INTRODUCTION
Free-living amoebae belonging to the genus Acanthamoeba are among the most prevalent protozoa in the environment (Schuster and Visvesvara, Reference Schuster and Visvesvara2004). Interest in the study of these protozoa is increasing due to their ability to infect humans and because they can act as a reservoir for other pathogens (Khan, Reference Khan2003). Acanthamoeba castellanii, a pathogenic species of Acanthamoeba, is the causative agent of amoebic keratitis (AK), granulomatous amoebic encephalitis (GAE) and cutaneous acanthamoebiosis (Torno et al. Reference Torno, Babapour, Gurevitch and Witt2000).
The pathogenesis of infections by Acanthamoeba is poorly understood. However, it is known that attachment of amoebae to the surface of the host tissue is a crucial step in the establishment of infection, which culminates in the death of the host cell (Serrano-Luna et al. Reference Serrano-Luna, Cervantes-Sandoval, Calderón, Navarro-García, Tsutsumi and Shibayama2006). Similarly, in several protozoan pathogens, such as Entamoeba histolytica, the infection process involves host cell adherence by recognition of glycolipids and/or glycoproteins on the host cell, followed by cellular lysis by secretion of proteases and phagocytosis (Martínez-Palomo et al. Reference Martínez-Palomo, González-Robles, Chávez, Orozco, Fernández-Castelo and Cervantes1985; Meza et al. Reference Meza, Talamás-Rohana and Vargas2006). On the surface of Acanthamoeba trophozoites, a transmembrane protein has been reported to interact with glycoproteins that are rich in mannose residues found on corneal epithelial cells (Yang et al. Reference Yang, Cao and Panjwani1997). However, the adhesion of pathogenic microorganisms is a complex process involving more than one protein in the interactions with the host cell (Karkowska-Kuleta et al. Reference Karkowska-Kuleta, Rapala-Kozik and Kozik2009).
In Acanthamoeba, the molecules and cellular events governing adherence to the host cell are not completely elucidated. In addition to the expression of surface molecules, actin polymerization plays an important role in various physiological processes, including cell adhesion, through the formation of lamellipodia and filopodia (Pollard and Borisy, Reference Pollard and Borisy2003). Structurally, Acanthamoeba trophozoites have multiple membrane projections called acanthopodia, which are primarily composed of fibres and bundles of actin (González-Robles et al. Reference González-Robles, Castañón, Hernández-Ramírez, Salazar-Villatoro, González-Lázaro, Omaña-Molina, Talamás-Rohana and Martínez-Palomo2008). The physiology of these structures is related to the adhesion process and the cytopathic effect, indicating the importance of the cytoskeleton in the establishment and spread of infection. However, the involvement of the acanthopodia in the early stages of interaction with the host cells is not well defined. Furthermore, the effect of actin depolymerization on the adhesion process is unknown. In the present study, we used a cellular and biochemical approach to identify surface molecules on the trophozoites of A. castellanii. We describe glycosidic molecules and those that have an affinity for neuronal and epithelial cells. Using confocal microscopy, we also describe the involvement of the acanthopodia in the early stages of interaction with epithelial and neural cells. Finally, the crucial involvement of the cytoskeleton in the infection process is determined using cytochalasin B (CB) and latrunculin B (LB), which depolymerize the actin cytoskeleton. These results contribute to our understanding of basic aspects that provide insights into the pathogenesis of infections caused by protozoa.
MATERIALS AND METHODS
Acanthamoeba castellanii cultures
Acanthamoeba castellanii trophozoites isolated from human cases of AK were kindly provided by Dr Simon Kilvington (Public Health Laboratory, Bath, UK). The amoebae (104 trop mL−1) were grown in axenic cultures in Chang's liquid medium supplemented with 10% v/v fetal bovine serum (GIBCO, Grand Island, NY). Trophozoites were cultured at 30 °C in 50 mL culture flasks (Corning, NY) until the exponential growth phase (72 h) according to a modified technique (Rivera et al. Reference Rivera, Medina, Ramírez, Alcocer, Vilaclara and Robles1984).
Host cell cultures
Monolayers of epithelial cells (L929, ATCC CCL-2) and neuronal cells (SH-SY5Y, ATCC CRL-2266) were grown (105 cells mL−1) in 50 mL culture flasks with D-MEM (Dulbecco's modified eagle's medium; Sigma, St. Louis, MO, USA). The medium was supplemented with 10% v/v fetal bovine serum, and cells were maintained in a 5% CO2 atmosphere at 37 °C for 72 h (Freshney, Reference Freshney2000).
