Published online by Cambridge University Press: 01 November 2004
In this study with the filarial model Litomosoides sigmodontis, we demonstrate that the worms ingest host red blood cells at a precise moment of their life-cycle, immediately after the fourth moult. The red blood cells (RBC) were identified microscopically in live worms immobilized in PBS at 4 °C, and their density assessed. Two hosts were used: Mongolian gerbils, where microfilaraemia is high, and susceptible BALB/c mice with lower microfilaraemia. Gerbils were studied at 12 time-points, between day 9 post-inoculation (the worms were young 4th stage larvae) and day 330 p.i. (worms were old adults). Only the very young adult filarial worms had red blood cells in their gut. Haematophagy was observed between days 25 and 56 p.i. and peaked between day 28 and day 30 p.i. in female worms. In males, haematophagy was less frequent and intense. Similar kinetics of haematophagy were found in BALB/c mice, but frequency and intensity tended to be lower. Haematophagy seems useful to optimize adult maturation. These observations suggest that haematophagy is an important step in the life-cycle of L. sigmodontis. This hitherto undescribed phenomenon might be characteristic of other filarial species including human parasites.
The fortuitous observation of red blood cells (RBC) in the gut of a young female Litomosoides sigmodontis filaria led us to question whether this was just an artefact caused by post-mortem haemorrhages of the host, or a more general trait of behaviour of this species. The nutritional needs of L. sigmodontis and other filarial worms are poorly known, and have been assessed by indirect ways such as modified diets given to the host (Storey, 1982; Sturchler et al. 1985; Beg, Fistein & Storey, 1995) or the composition of culture media (Chen & Howells, 1981; Pudney et al. 1988). The observation of intestinal contents is rare and is only feasible when solid elements are present. Among onchocercid filarial worms, we only know of one example where RBC were identified in the intestinal lumen of a rodent filaria (Bain & Guerrero, 2003).
In this study, we wished to eliminate the possibility of blood feeding occurring during the necropsy. The rodent was therefore placed on ice, in order to immobilize the filarial worms and block suction mechanisms. We studied haematophagy in 2 hosts, the Mongolian gerbil Meriones unguiculatus, where microfilaraemia reaches high levels, and the BALB/c mouse, a strain susceptible to L. sigmodontis (Petit et al. 1992) although with lower microfilaraemia than the gerbil. A high number of filarial worms of both sexes was examined at different stages of development from young 4th-stage larvae at day 9 post-infection to late adult stages at day 330 p.i. These observations showed that haematophagy was not accidental, but that L. sigmodontis ingests blood at specific periods of its development, thus raising a number of interesting questions. In the L. sigmodontis model major studies have been aimed at studying the role of immune factors in the regulation of the outcome of filarial infections (Allen et al. 2000; Martin et al. 2001; Saeftel et al. 2001; Le Goff et al. 2002; Babayan et al. 2003, 2004; Volkmann et al. 2003). This study suggests that other factors, such as feeding habits and nutrition, may also have a role in development of the parasite and its success as a parasite, and thus provide a starting point for further investigations.
Meriones unguiculatus: infection by L. sigmodontis is similar to the infection of the natural host, the cotton rat Sigmodon hispidus. Microfilaraemia reaches 13000 mfs/mm3 of blood. Gerbils are commonly used to maintain L. sigmodontis and they are bred in the MNHN animal facilities.
BALB/c mice: all mice inoculated with infective larvae harbour adult worms at day 60 p.i., and 62% of the mice become microfilaraemic with a mean density of 6·7 microfilariae/10 mm3 (Petit et al. 1992). BALB/c mice were purchased from Charles River, IFFA, CREDO and Harlan, France.
The infective larvae were collected from dissected mites and inoculated subcutaneously in the right lumbar region as previously described (Diagne et al. 1990; Babayan et al. 2003). The rodents received a dose of 40–60 larvae, which migrate through the lymphatic vessels and finally reach the pleural cavity.
