). This is important for parasitologists whose primary goal is to identify ways of controlling parasites. Finally, a Worm Community Forum and Blog allow researchers to stay connected and updated with new features and data.
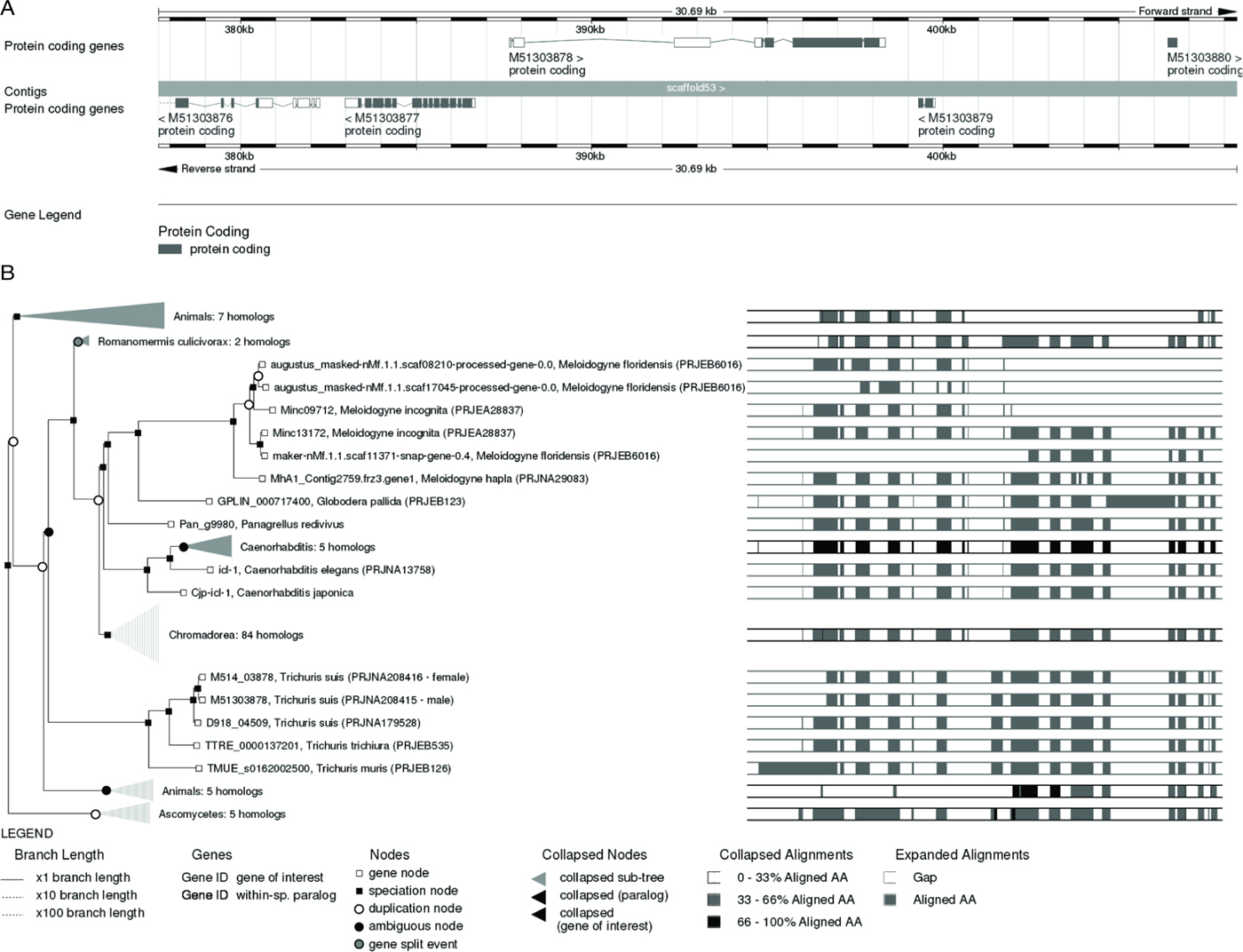
Fig. 2. WormBase and WormBase ParaSite: useful tools for nematode research. (A) Typical gene model provided by WormBase ParaSite. Gene model for bifunctional glyoxylate cycle protein isocitrate lyase-malate synthase from Trichuris suis (Gene ID: M51303878), projected from C. elegans orthologue icl-1. Detailed information about the gene is available at the gene page; it is possible to inspect the region for additional features or detailed information of the gene through friendly navigation options (e.g. zooming to see the nucleotide and protein sequences). (B) Gene tree provided by WormBase ParaSite. For any given gene WormBase ParaSite provides information on transcript variants (gene node, open square), orthologue genes (speciation node, black square) and paralogue genes (duplication nodes, open circle). Specific nodes can be collapsed or expanded according to particular needs. The information provides the gene models for the species selected. The gene tree for isocitrate lyase-malate synthase (icl-1) is shown.
