INTRODUCTION
The bovine lungworm Dictyocaulus viviparus causes severe economic damage concerning cattle farming in temperate zones. To avoid patent infections, potent anthelmintics such as macrocyclic lactones are used frequently. However, anthelmintic treatments, particularly those with residual activity, reduce the antigenic stimulus and thus the evoked immune response, leading to continuous susceptibility (Urquhart et al. Reference Urquhart, Jarrett, Bairden and Bonazzi1981; Schnieder et al. Reference Schnieder, Epe, von Samson-Himmelstjerna and Kohlmetz1996). Since the 1950s a commercial vaccine, composed of irradiated larvae (Bovilis® Huskvac, MSD Animal Health) which induces a strong protective immunity, is available for the prophylaxis of parasitic bronchopneumonia (McKeand, Reference McKeand2000). Nevertheless, there are several disadvantages: The vaccine is based on living D. viviparus third-stage larvae (L3) and thus requires experimentally infected cattle for larvae production, which raises ethical questions. Due to the necessity of living L3, the vaccine is not sterilizable. Furthermore, handling and storage of the vaccine is rather complex (McKeand, Reference McKeand2000) and it is marketed only in a few European countries. Therefore, the development of a recombinant vaccine would be beneficial in terms of ethical aspects regarding larvae production, cost effectiveness and biosecurity. Potentially essential proteins already expressed in preadult parasitic stages appear as promising vaccine targets since even early damage of the host might be avoided. In D. viviparus, transcription of legumains is upregulated in preadult parasitic compared to free-living pasture stages, leading to the assumption that they are probably essential for the parasite's adaptation to the cattle host (Strube et al. Reference Strube, Buschbaum and Schnieder2012). Legumains (also known as asparaginyl endopeptidases) are Clan CD cysteine proteases of the C13 superfamily (Chen et al. Reference Chen, Dando, Rawlings, Brown, Young, Stevens, Hewitt, Watts and Barrett1997; Caffrey et al. Reference Caffrey, Mathieu, Gaffney, Salter, Sajid, Lucas, Franklin, Bogyo and McKerrow2000), firstly discovered in plants (Kembhavi et al. Reference Kembhavi, Buttle, Knight and Barrett1993) and trematodes of the genus Schistosoma (Dalton et al. Reference Dalton, Hola-Jamriska and Brindley1995; Sajid et al. Reference Sajid, McKerrow, Hansell, Mathieu, Lucas, Hsieh, Greenbaum, Bogyo, Salter, Lim, Franklin, Kim and Caffrey2003; Krautz-Peterson and Skelly, Reference Krautz-Peterson and Skelly2008), before being described in vertebrates (Chen et al. Reference Chen, Dando, Rawlings, Brown, Young, Stevens, Hewitt, Watts and Barrett1997; Yamane et al. Reference Yamane, Takeuchi, Yamamoto, Li, Fujiwara, Nishi, Takahashi and Ohkubo2002) or the nematode Haemonchus contortus (Oliver et al. Reference Oliver, Skuce, McNair and Knox2006). Legumain of the trematode Schistosoma mansoni was suspected to transprocess other enzymes and therefore, participate in haemoglobin digestion but results of current studies are contradictory (Dalton and Brindley, Reference Dalton and Brindley1996; Sajid et al. Reference Sajid, McKerrow, Hansell, Mathieu, Lucas, Hsieh, Greenbaum, Bogyo, Salter, Lim, Franklin, Kim and Caffrey2003; Krautz-Peterson and Skelly, Reference Krautz-Peterson and Skelly2008; Dalton et al. Reference Dalton, Brindley, Donnelly and Robinson2009). A DNA-based vaccine against legumain of S. mansoni resulted in an anti-fecundity effect in female worms and increased weight gain of experimental animals. Thus, the authors discussed an anti-pathology effect of the vaccine (Chlichlia et al. Reference Chlichlia, Bahgat, Ruppel and Schirrmacher2001). Oliver et al. (Reference Oliver, Skuce, McNair and Knox2006) discovered similarities in pH-optimum and inhibitor profiles of H. contortus legumain compared with that of S. mansoni and therefore assumed H. contortus legumain to be involved in activation of cysteine proteases, making it an ideal drug target or vaccine candidate, as blocking of legumain would led to detrimental effects on the parasite. In the present study, legumain-1 (LEG-1) of the bovine lungworm D. viviparus was assessed for its enzyme characteristics in vitro and tested for its suitability as a recombinant vaccine in vivo.
MATERIALS AND METHODS
Phylogenetic classification of D. viviparus LEG-1 and secondary structure analysis
Phylogenetic analysis comprised 30 legumain amino acid sequences originating from parasites and vertebrates. Missing data positions were clipped resulting in a comparison of 343 amino acid positions per sequence in the final dataset (full length D. viviparus LEG-1 comprises 445 aa). Following sequence alignments with ClustalW, phylogenetic analysis was conducted using MEGA version 6.0 (Tamura et al. Reference Tamura, Stecher, Peterson, Filipski and Kumar2013). The phylogenetic tree was constructed by bootstrap test of phylogeny (1000 replicates) using the Maximum Parsimony method.
Secondary structure analysis of LEG-1 was conducted using the Phyre2 web portal for protein modelling by sequence alignment with human legumain (GenBank acc. no. Q99538) according to Dall and Brandstetter (Reference Dall and Brandstetter2016).
Recombinant expression of LEG-1 in the yeast Pichia pastoris
Dictyocaulus viviparus LEG-1 (GenBank accession number JQ828985) was recombinantly expressed in the yeast P. pastoris using the Pichia Pink™ Expression System (Invitrogen, Germany). Primer design included the LEG-1 sequence without signal peptide, a 3′-end linked thrombin cleavage site, hexa-histidine-tag (for protein affinity purification) and stop codon as well as SwaI (5′-terminal) and KpnI (3′-terminal) restriction sites for insertion into the P. pastoris expression vector. Primer sequences were: DvPichiaLEG-1_for 5′-ATTTAAATCCCTTAATCTACATGATTTTTTAACGC-3′ and DvPichiaLEG-1_rev 5′- GGTACCTTAGTGATGGTGATGGTGATGGGATCCACGCGGAACCAGCAAGAACTGTTTGGTAGCTGTTG-3′. The PCR reaction setup contained 40·5 µL deionized water, 5 µL 10× Advantage™ 2 PCR buffer, 1 µL forward and reverse primer (10 µ m each), 1 µL deoxyribonucleotide triphosphates (10 mm each), 0·5 µL Advantage® cDNA Polymerase Mix (BD Biosciences Clontech) and 1 µL cDNA of adult lungworms as template. Thermocycling conditions were as follows: 95 °C for 3 min for initial denaturation and polymerase activation, followed by 35 cycles of denaturation at 95 °C for 45 s, annealing at 60 °C for 1 min, and extension at 72 °C for 1 min. Final extension was performed at 72 °C for 10 min. The amplification product was inserted into the vector pCR®4-TOPO® (TOPO TA Cloning® Kit for Sequencing, Life Technologies) and transformed into One Shot® TOP10 chemically competent Escherichia coli (Life Technologies). Afterwards, it was subcloned into the vector pPinkα-HC (PichiaPink™ Expression System, Life Technologies), electroporated into the P. pastoris peptidase knockout strain PichiaPink Strain 4 (ade2, prb1 and pep4 deficient; Life Technologies) and recombinantly expressed as described by Kuerpick et al. (Reference Kuerpick, Schnieder and Strube2013). After three days expression at 28 °C, the supernatant containing rLEG-1 was sterile filtered and subsequently affinity purified with HisTrap™ HP columns operated by ÄKTA FPLC (GE Healthcare). The purified protein was visualized by sodium dodecyl sulphate-10% polyacrylamide gel electrophoresis (SDS–10% PAGE) and verified by custom matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometry (Functional Genomics Center Zurich, Switzerland).
Parasite material and anti-LEG-1-antibody production
Animal experiments were performed in accordance with the German Animal Welfare act in addition to national and international guidelines for animal welfare. Experiments were permitted by the Ethics Commission of the Institutional Animal Care and Use Committee (IACUC) of the German Lower Saxony State Office for Consumer Protection and Food Safety (Niedersaechsisches Landesamt für Verbraucherschutz und Lebensmittelsicherheit) under reference numbers mentioned below. Collection of different D. viviparus developmental stages for transcription and expression analysis was permitted under reference numbers AZ 33-42502-06/1160 and AZ 33.9-42502-04-09/1643. Baermann technique was used to isolate first stage larvae (L1) of D. viviparus (field isolate HannoverDv2010) from feces of experimentally infected calves. Thereafter, larvae were incubated in about 50 mL tap water for 14 days at room temperature, allowing development to L3. At day 1 to 6, 8, 10 and 14 of cultivation, L1 to L3 were collected. For L4 production, two male Holstein Friesian calves aged 3–5 months received 300 000 L3 each. Necropsy was performed 7 days post-infection (dpi) followed by perfusion of the lungs with tap water according to Wood et al. (Reference Wood, Amaral, Bairden, Duncan, Kassai, Malone, Pankavich, Reinecke, Slocombe, Taylor and Vercruysse1995). The washout was retained in 25 µ m sieves, rinsed with 0·9% sodium chloride solution and microscopically screened for L4 at 400-fold magnification. Preadult, hypobiotic preadult and adult worms were isolated as described by Laabs et al. (Reference Laabs, Schnieder and Strube2011). Adult lungworms for production of excretory/secretory (ES) antigen were obtained from experimentally infected Holstein-Friesian calves, permitted under reference number AZ 33.12-42502-05-13A321. Polyclonal antibody production in rabbits was permitted under reference number AZ 33.9-42502-05-12A294. A 14-weeks old New Zealand white rabbit (Charles River Laboratories, Germany) was injected subcutaneously in the neck with 100 µg of recombinant LEG-1 (rLEG-1) in 893 µL 10 mm Tris–HCl and an equal volume of Freund's incomplete Adjuvant (Sigma-Aldrich, Germany). Vaccination was repeated on days 22, 49 and 63 of the experiment with 50 µg of rLEG-1 in 581 µL 10 mm Tris–HCl and an equal volume of Freund's incomplete Adjuvant. The rabbit was euthanized on day 75 for harvest of serum.