The rodents were bled and killed humanely. The thoracic cage was isolated, skinned and placed on ice. The pleural cavity was opened ventrally under a stereo-microscope with an incision in the posterior ventral edge of the diaphragm, and the worms observed in situ. They were then collected one by one with a brush and placed in a Petri dish with PBS at 4 °C so that they remained extended and did not tangle. The thoracic cages, and peritoneal organs, were put in a decantation glass with PBS at 37 °C to allow any worms that might have missed sedimentation to be recovered.
Additionally, young worms at days 9–10 p.i. were collected by flushing the pleural cavity with cold PBS as described previously (Al-Qaoud et al. 1997; Babayan et al. 2003).
The filarial worms were immediately placed between slide and cover-slip in cold PBS, and observed live under a microscope. The RBC, naturally coloured by haemoglobin, were identified and counted at ×400 magnification (Fig. 1A–C). To ensure that all RBC had been observed, the worms measuring 70–90 mm in length were dissected; an incision of the cuticle near the area of interest causes the gut and RBC to extrude. A small number of worms were also fixed in hot 70% ethanol or 4% paraformaldehyde and examined after clearing in lactophenol, according to the usual protocol for systematic studies of nematodes. Some worms that had been examined alive were also fixed in 4% paraformaldehyde to serve as reference specimens. We noticed that fixing and clearing cause the RBC to loose their colour within a few days. However, they remained easy to identify, owing to the characteristic shape of their membranes, although lactophenol tends to dilate them slightly (Fig. 1D).

Fig. 1. Red blood cells in the intestine of young Litomosoides sigmodontis adult females. (A) From a BALB/c mouse, day 33 p.i., index 2 of RBC density (body with at vulva=80 μm). (B) Higher magnification of (A). (C) From a BALB/c mouse, day 30 p.i., index 3 of RBC density (body width=80 μm). (D) From a gerbil, day 28 p.i., worm fixed in 4% paraformaldehyde and cleared in lactophenol (diameter of RBC=10 μm). v, Vulva.
Sexes and stages of the worms were assessed on morphological characters (Maréchal et al. 1996). Some specimens were measured (length and width) and compared to previous observations on L. sigmodontis (Cross & Scott, 1947; Bain, Petit & Diagne, 1989; Maréchal et al. 1996; Martin et al. 2000a,b; Martin et al. 2001; Babayan et al. 2003).
RBC were roughly counted when they did not exceed 100, and the levels classed in 3 categories: up to 10 RBC, marked as 1; 11 to 100 RBC, marked 2, and >100 RBC, marked 3; if no RBC was found, 0.
One-way ANOVA and Tukey post-tests were performed to determine the evolution of haematophagy frequency and red blood cell density at different times post-inoculation. Additionally, when only 2 groups were compared, the non-parametric Mann Whitney U-test was used in all cases. P<0·05 was considered to be a significant difference. Values of prevalence and intensity of haematophagy are followed with standard errors.
The presence of RBC in the gut of filarial worms was first studied in Mongolian gerbils, and compared with that in BALB/c mice, which are classically used as susceptible hosts.
The first observation of RBC occurred in a young adult filarial worm. We thus chose time-points ranging between days 28 and 34 p.i. (11 gerbils), then extended the time-points around that period of the worms' life-cycle (Table 1). In total, 23 gerbils were necropsied, 249 filarial worms (143 females and 146 males) collected and examined alive.
Table 1. Analysis of Litomosoides sigmodontis haematophagy in Meriones unguiculatus (Mu) and BALB/c mice (Bc), at different days post-inoculation (No., number of filariae; % haem, prevalence of worms with red blood cells; Density, mean of indices of RBC on a scale from 0 to 3.)
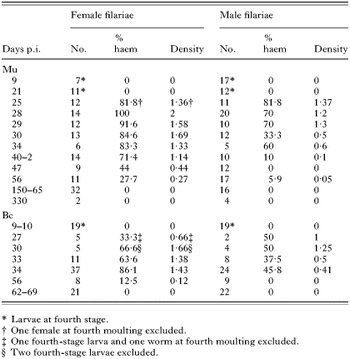
At day 9 p.i., the worms were young 4th-stage larvae (L4). At day 21 p.i. they were still L4s. At day 25 p.i., 82·62% were adult worms and at day 28 p.i. all were adult (Table 1). No RBC were found in the larvae but at day 21 p.i. small bodies (2 μm) with an irregular ‘spicky’ surface (possibly platelets), were observed in the gut of some female worms. RBC were only found in adult worms (Table 1), and were intact in the anterior region of the gut. In female adult worms, that region is situated between the vulva and the end of the oesophagus plus an equal length posterior to the vulva (Fig. 1A and B). Thus the length of that region corresponds to 6–10 times the width of the worm. The length of the gut analysed in the male was similar. RBC have also been seen occasionally in the lumen of the oesophagus.
