Introduction
Black sea bass, Centropristis striata, is an economically important species throughout the northwest Atlantic, occurring from the Gulf of Maine down to Florida and are most abundant in the middle Atlantic Bight, where they are an equally vital species for commercial and recreational fishing. In 2017, commercial landings of black sea bass north of Cape Hatteras, North Carolina were 3.8 million pounds and recreational landings were estimated at 4.2 million pounds (ASMFC, 2018). Their high market demand has also led to them being a prime candidate species for aquaculture (Watanabe, Reference Watanabe2011). Crustacean parasites have resulted in losses, including lost biomass from mortality and declined fish condition or unsightly injuries making fish unappealing for human consumption, in diverse marine fish species in the wild and in aquaculture (Kabata, Reference Kabata, Snieszko and Axelrod1970).
Copepods in the family Pennellidae are marine parasites requiring one or two hosts. Development to sexual maturity occurs on a single host, and females undergo metamorphosis into a much larger parasitic form in either the same host or in a new host (Lester and Hayward, Reference Lester, Hayward and Woo2006). For example, in northern Atlantic waters Lernaeocera branchialis, commonly known as cod worm, utilizes two different fish hosts, one supporting development from copepodids through four chalimus stages to adult copepods (Whitfield et al., Reference Whitfield, Pilcher, Grant and Riley1988). After sexual reproduction in the first host, the gills of a second host, most often an Atlantic cod Gadus morhua, are utilized by females for metamorphosis associated with severe enlargement of the parasite. Here the parasites posterior end and egg strings occur external to the fish from the gills and the parasite forms an attachment within the heart. Attachment and feeding on host blood from the heart causes reduced body condition, anaemia and mortality in the fish (Khan, Reference Khan1988). Infections have been known to cause reductions in wild Atlantic cod (Jones and Taggart, Reference Jones and Taggart1998) and are a threat to marine aquaculture, particularly cod mariculture (Khan et al., Reference Khan, Lee and Barker1990). In the northwest Atlantic, L. branchialis occurs in northern waters, is rare in the mid-Atlantic Bight (Sherman and Wise, Reference Sherman and Wise1961) and likely absent in more southern waters. In the mid-Atlantic, the mesoparasitic pennellid copepod in the genus Lernaeenicus is most common.
The genus Lernaeenicus is composed of about 30 valid species and was established based on the characteristics of metamorphosed females, which infect diverse marine fish hosts (Walter and Boxshall, Reference Walter and Boxshall2018). The full life cycle for many of the species in the genus has not yet been determined, except for Lernaeenicus sprattae and Lernaeenicus ramosus in Europe and Japan, respectively (Schram, Reference Schram1979; Izawa, Reference Izawa2019). In the northwest Atlantic, three species of Lernaeenicus are believed to occur based only on the morphology of metamorphosed females (Hogans, Reference Hogans2018). Lernaeenicus radiatus LeSueur 1824, which has previously only been documented in the metamorphosed female form, is described from at least 16 different marine fish hosts (Wilson, Reference Wilson1917; Voorhees and Schwartz, Reference Voorhees and Schwartz1979; Hogans, Reference Hogans2018). Metamorphosed females have a long slender abdomen with egg strings noticeable externally to the fish, while internal to the fish they are often deeply embedded in the muscle by way of an anchoring process made up of radiating cephalic horns (Wilson, Reference Wilson1917; Hogans, Reference Hogans2018). Though the adult post-metamorphosed female form of this parasite has long been known in the northwest Atlantic, the larval and adult stages prior to female metamorphosis have remained unknown until the present study.
The pre-metamorphosed stages of pennellids look very similar among genera, particularly between Lernaeenicus and Lernaeocera, despite the metamorphosed females being quite distinct from each other. Similarity of the pre-metamorphosed stages has led to difficulties in species identification. Previously, copepods described from the gills of black sea bass were believed to be a species of Lernaeocera and later suggested to be Lernaeenicus centropristi (Pearse, Reference Pearse1947; Kabata, Reference Kabata1965). We show herein that the conspecific copepods from black sea bass gills are in fact developmental and adult stages of L. radiatus, documenting the first known initial host for this parasite. The reported similarities in L. radiatus and L. branchialis led us to more comprehensively compare their morphology and genetics in the present investigation. Further we note gill pathology related to L. radiatus infections in black sea bass and consider life cycle patterns, developmental morphology and genetics to better understand the relation of L. radiatus to other pennellid copepods.
Materials and methods
Black sea bass collection and sampling
Black sea bass (n = 30) were collected off the coast of New Jersey in collaboration with the Bureau of Marine Fisheries, N.J. Division of Fish and Wildlife on July 18th, 2019 as part of the Ocean Stock Assessment Project. Fish were collected by biologists using pot traps set on an artificial reef located off the coast of southern New Jersey, USA (Lat 39°29.000′, Long 74°10.000′) (led by P. Clarke). This artificial reef was located about 10 km offshore with a depth of around 15 meters. Bottom water temperature on day of sampling was 11.4°C and was between 11.3 and 13.8°C in the month of July, as recorded by a temperature logger. The adult black sea bass, mean total length of 27 ± 3.5 cm (range of 21–36.5 cm), were maintained on ice and transferred to the Pequest Fish Health Laboratory and examined for parasites within 24 h. Gills were examined and photographed using a Zeiss Stemi-2000 stereomicroscope (Carl Zeiss, Jena, Germany) with a ProgRes Gryphax Arktur CMOS digital camera (Jenoptik AG, Jena, Germany). Copepods were counted on the first gill arch from the left side of the fish to generate an estimate of infection intensity. Infected gill filaments were dissected and wet mounts were prepared to examine copepod attachment to the gill; to examine the fine structures of the parasites, copepods were removed from the gills and wet mounts were prepared of fresh specimens in seawater. Microscopic examination and photography were done using differential interference contrast on a Zeiss Axioplan 2 research microscope equipped with the above-mentioned digital camera. For molecular sample collection, copepods were removed from the gill and immediately frozen at −80°C. The remaining gill arches were preserved in 10% neutral buffered formalin for histology.
For histology, tissues were routinely processed and 4 μm thick sections were cut and mounted on positively-charged slides. All tissue sections were stained with hematoxylin and eosin and select slides were stained with a Luna stain (Luna, Reference Luna1968), which is known to positively stain chitin, eosinophil granules, erythrocytes and cartilage.
