INTRODUCTION
The survival, population dynamics, and disease vector competency of ticks are a function of the ability of individuals to (1) survive while off the host until a host is found, and (2) once a host is found, to persist on the host for a sufficient period to gain a bloodmeal (Strickland et al. Reference Strickland, Gerrish, Hourrigan and Schubert1976; Allan, Reference Allan, Samuel, Pybus and Kocan2001). Factors that influence the ability of ticks to survive off-host and successfully infest and survive on a host are likely to be different. The former has been found to be a function of local site characteristics such as habitat and microclimate (Daniel, Reference Daniel1978; Lindström and Jaenson, Reference Lindström and Jaenson2003) or host diversity (LoGiudice et al. 2003). Thus, factors affecting the number of ticks that ultimately find a host are likely to be intrinsic to the local environment, with the host itself playing a secondary role. Once a host has been found, however, environmental effects may be of secondary importance relative to the density of ticks on a host (competition among ticks resulting in density dependency), and the host behavioural and physiological response to the attachment (factors intrinsic to the host). Together, these factors result in characteristics of hosts being correlated with parasite prevalence and abundance.
Differences due to sex and weight are well documented in a diversity of mammalian host-parasite associations, including helminths (Poulin, Reference Poulin1996; Morales-Montor et al. Reference Morales-Montor, Chavarria, De León, Del Castillo, Escobedo, Sánchez, Vargas, Hernández-Flores, Romo-González and Larralde2004), arthropods (Schalk and Forbes, Reference Schalk and Forbes1997), and unicellular parasites (Moore and Wilson, Reference Moore and Wilson2002). Male-biased rates of parasitism are primarily attributed to the immunosuppressive effects of male sex hormones (Zuk and McKean, Reference Zuk and McKean1996; Cox and John-Alder, Reference Cox and John-Alder2007), larger body sizes that support more parasites (Folstad and Karter, Reference Folstad and Karter1992), or differences in grooming or movement patterns (Mooring et al. Reference Mooring, McKenzie and Hart1996; Zuk and McKean, Reference Zuk and McKean1996). While sex biases in host parasitism have been detected for ticks (Gallivan et al. Reference Gallivan, Culverwell, Girdwood and Surgeoner1995; Mooring et al. Reference Mooring, McKenzie and Hart1996; Gompper, Reference Gompper, Sánchez-Cordero and Meddellín2004), few studies have examined patterns in the persistence of ticks once on a host, especially in free-ranging host populations. Yet such information is critical to understanding the likelihood of an individual tick obtaining a bloodmeal, and thereby surviving to reproduce (Wilson et al. Reference Wilson, Litwin, Gavin, Capkanis, MaClean and Spielman1990), as well as remaining on a host for sufficient time to act as a competent disease vector (Piesman et al. Reference Piesman, Mather, Sinsky and Spielman1987).
In addition to general correlations with host sex and size, 3 contrasting relationships between host age and parasite abundance have been described: a continual increase over the life of the host; an initial increase until the number of parasites reaches an asymptote and levels off; and an initial increase and peak, followed by a decline in older animals due to host immune response, death of heavily parasitized animals, or other age-related factors (Hudson and Dobson, Reference Hudson, Dobson, Grenfell and Dobson1995). A continual increase or leveling off of parasite burden with age has been observed for a variety of helminth species in mammals (Halvorsen, Reference Halvorsen1986; Quinnell, Reference Quinnell1992) and birds (Hudson, Reference Hudson1992), but few studies have documented an initial increase in young animals and subsequent decline in older animals (but see Gregory et al. Reference Gregory, Montgomery and Montgomery1992). Logistic difficulties associated with obtaining such data in the field may have contributed to the lack of such observations (Wilson et al. Reference Wilson, Bjørnstad, Dobson, Merler, Poglayen, Randolph, Read, Skorping, Rizzoli, Grenfell, Heesterbeek and Dobson2003).
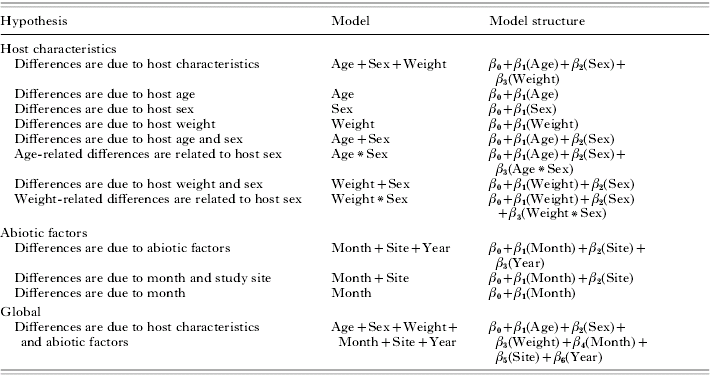
Correlation of parasitism to local environmental factors and to host-intrinsic factors such as age or sex and weight suggest the need to study these factors in concert to gain a more precise understanding of the likelihood of ticks persisting on hosts long enough to obtain a bloodmeal and fully act as a disease vector. To gain insights into biotic and abiotic factors that may underlie the likelihood of ticks persisting on hosts to obtain bloodmeals, we examined data on parasitism by the tick Dermacentor variabilis from 10 host populations of raccoons (Procyon lotor). Dermacentor variabilis is the largest and most abundant ectoparasite of raccoons in the Midwest U.S. (Whitaker, Reference Whitaker1982; Kollars et al. Reference Kollars, Oliver, Masters, Kollars and Durden2000). It is a 3-host tick that is only found on animals while feeding or mating. Once a bloodmeal is obtained, D. variabilis drop off the host, moult to the next stage or lay eggs and, if not an adult, seek another host. This life-history results in 2 distinct ‘habitats’ or ‘environments’ during each stage: free-living ticks that are seeking a host (questing) and ticks that have obtained a host and are attempting to mate or become fully engorged with blood (i.e. replete).