The proportion of haematophagous filariae and the quantity of ingested RBC differed between sexes (Table 1). In adult female worms, when considering time-point intervals of <day 25 p.i., days 25–40 p.i., days 42–56 p.i. and >day 56 p.i., prevalence of RBC peaked between days 25 and 40 p.i. (P=0·0098 between days 25–40 p.i. and days 42–56 p.i.). RBC prevalence was nil after day 56 p.i. (Fig. 2). The densities of RBC revealed finer time-frames: filarial worms ingested a maximum amount of RBC between days 28 and 30 p.i. (P<0·001) with a mean index of 1·8±0·1 out of 3. During that period, 88·4% of adult female worms contained RBCs (Table 2) and the haematophagy index reached the maximal value of 3 in a third of the females. The female worm lengths were then of 27±3 mm. In male worms, prevalence peaked between days 25 and 34 p.i. then decreased progressively and was nil after day 56 p.i. The comparison of densities here again showed greater resolution: RBC density peaked between days 25 and 29 p.i. (Tables 1 and 2). During this period, the mean density of RBC (1·3±0·1) was lower than in females (P=0·04) and 7% only of males reached a RBC density index of 3. Male length is about two thirds that of females.

Fig. 2. Haematophagy of female Litomosoides sigmodontis from the Mongolian gerbil: kinetics from 9 to 330 days p.i. Each time-point of Table 1 is presented. Ø, Absence of red blood cells. Arrow m, time of fourth moulting. Values are mean prevalence per time-point.
Table 2. Litomosoides sigmodontis haematophagy in female and male worms during the intensity peak in Mongolian gerbil and BALB/c mice (The results are expressed as the mean of frequency, or of intensity on a scale from 0 to 3, ±S.E.M. f, female worm; m, male worm.)

After the blood-feeding period, which lasts no more than 30 days, we often observed a thick translucent gel in the gut lumen (Fig. 3A).
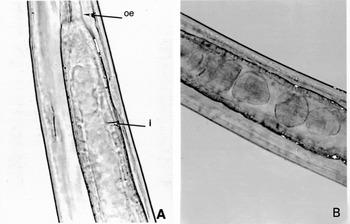
Fig. 3. Litomosoides sigmodontis intestinal contents of female worms after the haematophagous period. (A) From a Mongolian gerbil. (B) From a BALB/c mouse (body width=100–110 μm). oe, Oesophagus; i, intestine.
The time-points studied corresponded to 3 important phases of the L. sigmodontis life-cycle: (i) day 9–10 p.i., 13 mice, 37 worms all at the 4th larval stage; (ii) days 27, 30, 33 and 34 p.i., 19 mice, 118 worms and (iii) days 56, 62, 64 and 69 p.i., 9 mice, 70 adult worms. Filarial development is slower and less synchronized in mice than in gerbils and a few worms were still L4s or at the 4th moulting at day 30 p.i. (Table 1). Observations were usually made on live filariae. Haematophagy of worms in BALB/c mice followed the same kinetics as in gerbils. No RBC were identified in larvae nor in moulting worms, even 30 days after their inoculation, nor were RBC found in adult worms 62–69 days p.i. In female worms, the peaks of prevalence (79·3±5·3%) and of density (1·5±0·1) were observed between days 27 and 34 p.i. when they measured 20·6±5 mm in length (Babayan et al. 2003) (Fig. 3). The haematophagy index reached 3 in 14% of the females. Both prevalence and density decreased sharply after day 56 p.i. (P=0·0082). Haematophagy was less pronounced in male filarial worms than in females with a prevalence of 37·9±5·7%; (P<0·0001) and density of 0·5±0·1 (P<0·0001) during the peak (Table 2). No RBCs could be detected in the gut of male filariae at day 56 p.i. Gut contents were translucent masses after the haematophagous period (Fig. 3B).