Caenorhabditis elegans genetic tools and knowledge can serve nematode parasitologists in several ways
Caenorhabditis elegans has key attributes to understand biological processes from a genetic viewpoint, leading to a continuous development of powerful genetics tools (Corsi et al. Reference Corsi, Wightman and Chalfie2015). There are two C. elegans sexes: a self-fertilizing hermaphrodite (XX) and a male (XO). Males arise infrequently (0·1%) by spontaneous non-disjunction in the hermaphrodite germ line and at a higher frequency (up to 50%) through mating. Self-fertilization of the hermaphrodite allows for homozygous worms to generate genetically identical progeny, and male mating facilitates the isolation and maintenance of mutant strains as well as moving mutations between strains (http://www.wormatlas.org/hermaphrodite/introduction/Introframeset.html). The transparency of the organism and the ease at which this organism can be mutagenized and genetically manipulated by transgenesis has greatly helped to consolidate this model organism. Microinjection of DNA into the gonads leads to stable extrachromosomal arrays that are expressed and inherited during several generations after transformation. This has been extremely informative for studying the spatial and temporal expression of genes in a live organism, to dissect gene regulatory elements and to directly interrogate gene function. RNAi by feeding is also easy and convenient for interfering with gene expression.
New technologies are accelerating the pace: CRISPR/Cas9 is revolutionizing genetics and genome editing in C. elegans (Dickinson and Goldstein, Reference Dickinson and Goldstein2016) and Next-Generation Sequencing-based approaches being used for rapid mutation mapping and identification (Doitsidou et al. Reference Doitsidou, Jarriault and Poole2016). The resources available to researchers are unparalleled (Table 1). There is a wide and ever-increasing repertoire of vectors for different genetic studies. The existence of comprehensive RNAi libraries is another example. Worm strains can be requested at low cost from the Caenorhabditis Genetics Center, which is dedicated to collect, maintain and distribute C. elegans strains and host an enormous repository of mutant and reporter strains.