Reference samples of Lernaeocera branchialis
Reference samples of North American L. branchialis were collected off the coast of Newfoundland, Canada from adult lumpfish, Cyclopterus lumpus, to compare morphology and genetics. Lumpfish gills were provided by the Department of Ocean Sciences, Memorial University of Newfoundland, Canada (J. Fry and D. Boyce). Lethal gill samples were collected from a total of 23 lumpfish which were already being sampled as part of the cleaner fish broodstock program between July 8th–19th, 2019. Collection locations off the coast of Newfoundland, Canada included six fish from Baine Harbour, three from Champneys West (Trinity Bay), five from Gooseberry Cove (Trinity Bay), eight from Witless Bay and one from Holyrood (Conception Bay). The mean weight of the adult fish was 2635 ± 807 g (range of 585–4154 g). The first gill arch was collected from each fish and preserved in 96% ethanol. The gill samples were then shipped to the Pequest Fish Health Laboratory, NJ USA, to screen for L. branchialis. Gills were screened and photographed using the above-mentioned stereomicroscope and camera. Lumpfish from Champneys West and Holyrood, Conception Bay, had gills moderately infected with L. branchialis, having about 50–75 copepods/gill arch. Lernaeocera branchialis was removed from the gills and replaced to fresh 96% ethanol and kept at −20°C until further processing. Individual parasites were removed and wet mounts were prepared to examine microscopic structures using the above-mentioned microscope and camera. Histology was done on intact gill tissue containing parasites to better observe the attachment of the parasite to the gills; two gill samples that were moderately infected were rehydrated in a descending series of ethanols to water, followed by fixation for 24 h in 10% neutral buffered formalin and further processed for histology as described above.
For a reference genetic sample of L. branchialis from Europe, a tissue sample was taken from a museum specimen (# NTNU-VM-MI-73715) housed at the Norwegian University of Science and Technology, University Museum, Trondheim, Norway. This specimen was an adult metamorphosed female from the gill of Atlantic cod collected off the coast of Norway on July 17th, 2018 and preserved in 96% ethanol.
Parasite measurements and drawings
Measurements were made on parasites from digital images using the ProgRes CapturePro Software (Version 2.10). Measurements of copepod total length were from the anterior tip of the cephalothorax, not including the second antennae, to the caudal terminus, not including setae of caudal rami. For consistency, measurements of females were done prior to severe elongation of the genital segment, since the genital segment may expand greatly during early metamorphosis which may start to occur prior to females dropping off the gills of black sea bass. Cephalothorax measurements included the same anterior portion back to the end of the carapace where the first set of swimming legs began and width measured at its greatest width, where the pigmentation occurred. The total length and width of antennae were included; for the second antennae the width was measured at the terminal segment at its greatest width. The diameter of the mouth tube was an average of two perpendicular measurements, since at times the mouth was not perfectly circular. A minimum of 30 parasites of both sexes was examined to verify structures. Drawings were made based on a series of digital images taken at various focal planes of in-tact individuals using the Affinity Photo Software (version 1.6.9) on an IPad Pro.
Polymerase chain reaction and sequencing
Five parasite samples were evaluated, including two L. radiatus from black sea bass, two L. branchialis from lumpfish and a single sample of L. branchialis from Atlantic cod in Norway. Deoxyribonucleic acid (DNA) was purified from parasite samples using the DNeasy blood and tissue kit automated on a QIAcube (QIAGEN) according to the manufacturer's instructions for extracting DNA from animal tissues. Samples were first completely lysed in a solution of 180 μL Buffer ATL and 20 μL proteinase K by bead beating for 2 min at 20 Hz and incubating for 2 h at 56°C. Polymerase chain reaction (PCR) primers for the amplification of the 18S and 28S rRNA genes were as reported by Song et al. (Reference Song, Wang, Yao, Gao and Nie2008) and combined with general eukaryotic cell primers (Barta et al., Reference Barta, Martin, Liberator, Dashkevicz, Anderson, Feighner, Elbrecht, Perkins-Barrow, Jenkins, Danforth, Ruff and Profous-Juchelka1997; Whipps et al., Reference Whipps, Fournie, Morrison, Azevedo, Matos, Thebo and Kent2012; see Supplementary Table) to obtain nearly the full 18S sequence. Reactions were performed in 50 μL volumes containing 3 μL of template DNA, 1× PCR buffer, 1.5 mm MgCl2, 0.2 mm each dNTP, 0.4 μ m of each primer and 5U Platinum Taq DNA Polymerase (Invitrogen) and topped up with molecular grade water. Amplification was completed on a Veriti thermocycler (Applied Biosystems) with initial denaturation at 94°C for 5 min, followed by 35 cycles of 94°C for 30 s, 54°C for 30 s and 72°C for 1 min, followed by a final extension at 72°C for 5 min. Products were sequenced using the amplification primers.
A 710 bp fragment of the mitochondrial cytochrome c oxidase subunit I (COI) gene was amplified using the primers reported by Folmer et al. (Reference Folmer, Black, Hoeh, Lutz and Vrijenhoek1994) (see Supplementary Table). Reaction mixture volumes were the same as those listed above for the PCR. Cycling conditions amplified the COI under the following parameters: initial denaturation at 94°C for 5 min, followed by 38 cycles of 94°C for 45 s, 48°C for 45 s and 72°C for 80 s, followed by a final extension of 72°C for 7 min. Products were sequenced using the amplification primers.
Products were visualized in 1.2% agarose E-gels (Invitrogen) containing SYBR Safe DNA gel stain and under ultraviolet light to confirm product size and purity. Samples were enzymatically purified with ExoSAP-IT (Affymetrix) and diluted to ~2 ng of DNA μL−1 with molecular grade water plus 5 μ m of the respective amplification primer. Sequencing was done at GENEWIZ, Inc. (South Plainfield, NJ, USA) using ABI BigDye version 3.1 (Applied Biosystems), performed on an ABI 3730xl DNA analyser (Applied Biosystems). All sequences were checked for quality and base calls using Chromas (Version 2.6.6). The overlapping sequences were aligned using BioEdit (Version 7.2.5). Assembled sequences were queried in the National Center for Biotechnology Information's (NCBI) Basic Local Alignment Search Tool Nucleotide (BLASTn) to compare identities to other known species.