In contrast to the situation in Mongolian gerbils, the prevalence and densities of RBC ingested by male worms were lower (P=0·02 and P=0·0008, respectively) whereas there were no significant differences in the numbers of RBCs ingested by female worms recovered from mice and gerbils.
Morphological analysis of over 400 filarial worms, larvae and adults, revealed a new biological trait: haematophagy by L. sigmodontis. This does not appear to be accidental and has a precise chronology as it occurs only in the young adult worms, with a peak shortly after the last moult. The total length of the period during which the worms ingested blood did not exceed 30 days. Considering that L. sigmodontis can live 350 days in the Mongolian gerbil, this phase represents only 10% of the parasite's life-span. This might be a reason why haematophagy in this species (previously wrongly known as L. carinii, cf. Bain, Petit & Diagne, 1989) had never been described since its first experimental use 60 years ago. After the phase of haematophagy, intestinal contents suggest a new feeding behaviour.
Female L. sigmodontis worms ingested more blood than males, and both sexes tended to ingest less in BALB/c mice than in gerbils. Male worms are smaller than females (Cross & Scott, 1947; Bain et al. 1989), and the filarial worms of both sexes are smaller in BALB/c mice than in gerbils at equivalent time-points (Bain et al. 1989; Maréchal et al. 1996; Babayan et al. 2003), which may suggest a positive correlation between size and RBC uptake. However, size alone does not account for the peculiar kinetics of haematophagy, because this behaviour stops whereas the worm size is increasing.
Transcuticular feeding is usually hypothesized for filariae but the identification of red blood cells in the intestine suggests that the digestive tract is functional and the oesophageal pump is able to take up liquids and particles which are present in the vicinity of the filarial worms. Blood feeding can be carried out in two primary ways: directly from a blood vessel or of blood leaking from haemorrhages, which is named capillary and pool feeding respectively. We first hypothesized that the worms may feed by capillary feeding and that their cephalic extremities may be temporally intravascular. However, in situ observations showed that the worms lie unattached against the mucosal wall and their removal from this site does not induce haemorrhages. This suggests that worms ingest RBC by pool feeding. In this case a particular mechanism would have to drive red blood cells out from the capillaries into the pleural cavity, close to the filarial worms. An ongoing study shows that, in the pleural cavity, the sheaths of the moults are rapidly covered with leukocytes and those inflammatory processes take place in the vicinity of the filariae. These are intense and likely to induce small tissular haemorrhages, petechiae and purpura, a commonly described feature in histopathology (Robbins, Cotran & Kumar, 1994). However, this explanation is not completely satisfactory for the following two reasons. (i) The sheaths of the third moulting also induce an intense inflammatory process, but no red blood cells were ingested at that time. This may be explained by the small size (0·8 μm) of the buccal aperture of the 4th stage larvae compared to that of the young adult (1·6–2 μm). (ii) In the gerbil, the fourth moult is synchronous, begins on day 21 p.i. and is completed on day 25 p.i. The peak of haematophagy is observed 3 days later and lasts about 2 more weeks. This delay suggests a specific timing and requirement for blood feeding.
The next question is the significance of red blood cells in the intestine of young adult filariae: either blood ingestion is a fortuitous event or it is a necessary physiological step for an optimal development of the filaria. The fact that the RBC amounts are often quite small suggests that blood ingestion could be a by-product of taking up host body fluids. However, the number of RBC found in the intestine at the time of a necropsy does not reflect the total amount taken up over time. Indeed 100% of the female L. sigmodontis from gerbils are found with ingested blood at day 28 p.i., suggesting that blood feeding might be obligatory.
Haematophagy in arthropods, trematodes, hirudins, intestinal nematodes etc. is considered a favourable adaptation because haemoglobin has a high nutritional value and is frequently associated with completion of the reproductive cycle. This feeding adaptation requires involvement of specific enzymatic pathways to prevent clotting (Urata, Shojo & Kaneko, 2003) and to permit haemoglobin digestion (Brinkworth et al. 2001; Williamson et al. 2003).