In contrast to the advances made in C. elegans genetics, progress in genetics studies in parasitic nematodes has lagged behind (Ward, Reference Ward2015; Zamanian and Andersen, Reference Zamanian and Andersen2016). A major difficulty has been the impossibility to maintain parasite life cycles outside a host. The most advanced genetic studies in nematode parasites have been achieved in Strongyloides spp. and Parastrongyloides spp., which alternate parasitic and free-living generations; a thorough recent review on the genetics of these organisms is presented in (Streit, Reference Streit2017). It was soon realized that the toolkit of C. elegans required adjustments for performing genetics in these species (e.g. the use of specific 5′ and 3′ UTR (untranslated region) sequences to obtain effective transient transgene expression). Microinjection of DNA into the gonad of free-living Strongyloides females was achieved and expression observed after infection in an experimental host; this constituted a milestone that highlighted the potential of transgenesis for studying host–parasite interactions (Li et al. Reference Li, Shao, Junio, Nolan, Massey, Pearce, Viney and Lok2011). Although the arrays were inherited by several generations, silencing was observed after the F1 generation. The following key advance was the heritable and stable expression of an S. ratti transgene (Ss-act-2) integrated into the chromosome by means of the piggyBac system (Shao et al. Reference Shao, Li, Nolan, Massey, Pearce and Lok2012). Through alternation of host and culture passage with GFP selection, a stable transgenic line of S. ratti was established. The high efficiency of infection of this rat parasite was instrumental to this achievement. Recently, another milestone was achieved: a proof of principle of target-specific insertional mutagenesis via CRISPR/Cas9 was reported in Strongyloides stercoralis (Lok et al. Reference Lok, Shao, Massey and Li2017). Lok and coworkers succeed using this methodology inserting a 24 bp sequence containing stop codons in all frames of exon 5 of the insulin-transcription factor Ss-daf-16, resulting in a null mutation. In a recent study, heritable Ss-unc-22 mutations generated by CRISPR/Cas9 succeeded in passing a mutant F1 progeny through a laboratory host and collected F2/F3 nematodes with Ss-unc-22 phenotypes (Gang et al. Reference Gang, Castelletto, Bryant, Yang, Mancuso, Lopez, Pellegrini and Hallem2017). This mutant provides the same uncoordinated twitching phenotype as the C. elegans ortholog and this can be used as a marker to select for transgenic worms. Importantly, in this study the CRISPR/Cas9-induced double-strand breaks and the presence of a homology-directed repair template improved targeting efficiency and produced Ss-unc-22 homozygous knockouts (KO) in the F1 generation. This suggests a practical method to render the mutation homozygous, which a priori appeared as another bottleneck.
Strongyloides spp. with their key advantages and the most advanced toolkit still illustrates the difficulties in performing genetic studies in parasitic nematodes. The challenge ahead for most parasitic nematodes is enormous, and the techniques that have been established for C. elegans are not easily transferable to the research on other parasitic worms. For most parasites, a key problem is the inaccessibility to the gonad of the adult worms. Particle bombardment has been explored as an alternative, but in practice led to high mortality (Ward, Reference Ward2015). The thick cuticle of nematodes is a barrier for electroporation and chemical-mediated DNA transformation. The moulting of the cuticle can provide a window at which the organism may be more susceptible to the entrance of foreign DNA. The discovery of viruses that infect Caenorhabditis spp. (Felix and Braendle, Reference Felix and Braendle2010) may eventually offer a vehicle for gene delivery, but this has not been tried in the model organism, and remains speculative. Recent reviews have addressed in detail the limited advances in functional genomics and genome-editing techniques made in Brugia spp. and Haemonchus contortus (Ward, Reference Ward2015; Britton et al. Reference Britton, Roberts and Marks2016; Zamanian and Andersen, Reference Zamanian and Andersen2016). These two relevant human and livestock nematode parasites are among those in which research in genetics approaches have concentrated. RNAi has found limited application in these parasites, the translation of CRISPR/Cas9 technology to these parasites still appears distant. Some critical needs are: (i) the development of reliable and robust methods of transformation, (ii) appropriate vectors and markers that allow stable expression and easy selection as well as vectors that allow conditional or regulatable transgene expression for essential genes (Ward, Reference Ward2015).
Caenorhabditis elegans may provide a surrogate system to study parasite gene function. The characterization of parasite genes by heterologous expression in C. elegans provides a tool for studying the cellular functions of specific genes. If a parasite protein is similar enough to the C. elegans protein, it could be used to test recovery of a phenotype in a C. elegans KO strain. Caenorhabditis elegans mutant libraries can also be exploited by nematode parasitologists. The Million Mutation Project, a library of more than 2000 C. elegans strains with an average of eight non-synonymous mutations for all 20 000 C. elegans protein-coding genes (Thompson et al. Reference Thompson, Edgley, Strasbourger, Flibotte, Ewing, Adair, Au, Chaudhry, Fernando, Hutter, Kieffer, Lau, Lee, Miller, Raymant, Shen, Shendure, Taylor, Turner, Hillier, Moerman and Waterston2013). The Gene Knockout Project has generated over the years another useful library containing strains with loss of function mutations in more than 14 000 of the 20 000 worm's protein-coding genes (Hutter and Moerman, Reference Hutter and Moerman2015). The National Bioresource Project also stores and distributes its own C. elegans deletion mutants. These libraries can be readily exploited for many uses such as reverse genetic approaches to explore specific ligand (e.g. drug) binding sites (Mathew et al. Reference Mathew, Mathew, Miller, Simpson, Au, Garland, Gestin, Edgley, Flibotte, Balgi, Chiang, Giaever, Dean, Tung, Roberge, Roskelley, Forge, Nislow and Moerman2016) and forward genetics (i.e. random mutagenesis followed by screening selection).