Phylogenetic analysis
Mitochondrial COI nucleotide sequences were used to estimate the evolutionary history and phylogenetic relationships. A total of 23 sequences including L. radiatus and L. branchialis from this study, 20 other pennellid copepods from the NCBI database and Bomolochus cuneatus used as an outgroup, were represented. The sequences were aligned using Muscle and the best fit phylogenetic model was estimated using Mega X (Kumar et al., Reference Kumar, Stecher, Li, Knyaz and Tamura2018). The evolutionary history was inferred by using the Maximum Likelihood method and Hasegawa–Kishino–Yano model (Hasegawa et al., Reference Hasegawa, Kishino and Yano1985). There were a total of 698 positions in the final dataset. The tree with the highest log likelihood (−5604.55) is shown. The percentage of trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) was shown next to the branches; only bootstrap values over 0.50 were shown. Initial tree(s) for the heuristic search were obtained automatically by applying Neighbor-Join and BioNJ algorithms to a matrix of pairwise distances estimated using the Maximum Composite Likelihood approach, and then selecting the topology with superior log likelihood value. A discrete Gamma distribution was used to model evolutionary rate differences among sites [5 categories (+G, parameter = 0.4442)]. The rate variation model allowed for some sites to be evolutionarily invariable [(+I), 25.11% sites]. The tree is drawn to scale, with branch lengths measured in the number of substitutions per site. Codon positions included were 1st + 2nd + 3rd + noncoding.
Results
Gill observations and notes on L. radiatus development
All black sea bass gills were infected with L. radiatus with a mixture of developmental stages including chalimus stages, adult males and adult pre-metamorphic females. The copepods were found throughout the entire length of the gill filaments and did not have a preference for certain regions of the gill (Fig. 1A). The mean numbers of copepods on the first gill arch was 426 ± 514, with a range of 101–2946 copepods (n = 30). Grossly, gills appeared pale and epithelial hyperplasia was evident focally where parasites were attached (Fig. 1B). There was a total of four chalimus stages. The earliest stage observed was a stage I chalimus which had a relatively large cephalothorax in relation to the rest of the body (Fig. 2A). The paired first antennae were unsegmented with no rigidity and with small setae. The second antennae were non-chelate and held together anteriorly at the base of the attachment structure. Two sets of swimming legs were present, with a third set developing, though swimming setae were absent (Fig. 2A). Early formation of the attachment apparatus was observed at this stage (Fig. 2B). The main differences between the four chalimus stages were the further differentiation of swimming legs and development of plumes (Fig. 2C). When well-preserved, the chalimus stage was recognized by counting the remnants of previous larval cuticles around the attachment apparatus, which formed layers of hoods. These could not be confirmed in many of the stages. The stage IV chalimus had three previous hoods in addition to the intact one (Fig. 2D).

Fig. 1. Gills of black sea bass infected with L. radiatus (A) Gill with mature male and abundant female copepods. Scale 2 mm. (B) Two dissected gill filaments showing chalimus and adult copepods attached to the gill. Notice the pale areas around attached parasites indicative of epithelial hyperplasia and lamellar fusion. Scale 1 mm.
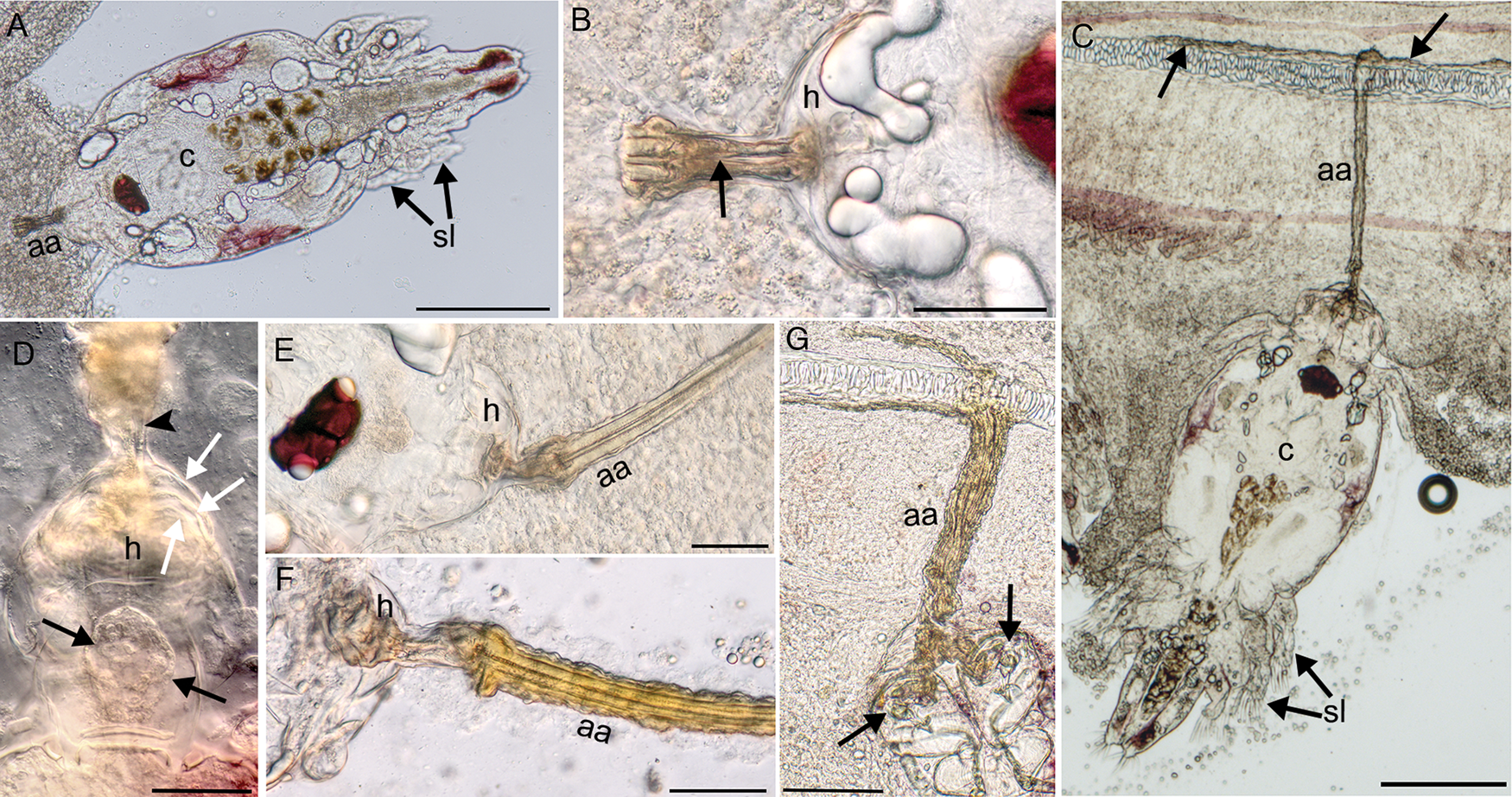
Fig. 2. Chalimus stages of L. radiatus in the gill of black sea bass showing the attachment apparatus (aa), hood (h), cephalothorax (c) and swimming legs (sl). (A) Chalimus I stage with large cephalothorax, swimming legs with no setae and the start of the attachment apparatus. Scale 200 μm. (B) Higher magnification of early attachment apparatus from (A) showing two parallel tubular structures originating from the hood, which appear to fuse (arrow) and then bifurcate. Scale 50 μm. (C) Chalimus IV stage with four swimming legs all containing setae and the attachment apparatus penetrating the gill and attaching to the cartilaginous gill filament. Notice the secretion over the cartilage to adhere the attachment (arrows). Scale 200 μm. (D) Attachment hood of a chalimus IV stage containing spherically arranged material (black arrows), one of the thin frontal filaments is visible (arrowhead) and the previous larval cuticles leaving three layers (white arrows) plus the intact hood. Scale 50 μm. (E) Attachment apparatus in chalimus II that is composed of two parallel tubular structures that bifurcate from the hood. Scale 50 μm. (F) Attachment apparatus of chalimus II with chitinous layers secreted over top of the tubular structures. Scale 50 μm. (G) Thickened tubular attachment apparatus in chalimus IV with multiple layers of secretions appearing to run through the second antennae (arrows). Scale 100 μm.