leg-1 transcription profile in D. viviparus throughout the life cycle
To analyse the transcription level of leg-1, all developmental stages [eggs, L1, L2, L3, hypobiosis induced L3 (L3i, chilled at 4 °C for 8 weeks), L4 as well as male, female and gender-mixed preadults, hypobiotic preadults as well as male and female adults] of the parasite were investigated using a probe-based quantitative real-time PCR (qPCR). cDNA synthesis of parasite material was performed as described previously (Strube et al. Reference Strube, Buschbaum, Wolken and Schnieder2008, Reference Strube, Buschbaum, von Samson-Himmelstjerna and Schnieder2009; Laabs et al. Reference Laabs, Schnieder and Strube2012). To enable calculation of transcript copy numbers in qPCR experiments, plasmid standards were used to generate standard curves. Plasmid standards containing partial leg-1 nucleotide sequences and the evaluated reference genes D. viviparus elongation factor 1α (ef-1α) and D. viviparus β-tubulin were produced as described by Strube et al. (Reference Strube, Buschbaum, Wolken and Schnieder2008). To prepare the leg-1 plasmid standard, primers leg-1_standard_for 5′-TGTTGCCCATGCTTACCACT-3′ and leg-1_standard_rev 5′-TTCACATGCTTCCAAATAGA-3′ were designed with Primer select software (DNASTAR, version 5.06; GATC Biotech, Germany). Primers and corresponding TaqMan™ minor groove binder (MGB) probes detecting ef-1α and β-tubulin transcripts were according to Strube et al. (Reference Strube, Buschbaum, Wolken and Schnieder2008, Reference Strube, Buschbaum, von Samson-Himmelstjerna and Schnieder2009). For leg-1 detection, primers and probe were designed with the AlleleID® software (version 7.01, PREMIER Biosoft International, USA). Resulting oligonucleotide sequences were: Primers leg-1_MGB_for 5′-GGT-ACAGATGTCTACAAAG-3′, leg-1_MGB_rev 5′-GAAGCAGATCAAACCTTC-3′ and the probe 5′-FAM-TTACAGTGGATCATCA-NFQ-3′. cDNA samples of all lungworm stages were analysed by qPCR as described by Laabs et al. (Reference Laabs, Schnieder and Strube2011). PCR efficiency correction, data normalization using reference genes and relative quantification using eggs as calibrator was done using the qBasePLUS software (version 1.3.5, Biogazelle, Belgium).
LEG-1 expression profile in D. viviparus throughout the life cycle
To determine expression of LEG-1, different developmental stages and organs of D. viviparus were analysed by immunoblots. Eggs, L1, L3, L4, male and female preadults as well as adults were homogenized followed by centrifugation at 2500 g for 5 min at 4 °C. Pellets were resuspended in 250 µL PBS and protein yields were measured using the NanoDrop ND-1000 spectrophotometer (Peqlab, Germany). For immunoblotting, samples were diluted to 1 µg µL−1 total protein in PBS and per lane, 15 µL of each sample was loaded and separated by SDS–10% PAGE. Additionally, rLEG-1 was loaded on the gel as positive control. Afterwards, proteins were transferred to a nitrocellulose membrane (Macherey-Nagel, Germany). Immunoblots were developed using polyclonal anti-rLEG-1-rabbit antiserum diluted 1:50 in Tris-buffered saline [(TBS: 10 mm Tris; 137 mm NaCl; pH 7·3) containing 0·05% Tween® 20 (Carl Roth, Germany)]. Binding was visualized by a polyclonal alkaline phosphatase (AP)-conjugated goat-anti-rabbit-IgG-antibody (1:6000 in TBS-Tween; Sigma-Aldrich, Germany) and 5-bromo-4-chloro-3′-indolyphosphate p-toluidine salt (BCIP®)/nitro-blue tetrazolium chloride (NBT) substrate solution (Carl Roth, Germany).
Expression of LEG-1 in lungworm organs
Cryosections of adult D. viviparus were analysed by immunohistochemistry as described by Strube et al. (Reference Strube, Haake, Sager, Schorderet Weber, Kaminsky, Buschbaum, Joekel, Schicht, Kremmer, Korrell, Schnieder and von Samson-Himmelstjerna2015), but with the amendment that polyclonal anti-rLEG-1 rabbit antiserum diluted 1:100 in 5% fetal calve serum (FCS, GE Healthcare, Germany) followed by fluorescein isothiocyanate (FITC)-labelled mouse monoclonal 2A9 anti-rabbit IgG heavy-chain antibodies (Abcam, UK) diluted 1:100 in PBS (final concentration 5 µg mL−1) were used for LEG-1 detection. For nuclear counterstaining 4′,6-diamidino-2′-phenylindol-dihydrochloride (DAPI, AppliChem GmbH, Germany) was used at 5 ng mL−1 in Mowiol®4–88 (Carl Roth, Germany). Slides incubated with rabbit serum obtained prior to immunization served as negative control.
Besides cryosections, organs of adult female and male worms obtained by microdissection were analysed by immunoblotting. Cuticles, oesophagi, guts, uteri, ovaries and testes were transferred into 0·5 mm glass bead 2 mL tubes (Süd-Laborbedarf, Germany) and homogenized in 500 µL PBS containing 0·1% Triton™ X-100 (Sigma-Aldrich, Germany) for 2× 1 min at 6500 rpm using the Precellys 24 homogenizer. The supernatants were transferred into 1·5 mL tubes (Eppendorf, Germany) and proteins were precipitated by adding 150 µL trichloroacetic acid (1 g mL−1) and subsequent incubation for 5 min on ice. After centrifugation for 15 min at 16 000× g and 2 °C, pellets were resolved in 300 µL (cuticles, oesophagi, ovaries and uteri) or 150 µL (guts and testes) 1·5 m Tris, respectively. After evaluation of the organ samples by Coomassie Brilliant Blue R250-stained SDS–PAGE, 20 µL of each sample was used for subsequent immunoblot analysis as described in the section above.
Detection of LEG-1 in D. viviparus ES product
Male and female adult lungworms were cultivated as described by Laabs et al. (Reference Laabs, Schnieder and Strube2011) with the modification that FCS-free Gibco® RPMI Medium 1640 with L-glutamine (Life Technologies, Germany) supplemented with 0·625 µg mL−1 amphotericin B (GE Healthcare, Germany), 100 IU ml−1 penicillin and 100 µg mL−1 streptomycin (GE Healthcare, Germany) was used for cultivation. ES products obtained after 24 and 48 h of cultivation were used. Part of the ES product was 100 times concentrated by precipitating 50 mL ES product with 500 mL acetone for 30 min at −20 °C. After centrifugation at 16 000× g and 4 °C for 30 min, the pellet was resolved in 500 µL 1× PBS. For SDS–PAGE and subsequent immunoblot, 4 µL 100 times concentrated and 30 µL unconcentrated ES product was loaded on the gel. Additionally, rLEG-1 as well as a medium control was loaded on the gel. Immunoblot analysis was done with either polyclonal anti-rLEG-1 rabbit antiserum as described above or a monoclonal mouse anti histidine tag antibody (1:500 in TBS-Tween; AbDSerotec, Germany) followed by an polyclonal AP-conjugated goat-anti-mouse-IgG/A/M-antibody (1:5000 in TBS-Tween AbDSerotec, Germany). Additionally, a second blot using the same samples, but rabbit serum prior to vaccination was performed to exclude cross-reactions of rabbit serum.
Activation of LEG-1
Since rLEG-1 showed insufficient auto-activation in 10 mm Tris–HCl, it was trans-activated with recombinant P. pastoris-expressed D. viviparus cathepsin L2 (rCPL-2; Genbank acc. no. AFM37362). Recombinant rCPL-2 expression and verification were performed as described for rLEG-1 above. Primer sequences were: DvPichiaCPL-2_for 5′-ATTTAAATCCAAGATCGGGACACCTAGAAAGC-3′ and DvPichiaCPL-2_rev 5′-GGTACCTTAGTGATGGTGATGGTGATGGGATCCACGCGGAACCAGGACGAGCGGATAACTCGCCTTCG-3′. An amount of 500 ng rLEG-1 and 50 ng recombinant rCPL-2 were incubated with an equal volume of buffer containing 250 mm NaCl, 50 mm sodium acetate, 0·16 mm Triton™ X-100, 1 mm dithiothreitol (DTT) and 2 mm ethylenediaminetetraacetic acid (EDTA) at pH 4·5 (cathepsin-assay-buffer) for 2 h at 39 °C and 400 rpm (Thermomixer® Comfort, Eppendorf, Germany). Afterwards, buffer with identical ingredients but pH 5·5 (legumain-assay buffer) was added to a final volume of 50 µL.
Enzymatic characterization of LEG-1
Unless stated otherwise, enzyme assays were performed with legumain-assay buffer pH 5·5 at 39 °C using the legumain-specific substrate N-benzyloxycarbonyl–alanyl–alanyl–asparaginyl–7-amido-4-methylcoumarin (Z–Ala–Ala–Asn–AMC; Bachem Holding, Switzerland). In 96-well black microtitre plates (VWR, Germany), 50 µL substrate solution diluted to 100 µ m in legumain-assay buffer was added to 50 µL activated rLEG-1, resulting in final concentrations of 50 µ m substrate and 141·3 nm rLEG-1 [based on a MW of 35·4 kDa for predicted mature LEG-1 after alignment with human Legumain (GenBank and UniProt accession no. Q99538)]. Substrate turnover was determined every 50 s for a total of 30 min using the FLx800™ multi-detection microplate reader (BioTek Instruments, Germany). The reaction was measured in kinetic mode and excitation/emission wavelengths of 360/460 nm. All reactions were set up as triplicates or quadruplicates. To ensure the accuracy of the assay, each run was replicated. To quantify the cleaved substrate, a serial dilution of 10 000, 5000, 2500, 1250, 625, 312·5, 156·25 and 78·13 nm AMC (Bachem Holding, Switzerland) was included on each plate. Furthermore, each plate contained a negative control including all reaction components (thus also rCPL-2; buffer-only control) except rLEG-1.
To determine enzyme kinetics of LEG-1, fluorescent substrates Z–phenylalanyl–arginyl–AMC (Z–Phe–Arg–AMC; cathepsin-specific substrate) and Z–arginyl–arginyl–AMC (Z–Arg–Arg–AMC; cathepsin B-specific substrate; all substrates were purchased from Bachem Holding, Switzerland) were used in addition to the legumain-specific substrate Z–Ala–Ala–Asn–AMC. K m (Michaelis constant) and k cat (turnover number) values were calculated using nonlinear regression of the Michaelis–Menten equation with starting values determined through the Lineweaver–Burk equation.