Interaction of A. castellanii with epithelial and neuronal cells
To establish the optimum incubation time for the adhesion of A. castellanii trophozoites to epithelial and neuronal cells, we carried out host–parasite interaction experiments. Confluent cell monolayers in Petri dishes (Corning, NY, 60×15 mm) were incubated with trophozoites (105 trop mL−1) of A. castellanii (ratio 1:1) at 37 °C in a 5% CO2 atmosphere (Shibayama et al. Reference Shibayama, Martínez-Castillo, Silva-Olivares, Galindo-Gómez, Navarro-García, Escobar-Herrera, Sabanero, Tsutsumi and Serrano-Luna2013). The interaction was carried out for different durations: 15, 30 and 45 min for epithelial cells and 10, 20 and 30 min for neuronal cells. After the interactions, we analysed morphological changes in the host cells and adhesion of the trophozoites.
Adhesion of A. castellanii to epithelial and neuronal cells
Once the host–parasite interaction experiments were completed, we evaluated the adhesion of trophozoites to the epithelial and neuronal cells. For this purpose, the culture medium containing unbound trophozoites was removed, and the cells were washed with phosphate buffer (PBS, pH 7·2). The amoebae attached to the cells were detached from the monolayer with 2 mm EDTA in PBS at 4 °C for 15 min; the amoebae were counted with a Neubauer camera (AO Scientific Instruments, NY, USA). The adhesion percentage was calculated from the average of three independent experiments in triplicate for each interaction time (n = 9; *P<0·05), relating the number of amoebae attached to the cell monolayers to the total number of amoebae applied (Lee and King, Reference Lee and King1983).
Actin cytoskeleton of A. castellanii
Trophozoites of A. castellanii were grown until the exponential growth phase and then collected by centrifugation at 1500 g for 2 min and fixed with 4% formaldehyde and 0·05% glutaraldehyde (Electron Microscopy Sciences, Washington, DC, USA). After 30 min, the amoebae were permeabilized with 0·5% v/v Triton X-100 in PBS for 4 min and washed three times with PBS. Actin filaments were stained with phalloidin-FITC (Sigma, St. Louis, MO, USA; 1:100) for 20 min at room temperature. The samples were mounted on cover slips using VECTASHIELD (Vector Laboratories Inc., Burlingame, CA, USA) for analysis with a fluorescence microscope (Nikon HFX-II, Japan) equipped with a UV filter (Exc = 400–420 nm) and with a confocal microscope (Spectral Laser Scanning Biological; Model FV-1000, Olympus, Japan).
Polyclonal antibody production against A. castellanii trophozoites
To specifically recognize trophozoites during their interaction with the cells, antibodies against trophozoites were obtained. Male New Zealand rabbits were injected intramuscularly with total protein extracts of A. castellanii prepared with Freud's complete and incomplete adjuvant (Sigma, St. Louis, MO, USA). Rabbits were injected four times at 12-day intervals according to the method of Grollo et al. (Reference Grollo, Chua, Jackson and Burns2005). The serum, which contained specific anti-A. castellanii polyclonal antibodies, was stored at −20 °C until use. The presence of antibodies was confirmed by Western blot assays using the pre-immune serum as control.
The rabbit used in this study was handled in accordance with the guidelines of the Institutional Animal Care and Use Committee. Our institution fulfils all the technical specifications for the production, care and use of laboratory animals and is certified by national law (NOM-062-ZOO-1999).
Analysis of the actin cytoskeleton during the interaction of A. castellanii with epithelial and neuronal cells
Epithelial and neuronal cells grown on cover slips were incubated with A. castellanii trophozoites (105 trop mL−1) at a 1:1 cell ratio. After 15 and 45 min of incubation with epithelial cells and 10 and 30 min with neuronal cells, the cells were fixed with 4% formaldehyde and 0·05% glutaraldehyde in PBS for 30 min. Samples were incubated for 1 h at room temperature with antibodies against A. castellanii trophozoites (1:20). Cells were washed three times with PBS and incubated with the appropriate rhodamine-labelled secondary antibody (goat-anti-rabbit; Sigma, St. Louis, MO, USA; 1:50) for 1 h at room temperature. For the labelling of actin filaments, the preparations were permeabilized with 0·5% v/v Triton X-100 for 4 min and washed with PBS. Actin filaments were stained with phalloidin-FITC (1:100) for 20 min at room temperature (Castillo-Romero et al. Reference Castillo-Romero, León-Avila, Pérez-Rangel, Cortes-Zarate, García-Tovar and Hernández2009). Cell nuclei were stained with 3·6 mm Vector-DAPI. The samples were mounted on cover slips for observation under a confocal microscope (Spectral Laser Scanning Biological; Model FV-1000, Olympus, Japan). Images were analysed using the program FV10ASW version 3.0.