Despite the advantages of C. elegans as a model, there are limitations. There is a large number of species-specific genes in parasitic nematodes with no homologues in C. elegans (and vice versa), precluding the use of C. elegans as a surrogate system for those cases. Approximately 25% of genes are unique to each species within the phylum Nematoda (Parkinson et al. Reference Parkinson, Mitreva, Whitton, Thomson, Daub, Martin, Schmid, Hall, Barrell, Waterston, McCarter and Blaxter2004). Furthermore, other genes have undergone lineage-specific duplications (Blaxter and Koutsovoulos, Reference Blaxter and Koutsovoulos2015). There are also inherent limitations of heterologous expression studies. Caenorhabditis elegans may not reproduce the native expression pattern of a parasite gene. Differences in regulatory elements may vary between species and lead to differences in tissue-specific expression or temporal regulation. These limitations illustrate the necessity for working with C. elegans and the parasite of interest in parallel.
Caenorhabditis elegans as a model in anthelmintic research
About one-quarter of the world population is estimated to be infected with parasitic nematodes, mainly in poor developing countries. In addition, food production is greatly reduced by animal and crop nematode infections causing large economic losses across the globe (Keiser and Utzinger, Reference Keiser and Utzinger2010; Lo et al. Reference Lo, Addiss, Hotez, King, Stothard, Evans, Colley, Lin, Coulibaly, Bustinduy, Raso, Bendavid, Bogoch, Fenwick, Savioli, Molyneux, Utzinger and Andrews2016). There is widespread resistance to currently used drugs in the veterinary field and concern that resistance may arise in humans parasitic nematodes (Besier, Reference Besier2007; Sutherland and Leathwick, Reference Sutherland and Leathwick2011). Thus, new anthelmintics and drug discovery projects are essential.
In this regard, C. elegans drug screenings present a valuable choice. However, there are some considerations to be made. As a free-living nematode, C. elegans has complex and developed detoxification mechanisms as well as a thick cuticle to prevent damage from the wide range of substances that might be present in changing environments as the soil. In order to circumvent this issue, there is a broad spectrum of screening design adjustments available. Some examples are to increase the concentration of the tested compounds, to improve the target accessibility by adding a mild detergent such as Triton X-100 or Tween-20, to sensitize worms with a low dose of a known anthelmintic or to use mutant worms, for example with modified xenobiotic defences or altered cuticle integrity (Burns and Roy, Reference Burns, Roy and Caffrey2012). Another issue is that as C. elegans lacks unique parasitic pathways, a considerable number of active molecules against parasites may be lost. Nonetheless, the advantages of using C. elegans as a primary screening filter (high-throughput capabilities, cost and ease of use) far outweigh its disadvantages.