Determination of the pH optimum was analysed using two buffers with a final pH of 4·0; 4·5; 5·0; 5·5 and 6·0. Both buffers contained 0·16 mm Triton™ X-100, 1 mm DTT and 2 mm EDTA and 50 mm of the buffer substances sodium acetate (pH 4·0–5·5) and MES (pH 5·0–6·0) were added to rLEG-1 directly before starting the assay. The temperature optimum for rLEG-1 was determined at temperatures of 25, 30, 35, 37, 39, 42, 45 and 50 °C.
The inhibitor profile (all inhibitors were purchased from Sigma-Aldrich, USA) of rLEG-1 was created by adding the cysteine protease inhibitors N-(trans-epoxysuccinyl)-L-leucine 4-guanidinobutylamide (E-64; 10 µ m), egg white cystatin or iodoacetic acid (100 µ m), the aspartyl protease inhibitor pepstatin A (10 µ m), the cathepsin B inhibitor N-(L-3-trans-[propylcarbamoyl]oxirane-2-carbonyl)-L-isoleucyl-L-proline (CA-074; 10 µ m) and the caspase inhibitor N-benzyloxycarbonyl-Val-Ala-Asp(OMe) fluoromethylketone (Z-VAD-FMK; 100 µ m) to the reaction setup.
Natural substrates of LEG-1
To identify natural LEG-1 substrates, 50 µ m bovine haemoglobin, fibrinogen, collagen type I from calf skin, collagen type II from bovine cartilage (all purchased from Sigma-Aldrich, USA), BSA (bovine serum albumin, Carl Roth, Germany) and 10 µ m bovine IgG (Sigma-Aldrich, USA) were incubated with 10 µg mL−1 activated rLEG-1 in legumain-assay-buffer at 39 °C and 400 rpm (Thermomixer® Comfort, Eppendorf, Germany). Samples were taken after 0, 30, 60, 90 and 120 min incubation.
A further substrate was bronchial mucus from lungworm-infected cattle (control animals of the vaccination trial described below), diluted to a final concentration of 1·4 g L−1 soluble protein. Samples were taken after 3 and 6 h of incubation. In this setup, rCPL-2 was completely inhibited after rLEG-1 activation through addition of E-64 to a final concentration of 100 µ m. In each experimental setup, a buffer-only negative control (see the section above) was included. Cleavage of substrates was visualized by SDS–10% PAGE.
Clinical vaccine trial
The vaccination trial was permitted by the above-mentioned ethics commission of the German Lower Saxony State Office for Consumer Protection and Food Safety under reference number AZ 33.9.-42502-04-11/0494. Ten parasite-naïve, 3-month-old Holstein Friesian cattle (mixed sex, 100–130 kg) were randomized into two groups of five calves each. The vaccine group was immunized three times (study day [s.d.] 0, 21, 42) intramuscularly with 100 µg rLEG-1 and 350 µg Matrix Q™ (Novavax, Sweden) as adjuvant dissolved in 10 mm Tris–HCl (pH 7·4) to a final volume of 1 mL. The control group received a respective volume of 10 mm Tris–HCl instead of rLEG-1 and the same amount of adjuvant. Challenge infection with the bovine lungworm isolate HannoverDv2010, maintained at the Institute for Parasitology, University of Veterinary Medicine Hannover, Germany, was performed on s.d. 63 and s.d. 64 (each with 1000 L3, total infection dose of 2000 L3). Measurement of weight gain and parasitological parameters (larvae shedding, worm burden and worm size) as well as analysis of immune response to rLEG-1 by enzyme-linked immunosorbent assay (ELISA) were performed as described previously (Joekel et al. Reference Joekel, Hinse, Raulf, Schicht, Baumer, Werling, Kremmer and Strube2015) with the modification that ELISA detection of immunoglobulin subclasses IgG, IgG1, IgG2, IgA and IgM was performed with 1:10 000 diluted secondary antibodies. In addition, immunoblots against whole adult lungworm lysate as antigen were performed as described by Joekel et al. (Reference Joekel, Hinse, Raulf, Schicht, Baumer, Werling, Kremmer and Strube2015) except that 64 µg crude antigen was loaded per lane and cattle sera were 1:25 diluted. As positive control, 0·2 µg rLEG-1 was detected with 1:50 diluted anti-rLEG-1 rabbit antiserum. Statistical analyses were done using unpaired t-test or, if data failed the normality test, Mann–Whitney test (GraphPad Prism software package, version 6, GraphPad Software, Inc., USA). A P value ⩽0·05 was considered statistically significant.
RESULTS
Phylogenetic classification of LEG-1 and secondary structure analysis
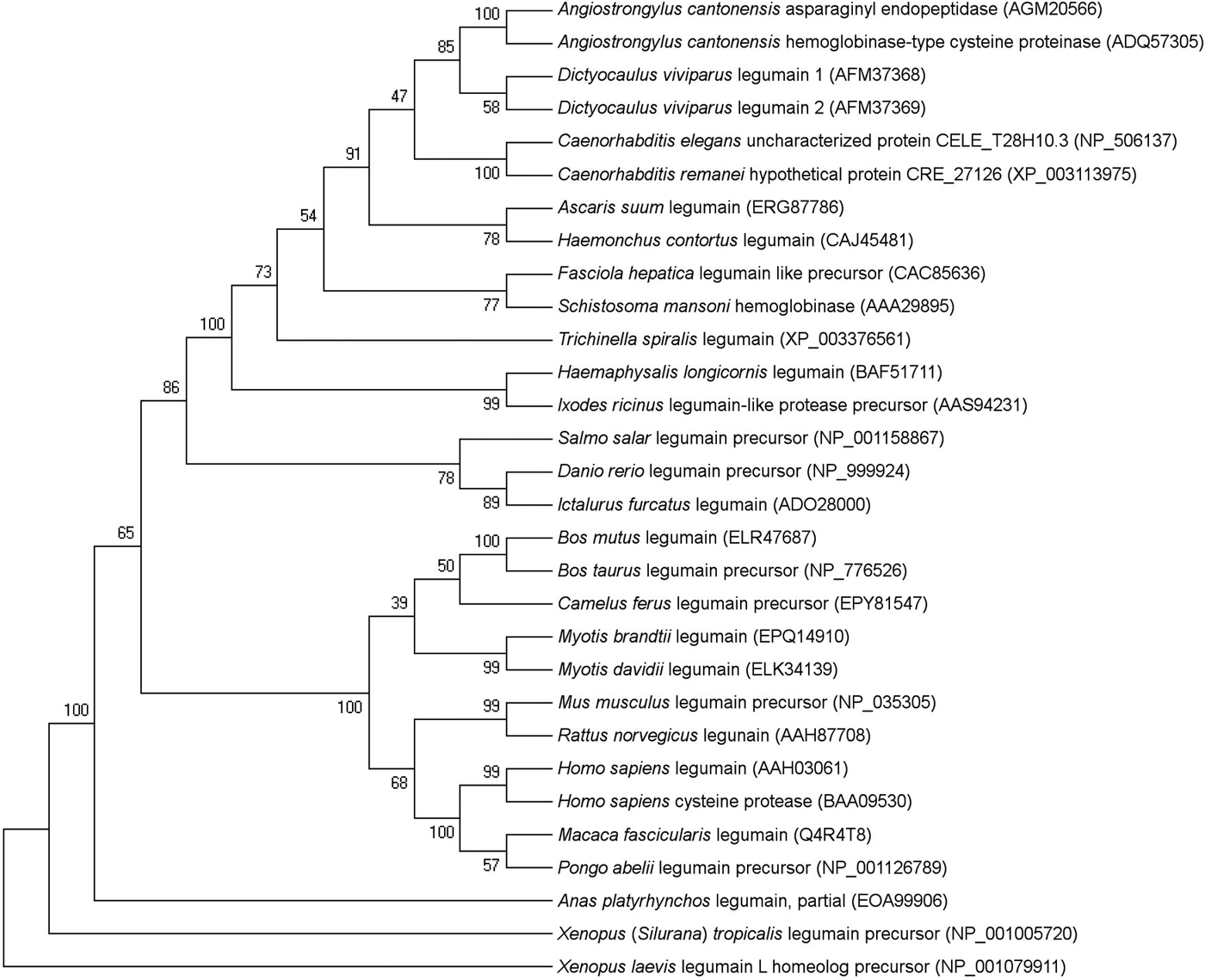
In the phylogenetic analysis, LEG-1 grouped together with the second D. viviparus legumain, LEG-2 (Fig. 1). Comparing the entire LEG-1 amino acid sequence to the LEG-2 sequence by BLASTP analysis revealed 58% amino acid sequence identity (99% query coverage). Dictyocaulus viviparus legumains grouped closest to an asparaginyl peptidase as well as haemoglobinase-type cysteine protease of the rat lungworm Angiostrongylus cantonensis (each 63% identity and 100% query coverage). Amongst helminths, D. viviparus LEG-1 showed the largest distance to Trichinella spiralis legumain (34% identity; 75% query coverage).