Treatment of A. castellanii with CB and LB
CB and LB were maintained as stock solutions of 5 mg mL−1 in 2% EtOH/H2O at −20 °C. Trophozoites of A. castellanii (105 mL−1) in the exponential growth phase were incubated with 15 μg mL−1 CB (Sigma, St. Louis, MO, USA) and 0·4 μg mL−1 LB (Sigma, St. Louis, MO, USA) for 30 min at room temperature (Ravdin et al. Reference Ravdin, Croft and Guerrant1980; Bellin et al. Reference Bellin, Kubicek, Frigault, Kamien, Steward, Barnes, DiGiacomo, Duncan, Edgerly, Morse, Park, Fredberg, Cheng and LeDuc2009). Subsequently, the trophozoites were washed with PBS and fixed with 4% formaldehyde and 0·05% glutaraldehyde for 30 min. The distribution of actin filaments after treatment with CB and LB was analysed by staining with phalloidin-FITC. Trophozoite nuclei were stained with 3·6 mm Vector-DAPI. The samples were observed under a fluorescence microscope (Nikon HFX-II) with UV Exc = 400–420 nm.
Interaction of epithelial cells with trophozoites of A. castellanii treated with CB
To evaluate the effect of actin filament depolymerization on the adhesion of Acanthamoeba, trophozoites were exposed to CB. After washing with PBS, interaction with epithelial cells for 45 min was performed. The control for these experiments consisted of trophozoites that were untreated or treated with 2% EtOH/H2O and incubated with epithelial cells (Bracha and Mirelman, Reference Bracha and Mirelman1983). The amoebae attached to the cells were detached from the monolayer with 2 mm EDTA in PBS at 4 °C for 15 min and counted with a Neubauer camera. The adhesion percentage was calculated from the average of three independent experiments conducted in triplicate for each interaction time (n = 9; *P<0·0003), relating the number of attached amoebae to the total number of amoebae applied.
Labelling of surface proteins and glycoproteins of A. castellanii
For specific labelling of membrane proteins, trophozoites were washed three times with PBS and 1 mm PMSF (Sigma, St. Louis, MO, USA). Next, 2×106 trophozoites mL−1 were incubated for 1 h with 2 mm biotin (EZ-Link Sulfo-NHS-LC-Biotinylation Kit; PIERCE, Rockford, IL, USA) and dissolved in PBS with protease inhibitors (1 mm PMSF; 0·1 mm protease inhibitor cocktail Mini complete; Roche) at room temperature with constant stirring.
Labelling of membrane proteins containing carbohydrate residues was performed by incubating the trophozoites with biotinylated lectins. Concanavalin A (Sigma, St. Louis, MO, USA) is specific for α-D-mannosyl and α-D-glucosyl, and wheat germ agglutinin (Sigma, St. Louis, MO, USA) is specific for N-acetyl glucosamine. Lectins were dissolved 1:100 in PBS with protease inhibitors (1 mm PMSF; 0·1 mm protease inhibitor cocktail Mini complete; Roche). The samples were incubated for 1 h at room temperature with constant stirring. To exclude the presence of endogenous biotin, the negative control consisted of trophozoites without exposure to biotin, revealed with streptavidin-peroxidase at a 1:3000 dilution in PBS (Sandoval-Bernal et al. Reference Sandoval-Bernal, Barbosa-Sabanero, Shibayama, Perez-Torres, Tsutsumi and Sabanero2011).
Extraction and detection of surface biotinylated proteins and glycoproteins
After labelling with biotin and biotinylated lectins, trophozoites were collected by centrifugation at 1500 g for 10 min. The cell pellet was washed three times with PBS, solubilized in 2% SDS in the presence of protease inhibitors (1 mm PMSF; 0·1 mm protease inhibitor cocktail Mini complete; Roche) and lyophilized. Cell protein concentrations were quantified by Lowry's method (Lowry et al. Reference Lowry, Rosebrough, Farr and Randall1951). Subsequently, the proteins (30 μg of protein per lane) were fractionated by SDS-PAGE (10% gel) under reducing conditions. The transfer to nitrocellulose membranes (PROTAN, Schleicher & Schuell, Germany) was performed according to the method described by Laemmli (Reference Laemmli1970) and Towbin et al. (Reference Towbin, Staehelin and Gordon1979). The membranes were treated overnight with 5% fat-free milk in PBS at 4 °C. Biotinylated proteins and glycoproteins were detected with streptavidin-peroxidase conjugate (Sigma, St. Louis, MO, USA) at 1:3000 dilution in PBS and incubated for 1 h at room temperature with constant stirring. Blots were washed three times with PBS-T, and reactive bands were revealed with hydrogen peroxide and 4-chloro-1-naphthol as the chromogenic reagent (Casanova et al. Reference Casanova, Lopez-Ribot, Martinez and Sentandreu1992).