In the past years, multiple approaches have been carried out; some recent key studies are reviewed in this section. A notable work in this regard was the performance of an extensive C. elegans screen of chemical compounds and re-screen of the active compounds against Cooperia oncophora and H. contortus (Burns et al. Reference Burns, Luciani, Musso, Bagg, Yeo, Zhang, Rajendran, Glavin, Hunter, Redman, Stasiuk, Schertzberg, Angus McQuibban, Caffrey, Cutler, Tyers, Giaever, Nislow, Fraser, MacRae, Gilleard and Roy2015). Molecules that killed C. elegans were 15 times more likely to also kill these parasitic nematodes compared with randomly chosen molecules. In addition, almost 40% of the C. elegans lethal molecules were also lethal to both of the other nematodes tested. This confirms the usefulness of C. elegans for anthelmintic drug discovery. Moreover, the authors proposed a pipeline for the primary screening process, starting with C. elegans as a primary filter, continuing with parasitic nematodes (e.g. C. oncophora, H. contortus) to confirm the nematocidal activity and ending with vertebrate models (e.g. HEK293 cells, zebrafish) to address possible host toxicity.
Several different C. elegans assays have been developed. Changes in worm behaviour and metabolism are the most used readouts, which are usually automated. In the case of metabolism analysis, colorimetric and even fluorescence-based assays have been reported (James and Davey, Reference James and Davey2007; Ferreira et al. Reference Ferreira, Mendes, Bueno, de Araujo, Bartholomeu and Fujiwara2015). Regarding behaviour, the range of approaches is broader. Mathew et al. reported the usage of a scanner to identify decreased motility (Mathew et al. Reference Mathew, Mathew, Miller, Simpson, Au, Garland, Gestin, Edgley, Flibotte, Balgi, Chiang, Giaever, Dean, Tung, Roberge, Roskelley, Forge, Nislow and Moerman2016). The light intensity produced by the scanner was enough to produce negative phototaxis, which allowed worm paralysis to be detected. Recently, Weaver et al. proposed the implementation of a health-rating system to prevent the oversight of possible active drugs in binary dead/alive approximations (Weaver et al. Reference Weaver, May and Ellis2017). There are numerous automatic systems to analyse behaviour changes, including worm tracking by beam interruption or camera displays, targeting specific or general behaviour, microfluidics, among others (Buckingham et al. Reference Buckingham, Partridge and Sattelle2014).
Although C. elegans is a good model organism for anthelmintic screening, the selection of what to screen is equally important. In this regard, there are several options: natural products, chemically synthesized libraries, Food and Drug Administration (FDA)-approved drug libraries, among others. The complexity and molecular diversity that can be obtained from natural sources are vast. Moreover, the most effective anthelmintic family, the avermectins, has arisen from this origin (Cragg and Newman, Reference Cragg and Newman2013). Chemically synthesized libraries, on the other hand, can easily reach huge sizes, allowing the study of more molecules. Besides, active compounds usually can be readily identified and synthesized in great quantities. FDA-approved drug libraries have the advantage that their compounds are known in detail, accelerating future regulatory studies (Panic et al. Reference Panic, Duthaler, Speich and Keiser2014).
In addition to being a model for drug discovery, C. elegans has also been important in defining molecular targets of several nematocidal drugs and understanding the complex phenomena of resistance to anthelmintics. The ability to generate random mutants in the laboratory followed by selection with anthelmintics simulates the natural process that gives rise to the emergence of resistance. The identification of the mutated gene (or genes) that generates resistance creates a link, either direct or indirect, between the gene and the resistance mechanism. This approach used in C. elegans has been important in identifying anthelmintic targets, downstream signalling, metabolization and detoxification (Sloan et al. Reference Sloan, Reaves, Maclean, Storey and Wolstenholme2015; Duguet et al. Reference Duguet, Charvet, Forrester, Wever, Dent, Neveu and Beech2016). A review of the contributions that C. elegans research has made to this specific field has recently been provided in (Holden-Day and Walker, Reference Holden-Day and Walker2014). For instance, C. elegans was instrumental in identifying the target and mutations conferring resistance to monepantel, one of the most recent nematocidal drugs (Kaminsky et al. Reference Kaminsky, Ducray, Jung, Clover, Rufener, Bouvier, Weber, Wenger, Wieland-Berghausen, Goebel, Gauvry, Pautrat, Skripsky, Froelich, Komoin-Oka, Westlund, Sluder and Mäser2008). Caenorhabditis elegans is also useful to uncover new drug targets. Recently, nematode-specific acetylcholine-gated chloride channels have been validated as anthelmintic targets in a C. elegans-based study (Wever et al. Reference Wever, Farrington and Dent2015). This was determined by ectopically expressing AVR-15, an ivermectin-gated chloride channel, in tissues that endogenously express acetylcholine-gated chloride channels and using ivermectin to predict the effect of an agonist drug on them.