Our objective was to measure the relative influence of abiotic variables and host characteristics on 2 components of the tick population, male and non-replete female ticks (hereafter referred to as non-replete) and replete female ticks. Adult female D. variabilis must feed continuously for 7–10 days to become fully engorged and markedly distended (Atwood and Sonenshine, Reference Atwood and Sonenshine1967). We hypothesized that different factors affect non-replete and replete female tick abundance on raccoons. Because free-living adult D. variabilis ticks are primarily influenced by local site conditions (Campbell and MacKay, Reference Campbell and MacKay1979; Sonenshine, Reference Sonenshine1991) and time of collection (Kollars et al. Reference Kollars, Oliver, Masters, Kollars and Durden2000), we predicted that non-replete infestation will primarily be related to abiotic factors such as site or month of data collection that influence questing ticks. Conversely, we predicted that the ability of a female tick to remain on their host and become fully engorged is more likely to be influenced by host characteristics such as age or sex. Such effects are expected to be particularly important if testosterone is suppressing the immune system, if acquired immunity is occurring, or if other age-related factors predict the extent of parasitism.
MATERIALS AND METHODS
Host species and tick quantification
Raccoons were sampled during summer 2005 and 2006 at 10 locations in central Missouri. All sites were located on state, federal, or university conservation or research areas within 60 km of Columbia, MO. Sites consisted of second growth oak (Quercus spp.) and hickory (Carya spp.) forest with a maple (Acer spp.) and cedar (Juniperus virginiana) understory. All sites were >10 km apart, with the exception of 2 areas that had 2 sites each, that were 4 km apart. To reduce confounding effects of seasonal fluctuations in tick abundance, only animals captured between 20 May and 5 August were included in analyses. Our trapping extended beyond these dates, but tick infestations were not consistently observed across all study sites before mid-May or after early August in 2005 or 2006. This is consistent with other studies of raccoons and D. variabilis in Missouri (Kollars et al. Reference Kollars, Oliver, Masters, Kollars and Durden2000), and we assumed that all animals included in analyses were susceptible to infestation during this time period. Data from recaptured animals were not included in analyses. Research was carried out under Missouri Department of Conservation permit &num1;12869 and University of Missouri Animal Care and Use Protocol #3927.
Trapping occurred at all 10 areas in 2005 but we only included data from 4 of the areas in 2006 because food supplementation treatments were initiated in the other 6 areas as part of a separate study. These areas were not included because raccoon behaviour and resource availability in these areas were altered, which may influence parasite prevalence and abundance (Wright and Gompper, Reference Wright and Gompper2005). Traps were baited with mackerel and checked daily. Raccoons were immobilized with an injection of ketamine hydrochloride and xylazine (Evans, Reference Evans and Heard2002), marked with metal ear tags, weighed, sexed, and aged by body size, genital morphology, and tooth eruption and wear (Grau et al. Reference Grau, Sanderson and Rogers1970; Larson and Taber, Reference Larson, Taber and Schemnitz1980). Grau et al. (Reference Grau, Sanderson and Rogers1970) provided tooth wear patterns for 5 age classes; I=0–14 months, II=15–38 months, III=39–57 months, IV=58–86 months, and V=over 86 months. We combined ages IV and V due to difficulty distinguishing between these groups (hereafter referred to as IV+), and included a cub category (0–5 months) based on date of capture, weight, and dental characteristics.
Adult D. variabilis ticks are distinct, relatively large (3–5 mm in length), and readily found and identified on animals in the field without magnification. We quantified adult D. variabilis by a thorough search of the entire body (Kollars et al. Reference Kollars, Oliver, Masters, Kollars and Durden2000; Kollars and Kengluecha, Reference Kollars and Kengluecha2001), and classified each tick as non-replete (i.e. not engorged with blood), replete (engorged), or semi-replete. We considered ticks to be non-replete if they were not discolored and similar in width (2–3 mm) to questing adult D. variabilis ticks found off host. We considered a tick to be replete when it was obviously engorged with blood, ⩾5 mm width, and discolored. Ticks that were not clearly in either category were noted as semi-replete and not included in analyses.
Prevalence and abundance were estimated, respectively, as the number of animals infested by ticks divided by the total number of animals examined, and as the total number of ticks observed on each individual animal (Bush et al. Reference Bush, Lafferty, Lotz and Shostak1997). We also calculated the ratio of replete:non-replete ticks for each individual host; this measure approximates a proportion of ticks that encounter a host that persist to the point of fully obtaining a bloodmeal. We used Mann-Whitney U tests to examine sex-related differences in tick abundance in each age category. Separate comparisons were conducted within age classes to ensure statistical differences due to sex would not be masked or biased by divergent patterns in older or younger individuals and reveal the nature of sex*age interactions. Because there were 5 age-class analyses for each of non-replete tick abundance, replete tick abundance, and the ratio of replete to non-replete abundance, we used a Bonferroni correction (α=0·05/5=0·01) to qualify statistically supported relationships. Thus, values of P⩽0·01 were deemed significant, and values of 0·05>P>0·01 were deemed weakly significant.