Detection of A. castellanii surface proteins with affinity for epithelial and neuronal cells
Biotin-labelled trophozoites were collected by centrifugation at 1500 g for 10 min and washed three times with PBS. The cell pellet was resuspended in 250 μL of PBS in the presence of protease inhibitors (1 mm PMSF; 0·1 mm protease inhibitor cocktail Mini complete; Roche), and an equal volume of 425–600 μm glass beads (Sigma, St. Louis, MO, USA) was added. The trophozoites were disrupted by five periods of 1 min in vortex and 1 min cooling in an ice bath. The cell lysate was centrifuged at 14 000 g for 10 min, and the supernatant was treated with Sephadex G25 (Sigma, St. Louis, MO, USA). After a second centrifugation at 1500 g for 2 min, the supernatant was added to confluent monolayers of epithelial and neuronal cells. The host–parasite protein interaction was performed for 30 min at 37 °C in an atmosphere of 5% CO2. After the interaction, the cells were washed three times with PBS and solubilized with 2% SDS. The extracted proteins were lyophilized, subjected to SDS-PAGE (10%, 30 μg of protein per lane) and transferred to nitrocellulose membranes. The biotinylated proteins of Acanthamoeba that interacted with the epithelial and neuronal cells were revealed with streptavidin-HRP conjugate (10 μg mL−1) dissolved in PBS (Sigma, St. Louis, MO, USA).
RESULTS
Adhesion of trophozoites of A. castellanii to epithelial and neuronal cells
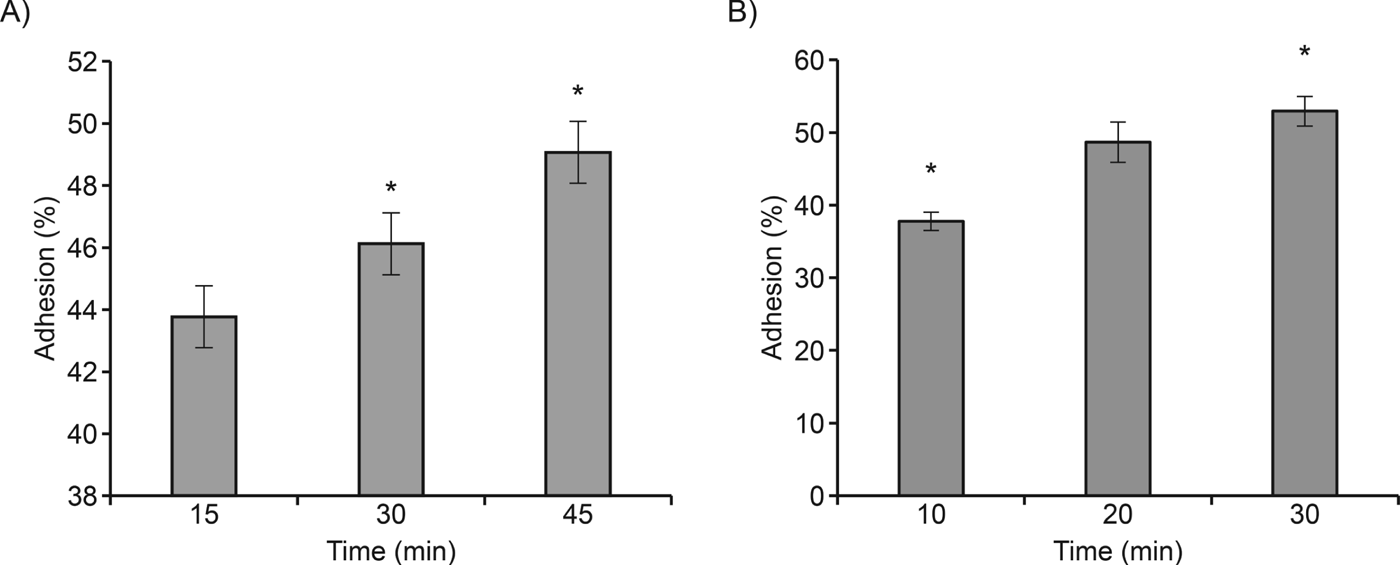
The host–pathogen interaction experiments indicated that adhesion of A. castellanii to epithelial and neuronal cells occurs in the first few minutes of interaction. At 15 min of interaction with epithelial cells, 43% of trophozoites were attached to the cell surface. Neuronal cell adhesion was faster than with epithelial cells; 37% of trophozoites were adhered in the first 10 min (Fig. 1A and B). After 30–45 min of interaction with epithelial cells and 20–30 min with neuronal cells, the number of trophozoites attached to the cell surface increased slightly (from 46–49% for epithelial cells and from 49–53% for neuronal cells; Fig. 1A and B).