Caenorhabditis elegans as a model to study helminth metabolism: a neglected area ready to advance
Caenorhabditis elegans offers an excellent model to understand nematode biochemistry at different organizational levels (cell, tissue, organ and organism). A limitation has been the isolation of large quantities of particular tissues. The development of sensitive metabolomics techniques for analysing small-volume samples [mass spectrometry (MS), gas–liquid chromatography–MS, capillary electrophoresis-MS and nuclear magnetic resonance spectroscopy] should help to reduce this problem. Caenorhabditis elegans reporters for specific genes and pathways would be particularly useful to understand cell/tissue/organ metabolic specializations and contribute to the understanding of this unexplored area. For nematode parasitologists to understand metabolism is also important from a practical viewpoint. Knowing how parasites make a living in the host can provide pharmacological targets to treat infections. In this section, we will focus on key intermediary metabolic and other unique aspects of nematode metabolism that C. elegans shares with parasitic nematodes, but not with the host.
Malate dismutation is a pathway that serves parasitic nematodes (and also platyhelminths) to harvest energy under hypoxic conditions, such as those found in the gastrointestinal tract (Tielens and Van Hellemond, Reference Tielens and Van Hellemond1998). This mitochondrial pathway uses an alternative electron transport chain (ETC) in which fumarate instead of oxygen is the final electron acceptor, and rhodoquinone instead of ubiquinone is an electron carrier (Fig. 3A) (Van Hellemond et al. Reference Van Hellemond, Klockiewicz, Gaasenbeek, Roos and Tielens1995; Kita and Takamiya, Reference Kita and Takamiya2002). Although ubiquinone and rhodoquinone share some steps in their biosynthetic route, rhodoquinone synthesis has not been completely elucidated (Lonjers et al. Reference Lonjers, Dickson, Chu, Kreutz, Neacsu, Anders and Shepherd2012; Mentel et al. Reference Mentel, Rottger, Leys, Tielens and Martin2014). Due to its size, Ascaris suum has been a model nematode for biochemistry. Studies in A. suum have shown that exchange of complex II subunits occurs from the L3 free-living stage to the adult worm, which resides in the pig intestine where there is low oxygen tension (Kita and Takamiya, Reference Kita and Takamiya2002; Iwata et al. Reference Iwata, Shinjyo, Amino, Sakamoto, Islam, Tsuji and Kita2008). This subunit exchange appears to be responsible for allowing complex II to work in reverse direction to the conventional ETC (Fig. 3A). The fact that adjustments in ligand recognition (rhodoquinone vs ubiquinone) and/or redox potentials may be required in complexes I and II might explain that some identified nematocides appear to target these complexes without affecting their mammalian counterpart (Kita, Reference Kita2016). The key metabolite rhodoquinone and the malate dismutation pathway are present in C. elegans where it is used, at least in the dauer stage, providing a model to elucidate this partially understood pathway.