Attachment of L. radiatus and associated gill pathology
The attachment apparatus was a long tubular appendage that selectively adhered to the cartilage of the gill filament. The synthesis of the attachment apparatus occurred from the hood located dorso-anteriorly between the first antennae and dorsal to the second antennae. The hood structure contained material in a spherical arrangement (Fig. 2D), which was likely the source from which the attachment apparatus was synthesized. Rarely, frontal filaments were seen originating from the spherical structure and leading to the base of the attachment apparatus (Fig. 2D). The attachment apparatus was present in the stage I chalimus, in which early development was observed. Two tubular structures running parallel to each other originated from the hood (Fig. 2B), which appeared to fuse with each other and then differentiate again into two tubular structures that extended into gill tissue until anchoring to the cartilage of the gill filament (Fig. 2C and E). A secretion of material occurred over top of the tubular elements to reinforce the attachment apparatus, giving it a much thicker diameter (Fig. 2F and G). When the tubular attachment terminated at the gill cartilage, this secreted material covered the surface of the cartilaginous filament to adhere to the gill cartilage (Fig. 2G). The tubular attachment apparatus varied in length depending on proximity to the cartilage of the gill filament; the longest attachment apparatus observed was 665 μm in length. Females remained attached at least until copulation, evidenced by attached females having adhered sperm sacs to their genital segment. Adult males no longer had this attachment.
In H&E stained histology, the tubular structure of the attachment apparatus originated from the hood (Fig. 3A), from which it appeared to bifurcate into two tubular elements (Fig. 3B). In cross sections of the tubular attachment apparatus, it was composed of a central core containing two tubular structures in a lightly basophilic-stained matrix containing refractive material when stained with H&E (Fig. 3C and D). The core was surrounded by 1 to 4 layers of eosinophilic material (Fig. 3C and D). The entire attachment apparatus stained positively with Luna stain, with the most intense staining occurring in the tubular structures in the core and the surrounding layers, indicating that these likely were composed of chitin (Fig. 3E). Luna stain is known to positively-stain erythrocytes, eosinophil granules, cartilage and chitin. In the longitudinal section, the core tubular structure was surrounded by multiple eosinophilic-staining layers, and the entire attachment terminated at the cartilage of the gill filament adhered by the secreted material (Fig. 3F).

Fig. 3. Histology of the attachment apparatus of L. radiatus from gill of black sea bass; H&E stain in all except E which is stained with Luna. Scale (A, F) 50 μm; (B–E) 10 μm. (A) Hood of a chalimus III stage showing tubular appendage, which in cross section (arrow) showed two tubular structures. Notice chitinous secretions on both sides (arrowheads). (B) Anterior segment of attachment apparatus showing bifurcation into two tubular structures. (C) Cross section of the early attachment apparatus containing two incomplete tubular structures surrounded by a single chitinous layer. (D) Cross section of a mature attachment apparatus containing two tubular structures in a light staining matrix surrounded by four layers of chitinous secretions. (E) Cross section of attachment apparatus staining positively with Luna stain. (F) Longitudinal section of the attachment to gill cartilage showing the central tubular structures surrounded by multiple layers of secretions.
The invasive attachment apparatus caused pathologic changes in the gill including epithelial hyperplasia and branchitis. In heavy infections, much of the gill tissue was impacted and a large portion of the lamellar surface area was reduced as a result of epithelial hyperplasia and lamellar fusion (Fig. 4A). Lamellar fusion occurred adjacent to attached parasites and around the attachment apparatus (Fig. 4B). The attachment apparatus breached the basement membrane separating the lamellae and the gill filament (Fig. 4C). An inflammatory infiltrate containing monocytes, macrophages and neutrophils occurred around the attachment in the gill filament, leading to swelling. Following detachment of adult males and females, the attachment apparatus remained in the gill, leaving the host to break down and eliminate the structure (Fig. 4D). Pathologic changes were directly associated with the parasite and attachment.

Fig. 4. Gill pathology related to L. radiatus in gills of black sea bass, H&E stain. (A) Gills with regions of lamellar fusion and epithelial hyperplasia reducing the lamellar surface, notice the sections of copepods (*). Scale 500 μm. (B) Copepod (*) attached to the gill with lamellar fusion near attachment and in areas that contain sections of the attachment apparatus (arrows). Scale 200 μm. (C) Attached female L. radiatus with the attachment apparatus breaching the basement membrane between the lamellar epithelium and the gill filament (arrows). Scale 200 μm. (D) Attachment apparatus remaining in gill following detachment of copepod, notice the lamellar fusion with abundant mucus cells arranged in the periphery (arrowheads) and inflammatory cells lifting the attachment apparatus from the gill cartilage (arrow). Scale 200 μm.
Morphology of adult Lernaeenicus radiatus from black sea bass gills
The morphology of parasites was consistent with previous descriptions of the copepod reported by Kabata, (Reference Kabata1965), with additional morphological features reported herein.