Fig. 1. Adhesion kinetics of A. castellanii to epithelial (A) and neuronal (B) cells. Adhesion of Acanthamoeba to host cells was time-dependent, and a high percentage of attached trophozoites was observed. Values represent the mean±s.d. of three independent experiments conducted in triplicate. Data were analysed by an ANOVA, *P<0·05.
Actin cytoskeleton of A. castellanii
Trophozoites of A. castellanii (control) stained with phalloidin-FITC showed a distribution of actin filaments in the cortical region of the cytoplasm. A strong fluorescent signal on fine spiculated structures, known as acanthopodia, was clearly observed. This indicates enrichment of F-actin in these structures (Fig. 2).

Fig. 2. Actin cytoskeleton of A. castellanii. Epifluorescence (upper panel) and confocal microscopy (lower panel) show the distribution of trophozoite actin filaments stained with phalloidin-FITC. Note the strong reaction to the fluorescent probe in the cortical regions of the cytoplasm and acanthopodia. Scale bar = 10 μm.
Actin cytoskeleton during the interaction of A. castellanii with epithelial and neuronal cells
Polyclonal antibodies against the amoeba recognized trophozoites (Fig. 3A) without showing non-specific reactivity against host cells. These antibodies were used for the analysis of the actin cytoskeleton during the interaction of A. castellanii with both cell lines. Analysis of the cytoskeleton at the beginning of the interaction with epithelial cells (15 min) showed the trophozoite actin filaments at the periphery of the cell membrane and in the acanthopodia. This localization of cytoskeleton was maintained until the end of the interaction at 45 min (Fig. 3A).

Fig. 3. Actin cytoskeleton during the interaction of A. castellanii with epithelial and neuronal cells. (A) Cell actin (green) and DNA (blue) during the interaction of trophozoites (red, marked with anti-trophozoite antibody) with host cells at different times. The actin filaments of trophozoites colocalized with the membrane and acanthopodia. The cytoskeleton from host cells began to depolymerize at the end of the interaction (30 and 45 min). No apparent damage to the nuclei of host cells was observed. (B) Actin cytoskeleton (green) in the acanthopodia of the trophozoite (arrow) and (C) Actin cytoskeleton (green) in the neuronal cell. Note the close contact of acanthopodia to the surface of neuronal cells and the adhesion near intercellular junctions (arrow). Scale bar = 10 μm.
With respect to the epithelial cells, at the beginning of the interaction (15 min), the actin cytoskeleton maintained a normal distribution in the cytoplasmic region of the cells. However, at 45 min, there was a structural alteration of the cytoskeleton in the epithelium (Fig. 3A).
In contrast, when amoebae were co-incubated with neuronal cells for 10 min, a strong fluorescent reaction in the cortical region of the trophozoite cytoplasm was observed, indicating that F-actin was forming the acanthopodia (Fig. 3A). The amoeba cytoskeleton was unchanged at the end of the interaction (30 min). Neuronal cells maintained a fibrillar cytoskeleton pattern at 10 min of interaction; however, after 30 min, the filaments began to depolymerize (Fig. 3A).
At the analysed time of interaction, no apparent damage to the nuclei of epithelial cells or neuronal cells was observed (Fig. 3A).
During interaction with both cell lines, there was no change in the distribution of actin filaments in the trophozoites. It is evident that the formation of acanthopodia is vital for the establishment of infection. These membrane projections allow the adherence of amoebae to the cell surface, primarily in regions near of the intercellular junctions. In these contact zones, concentrated polymerized actin was found (Fig. 3B).