Fig. 3. Nematode metabolic pathways for harvesting energy. (A) Schematic cell depicting the malate dismutation pathway. In this pathway, part of the malate is oxidized to acetate and part is reduced to succinate, using an alternative electron transport chain that involves rhodoquinone (R) instead of ubiquinone (Q). This pathway harvests energy (5 ATP/glucose) during dauer larval stage (a resistance larval form) and it is also thought to be important under hypoxic conditions and possibly in certain tissues or cells under normoxic conditions. Lactic and ethanol fermentation (mainly lactic) occur concomitantly to malate dismutation with a lower energy yield (2ATP/glucose). (B) Schematic cell depicting the glyoxylate shunt. This shunt is a shortcut to TCA that produces succinate from 2 AcCoA, allowing fatty acids to be oxidized and to harvest energy and support gluconeogenesis. It is thought to be important during embryonic development, and it has also been reported to be active in dauer metabolism. In parasitic nematodes, this pathway has not been studied in detail, but it is known to be present in several nematodes. C. Schematic cell depicting aerobic respiration. This pathway is functional in normoxia conditions, during the reproductive cycle of C. elegans. The rectangles within the cells represent the inner and outer mitochondrial membranes. In parasitic nematodes, conventional respiration occurs in normoxia (free-living stages) and malate dismutation under hypoxia. In all cells, the trehalose shunt is shown. Trehalose is important for a variety of environmental stresses (osmotic, anoxic, temperature), as an energy reserve and connects carbohydrate and lipid reserves. (TCA, tricarboxylic acid cycle; R, rhodoquinone; U, ubiquinone; C, cytochrome C).
Another key pathway that is absent in mammalian hosts, but present in C. elegans and parasitic nematodes is the glyoxylate cycle that consumes Acetyl-CoA derived from fatty acid oxidation and produces succinate (Fig. 3B) (Braeckman et al. Reference Braeckman, Houthoofd and Vanfleteren2009). This cycle, when functioning together with TCA (tricarboxylic acid cycle) enzymes generates reducing power and ATP, and fuels gluconeogenesis and trehalose (a glucose disaccharide) synthesis (see below). In C. elegans the glyoxylate cycle is important during embryogenesis and in the dauer stage (Braeckman et al. Reference Braeckman, Houthoofd and Vanfleteren2009; Erkut et al. Reference Erkut, Gade, Laxman and Kurzchalia2016). These dissimilar scenarios (development and arrested development, respectively) have no access to food, and thus depend on lipid and carbohydrate reserves. This pathway has not been much studied in parasitic nematodes, but its gene signature is present in several nematodes (Fig. 2). How the glyoxylate cycle can function linked to malate dismutation in dauer larva (or other conditions) is not clear, particularly considering that they function in opposite directions (i.e. one generates glucose, while the other consumes glucose). One possibility is that these two pathways are separated in space, with the glyoxylate cycle generating glucose in some cells (likely with some oxygen supply), and malate dismutation catabolizing glucose in other cells (without reserves or oxygen supply). There is a need to fully characterize these pathways in nematodes, particularly regarding cell/tissue metabolic specializations.
The trehalose shunt is another difference between parasitic nematodes and their hosts (Fig. 3). This shunt allows production, storage and use of trehalose (Braeckman et al. Reference Braeckman, Houthoofd and Vanfleteren2009). Trehalose is essential as a protective metabolite against different environmental stresses such as desiccation, osmotic stress, anoxia, heat, cold, freezing and as a ready to use saccharide reserve (Braeckman et al. Reference Braeckman, Houthoofd and Vanfleteren2009; Farelli et al. Reference Farelli, Galvin, Li, Liu, Aono, Garland, Hallett, Causey, Ali-Reynolds, Saltzberg, Carlow, Dunaway-Mariano and Allen2014; Erkut et al. Reference Erkut, Gade, Laxman and Kurzchalia2016). Nematodes lack glucose 6-phosphatase (Farelli et al. Reference Farelli, Galvin, Li, Liu, Aono, Garland, Hallett, Causey, Ali-Reynolds, Saltzberg, Carlow, Dunaway-Mariano and Allen2014), a key enzyme in conventional gluconeogenesis and glycogen→glucose conversion. Thus, the trehalose shunt is a key pathway to obtain glucose from carbohydrate reserves or lipid reserves by gluconeogenesis (Fig. 3B). Trehalose-6-phosphate phosphatase is essential in C. elegans, since its absence leads to toxic accumulation of trehalose 6-phosphate (Kormish and McGhee, Reference Kormish and McGhee2005; Erkut et al. Reference Erkut, Gade, Laxman and Kurzchalia2016), and is a promising pharmacological target for some nematodes (Farelli et al. Reference Farelli, Galvin, Li, Liu, Aono, Garland, Hallett, Causey, Ali-Reynolds, Saltzberg, Carlow, Dunaway-Mariano and Allen2014).