Adult female
Despite not having fully developed ovary, females at this stage were considered adults, since copulation occurred. This interpretation is similar to that of Sproston (Reference Sproston1942) for L. branchialis. Copulation was evidenced by adhered sperm sacs over the orifices posterior to the seminal receptacles of the genital segment. Females with attached sperm sacs were still attached to the gill via the frontal filament. Females were larger than males and with a more rectangular shaped cephalothorax (Fig 5A). The genital segment of females had variable lengths. Measurements of copepods and select structures are summarized in Table 1. The longest total length of a post-mated female in the gill after the expansion of the genital segment was 3.5 mm. Pigmentation in fresh specimens was dark red in colour and occurred on the lateral sides of the carapace, base of the swimming legs, around the eyes and on the posterior end of the genital segment anterior to the caudal rami (Fig. 5). There were four pairs of swimming legs with the first two biramous and the second two uniramous. Leg 1 exopod with six plumose setae plus 1 spine, endopod with seven plumose setae; leg 2 was like leg 1; leg 3 uniramous with five plumose setae plus 1 spine; leg 4 with four plumose setae plus 1 spine (Fig. 5F–I). The first antennae were indistinctly 4-segmented. Setae occurred mainly on the anterior side (Fig. 5E). Second antennae were made up of three segments, including the chelae on the distal segment (Fig. 6A). The mouth cone was made up of a buccal tube comprised three rings as previously reported by Kabata, (Reference Kabata1965). The diameter of the buccal tube was larger in the female than in the male (Fig. 6A and B, respectively). A band with blade-like lamellae at the base of the mouth cone was observed when the buccal tube was in profile. Notice the three teeth-like structures (arrow pointing to one of the teeth in Fig. 6C) that make up the lamellae. This was difficult to appreciate in most specimens. The maxilliped comprised two segments, with the basal segment armed with two spines and the distal segment with a curved tip (Fig. 6A). The cuticle of the genital segment was heavily wrinkled. The most obvious structures in the genital segment were the paired seminal receptacles. The ducts connecting the seminal receptacles to the external orifices were frequently looped over each other; in three females these were not looped over each other, though they were bent in an S-shape (Fig. 6D and E). Caudal rami were on both sides of the posterior tip, each had five short plumose setae, a pair on the lateral side and three medially (Fig. 5C).

Fig. 5. Drawings representing L. radiatus from gills of black sea bass showing major morphological features and pigmentation. (A) Mature female; (B) mature male. Scale (A, B) 200 μm, (C) Caudal end of female; (D) caudal end of male; (E) first antenna; (F) first swimming leg; (G) second swimming leg; (H) third swimming leg and (I) fourth swimming leg. Scale (C–I) 50 μm.

Fig. 6. Lernaeenicus radiatus from gills of black sea bass. (A) Ventral view of female showing first antennae (*), robust second antennae (a), mouth tube (arrowhead) and maxillipeds (arrows). (B) Ventral view of male showing mouth tube, first maxillipeds (arrow) and second maxillipeds (arrowhead). (C) Side view of mouth tube with a layer of teeth-like lamellae at the base (arrow). (D, E) Genital segment of female with two sperm sacs adhered near external orifices (arrows), ducts leading to seminal receptacles were often looped over each other and rarely not, but in an S configuration (arrowheads). Scale for all 50 μm.
Table 1. Dimensions and standard deviation of select structures of Lernaeenicus (Le.) radiates and Lernaeocera (Lo.) branchialis from gills of the first fish host. Dimensions and morphology are compared to previously reported Lo. branchialis, Le. sprattae and Le. ramosus. Some dimensions (*) were taken based on parasite drawings.

Adult males
The total length and cephalothorax dimensions were smaller than in the female (Table 1); cephalothorax was more rounded and eyes localized slightly more posterior than the female (Figs 5B). Similar dark red pigmentation as in the female (Fig. 5). Swimming legs and first antennae were like those in the female, though the first antennae were marginally longer in the male. The second antennae were chelate and much less robust, with nearly half the width as those in the female. Mouth cone and buccal tube like that of the female, though with a smaller diameter (Table 1). The first maxilliped was like that of the female except the basal segment was not armed with spines. Unlike the female, males had a second pair of maxillipeds which were bi-segmented, with the terminal segment ending as a curved claw (Fig. 6B). The genital segment was much shorter than female and had paired extensions of the vas deferens directly anterior to the prominent paired spermatophore sacs. Caudal rami were more prominent and larger than in females each with a total of six plumose setae, also much longer and prominent than those in the female (Fig. 5D, compare to female caudal ramus in Fig. 5C). Each caudal ramus had paired shorter setae located laterally, and four medially with the central plumose setae considerably longer than the other three (Fig. 5D).
General morphology and gill attachment of L. branchialis in gills of lumpfish
Lernaeocera branchialis development had been previously described from North America (Wilson, Reference Wilson1917) and the European side of the Atlantic (Sproston, Reference Sproston1942; reviewed by Brooker et al., Reference Brooker, Shinn and Bron2007), thus only general features and parasite dimensions were reported herein to compare to L. radiatus (Table 1). A primary focus was to detail the attachment of the parasite compared to L. radiatus in the current study. Gills of lumpfish contained chalimus stages and adult males and females, with an infection intensity of about 50–75 copepods within the first left gill arch. They occurred throughout the gill, though were primarily localized on the tips of gill filaments. The copepods were relatively heavily pigmented with a black pigment (Fig. 7A and B). Comparing total length, cephalothorax dimensions, second antennae and diameter of mouth tube, all were larger than that of L. radiatus (Table 1). Parasites and gill were fixed in 96% ethanol thus much of the gill colour had become pale. Despite this, attachment of L. branchialis was associated with a small paler area in the gill directly around the attachment. No filament or attachment apparatus was observed grossly or with a stereomicroscope. In histology, the chalimus stages were superficially attached to the gill, which had foci of epithelial hyperplasia associated with the attachment area. Attachment was comprised of two thin frontal filaments that widened within the tissue. From the frontal filaments there was a secretion of eosinophilic material that adhered the parasite within the gill epithelium. This attachment generally reached about 6–10 cells deep into the gill epithelium (Fig. 7C and D). The secreted material making up the attachment stained positively with Luna stain (Fig. 7E). The attachment organ and general morphology of gill were relatively well preserved despite initial fixation in 96% ethanol prior to formalin fixation. Shrinkage of gill epithelial cells was the main artifact caused by initial ethanol fixation.