F-actin distribution in A. castellanii trophozoites after treatment with CB and LB
Visible changes in the actin cytoskeleton were detectable after treatment of trophozoites with CB and LB. Small actin aggregates were observed in heterogeneous regions of the plasma membrane. Actin filament depolymerization led to the disorganization of the acanthopodia after treatment with both drugs. Moreover, the typical amoeboid morphology was lost, and the trophozoites became round (Fig. 4A). After exposure to LB, some trophozoites displayed enucleation. This effect did not occur in trophozoites exposed to CB (Fig. 4A).

Fig. 4. Effect of CB and LB on A. castellanii trophozoites. (A) Treatment of trophozoites with CB and LB showed depolymerization of actin filaments and subsequent disorganization of the acanthopodia. Trophozoites exposed to LB showed cell enucleation (arrow). Enucleation did not occur after treatment with CB. Scale bar = 10 μm. (B) Adhesion of trophozoites treated with CB to epithelial cells. Treatment with CB decreased the adhesion of trophozoites up to 70% compared with control trophozoites not exposed to the drug. Data were analysed using a Kruskal–Wallis test, *P = 0·0003.
Effect of treatment with CB on the adhesion of A. castellanii to epithelial cells
After treatment of trophozoites with CB, we examined the effect of depolymerization of the actin cytoskeleton and disorganization of acanthopodia on the trophozoite adherence to epithelial cells. After 45 min of interaction, only 10% of trophozoites treated with CB adhered to the epithelial cells (Fig. 4B). In contrast, control trophozoites (no treatment with CB) showed 89% adherence to epithelial cells (Fig. 4B), indicating that treatment with CB inhibited the adhesion of trophozoites by 70%. Trophozoite viability was evaluated by trypan blue staining, which indicated that 95% of the trophozoites were viable. Moreover, treatment of trophozoites with 2% EtOH/H2O did not affect their cell adhesion (data not shown).
Biochemical analysis of surface proteins and glycoproteins of A. castellanii
Using biotin labelling of proteins and glycoproteins on the surface of trophozoites, we detected 16 protein bands with molecular weight MW⩾129, 124, 112, 106, 84, 77, 67, 54, 46, 36, 31, 27, 23, 19, 18 and 17 kDa (Fig. 5A, lane 2). Analysis of the glycosidic nature of the protein bands indicated the presence of at least 8 glycoproteins with GlcNAc residues with MW⩾112, 106, 77, 54, 46, 31, 19 and 18 kDa (Fig. 5B, lane 1) and 8 glycoproteins with Man residues with MW⩾129, 106, 84, 77, 67, 31, 19 and 17 kDa (Fig. 5B, lane 2).

Fig. 5. Surface proteins and glycoproteins of A. castellanii. (A) Total protein (lane 1) stained with Coomassie blue and surface proteins (lane 2) labelled with biotin and detected with streptavidin-peroxidase. Molecular weight markers are shown in M. *Indicates proteins detected with greater intensity. (B) Detection of surface glycoproteins by labelling with biotinylated lectins, which showed the surface glycoproteins with GlcNAc residues (lane 1) and Man residues (lane 2).
Identification of proteins of A. castellanii with affinity for epithelial and neuronal cells
After collection of the trophozoite surface protein profile, we identified the proteins that presented an affinity for epithelial and neuronal cells, particularly in the early stages of infection. The A. castellanii trophozoites had at least 10 membrane proteins with MW⩾129, 84, 67, 54, 46, 36, 31, 27, 23 and 19 kDa showing affinity for neuronal cells (Fig. 6, lane 2) and 9 membrane proteins with MW⩾129, 106, 84, 67, 54, 46, 36, 23 and 19 kDa showing affinity for epithelial cells (Fig. 6, lane 3).

Fig. 6. Identification of A. castellanii proteins with affinity for epithelial and neuronal cells. 1. Total protein stained with Coomassie blue; 2. Biotinylated trophozoite proteins showing affinity for neuronal cells and 3. Biotinylated trophozoite proteins showing affinity for epithelial cells; M. molecular weight markers. *Indicates proteins detected with greater intensity.
Table 1 summarizes the MW of the trophozoite surface proteins and those that were detected in the interaction with both cell types. The table also indicates the surface proteins that contained GlcNAc and Man residues.
Table 1. A. castellanii surface proteins with affinity for epithelial and neuronal cells

MW = Molecular weight of trophozoite surface proteins.
✓Trophozoite surface proteins with affinity for epithelial and neuronal cells.
* Surface glycoproteins with Man residues.
°Glycoproteins with GlcNAc residues.