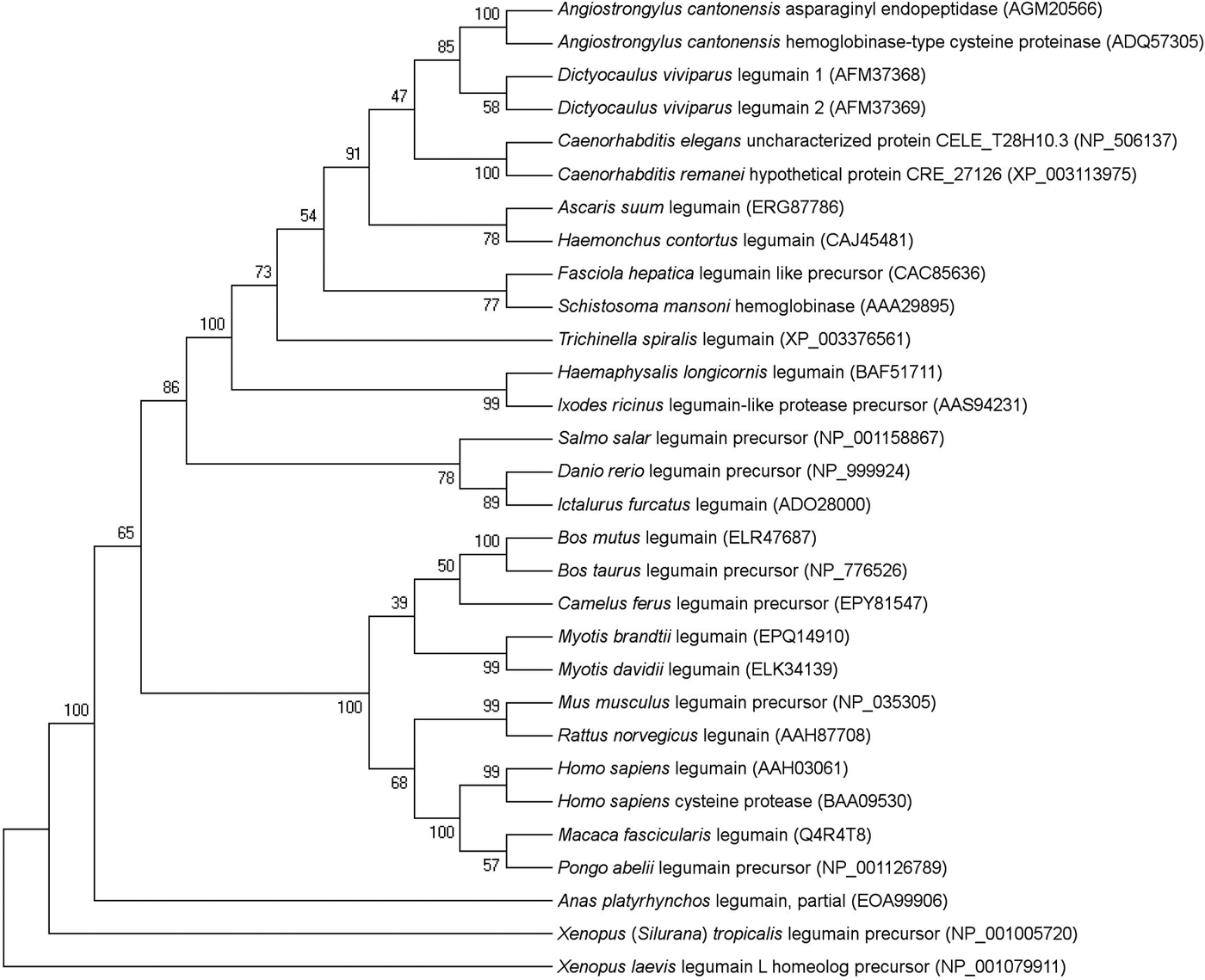
Fig. 1. Evolutionary relationships of legumain amino acid sequences of helminths, arthropods and vertebrates. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) is shown next to the branches. GenBank accession numbers are given in brackets.
Predicted secondary structure as assessed by alignment with human legumain is shown in Fig. 2.

Fig. 2. Secondary structure analysis of D. viviparus LEG-1 (GenBank acc. no. JQ828985) and human legumain (GenBank acc. no. Q99538) was performed using Phyre2 web portal for protein modelling. Structure elements are indicated for human legumain according to Dall and Brandstetter (Reference Dall and Brandstetter2016). Note: the alignment is shown without signalpeptide and N-terminal pro-peptide. C-terminal autocatalytic processing sites are indicated with arrows, catalytic residues with stars, glycosylation sites with triangles, S1-specificity residues with diamonds, the double arginine-motif (Arg324, Arg403) with square, the ubiquitination site Lys318 with a circle, the RGD120 motif, the TRAF6-binding site (A210NPRESSY217) and the KRK289–KRK318 motifs are indicated by boxes. Secondary structure elements are based on the crystal structure of human prolegumain (pdb entry 4fguB), whereby arrows stand for beta-strands, faint lines for coils, helices for alpha-helixes, the letter G for three-turn helix, T for hydrogen bonded turn and B for residue in isolated beta-bridge. The catalytic domain corresponds to dark grey, activation peptide to light grey and the LSMA (Legumain Stabilization and Activity Modulation) domain to the black elements.
leg-1 transcription profile in D. viviparus throughout the life cycle
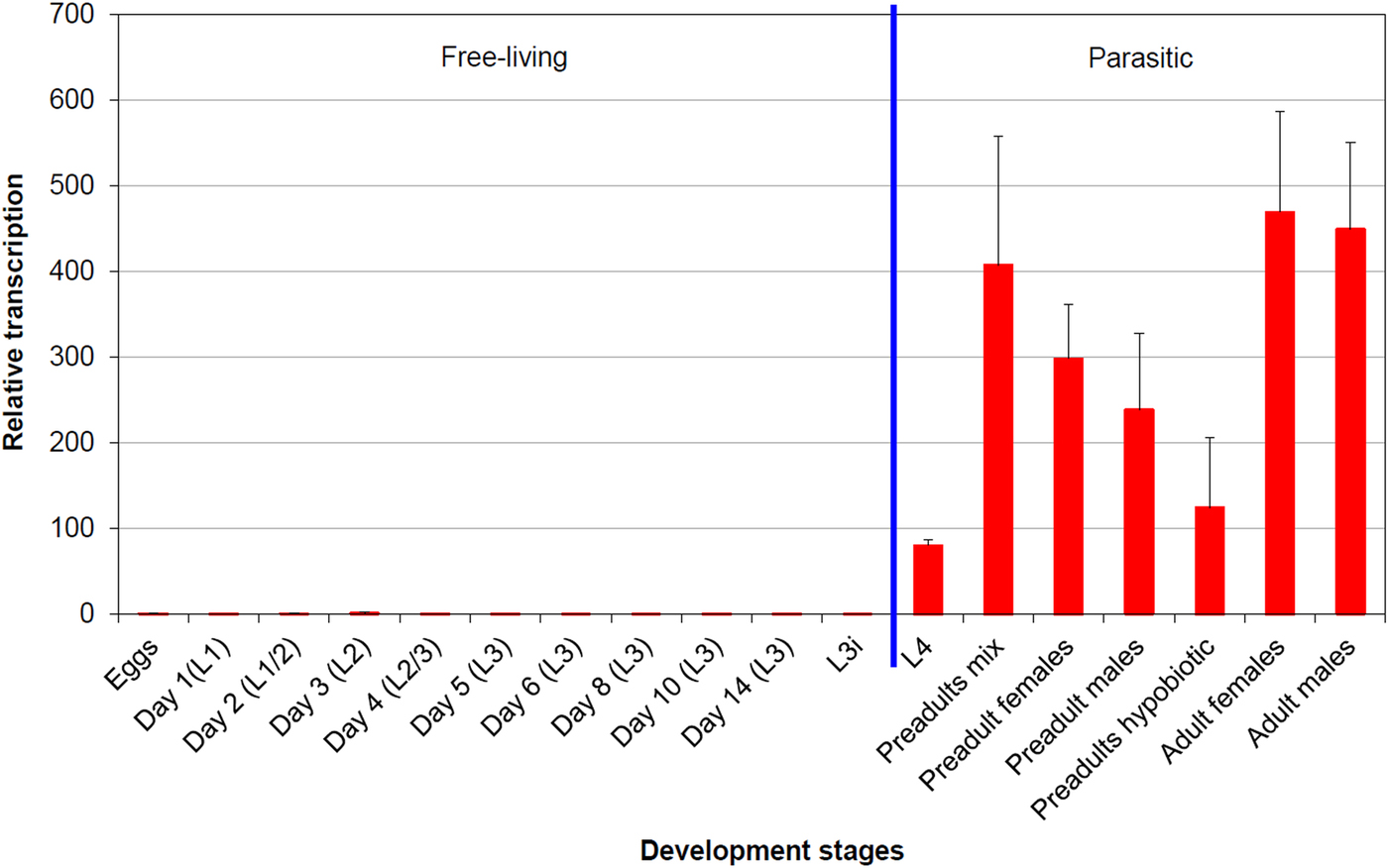
In qPCR analysis, the average squared correlation coefficients (R 2) as well as amplification efficiencies based on the corresponding standard curve were as follows: 0·997 (R 2) and 93·1% (amplification efficiency) for leg-1 (gene of interest); 1·0 and 96·2% for ef-1α (reference gene); and 1·0 and 99·0% for β-tubulin (reference gene). Figure 3 shows the efficiency corrected, normalized transcription profile of leg-1 relative to eggs as calibrator, whose transcription level was set to 1. Transcription of leg-1 was almost exclusively detected in parasitic lungworm stages. In detail, L4 showed approximately 80 times higher transcription levels than eggs, whereas non-hypobiotic preadult stages showed about 240–400 times and hypobiotic preadults about 120 times higher transcription levels. Highest leg-1 transcription levels were found in adult male and female worms showing approximately 450 and 470 times higher transcription levels than eggs, respectively.
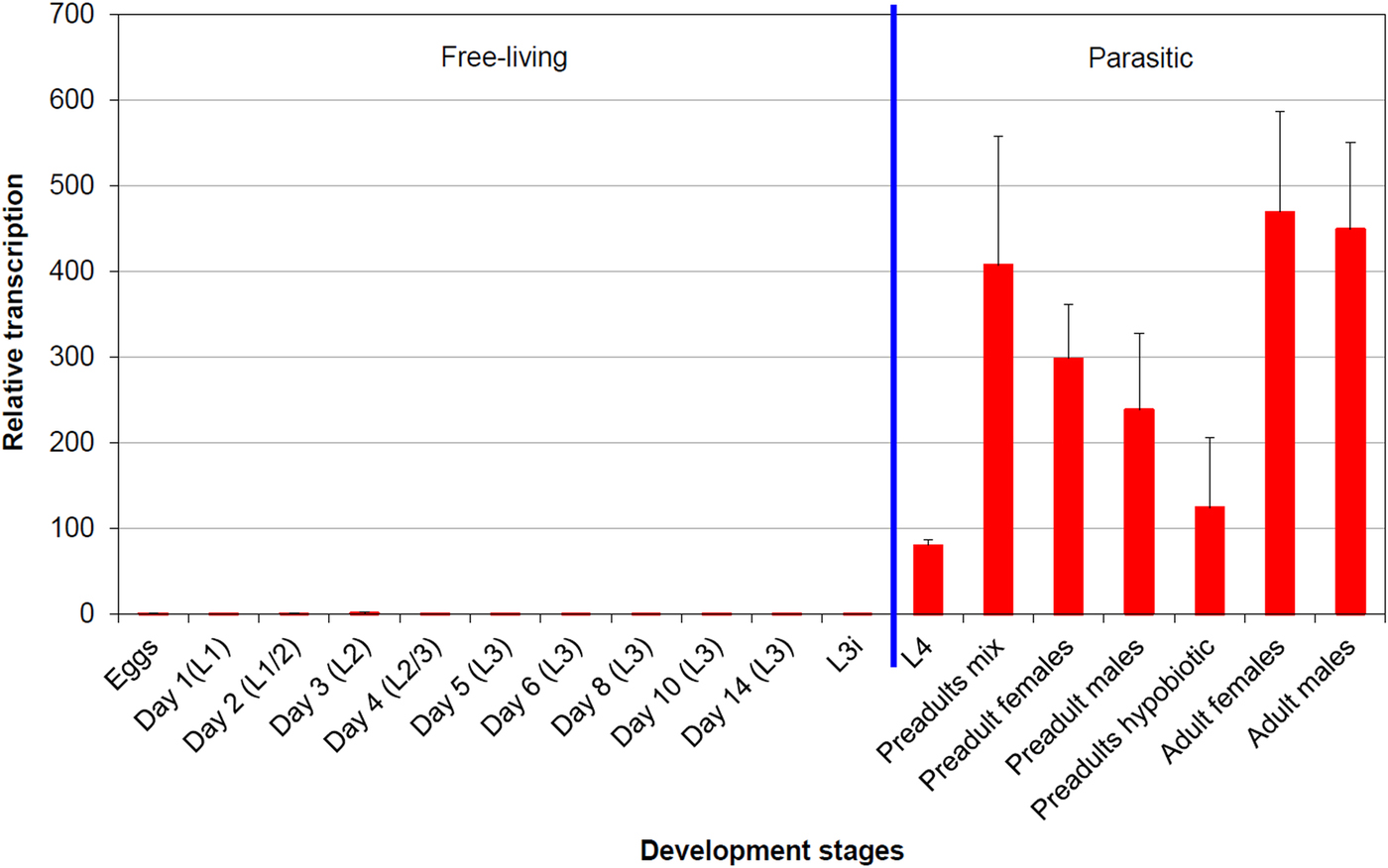
Fig. 3. leg-1 transcription profile throughout the D. viviparus life cycle. The transcription level of eggs was used as calibrator and set to 1. Days in brackets mean days after fecal shedding, L3i are hypobiosis induced L3 (chilled for 8 weeks at 4 °C).