In L. sigmodontis, only young adults ingest blood. This corresponds to the period at which the sexual organs mature, where extensive cell divisions occur in male and especially in female worms, which also continue growing exponentially. Haematophagy could therefore fulfil the increased nutritional needs of L. sigmodontis. Platelets present in the blood could also provide an external source of platelet-derived growth factor (PGDF) (Claesson-Welsh, 1994a,b; Meyer-Ingold & Eichner, 1995) and transforming growth factor beta (TGF-β). TGF-β and its homologues have been implicated in development of Caenorhabditis elegans (cf.Tissenbaum et al. 2000), that of Ancylostoma caninum (cf.Arasu, 2001), and found in Brugia filariae (Gomez-Escobar, Gregory & Maizels, 1998; Gomez-Escobar, Lewis & Maizels, 2000). An amphidial neuroendocrine TGF-β pathway is documented in C. elegans and A. caninum (Tissenbaum et al. 2000). However, as suggested by studies on suckling rats (Zhang et al. 2001), other pathways might occur in the digestive tract. A correlation between IL-5 production and growth (Martin et al. 2000a,b; Babayan et al. 2003) had been suggested during the larval stages. Haematophagy, however, seems to be a characteristic of the adult stage and may contribute to sexual maturation and thus the fitness of L. sigmodontis with its hosts.
In this study, haematophagy is evidenced with one species, L. sigmodontis, and appears to be associated with successful development. This specialized trophic behaviour might be more common, even perhaps obligatory in onchocercid filariae given their zoological uniformity (Bain, 2002; Bain & Babayan, 2003). The ferric pigments which were identified in the intestinal cells of Onchocerca volvulus adult worms (Franz & Buttner, 1983) are consistent with this hypothesis. An haematophagous phase, even brief, might represent an interesting target to weaken filariae and reduce their virulence and fitness. Vaccination against hidden antigens expressed by intestinal epithelium have been initiated with an haematophagous gut nematode from sheep, Haemonchus contortus, and have resulted in inducing particularly good protection (Newton & Munn, 1999). The enzymatic systems linked to haematophagy are likely to be conserved (Williamson et al. 2003) and antibodies targeting the digestive enzymes of haemoglobin might lead to a reduction of filarial worm burden and pathology. Studies on haematophagous intestinal nematodes of veterinarian importance are providing a number of vaccine candidates (Knox et al. 2001; Smith et al. 2003), and some of which implicated in haematophagy might point to homologues in human filariasis as novel targets of vaccination.
This work was supported by European Community grant ICA4-CT-1999-10002. W. J. Kozek would like to express his appreciation for the support obtained from the Museum National d'Histoire Naturelle, Paris and the Deanship of Biochemical Sciences Medical Sciences Campus, University of Puerto Rico. We express our gratitude to Dr G. Snounou for his valuable comments, and S. Dassault for his kind support of T.A.

Fig. 1. Red blood cells in the intestine of young Litomosoides sigmodontis adult females. (A) From a BALB/c mouse, day 33 p.i., index 2 of RBC density (body with at vulva=80 μm). (B) Higher magnification of (A). (C) From a BALB/c mouse, day 30 p.i., index 3 of RBC density (body width=80 μm). (D) From a gerbil, day 28 p.i., worm fixed in 4% paraformaldehyde and cleared in lactophenol (diameter of RBC=10 μm). v, Vulva.

Table 1. Analysis of Litomosoides sigmodontis haematophagy in Meriones unguiculatus (Mu) and BALB/c mice (Bc), at different days post-inoculation

Fig. 2. Haematophagy of female Litomosoides sigmodontis from the Mongolian gerbil: kinetics from 9 to 330 days p.i. Each time-point of Table 1 is presented. Ø, Absence of red blood cells. Arrow m, time of fourth moulting. Values are mean prevalence per time-point.

Table 2. Litomosoides sigmodontis haematophagy in female and male worms during the intensity peak in Mongolian gerbil and BALB/c mice

Fig. 3. Litomosoides sigmodontis intestinal contents of female worms after the haematophagous period. (A) From a Mongolian gerbil. (B) From a BALB/c mouse (body width=100–110 μm). oe, Oesophagus; i, intestine.