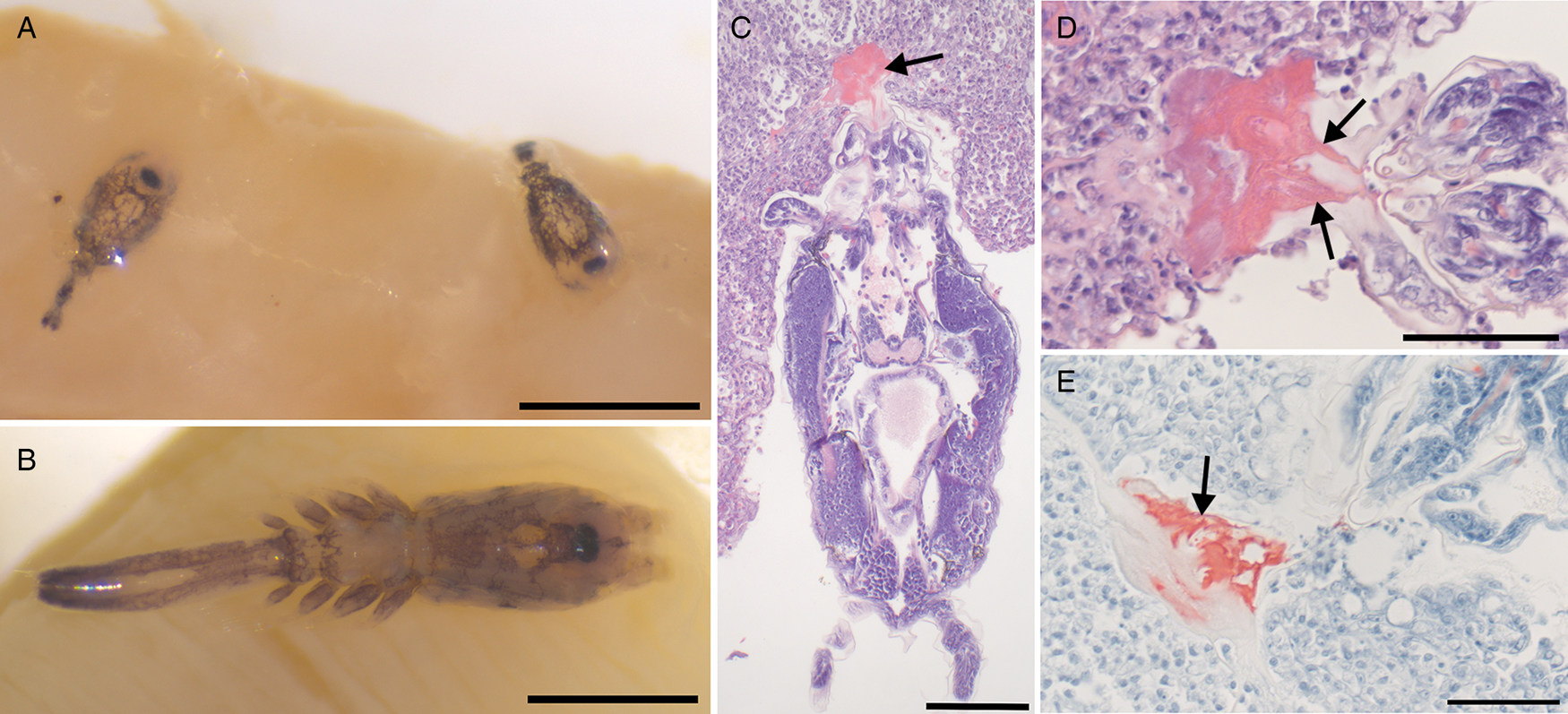
Fig. 7. Lernaeocera branchialis from gills of lumpfish. (A) Chalimus stages and (B) adult females attached to the gill, both with heavy dorsal pigmentation composed of black pigment. Scale 500 μm. (C–E) Histology showing attachment to the gill epithelium (arrows). Attachment is composed of two frontal filaments that widen in the tissue with secretion limited to epithelium. (C, D) H&E stain; (E) Luna stain; scale (C) 100 μm and (D, E) 50 μm.
Genetic characterization of L. radiatus and L. branchialis
Mitochondrial cytochrome c oxidase subunit I (COI)
Lernaeenicus radiatus from gills of black sea bass yielded a 678 nt sequence of the COI. GenBank accession numbers are stated in Table 2. This sequence shared identity with L. radiatus from GenBank, 100% to MH235914 and 99.85% to MH235827, which corresponded to museum specimens of L. radiatus held in the Smithsonian National Museum of Natural History Invertebrate Zoology (USNM:IZ) collection deposited under catalog numbers 1463159 and 1463158, respectively. These specimens were collected from Chesapeake Bay, Maryland, USA as part of the Smithsonian Chesapeake Bay Barcode Initiative.
Table 2. Identities within COI nucleotide and translated protein sequences (bold) from the current study (*) compared with closely related species. Percent identity is reported; GenBank accession numbers reported under species names. Lernaeenicus (Le.); Lernaeocera (Lo.) and identical (ID)

Lernaeocera branchialis from lumpfish gills from Canada and L. branchialis from Atlantic cod from Norway were identical to each other. Nucleotide divergence in the COI between L. radiatus, L. branchialis and other closely related species was between 15–20%. Translated protein sequences were more conserved and demonstrated high identity of L. radiatus to Haemobaphes spp. and L. branchialis (98–100%) and less identity to other Lernaeenicus spp. (95–96%) (Table 2).
Phylogenetic analysis of the COI gene showed that the genus Lernaeenicus was polyphyletic. Lernaeenicus radiatus formed a grouping within a clade containing Lernaeocera and Haemobaphes spp. (Fig. 8). Lernaeenicus sprattae and L. ramosus were closely related to each other in a grouping in a basal position compared to L. radiatus and L. branchialis. The clade for these species was poorly supported based on the low bootstrap values, and further taxon sampling will be necessary to better resolve the clades. Several species of Lernaeenicus (L. seeri, L. alatus and L. hemirhamphi) described from marine fish in India formed a clade basal to all other pennellid species included in the analysis (Fig. 8).

Fig. 8. Phylogenetic tree based on Maximum likelihood analysis of the COI gene showing Lernaeenicus is polyphyletic and that L. radiatus groups close to a clade containing Lernaeocera and Haemobaphes spp. Species from this study are boxed in. Bootstrap values above 0.50 are shown next to the branches.
Ribosomal DNA (rDNA) sequences
GenBank accession numbers for sequences are noted in Tables 3 and 4. Identities showed that the 18S subunit was the most conserved gene between species and L. radiatus showed closest identity to L. branchialis (99.32%), and less identity to other Lernaeenicus spp. in GenBank (Table 3). Lernaeocera branchialis from Canada and Norway were identical to each other. The 18S sequences from L. branchialis had 99.4% identity to a previous GenBank submission of L. branchialis from Europe accession # AY627030 (Table 3). The partial sequence of the 28S region was 760 bp long for both L. radiatus and L. branchialis. The highest identities were between L. radiatus, H. pannosus and L. branchialis (Table 4).