Model selection
We used information-theoretical model selection to test a priori models predicting non-replete tick abundance and replete tick abundance. Model selection refers to the process of using the observational data to evaluate a suite of models that represent hypotheses. Models are ranked and weighted to evaluate the probability that the model in question is the best fitting model. Although rarely applied in ecological parasitology, model selection has been increasingly used in the broader ecological literature over the last 20 years (Johnson and Omland, Reference Johnson and Omland2004), and can provide a powerful tool to identify the most relevant ecological factors influencing dependent variables such as parasite abundance.
We erect a series of explicit hypothesis-based models that relate abiotic and host characteristics to the abundance of parasitizing ticks (Table 1), and rank the fit of these models using an information-theoretical approach. Such an approach allows us to identify not only the support for an array of hypotheses, but also to rank the hypotheses and thereby discern the model with the greatest support in explaining parasitism by ticks. Model covariates included 3 abiotic (site, month, year of collection) and 3 biotic variables (sex, age, weight of host). The same set of models were used to predict non-replete and replete abundance to evaluate differences in predictive abilities across tick populations as a whole. The number of non-replete ticks was not included as an independent parameter in models of replete tick abundance as we were specifically interested in whether and how biotic and abiotic factors influence replete tick abundance, and including non-replete ticks as a parameter for replete tick models would likely confound the test of the hypothesis that temporal and spatial abiotic factors are important drivers of tick abundance (Burg, Reference Burg2001; see below).
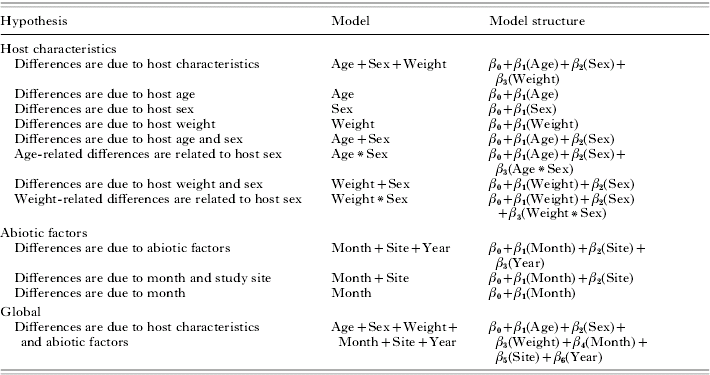
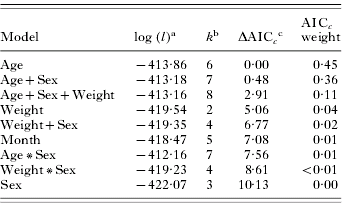
Table 1. A priori models used to estimate non-replete and replete Dermacentor variabilis tick abundance of raccoons in Missouri
(β0=intercept, βi(X) are the parameters of independent variables.)
We tested whether non-replete and replete ticks differed from a negative binomial distribution using the maximum-likelihood method of Bliss and Fisher (Reference Bliss and Fisher1953) with the program Quantitative Parasitology 3.0 (Rozsa et al. Reference Rozsa, Reiczigel and Majoros2000). We used generalized linear models with a negative binomial distribution for model selection. Tick abundance of individual raccoons was the sample unit and all covariates were fixed effects. We calculated Akaike's Information Criterion (corrected for small sample size; AICc) to rank the models and calculated the difference between the best approximating model (i.e. the model with the lowest AICc) and all other models (ΔAICc) in the candidate set. Only models with an AICc value within 2 points of the best-fitting model were considered to have substantial empirical support as a best-fitting model (Burnham and Anderson, Reference Burnham and Anderson2002).
RESULTS
Quantitative patterns
We captured 177 individual raccoons, with an average of 17·7±2·63 (s.e.) animals caught per study area. Prevalence of non-replete ticks did not vary between males and females (92% for both) and, with the exception of cubs (56%), was ⩾85% for all age classes. Prevalence of replete ticks was also similar among males (63%) and females (65%). Prevalence of replete ticks increased in older animals, ranging from 11 to 38% for cubs and age class I animals, and 75 to 78% for age class II and above (Table 2).
Table 2. Prevalence (%) of Dermacentor variabilis on raccoons
(Raccoon age class designations are cub=0–5 months, I=5–14 months, II=15–38 months, III=39–57 months, IV+=⩾58 months. Sample sizes (n) represent number of individual raccoons.)