LEG-1 expression profile in D. viviparus throughout the life cycle
Immunoblot analysis revealed a visible LEG-1 band in male and female preadults and adults, while no band appeared in L4 or earlier life cycle stages (data not shown).
LEG-1 expression in organs and ES product
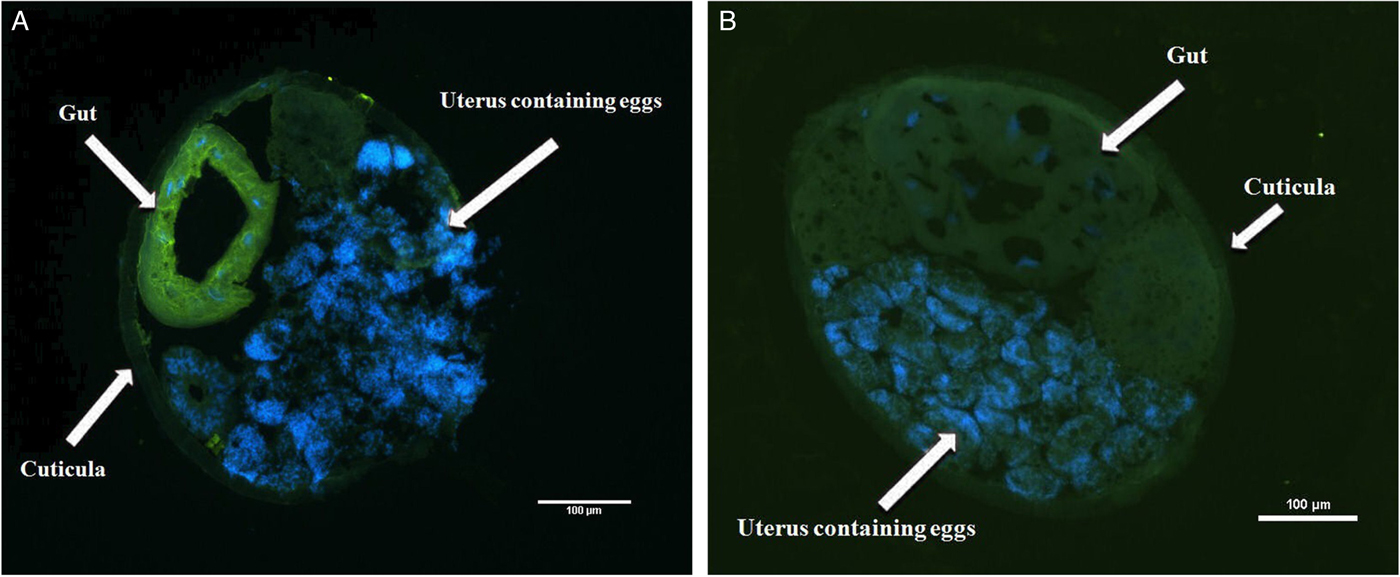
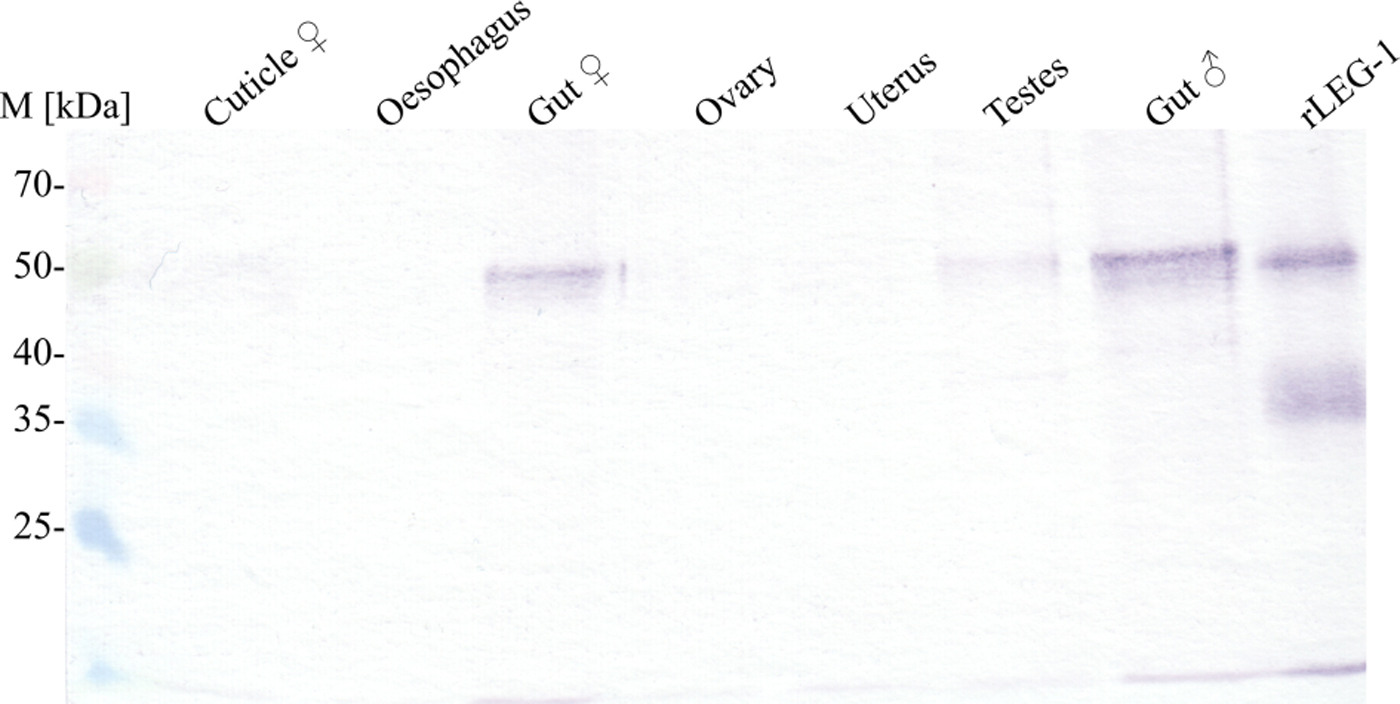
Immunohistochemistry located LEG-1 in the worm's intestine (Fig. 4). Immunoblot analysis with isolated organs not only confirmed intestinal expression, but also showed a faint LEG-1 band in the testes (Fig. 5). In addition to somatic location, LEG-1 was detected in D. viviparus ES product (Fig. 6).
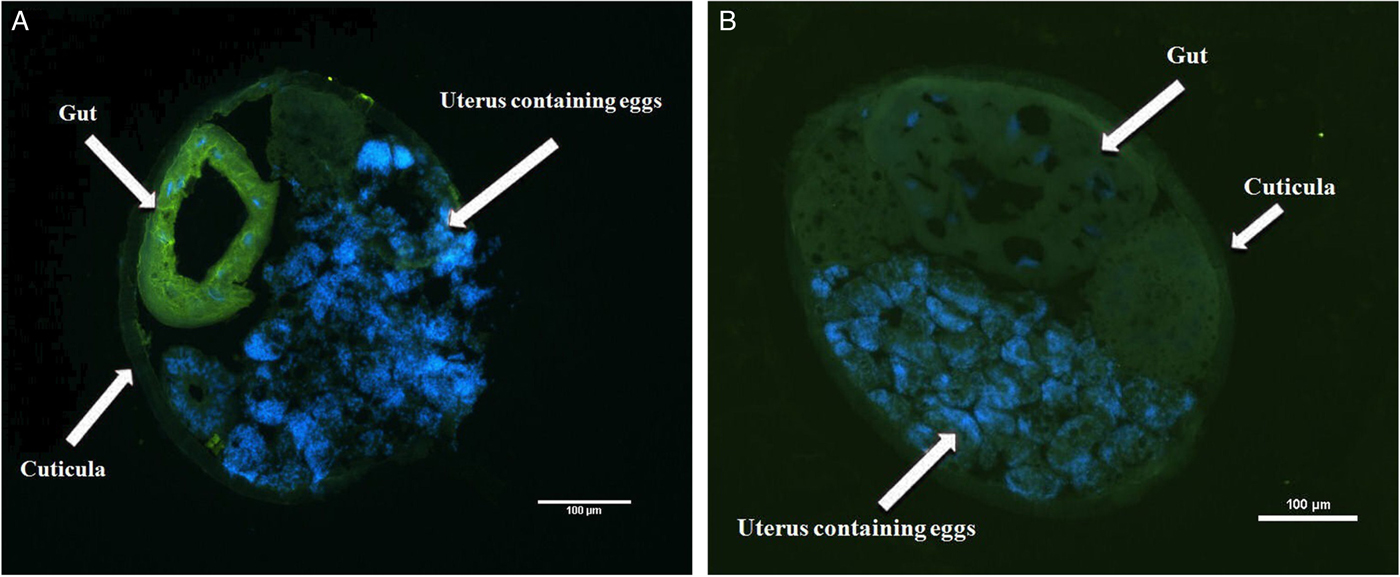
Fig. 4. Immunohistochemical localization of LEG-1 in D. viviparus. (A) Localization of LEG-1 in the intestine of a female worm with anti-rLEG-1 rabbit antiserum. (B) Control section with rabbit serum prior to immunization with rLEG-1 to exclude potential cross-reactions. FITC staining: green colour, DAPI staining: blue colour.