DISCUSSION
The expression of glycoprotein surface molecules and cytoskeletal dynamics are linked to the adhesion process, which is essential for the colonization of pathogens in various tissues (Karlsson, Reference Karlsson1989; Moore et al. Reference Moore, Ubelaker, Martin, Silvany, Dougherty, Meyer and McCulley1991). In A. castellanii, the cellular processes and molecules involved in the recognition and adhesion to the host tissue are relatively unknown.
The cytoskeleton, particularly actin filaments, plays a crucial role in the establishment of infection by various protozoan pathogens. Burchard and Bilke (Reference Burchard and Bilke1992) showed that disruption of the filament system of E. histolytica using CB inhibits the adhesion of trophozoites to polymorphonuclear cells. Similarly, in human fungal pathogens, such as Cryptococcus neoformans, phagocytosis of the fungus by endothelial cells is facilitated by actin cytoskeleton reorganization; this reorganization also affects the permeability of tight junctions (Chen et al. Reference Chen, Stins, Huang, Chen, Kwon-Chung, Chang, Kim, Suzuki and Jong2003). In A. castellanii, the presence of actin filaments has been reported in the acanthopodia (González-Robles et al. Reference González-Robles, Castañón, Hernández-Ramírez, Salazar-Villatoro, González-Lázaro, Omaña-Molina, Talamás-Rohana and Martínez-Palomo2008). These structures are especially important in the infection process. In interaction experiments with corneal epithelial cells, acanthopodia allow the trophozoite to interact with the cell surface (Omaña-Molina et al. Reference Omaña-Molina, Navarro-García, González-Robles, Serrano-Luna, Campos-Rodríguez, Martínez-Palomo, Tsutsumi and Shibayama2004). Moreover, Taylor et al. (Reference Taylor, Pidherney, Alizadeh and Niederkorn1995) demonstrated the involvement of actin filaments in the cytopathic effect of A. castellanii trophozoites in ocular melanoma cells; they suggested that the decrease in cytolysis was due to reduced binding sites on the host cells.
In this work, we used epifluorescence and confocal microscopy to show the distribution of actin filaments in trophozoite acanthopodia. We used specific antibodies against the trophozoites of A. castellanii. Confocal microscopy showed that the arrangement of actin filaments forming acanthopodia remains stable after adhesion to the host cells (up to 45 min). Furthermore, the use of CB and LB allowed us to test the active participation of filaments in the adhesion process to the host cell. The main mechanism of action of cytochalasins and latrunculines is to decrease the rate of polymerization of actin. This effect is caused by the formation of a complex between the drug molecules and G-actin monomers, preventing polymerization into F-actin (Beckerle, Reference Beckerle1998; Fürstner et al. Reference Fürstner, Kirk, Fenster, Aïssa, De Souza and Müller2005). After treatment of A. castellanii with CB and LB, the normal distribution of actin filaments was changed, and the acanthopodia of the amoeba were disintegrated. This effect markedly inhibited the adhesion of trophozoites to epithelial cells. Thus, the cytoskeleton actively participates in the adhesion of trophozoites to the host cell.
Cytoskeletal participation in infectious processes is not limited to the formation of membrane structures, such as lamellipodia and filopodia. Adhesins in the membrane have been reported to strongly anchor to the cytoskeleton network. This anchorage regulates the physiology of the adhesion molecules and transduces signals to the cytoplasm and nucleus. The cytoskeleton–membrane relationship plays a crucial role in the establishment of infections by protozoan pathogens. With respect to the nature of adhesin proteins, studies on various protozoan pathogens, such as E. histolytica, have shown that adherence to host tissue is mediated by the interaction of surface proteins containing carbohydrate residues (Bailey et al. Reference Bailey, Day and Gasque1985; Pacheco-Yépez et al. Reference Pacheco-Yépez, Campos-Rodríguez, Rojas-Hernández, Serrano-Luna, Rivera-Aguilar, Villa-Treviño, Martínez-Palomo, Tsutsumi and Shibayama2009). Analysis of the surface composition of Naegleria trophozoites showed differences in glycoconjugates between pathogenic and non-pathogenic species. This suggests that glycoconjugates of D-mannose and L-fucose are involved in the adhesion of Naegleria fowleri to MDCK cells (Cervantes-Sandoval et al. Reference Cervantes-Sandoval, Serrano-Luna, Pacheco-Yépez, Silva-Olivares, Tsutsumi and Shibayama2010).