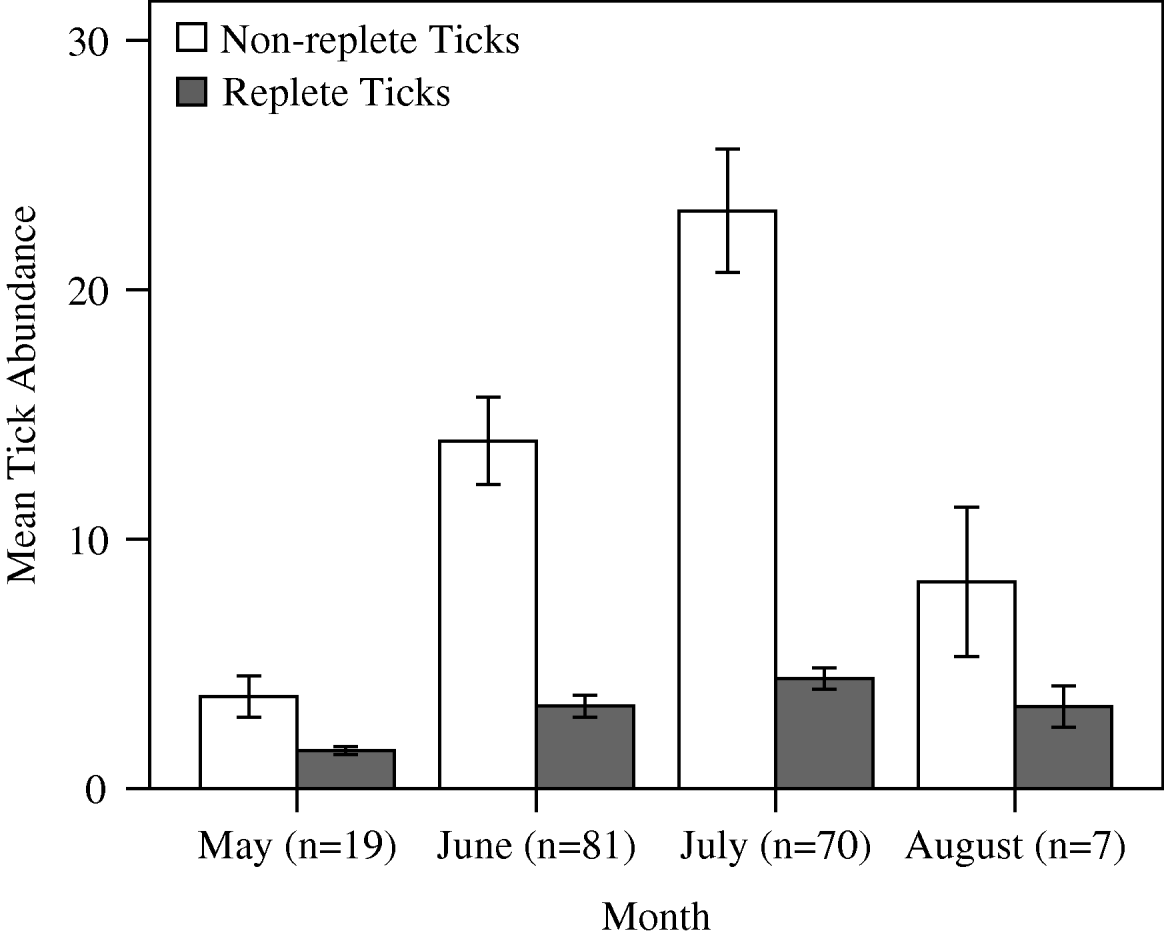
Tick distributions did not differ from a negative binomial distribution (non-replete χ2=21·67, d.f.=22, P=0·520; replete χ2=10·09, d.f.=13, P=0·310). The exponent of the negative binomial (k) indicated that both non-replete (k=0·890) and replete (k=0·600) ticks were highly aggregated on raccoons. Replete ticks displayed a greater degree of aggregation, with 10% (n=18) of hosts harbouring 51% of the replete D. variabilis (Fig. 1). Non-replete tick abundance across all sites combined ranged from 0 to 95 and averaged 16·27±1·35 (s.e.). Replete tick abundance ranged from 0 to 21 and averaged 2·54±0·28. Across sites, the range of means for ticks per animal was 3·63 to 30·23 for non-replete and 0·42 to 3·69 for replete ticks. There were temporal differences in the mean abundance for non-replete and replete ticks; both peaked in July, but replete abundance displayed less variation than non-replete abundance (Fig. 2). The ratio of replete:non-replete did not differ by month (Kruskal-Wallis H′=5·990, d.f.=3, P=0·112).

Fig. 1. Number of non-replete and replete Dermacentor variabilis adult ticks infesting raccoons in mid-Missouri. Note: 15 raccoons had >40 non-replete ticks (range=41 to 95). These data were removed for figure clarity.