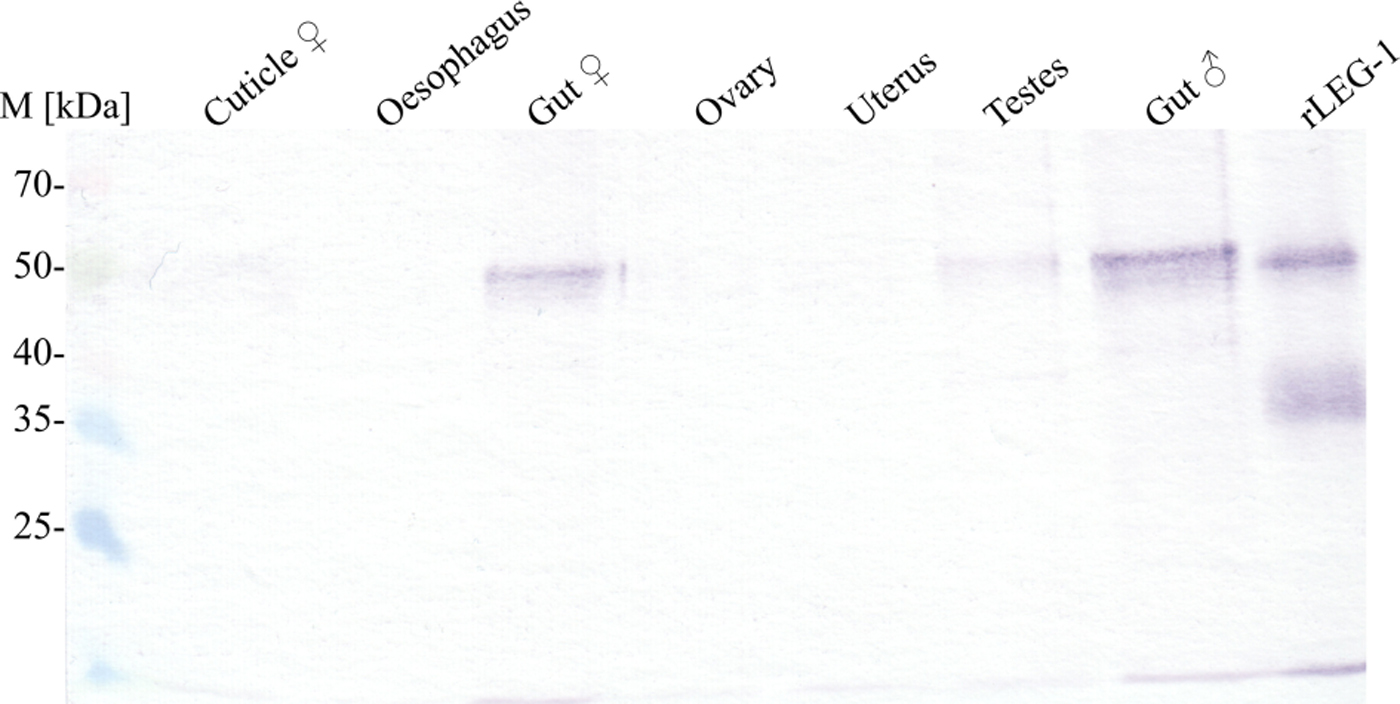
Fig. 5. Immunoblot of LEG-1 detection in different D. viviparus organs. In this qualitative approach, organs of adult female and male worms were homogenized and supernatants were precipitated by trichloroacetic acid. An equal volume of each sample was resolved by SDS–10% PAGE for Western blot analysis with anti-rLEG-1 rabbit serum. M: Spectra™ Multicolor Broad Range Protein Ladder (Thermo Scientific).

Fig. 6. Immunoblot of LEG-1 detection in adult lungworm ES product collected after 24 and 48 h of cultivation. (A) Incubation of ES product with anti-rLEG-1 rabbit antiserum for 1 h revealed the presence of LEG-1 (band at approx. 50 kDa). (B) Negative control incubation with rabbit serum prior to immunization with rLEG-1 excludes potential cross-reactivity. M: Spectra™ Multicolor Broad Range Protein Ladder (Thermo Scientific), rLEG-1: recombinant legumain-1 as positive control (5 µg 1st lane, 0·25 µg 2nd lane), anti-His-mab: monoclonal anti-histidine antibody, ES: unconcentrated ES product (30 µL/lane), ES-100×: 100 times concentrated ES product (4 µL/lane), MED-100×: 100 times concentrated worm-free cultivation medium as negative control (4 µL/lane).
Enzymatic characterization of LEG-1
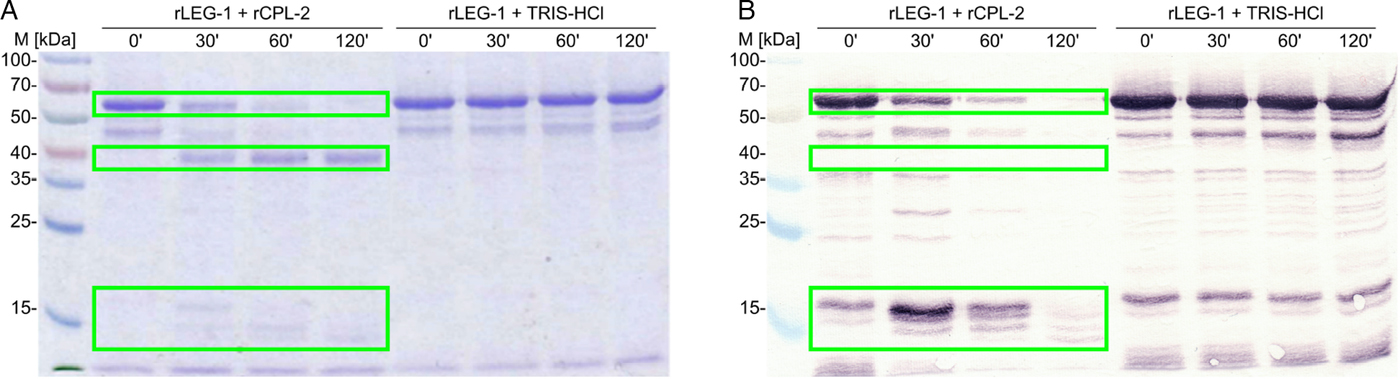
C-terminal hexa-His-tagged D. viviparus LEG-1 recombinantly expressed in the yeast P. pastoris showed the expected apparent molecular weight (MW) of approximately 50 kDa (calculated MW: 48·31 kDa). The protein was confirmed by MALDI-TOF mass spectrometry analysis. Auto-activation of rLEG-1 was insufficient, thus, trans-activation was required. Activation was successful with rCPL-2. During trans-activation, a pro-peptide of approximately 10 kDa was cleaved off from the C-terminus. C-terminal localization was obvious from immunoblot analysis, since the mature LEG-1 was not detectable with an anti-His-antibody (Fig. 7). Thus, the C-terminal hexa-His-tag was cleaved off together with the pro-peptide.
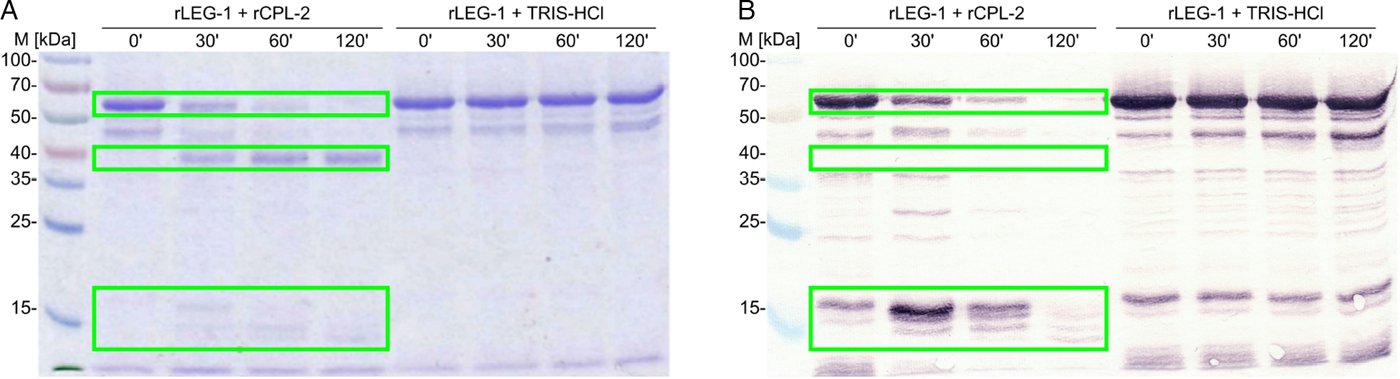
Fig. 7. Cleavage of rLEG-1 through rCPL-2 over 120 min visualized by (A) Coomassie Brilliant Blue R250-stained SDS–10% PAGE and (B) immunoblot using a monoclonal anti-histidine antibody. Self-cleavage (auto-activation) was tested by incubation of rLEG-1 in 10 mm Tris–HCl buffer (rLEG-1+TRIS–HCl) without rCPL. Legumain is indicated by boxes (pro-legumain at approx. 50 kDa, mature legumain at approx. 40 kDa, pro-peptides at approx. 14 and 16 kDa). Note that the mature protein is not visible in the immunoblot as the C-terminal hexa-His-tag was cleaved with the pro-peptide. M: Spectra™ Multicolor Broad Range Protein Ladder (Thermo Scientific). ’: minutes.
Of the tested artificial enzyme substrates, rLEG-1 solely cleaved the legumain-specific substrate Z-Ala-Ala-Asn-AMC. Determination of K m (Michaelis constant) and k cat (turnover number) resulted in K m = 51·95 µ m and k cat = 0·33 s−1. Optimum pH for rLEG-1 was pH 5·5, the optimum reaction temperature ranged from 35 to 39 °C. rLEG-1 was fully inhibited by the general caspase-inhibitor Z-VAD-FMK (0·23% remaining activity compared with the negative control setup without addition of the inhibitor). Slight to moderate inhibition was observed with the aspartyl protease inhibitor pepstatin A (86·9% remaining activity) as well as the cysteine protease inhibitors iodoacetic acid (82·4% remaining activity) and cystatin (90·5% remaining activity), whereas the cysteine proteinase inhibitor E-64 as well as the specific cathepsin-B inhibitor CA-074 did not reveal any rLEG-1 inhibition (100·1% and 101·8% remaining activity, respectively).