Table 3. 18S rDNA sequence identity table with sequences from this study (*) compared to closely related species from GenBank. Percent identity is reported; GenBank accession numbers reported below species names. Lernaeenicus (Le.); Lernaeocera (Lo.) and identical (ID)

Table 4. 28S rDNA sequence identities from the current study (*) compared to closely related species from GenBank. Percent identity is reported; GenBank accession numbers are reported below species names. Lernaeenicus (Le.); Lernaeocera (Lo.) and identical (ID)

Discussion
Metamorphosed female forms of L. radiatus have been reported in at least 16 different fish hosts, some of which include Atlantic menhaden, Brevoortia tyrannus, Bay anchovy, Anchoa mitchilli, white perch, Morone americana, tomcod, Gadus tomcod, Atlantic croaker, Micropogonias undulatus, killifish, Fundulus heteroclitus, blueback herring, Alosa aestivalis and American eel, Anguilla rostrata (Wilson, Reference Wilson1917; Voorhees and Schwartz, Reference Voorhees and Schwartz1979; Hogans, Reference Hogans2018). Until the present study, a host supporting development and sexual reproduction of L. radiatus had not been identified. Some pennellid copepods utilize a two-host life cycle, a first host (definitive host) that supports copepod development and sexual reproduction and a second host supporting metamorphosis of females, while other pennellids complete their life cycle within a single host. The study herein was the first to identify the first (definitive) host for L. radiatus, which supports larval to adult copepod development and sexual reproduction. Gill infections of larval L. radiatus have not been previously reported in any other species, thus presently black sea bass is the only species known with developmental and adult stages of L. radiatus in the gill, suggesting that this parasite has a two-host life cycle starting with black sea bass. The host specificity of L. radiatus to black sea bass as a first host will still need confirmation by further examining gills of other fish species.
This was not the first description of this copepod in the gills of black sea bass, though identification of the species was difficult due to its remarkable similarity to Lernaeocera and highly conserved morphology of the mature copepods in their first host. The conspecific parasite to the one herein was identified in North Carolina and described as Lernaeocera centropristi (Pearse, Reference Pearse1947). Following this, Kabata, (Reference Kabata1965) reevaluated the same parasite and concluded it more closely resembled Lernaeenicus spp. and thus renamed it L. centropristi. We confirmed that this parasite from black sea bass is in fact a larval and adult form of L. radiatus prior to female metamorphosis. Linking the developmental stages in the black sea bass gill to L. radiatus was confirmed in the present investigation by sequencing the COI gene, which is the main target gene for barcoding of invertebrates (Folmer et al., Reference Folmer, Black, Hoeh, Lutz and Vrijenhoek1994; Raupach et al., Reference Raupach, Barco, Steinke, Beermann, Laakmann, Mohrbeck, Neumann, Kihara, Pointner, Radulovici, Segelken-Voigt, Wesse and Knebelsberger2015; Castro-Romero et al., Reference Castro-Romero, Montes, Martorelli, Sepulveda, Tapia and Martínez-aquino2016). This finding suggests that the species L. centropristi should be abolished, as these are in fact stages of L. radiatus. Future genetic studies and barcoding of diverse crustacea using the COI gene will be invaluable in linking the life cycle of species within the Pennellidae to better understand their life history.
A notable finding herein was the unique attachment apparatus for L. radiatus larvae in the gills, which had not been previously documented. Since larval development has not been described for many pennellid species, previous descriptions of the attachment apparatus from diverse species are lacking. Attachment of developing L. branchialis (Sproston, Reference Sproston1942) and L. sprattae (Schram, Reference Schram1979) have been shown to be similar to each other, comprising of two frontal filaments that superficially attach to epithelium. These previous descriptions were from parasites separated from the gill tissue, making it difficult to note on the attachment to the host. For this reason, we investigated the attachment of L. branchialis intact within the gill by histology and confirmed that the parasite attached superficially in the epithelium by way of two thin frontal filaments and a secretion to reinforce the attachment. The attachment apparatus in L. radiatus formed a long tubular appendage that deeply penetrated the gills and selectively attached to the cartilage of the gill filament. The relevance for this major difference may be related to the activity and life history of the host fish species. Perhaps higher activity levels in black sea bass related to foraging and mating require L. radiatus to form a more secure attachment compared to that of L. branchialis in lumpfish and flounder. The cartilaginous gill filament is a stronger, more rigid structure for attachment compared to the gill epithelium which has a high cell turnover rate. With this notable difference in attachment, this is a useful morphological characteristic to make note of in future descriptions of pennellid copepods.
The invasive attachment of L. radiatus caused considerable gill pathology. Larval development of L. branchialis in lumpfish and flounder has been reported to have minimal health impacts on their hosts despite heavy infections (Templeman et al., Reference Templeman, Hodder and Fleming1976). This may be related to the superficial attachment within the epithelium and thus less detrimental pathology (Templeman et al., Reference Templeman, Hodder and Fleming1976; current study). This contrasted to the more invasive attachment of L. radiatus in black sea bass gills which occurred throughout the entire gill, with attachment associated with epithelial hyperplasia, lamellar fusion and branchitis. With 100% prevalence and heavy infections ranging from hundreds to thousands of attached copepods per gill arch, the health impact caused by these copepods is an important consideration. Gills are a multi-functional organ with a high surface area responsible for respiration, osmoregulation and excretion of metabolic waste products (Speare and Ferguson, Reference Speare, Ferguson and Ferguson2006). The fish collected in the study herein were not apparently ill and perhaps have a high tolerance to gill damage caused by these infections.
It is possible that gill damage induced by parasites may be overcome under normal metabolic processes in relatively good water quality. However, these infections may impact how fish handle environmental stressors and activity that requires a higher metabolic scope. This is particularly important for black sea bass populations, known to experience hypoxic or anoxic conditions in the coastal habitat during the summer (Hales and Able, Reference Hales, Able, Secor, Dean and Campana1995), which is when heavy infections with this parasite occurred in the present study. Other gill diseases in marine fish have been shown to impair gill function. For example, amoebic gill disease in Atlantic salmon results in decreased metabolic scope and compromises ion regulation (Hvas et al., Reference Hvas, Karlsbakk, Mæhle, Wright and Oppedal2017). Additionally, metabolic scope is impaired by microsporidial gill infection in Atlantic cod (Powell and Gamperl, Reference Powell and Gamperl2016). It has been shown that fish can overcome some of the negative effects of gill disease by increasing the efficiency of oxygen extraction and enhancing capacity for anaerobic metabolism (Powell and Gamperl, Reference Powell and Gamperl2016). How L. radiatus impacts gill health of wild black sea bass populations is an important consideration and avoidance of this parasite in an aquaculture setting would be particularly important for maintaining optimal fish health.
Several morphologic features of the copepods have been described herein that were not previously shown and aid in distinguishing L. radiatus from other species. The most significant of these was the unique attachment apparatus discussed above. Additionally, the overall pigmentation of L. radiatus differed from other species in that the pigment was a dark-red colour, and generally there was less pigment making the parasite relatively translucent. We summarized the main distinguishing features and dimensions of select structures between L. radiatus and L. branchialis from this study compared to other Lernaeenicus spp. from previous studies (Table 1). We also confirmed the finding from Kabata, (Reference Kabata1965) showing that in females the ducts to the seminal receptacles often crossed over each other, which did not occur in L. branchialis. This does appear to be a distinguishing feature between L. radiatus and L. branchialis, though it may be related to the shifting of the seminal receptacles making the ducts cross over. Nonetheless, it is a feature not reported in L. branchialis.