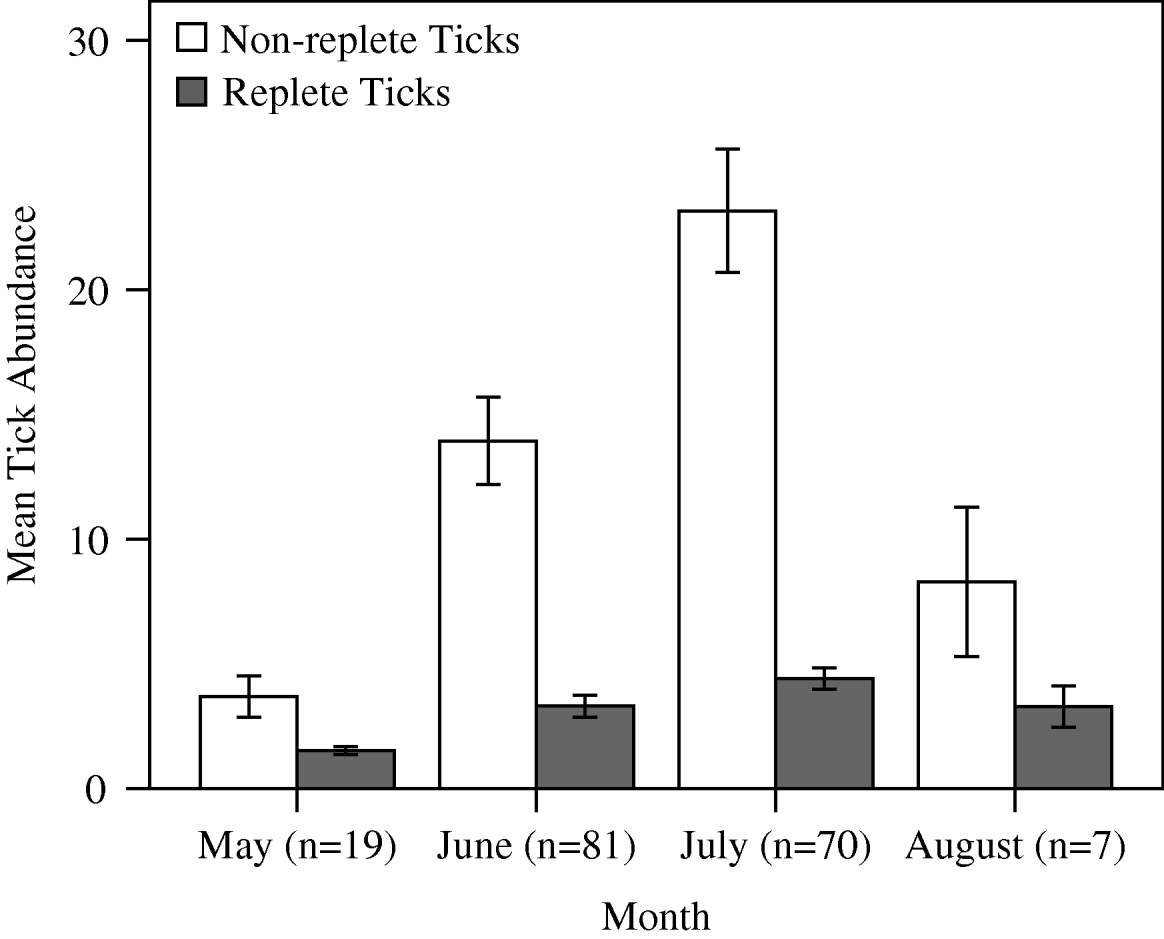
Fig. 2. Mean (±s.e.) seasonal abundance of Dermacentor variabilis adult ticks on raccoons in Missouri during 2005 and 2006. Sample sizes represent number of animals captured in each month.
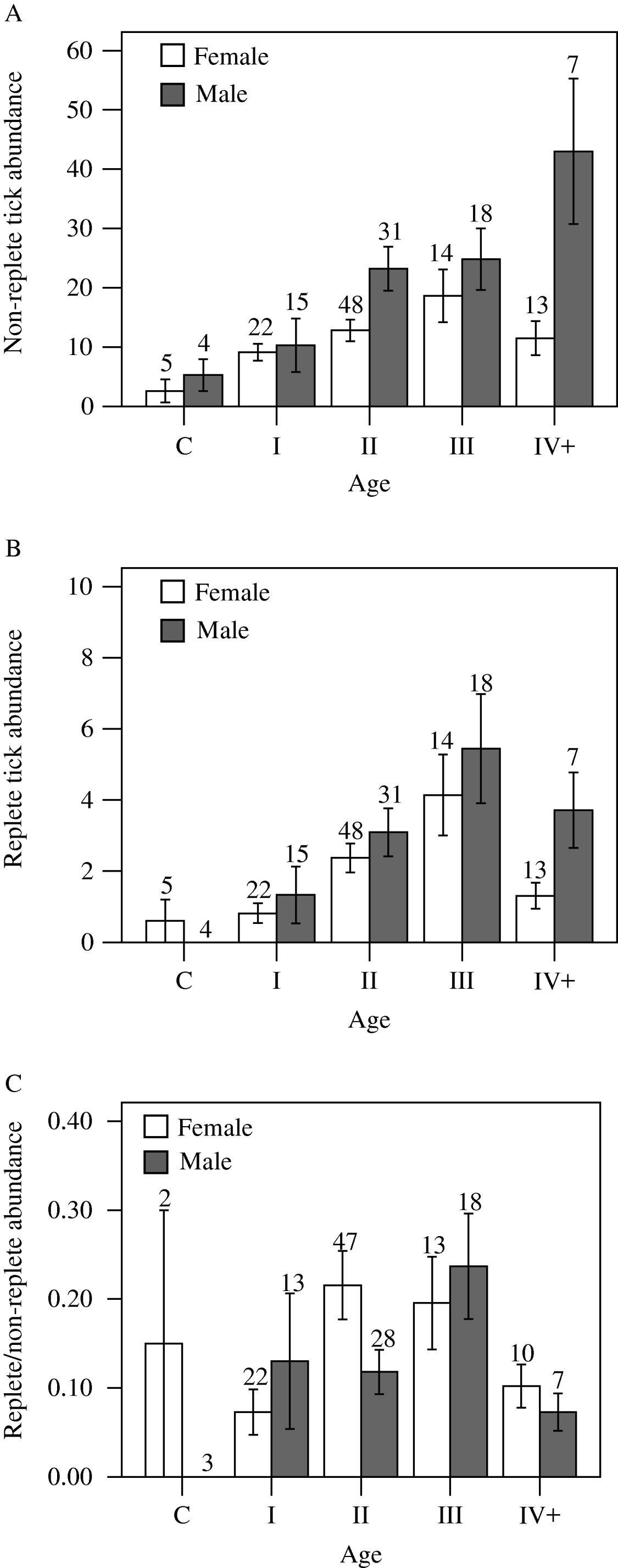
Non-replete tick abundance increased with age, with the exception of females in age class IV+ (Fig. 3A). In age class II, males supported a greater number of non-replete ticks than females (Mann-Whitney U-test, P=0·001), and there was a weakly significant pattern (P=0·037) for age class IV+. No other sex-related differences were detected among non-replete ticks (P⩾0·160 for all other comparisons), although males consistently had higher tick burdens. Replete tick abundance displayed an increasing trend with age of host; however, both females and males in age class IV+ exhibited declines in replete tick abundance when compared to age class III (Fig. 3B). No sex-related differences were detected among replete tick abundance in any age class (P⩾0·181 for all comparisons), although as for non-replete ticks, males consistently had higher tick burdens (excepting cubs).