Caenorhabditis elegans can also serve as a model to study certain pathways that are present in nematodes, but not in their mammalian hosts. Unlike vertebrates, nematodes lack the enzymes needed for haeme synthesis, yet haeme is essential and therefore must be acquired from the diet (Rao et al. Reference Rao, Carta, Lesuisse and Hamza2005; Luck et al. Reference Luck, Yuan, Voronin, Slatko, Hamza and Foster2016). Furthermore, nematodes possess an unusually high number of haeme-containing globins, and require haeme for several protein families such as guanylate cyclases, adenylate cyclases and cytochromes. This auxotrophy may be exploited to develop drugs that interfere with haeme uptake and homeostasis in parasites. Haeme transporters and several haeme-responsive genes have been characterized in C. elegans, and a recent study has shown that at least some of them are expressed in B. malayi (Luck et al. Reference Luck, Yuan, Voronin, Slatko, Hamza and Foster2016). Another auxotrophy of nematodes is cholesterol. Specific cholesterol-modifying enzymes (absent in vertebrates) have been reported to be important in C. elegans development (Chitwood, Reference Chitwood1999).
A number of small molecules such as ascarosides, dafachronic acids, and nemamides act as pheromones and hormones that control nematode development. Research in C. elegans and the advances in metabolomics have been important in elucidating the chemical nature and biosynthesis of these signals in recent years. The advances made in nematode small-molecule identification and biosynthesis is fully discussed in a recent perspective article (Butcher, Reference Butcher2017). The biosynthesis of these molecules offers potential drug targets, and analogues of these metabolites may function as potential anthelmintics. Another specific metabolic activity of nematodes, absent in their mammalian hosts, is the synthesis and turnover of the cuticle. Much of what is known about the nematode cuticle is due to research in C. elegans and this has recently been reviewed (Lazetic and Fay, Reference Lazetic and Fay2017).
The development of more amenable axenic culture is a pending issue for C. elegans biochemical studies. Nevertheless, Escherichia coli (the diet of the worm in the lab) can be genetically modified, which may be useful for some specific metabolic studies.
Conclusions and perspectives
Although the phenomenon of parasitism cannot be fully understood with a free-living nematode, C. elegans is a powerful model to address many biological questions of parasitic nematodes. The wealth of knowledge on C. elegans that has accumulated over the years provides key information on numerous genes, gene products and biological processes. This information is extremely well organized and is being exploited by nematode parasitologists.
As stated by Sidney Brenner, progress in science depends on new techniques, new discoveries and new ideas, probably in that order. RNAi and the use of GFP as an in vivo gene reporter, pioneered in C. elegans, clearly illustrate this concept. The powerful genetic tools developed for C. elegans research are helping helminth research in different ways, notably as a surrogate system. Yet, there is a need to invest efforts in appropriate genetics techniques and tools to advance the helminth parasitology field. Microfluidics is another technology in which C. elegans advances can fuel helminth research. This technology enables experiments that are otherwise impossible with conventional methods. Indeed, microfluidics is becoming very useful in some C. elegans research areas where precise environmental conditions and worm handling is needed (e.g. imaging, drug studies, behavioural studies, signalling and sorting screening) (San-Miguel and Lu, Reference San-Miguel and Lu2013). Fostering interactions with researchers at the intersection of C. elegans biology and parasitology is important for cross-fertilization of both fields.
Acknowledgements
We thank all members of the Worm Biology Lab for helpful discussions (Institut Pasteur de Montevideo).
Financial Support
This work has been funded by FOCEM (MERCOSUR Structural Convergence Fund, [COF 03/11]), Agencia Nacional de Investigación e Innovación [ANII, (FCE_1_2014_1_104366)] and Universidad de la República. G.R. was the recipient of postgraduate study fellowships from ANII [POS_NAC_2015_1_109854].