In addition, the adhesion of clinical isolates of Acanthamoeba polyphaga, Acanthamoeba culbertsoni and A. castellanii to corneal epithelial cells involves binding to carbohydrate groups present on the cell surface (Morton et al. Reference Morton, McLaughlin and Whiteley1991). Particularly, in A. castellanii, the presence of a glycoprotein with mannose residues on the surface of trophozoites has been reported; this protein, known as mannose binding protein (MBP), recognizes mannosylated glycoproteins in the membrane of the corneal epithelial cells and adheres to them (Garate et al. Reference Garate, Cubillos, Marchant and Panjwani2005, Reference Garate, Marchant, Cubillos, Cao, Khan and Panjwani2006). However, adherence to the host cell is a complex process involving more than one protein in the host–pathogen interaction. In other pathogens, such as Candida albicans, different adhesins on the surface have been reported to facilitate the first stage of infection. This diversity of adhesins provides the fungus with great flexibility and adaptability to the host cell (Karkowska-Kuleta et al. Reference Karkowska-Kuleta, Rapala-Kozik and Kozik2009). Monoclonal antibodies provide evidence of an adhesion molecule other than the MBP that also binds mannose in A. castellanii (Kennett et al. Reference Kennett, Hook, Franklin and Riley1999). In this work, using both biotin labelling and biotinylated lectins, 16 surface protein bands were detected, of which at least 8 were mannose glycoproteins. The interest in understanding Acanthamoeba mannoproteins stems from previous reports that have indicated that epithelial cell adhesion may be inhibited by mannose and methyl-mannopyranoside but not by other sugars (Morton et al. Reference Morton, McLaughlin and Whiteley1991). Furthermore, our results showed the presence of 8 glycoproteins with N-acetyl glucosamine residues on the surface of trophozoites. This result is of particular interest because it has been reported that corneal epithelial cells, a potential target of Acanthamoeba infection, express proteins with GlcNAc and Man residues on their cell surface (Panjwani et al. Reference Panjwani, Ahmad and Raizman1995), indicating that the trophozoite of Acanthamoeba has a complex composition of surface proteins and glycoproteins that can potentially act as receptor adhesins to host cells. We performed experiments in which biotinylated trophozoite surface proteins interacted with neural and epithelial cells. We detected at least 10 trophozoite proteins involved in the interaction with neuronal cells and 9 with epithelial cells. Our results indicated that these molecules play an important role in the process of adherence to epithelial and neuronal cells. These results are of great importance, as MBP is the only molecule that has been reported to regulate the adhesion of A. castellanii to corneal cells. The interacting proteins reported in this work therefore represent a promising discovery, suggesting that a variety of proteins govern Acanthamoeba adhesion to a wide diversity of host cells. We are now performing immunological experiments and sequence analysis to elucidate the specific role of each of the interacting proteins important for adhesion of A. castellanii to epithelial and neuronal cells.
In summary, the results presented here show that adhesion of A. castellanii trophozoites to the host cell is a complex process. At the cellular level, adhesion involves cytoskeletal elements that form structures such as filopodia. These structures establish close contact with the host cell. At the molecular level, glycoproteins on the surface of the trophozoite form molecular bonds with proteins on the host cell, resulting in the establishment and spread of infection through virulence factors that are dependent and independent of contact (Fig. 7A). When the elements involved in the establishment of infection are altered, it is possible to reduce the damage to the host cell. Disruption of the trophozoite filament system results in the disintegration of the acanthopodia of the amoeba. This reorganization of the cytoskeleton deregulates the physiology of the adhesion molecules anchored to the trophozoite membrane, with a subsequent decrease in the adhesion to the host cell surface (Fig. 7B). Thus, a reduction in the cytopathic effect is observed.

Fig. 7. Interaction of A. castellanii with host cells. (A) The acanthopodia are composed of actin and recognize receptors on the host cell via surface glycoproteins, allowing the trophozoite begin the adhesion. (B) Depolymerization of the cytoskeleton of A. castellanii by CB and LB leads to the disorganization of surface molecules. This effect decreases the ability to adhere to host cells and likely reduces the cytopathic effect.
Our results provide insight into the biochemical and cellular mechanisms of the Acanthamoeba infection process. This knowledge can be utilized in the generation of targeted therapies against different infections caused by this species of amoeba.
ACKNOWLEDGEMENTS
We are grateful to Dr Félix Gutierrez Corona and to Dr Luis Manuel Orozco Castellanos for their valuable support with the fluorescence microscope and Animal Research Facility respectively, University of Guanajuato.
FINANCIAL SUPPORT
This work was supported by a CONACyT grant (number 157577) to M. Sabanero and a student fellowship from CONACyT (number 17279) to Karla J. Soto-Arredondo. We would like to thank Dirección de Apoyo a la lnvestigación y al Posgrado, Universidad de Guanajuato.