Natural LEG-1 substrates
As potential natural substrates, bovine IgG, BSA, bovine haemoglobin and fibrinogen where not cleaved by rLEG-1 within 120 min. By contrast, bovine collagen types I and II were almost completely cleaved by activated rLEG-1 within 15 min (Fig. 8). Collagen bands also decreased slightly in the control reactions, probably caused by contamination of collagens with proteases. Furthermore, the controls contained also a small amount of the trans-activator rCPL-2, which could have caused some cleavage. However, we regard this as rather unlikely since no cleavage of BSA, which is a natural substrate of rCPL-2 (unpublished data), was observed in the control setup (Fig. 8C). This indicates that the amount of rCPL-2 present in these assays was too small to have a visible effect.

Fig. 8. Cleavage of bovine collagen and bovine serum albumin by rLEG-1 over an incubation time of up to 240 min visualized by Coomassie Brilliant Blue R250-stained SDS–10% PAGE. 50 µ m collagen type I from calf skin (A), collagen type II from bovine cartilage (B) and bovine serum albumin (C) were incubated with 10 µg/mL activated rLEG-1 in legumain-assay-buffer at 39 °C. Cleavage bands are indicated by boxes. Negative controls were incubated under the same conditions including all reaction components except rLEG-1. M: Spectra™ Multicolor Broad Range Protein Ladder (Thermo Scientific), Col-I: bovine collagen type I, Col-II: bovine collagen type II, ′: minutes.
Incubation of activated rLEG-1 with bronchial mucus resulted in the cleavage of two distinct bands in SDS–PAGE at about 90 kDa and 40 kDa (Fig. 9). MALDI-TOF mass spectrometry analysis revealed the 90 kDa band as cattle heat shock protein 90 alpha, but could not differentiate between glutathione-S-transferase P and peroxyredoxin for the 40 kDa band. As for bovine collagen, there was a slight decrease of bands also in the control setup, in which rCPL-2 was inactivated by the cysteine protease inhibitor E-64.

Fig. 9. Cleavage of 1·4 g L−1 soluble bronchial mucus protein from lungworm-infected cattle through 10 µg mL−1 activated rLEG-1 (bronchial mucus+rLEG-1) at 39 °C over 6 h, visualized by Coomassie Brilliant Blue R250-stained SDS–10% PAGE. Cleavage bands at approximately 90 and 40 kDa are indicated by boxes. Negative controls (bronchial mucus + TRIS–HCl) were incubated under the same conditions including all reaction components except rLEG-1. M: Spectra™ Multicolor Broad Range Protein Ladder (Thermo Scientific).
Clinical vaccine trial
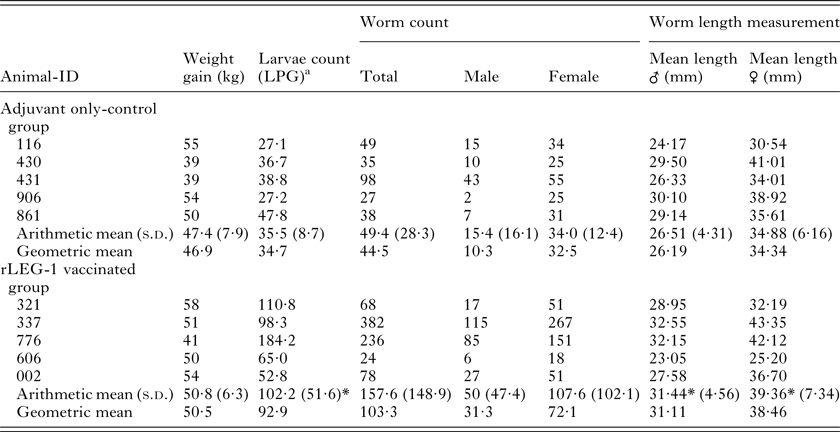
All animals were in good physical condition and health during the whole vaccination trial. Weight gain of vaccinated animals compared with the control group revealed no statistically significant differences. Regarding worm burden, no statistically significant difference was observed between the rLEG-1-vaccinated and the adjuvant-only control group. Unexpectedly, male and female worms of rLEG-1-vaccinated animals were significantly longer than those of the control group (P ⩽ 0·0001) and rLEG-1-vaccinated cattle showed significantly higher cumulative values of larvae numbers per gram feces (LPG; P = 0·004). Detailed results are listed in Table 1.
Table 1. Vaccination parameters weight gain, larvae counts, worm burden and worm length measurements of calves vaccinated with rLEG-1 and the adjuvant only-control group
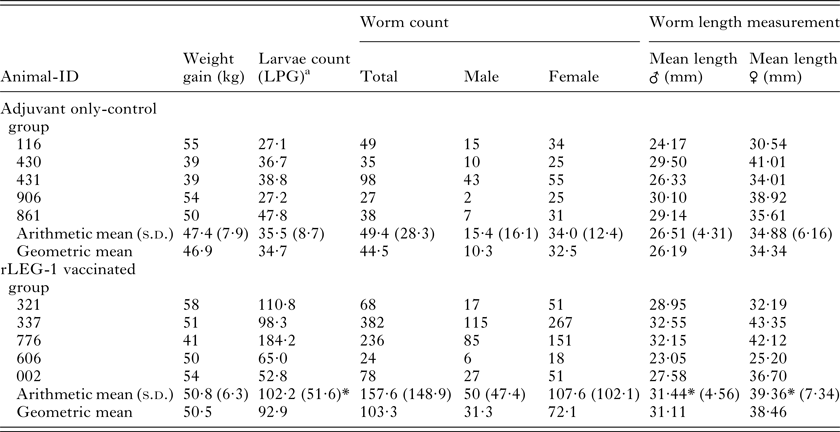
a Larvae per gram feces (cumulative values of larvae counts on study days 84, 87, 91, 94 and 98)
*P ⩽ 0·05.
Regarding antibody response (Fig. 10), IgG and IgG1 antibody titres had already increased in rLEG-1-vaccinated cattle on day 7 after first immunization, except for animal 606 showing a distinct increase of IgG1 antibody titres only after second immunization. IgG2 antibody titres increased slightly after first vaccination. Following second immunization on study day (s.d.) 21, ELISAs revealed a further increase of IgG, IgG1 and IgG2 antibody titres in vaccinated animals. As antibody titres remained high for IgG and IgG1, no further increase was observed following third immunization at s.d. 42. No further increase of antibody titres occurred after challenge infection (s.d. 63 and 64) for mentioned immunoglobulin classes. However, IgA antibody titres increased mildly in vaccinated animals starting s.d. 77 until necropsy at s.d. 98. IgM antibody titres fluctuated both in control and in vaccinated animals and showed no increase upon vaccination, whereas IgE could not be detected at all. Animals of the control group showed no significant increase in antibody titres during the whole study period.

Fig. 10. Anti-rLEG-1-antibody development against during the vaccine trial detected by ELISAs using rLEG-1-coated plates. Serum was taken weekly from study day 0 until necropsy on day 98. Optical density (OD) values are displayed for immunoglobulin (Ig) classes A, G, G1, G2, E and M of individual study animals. The graph legend lists individual animal IDs of each group.
In immunoblot analysis, serum of vaccinated animals detected LEG-1 in adult lungworm lysate as of 1 week after second vaccination (s.d. 28). However, sera of control animals did not recognize LEG-1 at any time point of the study. Immunoblot results are exemplarily pictured in Fig. 11.

Fig. 11. Immunoblot detection of LEG-1 (band at approx. 50 kDa) in whole adult lungworm lysate by serum antibodies of vaccinated cattle. Per lane, 64 µg crude antigen from female and male worms was loaded. (A) Serum of an exemplary rLEG-1+Matrix-Q™ vaccinated calf, (B) serum of an exemplary Matrix-Q™-only control calf. M: PageRuler™ Plus Prestained Protein Ladder (Thermo Scientific), lane 1: Day 0 (1st vaccination), lane 2: Day 14, lane 3: Day 28, lane 4: Day 45, lane 5: Day 63 (challenge infection), lane 6: Day 77, lane 7: Day 84, lane 8: Day 98 (necropsy), lane 9: 0·2 µg rLEG-1 as positive control (detected with anti-rLEG-1 rabbit antiserum).
DISCUSSION
Since natural infection with D. viviparus evokes a protective immunity in the cattle host, the development of a recombinant subunit vaccine appears well-suited and would be highly beneficial for prophylaxis of parasitic bronchitis. Previous vaccine trials with recombinant D. viviparus paramyosin have proved promising regarding their impact on worm burden, worm length and thus worm development, and larvae shedding (Joekel et al. Reference Joekel, Hinse, Raulf, Schicht, Baumer, Werling, Kremmer and Strube2015; Strube et al. Reference Strube, Haake, Sager, Schorderet Weber, Kaminsky, Buschbaum, Joekel, Schicht, Kremmer, Korrell, Schnieder and von Samson-Himmelstjerna2015). The presented study aimed to investigate the suitability of recombinantly expressed LEG-1 as a bovine lungworm vaccine candidate. Legumains are asparaginyl peptidases and have been described previously in a variety of species (Kembhavi et al. Reference Kembhavi, Buttle, Knight and Barrett1993; Dalton et al. Reference Dalton, Hola-Jamriska and Brindley1995; Chen et al. Reference Chen, Dando, Rawlings, Brown, Young, Stevens, Hewitt, Watts and Barrett1997; Yamane et al. Reference Yamane, Takeuchi, Yamamoto, Li, Fujiwara, Nishi, Takahashi and Ohkubo2002; Oliver et al. Reference Oliver, Skuce, McNair and Knox2006; Horn et al. Reference Horn, Nussbaumerová, Šanda, Kovářová, Srba, Franta, Sojka, Bogyo, Caffrey, Kopáček and Mareš2009). As expected, phylogenetic analysis showed that D. viviparus LEG-1 is closely related to legumains of other nematodes, followed by those of trematodes and finally vertebrates. The functional conservation of different asparaginyl peptidases amongst a variety of organisms suggests a fundamental biological function (Chen et al. Reference Chen, Dando, Rawlings, Brown, Young, Stevens, Hewitt, Watts and Barrett1997; Nguyen et al. Reference Nguyen, Srihari and Leong2013). Gene transcriptional analysis revealed that leg-1 is almost exclusively transcribed after transition from free-living lungworm stages to parasitic stages inside the host, which was confirmed by expression analysis. These findings enhance the presumption that this enzyme is involved in typical purposes of parasitic lifestyle such as feeding as proposed by Strube et al. (Reference Strube, Buschbaum and Schnieder2012).