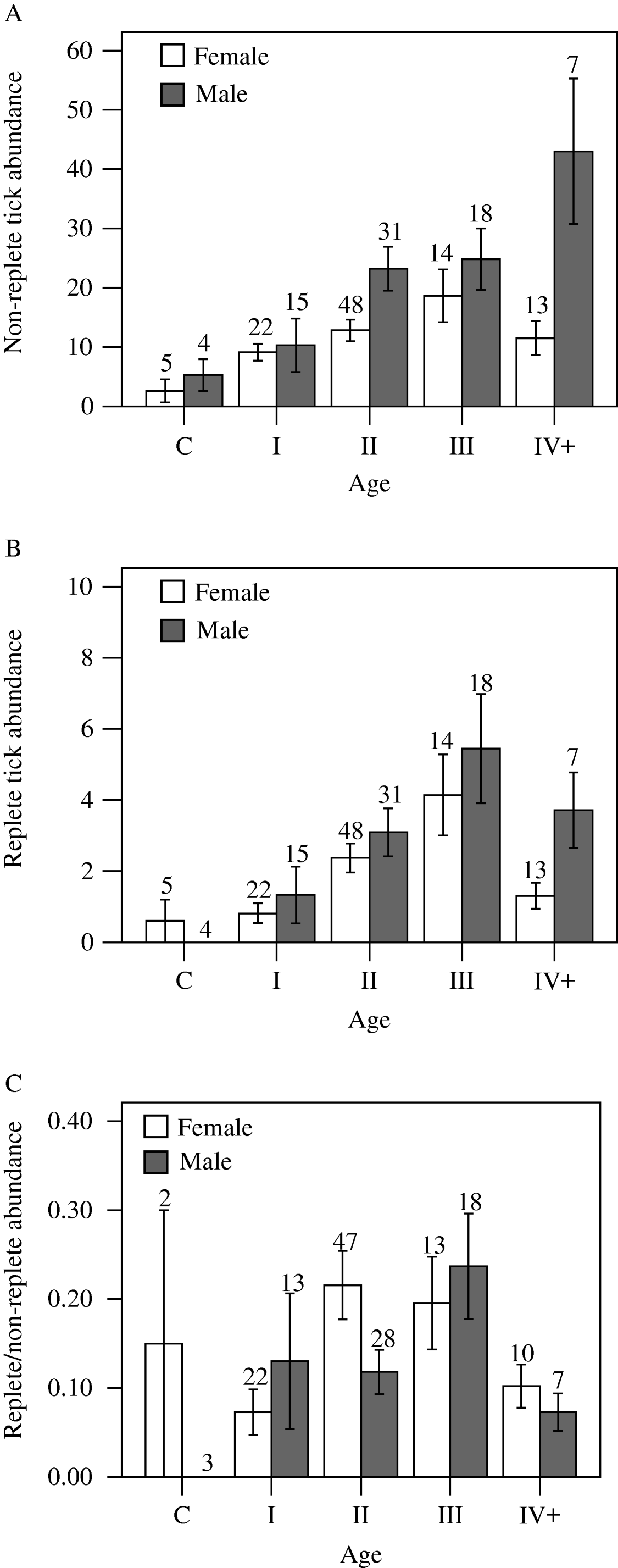
Fig. 3. Mean (±s.e.) abundance of non-replete (A) and replete (B) Dermacentor variabilis ticks; and the ratio (±s.e.; only animals with at least 1 non-replete tick are included) of replete to non-replete Dermacentor variabilis ticks (C) on raccoons. Raccoon age class designations are c=cub, I=5–14 months, II=15–38 months, III=39–57 months, IV+=⩾58 months Sample sizes are shown above s.e. bars.
There was a significant difference among age classes in the ratio of replete to non-replete ticks (Kruskal Wallis H′=18·54, d.f.=4, P=0·001) (Fig. 3C). Post-hoc comparisons indicated significant differences between age classes I and II (Mann-Whitney U-test, P=0·001) and I and III (P=0·001), and weakly significant differences between age classes III and IV+ (P=0·025). Within each age class, no sex-related differences were detected for the ratio of replete to non-replete ticks (Mann-Whitney U-test, P⩾0·054 for age class II and P⩾0·432 for all other age classes).
Model selection
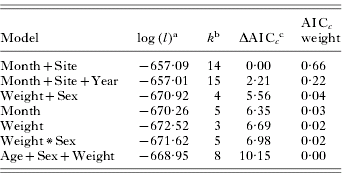
Non-replete tick abundance was best predicted by abiotic models (Table 3). The non-repletemonth+site model was the best fitting model of the data, with no other models falling within 2 AICc units; thus this model represents the only model considered to have substantial empirical support as the best fitting model. The weight of evidence (i.e. probability) in favour of non-repletemonth+site being the best model was 0·66. The non-repletemonth+site+year model ranked second (ΔAICc.=2·21) and had a weight of 0·22. Global and host characteristic (biotic) models had little support as a best-fitting model (AICc weight ⩽0·04 in all cases; Table 3).
Table 3. Ranking of a priori models estimating abundance of non-replete Dermacentor variabilis adult ticks on raccoons
(Rankings are based on Generalized Linear Models with a negative binomial distribution; n=177.)
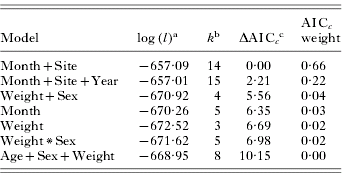
a Maximized log-likelihood value.
b The number of model parameters.
c The lowest AICc score was 1342·41.