Genetic comparisons herein showed Lernaeenicus to be a polyphyletic genus and that L. radiatus had higher identity to L. branchialis and Haemobaphes spp. than to other Lernaeenicus spp. The identical genetics of L. branchialis collected from Norway and Canada supports that these are the same species, as proposed by Kabata, (Reference Kabata1961) who showed identical morphology in these, despite the geographic separation and different host preferences. The 18S rRNA gene, a highly conserved gene previously used to understand phylogeny of copepods (Khodami et al., Reference Khodami, McArthur, Blanco-Bercial and Arbizu2017), showed the smallest differences between the species herein. Interestingly, L. radiatus showed considerable divergence from L. sprattae (the only other species in the genus with 18S rDNA sequence available) with 2.21% divergence in the sequence, whereas it only diverged by 0.68% from L. branchialis collected from Canada and Norway. The identities within the 28S rDNA sequences supported a similar pattern. The COI gene had the most representation for pennellid copepods because this is the target gene for barcoding of invertebrates (Folmer et al., Reference Folmer, Black, Hoeh, Lutz and Vrijenhoek1994; Raupach et al., Reference Raupach, Barco, Steinke, Beermann, Laakmann, Mohrbeck, Neumann, Kihara, Pointner, Radulovici, Segelken-Voigt, Wesse and Knebelsberger2015) and it has been shown to be a useful marker for taxonomy within Pennellidae (Castro-Romero et al., Reference Castro-Romero, Montes, Martorelli, Sepulveda, Tapia and Martínez-aquino2016). Because of high nucleotide divergence rates between species, we compared translated protein sequences, since amino acids are more highly conserved than nucleotides in evolution. Translated protein sequences supported the pattern of high identity of L. radiatus to Haemobaphes spp. and L. branchialis, while more diverged from the other Lernaeenicus species. Lernaeenicus radiatus formed a separate grouping from L. sprattae and L. ramosus, and both grouped separately from the Lernaeenicus spp. described from India (Raja et al., Reference Raja, Saravanakumar, Gopalakrishnan, Vijayakumar, Hwang and Maran2016). Considering that the morphology of metamorphosed females has historically been used for taxonomy, the polyphyletic phylogeny suggests that other characters must be used to understand the evolution and taxonomy of pennellid copepods.
Current taxonomy of pennellids based on the morphology of metamorphosed females (Wilson, Reference Wilson1917) has been out of necessity, since relatively few complete life cycles are known in this group. A difficulty in using metamorphosed females in taxonomy is the apparent variation of morphology in metamorphosed females even within individual species, as pointed out by Castro-Romero et al. (Reference Castro-Romero, Montes, Martorelli, Sepulveda, Tapia and Martínez-aquino2016). This morphologic plasticity has been documented for Lernaeocera and has been attributed to host size and location of infection (Kabata, Reference Kabata1979; Van Damme and Ollevier, Reference Van Damme and Ollevier1995). Similar morphologic plasticity had been reported for L. radiatus with the morphology of developing horns being influenced by the type of tissue infected, i.e. bony operculum vs muscle (Wilson, Reference Wilson1917; Hogans, Reference Hogans2018), thus it is common to see L. radiatus with less than the typical five horns. This plasticity in morphology makes it less useful for taxonomic descriptions and it appears that starkly different body forms can be a product of tissue tropisms. Future taxonomic studies carefully evaluating the male and pre-metamorphic females will be very useful, since these stages are more conserved and show less variability within species.
Life cycle patterns are important characters in the taxonomy of parasites. The life cycle of L. radiatus, including heavy infection of the gills of their first host, is like that described for L. branchialis, which heavily infect lumpfish gills in North America (Templeman et al., Reference Templeman, Hodder and Fleming1976) and flounder gills in Europe (Whitfield et al., Reference Whitfield, Pilcher, Grant and Riley1988). The work herein suggests a two-host life cycle is likely for L. radiatus which includes black sea bass as a first host and a range of other marine fish species as second hosts. This is similar to the two-host life cycle in L. branchialis. Life cycles within the genus Lernaeenicus have thus far shown to include only a single host, though this is only based on the known life cycles of two species. Lernaeenicus sprattae completes its entire life cycle within the European sprat, Sprattus sprattus (Schram, Reference Schram1979) and L. ramosus was shown to complete its entire life cycle within the Hong Kong grouper, Epinephelus akaara (Izawa, Reference Izawa2019). Both life cycles occurred within a single host species, though with a tissue tropism shift for the metamorphosis of the females. For L. sprattae, larval development occurred in the skin and fins and females migrated to the eye where metamorphosis occurred (Schram, Reference Schram1979), whereas in L. ramosus larvae were found in gills and metamorphic females predominantly on the fins (Izawa, Reference Izawa2019). Clear differences may be seen in the utilization of hosts in the life cycle of pennellid copepods. One species, Peniculus minuticaudae, has been shown to complete its entire life cycle within a single host and without a change in tissue tropism (Ismail et al., Reference Ismail, Ohtsuka, Maran, Tasumi, Zaleha and Yamashita2013). Future taxonomic revisions within pennellid copepods should focus on understanding life cycle patterns, morphology of adult stages prior to female metamorphosis and genetic data to best understand the evolution of this group of copepods.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0031182019001781.
Acknowledgements
We are grateful for the assistance of Peter Clarke and Greg Hinks from the Bureau of Marine Fisheries, N.J. Division of Fish and Wildlife, who provided the fish for this study, and Dr Ken Able and Maggie Shaw from the Rutgers University Marine Field Station. We also thank Danny Boyce, Jess Fry and Karstein Hårsaker for providing reference samples of L. branchialis. We'd like to thank Bridget Giblin and Vincent Nicoletta for assistance with fish sampling in the fish health lab. Lastly, we are grateful for the laboratory support from the Animal Health Diagnostic Laboratory, N.J. Department of Agriculture.
Financial support
Funding for this study was provided by the Federal Aid in Sport Fish Restoration Act (Project # FW-69-R-20) and the New Jersey Hunter and Anglers Fund.
Conflict of interest
None.
Ethical standards
Not applicable. Fish sampling was done in accordance with approved methods in the Wildlife and Sportfish Restoration Program, Fish and Wildlife Health Project.