Immunohistochemical staining of adult D. viviparus sections revealed that LEG-1 is localized in the worm's intestine. In this context, it needs to be considered that staining might have also included D. viviparus LEG-2. Even though the identity between both proteins is only 58%, some cross-reactivity cannot entirely be excluded (which also applies to detection in different developmental lungworm stages and organs). Legumains have been found in the gut of several parasites, including ticks, trematodes and the nematode H. contortus, where they are localized on the microvillar surface of the intestine and are suspected to act in food protein degradation (Dalton and Brindley, Reference Dalton and Brindley1996; Sajid et al. Reference Sajid, McKerrow, Hansell, Mathieu, Lucas, Hsieh, Greenbaum, Bogyo, Salter, Lim, Franklin, Kim and Caffrey2003; Oliver et al. Reference Oliver, Skuce, McNair and Knox2006; Horn et al. Reference Horn, Nussbaumerová, Šanda, Kovářová, Srba, Franta, Sojka, Bogyo, Caffrey, Kopáček and Mareš2009). Such microvillar surface location seems reasonable for D. viviparus LEG-1 as well, where it may work as part of an enzyme cascade. For H. contortus it has been shown that membrane-bound thiol Sepharose-binding fractions localized at the surface of the parasite gut were enriched for cysteine protease activity (Redmond and Knox, Reference Redmond and Knox2004; Knox et al. Reference Knox, Smith, Redmond and Smith2005). As potential natural substrates, D. viviparus rLEG-1 cleaved bovine collagen types I and II as well as two distinct protein bands when incubated with bronchial mucus. This enhances the presumption that D. viviparus feeds on bronchial mucus as it was shown for lungworms of the genus Metastrongylus (Porter, Reference Porter1936).
A small degree of rLEG-1 auto-activation was present; however, the amount of cleaved protein did not increase over time (cf. Fig. 7). For sufficient functionality in the assays, D. viviparus rLEG-1 needed trans-activation through cathepsin L-2. Several activation mechanisms are known (Dall and Brandstetter, Reference Dall and Brandstetter2016), but activation by cathepsins has not been described before. Thus, this result was unexpected and indicates a novel mechanism of legumain activation. Upon activation, cleavage of the C-terminal pro-peptide was observed. According to Dall and Brandstetter (Reference Dall and Brandstetter2016), legumains possess also a very small N-terminal pro-peptide. Secondary structure analysis shows that, D. viviparus LEG-1 possesses an approx. 2 kDa N-terminal pro-peptide. However, from the assays used in this study no conclusion can be drawn whether it has been cleaved, because a hexa-His-tag was only linked C-terminally.
rLEG-1 was active at tested pH values 4·0–6·0 with an optimum at pH 5·5. This supports a function inside the acidic environment of the parasite intestine rather than in the cattle's lungs, where a pH ⩾7 is expected. However, since LEG-1 was also detected in the parasite's ES product, it cannot entirely be excluded that this enzyme also plays a role in tissue invasion and maintenance of infection in the lungs. Regarding temperature dependency, the enzyme worked best at temperatures ranging from 35 to 39 °C, which corresponds to the natural body temperature of cattle.
Specific enzymatic classification of LEG-1 by creation of a protease inhibitor profile assumes a caspase-related asparaginyl peptidase, because the general caspase inhibitor Z-VAD-FMK led to complete inhibition of LEG-1 activity. Lacking inhibition by the general cysteine protease inhibitor E-64 is considered characteristic for the class of legumains (Dalton et al. Reference Dalton, Hola-Jamriska and Brindley1995; Caffrey et al. Reference Caffrey, Mathieu, Gaffney, Salter, Sajid, Lucas, Franklin, Bogyo and McKerrow2000; Yamane et al. Reference Yamane, Takeuchi, Yamamoto, Li, Fujiwara, Nishi, Takahashi and Ohkubo2002; Oliver et al. Reference Oliver, Skuce, McNair and Knox2006; Adisakwattana et al. Reference Adisakwattana, Viyanant, Chaicumpa, Vichasri-Grams, Hofmann, Korge, Sobhon and Grams2007; Horn et al. Reference Horn, Nussbaumerová, Šanda, Kovářová, Srba, Franta, Sojka, Bogyo, Caffrey, Kopáček and Mareš2009) and was also observed for D. viviparus rLEG-1. Moreover, the cathepsin B-specific inhibitor CA-074 did not affect rLEG-1 activity. While various authors have not observed any inhibitory effect of the aspartyl protease inhibitor pepstatin A on legumains of the tick Ixodes ricinus or the trematodes S. mansoni and F. gigantica (Caffrey et al. Reference Caffrey, Mathieu, Gaffney, Salter, Sajid, Lucas, Franklin, Bogyo and McKerrow2000; Adisakwattana et al. Reference Adisakwattana, Viyanant, Chaicumpa, Vichasri-Grams, Hofmann, Korge, Sobhon and Grams2007; Horn et al. Reference Horn, Nussbaumerová, Šanda, Kovářová, Srba, Franta, Sojka, Bogyo, Caffrey, Kopáček and Mareš2009), a partial slight inhibition of the nematode H. contortus legumain has been reported by Oliver et al. (Reference Oliver, Skuce, McNair and Knox2006). Similarly, D. viviparus rLEG-1 was slightly inhibited in the presence of pepstatin A. Slight inhibition resulted also from the cysteine protease inhibitor cystatin, while complete inhibition is described for the legumains of I. ricinus and S. mansoni (Sajid et al. Reference Sajid, McKerrow, Hansell, Mathieu, Lucas, Hsieh, Greenbaum, Bogyo, Salter, Lim, Franklin, Kim and Caffrey2003; Horn et al. Reference Horn, Nussbaumerová, Šanda, Kovářová, Srba, Franta, Sojka, Bogyo, Caffrey, Kopáček and Mareš2009). S. mansoni legumain was also completely inhibited in the presence of the cysteine protease inhibitor iodoacetic acid (Caffrey et al. Reference Caffrey, Mathieu, Gaffney, Salter, Sajid, Lucas, Franklin, Bogyo and McKerrow2000), whereas D. viviparus rLEG-1 was only moderately inhibited.
The probable digestive function of bovine lungworm LEG-1 – potentially triggered by activation of other cysteine proteases – makes it a relevant candidate for vaccine development. Vaccine-derived antibodies directed against rLEG-1 might block legumains in the gut and, therefore, interfere with the worm's nutrition. Indeed, Murray and Smith (Reference Murray and Smith1994) found host immunoglobulins in homogenates of adult D. viviparus, indicating that the worms ingest antibodies while feeding. Although the recombinantly expressed LEG-1 was functionally active and therefore most likely correctly folded, vaccination of cattle had no success, as vaccinated cattle had higher mean worm intensity and larvae excretion values than the adjuvant-only control group. Furthermore, worms of vaccinated animals were significantly longer than those of the control group, which was entirely unexpected. One possible explanation might be a preferential Th1 response, which may lead to an exacerbation of the challenge infection (Newton and Munn, Reference Newton and Munn1999). However, the used adjuvant Matrix-Q™ provides a strong Th1, Th2 and effective cytotoxic T-lymphocyte response, if the antigen is correctly incorporated (Cox and Coulter, Reference Cox and Coulter1997). Moreover, all animals, including the controls, received the adjuvant. Thus, an exacerbation due to an increased Th1-response is not plausible. The longer worms in the vaccinated group seem to be caused rather by the fact that worms of the control group were unusually short in the presented study. Since vaccine-group and control-group cattle were housed separately, manifold environmental influences cannot completely be excluded. Moreover, it might be possible that the reduced function of LEG-1 due to vaccination is compensated by other proteases with similar functions, such as LEG-2.
While increasing serum antibody titres of the immunoglobulin subclasses IgG, IgG1 and IgG2 were observed in rLEG-1 vaccinated cattle, no IgA response was induced by the vaccinations. As antibodies play a significant role in protective immunity against D. viviparus (Jarrett et al. Reference Jarrett, McIntyre, Jennings and Mulligan1957), this missing Ig subclass may have contributed to the failure of rLEG-1 vaccination. Increased IgA levels have been found in bronchoalveolar lavage fluid of D. viviparus-infected calves, suggesting that this Ig subclass may play an important role in local immunity (Scott et al. Reference Scott, McKeand and Devaney1996). IgM values fluctuated both in the vaccinated and control animals, which might be due to an immunological response against copurified P. pastoris antigens or an unspecific antibody binding to rLEG-1 and/or the hexa-His-tag as discussed by Joekel et al. (Reference Joekel, Hinse, Raulf, Schicht, Baumer, Werling, Kremmer and Strube2015). Regarding IgE, no titre was measurable at all, which corresponds to the findings of Strube et al. (Reference Strube, Haake, Sager, Schorderet Weber, Kaminsky, Buschbaum, Joekel, Schicht, Kremmer, Korrell, Schnieder and von Samson-Himmelstjerna2015) and Joekel et al. (Reference Joekel, Hinse, Raulf, Schicht, Baumer, Werling, Kremmer and Strube2015) who suspect IgE to be bound on surfaces of, e.g. basophilic granulocytes and consequently not measurable in serum. Interestingly, no natural boosting occurred after challenge infection. Additionally, control animals did not recognize LEG-1 in adult worm lysate after challenge infection, suggesting the hypothesis that bovine lungworm LEG-1 is a hidden antigen. This seems contradictory to the detection of LEG-1 in D. viviparus ES products; however, it is possible that proteolytic enzymes contained in the bronchial mucus degrade the protein so that it cannot trigger the antibody response. A combination of rLEG-1 with other promising vaccine candidates like other cysteine proteases may have additive or synergistic effects leading to substantial effects against D. viviparus.
ACKNOWLEDGEMENTS
The authors would like to thank Novavax AB, Sweden, for kindly providing the adjuvant Matrix-Q™. Furthermore, the excellent technical assistance of Sandra Buschbaum, Sabine Haendel and Ursula Kuettler is gratefully acknowledged.
FINANCIAL SUPPORT
The study has received funding from the European Union Seventh Framework Programme (FP7/2007-2013) under grant agreement number KBBE-2010-4-265862 and from the German Research Foundation under Grant STR 1171/2-1. The adjuvant Matrix-Q™ was kindly provided by Novavax AB, Sweden.