Conversely, biotic models provided a better fit for replete tick data. The top 5 models were based on factors intrinsic to the host, with repleteage and repleteage+sex both having substantial empirical support as the best fitting model (Table 4). The AICc values for these models differed little and both had similar weight of evidence as the best fitting model (repleteage AICc weight=0·45, repleteage+sex AICc weight=0·36). The only other model with substantial support based on weight of evidence was repleteage+sex+weight (AICc weight=0·11). None of the abiotic models had an AICc weight >0·01 (Table 4).
Table 4. Ranking of a priori models estimating abundance of replete female Dermacentor variabilis adult ticks on raccoons
(Rankings are based on Generalized Linear Models with a negative binomial distribution; n=177.)
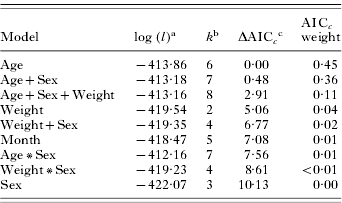
a Maximized log-likelihood value.
b The number of model parameters.
c The lowest AICc score was 838·08.
DISCUSSION
We observed clear differences in factors predicting the abundance of non-replete and replete D. variabilis on raccoons. Non-replete abundance was primarily a function of month and site of collection and, while a relationship between abundance and host age and sex was observed, these relationships were of secondary importance in predicting tick abundance on hosts. In contrast, replete tick abundance was best predicted by biotic covariates, in particular host age and sex. Such differences are surprising because age profiles of non-replete and replete abundance were similar, both displaying a positive relationship with age. However, seasonal fluctuations of non-replete abundance were >400% greater than replete abundance. This suggests that for non-replete ticks seasonal and site-specific effects overshadow age and sex-specific differences in susceptibility to tick attachment, but factors intrinsic to the host are more important for predicting the abundance of ticks that are able to both reach a host by questing and subsequently persist on the host long enough to become fully engorged.
Previous research had found that host-seeking adult D. variabilis display large seasonal fluctuations in abundance and are aggregated in habitats and locations where the likelihood of survival and host attachment are best. Kollars et al. (Reference Kollars, Oliver, Masters, Kollars and Durden2000) sampled free-living adult D. variabilis in Missouri and observed a peak in July with similar magnitudes of difference between months to that observed in this study. These patterns are also consistent with those observed in northeastern North America (Campbell, Reference Campbell and Rodriguez1979; McEnroe, Reference McEnroe and Rodriguez1979; McEnroe and Specht, Reference McEnroe and Specht1987). In contrast, adult D. variabilis in Southeast U.S. display bimodal or multimodal activity patterns due to the large numbers of adult ticks that successfully overwinter and attach to hosts in spring (Sonenshine and Stout, Reference Sonenshine and Stout1971; McEnroe, Reference McEnroe1974; Sonenshine, Reference Sonenshine and Rodriguez1979; Newhouse, Reference Newhouse1983; Carroll and Nichols, Reference Carroll and Nichols1986; McEnroe and Specht, Reference McEnroe and Specht1987; Burg, Reference Burg2001). Based on seasonal fluctuations in this study and that of Kollars et al. (Reference Kollars, Oliver, Masters, Kollars and Durden2000), Missouri populations appear to primarily consist of overwintering larvae that result in summer cohorts of adults. Burg (Reference Burg2001) suggested that summer cohorts of D. variabilis may have greater energy reserves than overwintering adults. Future research should determine whether this results in higher rates of host attachment, feeding success, or transovarial disease transmission via greater egg mass size and zoonotic amplification of tick-borne pathogens such as Rocky Mountain spotted fever (Rickettsia spp.).
Site and month of collection were the primary influence on non-replete tick abundance, but there were consistent increases in tick burden through age class III (declines in age class IV+ are discussed below). This suggests that there may be age-related behavioural or physiological factors that influence tick infestation (as there are with sex-related differences). The most likely explanation is that the youngest animals (age classes C and I) may receive the benefits of grooming from adult females that they den and travel with, while for older animals (age classes II–IV+) greater movements may increase susceptibility to infestation. Therefore, on a smaller scale (within a site vs between sites), age is of greater relative importance than sex when considering tick infestation in general. A variety of patterns have been described for helminths and mammals (e.g. Halvorsen, Reference Halvorsen1986; Gregory et al. Reference Gregory, Montgomery and Montgomery1992; Quinnell, Reference Quinnell1992); however, we know of no studies that have examined the influence of age on tick acquisition in free-living mammals. Hudson (Reference Hudson1992) found that tick intensity declined in red-grouse chicks after 2 weeks when Louping ill virus was present, but continually increased in the absence of this disease. Further investigation is needed to determine whether such relationships are common.
The finding that the best-fitting models of replete tick abundance included age and sex and no abiotic covariates suggests that once a tick has reached a host, host-parasite interactions are the primary determinant of full engorgement by female ticks, independent of where the host is found. This is also supported by the observations that replete tick abundance exhibited little difference between months, and a greater degree of aggregation than non-replete abundance. Several mechanisms may underlie the prominent role of host characteristics, including acquired immunity, tick-associated mortality of hosts, or the effect of density dependence on numbers of ticks on a host. Previous research has found a variety of mammals to acquire resistance to tick infestation (Wikel, Reference Wikel1996; Hughes and Randolph, Reference Hughes and Randolph2001; Castagnolli et al. Reference Castagnolli, de Figueiredo, Santana, De Castro, Romano and Szabó2003), including D. variabilis (Trager, Reference Trager1939; denHollander and Allen, Reference denHollander and Allen1985). Raccoons have been found to develop immunity to Ixodes scapularis (Craig et al. Reference Craig, Norris, Sanders, Glass and Schwartz1996), but this has not been examined for Dermacentor spp. Our results do not, however, support acquired immunity as underlying our models, for although we saw a decline in the absolute number and proportion of replete tick abundance in older age classes, acquired immunity is predicted to occur at a much earlier age. Craig et al. (Reference Craig, Norris, Sanders, Glass and Schwartz1996) found a significant decline in the proportion of engorged Ixodes scapularis larvae within 2 months of initial tick infestation, and antibody production displayed a 2 to 10-fold increase within weeks of infestation. Other studies also suggest that immunity to ticks develops in mammals and birds within weeks or months (Hudson, Reference Hudson1992; Hughes and Randolph, Reference Hughes and Randolph2001; Castagnolli et al. Reference Castagnolli, de Figueiredo, Santana, De Castro, Romano and Szabó2003). Acquired immunity might be perceived as underlying the decline in the abundance of replete compared to non-replete ticks observed between months (excepting August for which sample sizes were small). However, these data disassociated the abundance of these 2 classes of ticks on individual hosts. When the ratio of replete:non-replete ticks on individual hosts is examined, there is no temporal decline in the likelihood of a female tick persisting to become engorged.
The decline of replete ticks in the oldest age class suggests that the most heavily infested older animals may die earlier, perhaps due to persistently high tick burdens or due to causes that correlate with, or result in, higher tick burdens. Dermacentor spp. are known to cause tissue damage, anaemia, and paralysis in domestic animals (Strickland et al. Reference Strickland, Gerrish, Hourrigan and Schubert1976; Allan, Reference Allan, Samuel, Pybus and Kocan2001). Hawlena et al. (Reference Hawlena, Abramsky and Krasnov2006) observed age-related differences in flea-induced mortality of rodents; thus while tick-driven effects have not been evaluated in natural hosts, older animals are likely to be more susceptible to such impacts. Alternatively, ticks may be more abundant on less healthy animals that have higher mortality rates. Ticks may also have indirect effects by transmitting diseases or creating sites suitable for bacterial infection. Problematically for the explanation that declines in replete tick abundances in older age classes may be a function of host mortality, however, is the finding that non-replete tick burdens also decline among the oldest females (but not males). Such a pattern seems unlikely to derive from host mortality.
Lack of variation in replete compared to non-replete abundance may be due to ticks reaching their carrying capacity and exhibiting density dependence due to limited host resources or intraspecific interactions. Competition between ectoparasites is not expected to have a significant influence on rates of infestation because many species spend long periods of time off the host, and thus it has received little research attention (Krasnov et al. Reference Krasnov, Burdelova, Khokhlova, Shenbrot and Degen2005). Adult Dermacentor ticks exhibit high prevalence (Kollars et al. Reference Kollars, Oliver, Masters, Kollars and Durden2000; this study), however, and are located almost exclusively on the head of raccoons, primarily on the back of the neck and in and around the ears (R. Monello, unpublished observation). Raccoons can presumably self-groom all other locations and thus space may be limited for tick attachment sites. In addition, several studies have documented the effect of density dependence on numbers of ticks on hosts that can develop acquired immunity (Randolph, Reference Randolph1994) and competition for food between and within haematophagous flea species (Tripet and Richner, Reference Tripet and Richner1999; Krasnov et al. Reference Krasnov, Burdelova, Khokhlova, Shenbrot and Degen2005).
Although differences in parasitism between male and female raccoons were not statistically significant within most age classes, the consistent trend of greater tick burdens in males was important in model formulation. The lack of large sex-related differences within age classes is not surprising as male raccoons in Missouri are only ∼12% larger than females (Lotze and Anderson, Reference Lotze and Anderson1979) and the degree of male-biased parasitism is often associated with sexual dimorphism (Moore and Wilson, Reference Moore and Wilson2002). In addition, D. variabilis parasitize raccoons pre- and post-parturition, and the immunosuppressive effects of hormones or energy required during pregnancy or lactation can increase rates of parasitism in females (Festa-Bianchet, Reference Festa-Bianchet1989; Dobson and Meagher, Reference Dobson and Meagher1996).
Factors that influence the ability of female ticks to feed to repletion may impact tick population and disease dynamics because female egg mass is related to the amount of blood ingested (Strickland et al. Reference Strickland, Gerrish, Hourrigan and Schubert1976; Allan, Reference Allan, Samuel, Pybus and Kocan2001), and transovarial pathogen maintenance of Rickettsia spp. (including R. rickettsii, i.e., Rocky Mountain spotted fever) and Francisella tularensis has been documented in Dermacentor variabilis (Macaluso et al. Reference Macaluso, Sonenshine, Ceraul and Azad2002; Goethert and Telford, Reference Goethert and Telford2005; Parola et al. Reference Parola, Paddock and Raoult2005). Thus, while environmental factors are of importance during free-living stages, host population structure may have a large influence on potential cohort size of ticks by reducing or increasing the total number and proportion that can become engorged and moult or lay eggs. Such effects may be particularly important when the host is the primary feeding source for the tick and when individuals exhibit large differences in susceptibility or response to parasitism as a function of sex and age. Further research on host-tick interactions should focus on mechanisms underlying these patterns and the ability of ticks to become engorged.
This work was supported by an NSF grant (DEB-0347609) to M. Gompper. We thank J. Millspaugh for assistance with model analyses and B. Cook, T. Hamilton, K. McBride, T. McVicker, A. Wiewel, and J. Wisdom for assistance in the field.