INTRODUCTION
The oxyuroid nematode Aspiculuris tetraptera (Nitzsch, Reference Nitzsch1821) is a well-known contaminant of laboratory mice, a pest that is easily transmitted in conventional animal houses and a major headache for animal house staff breeding specific pathogen-free mice for research projects (Flynn, Reference Flynn1973; Taffs, Reference Taffs1976). It is also well known as a parasite of wild house mice Mus musculus Linnaeus, 1758 (both Mus musculus musculus and M. m. domesticus Rutty, 1772; see Suzuki et al. Reference Suzuki, Nunome, Kinoshita, Aplin, Vogel, Kryukov, Jin, Han, Maryanto, Tsuchiya, Ikeda, Shiroishi, Yonekawa and Moriwaki2013), occurring widely in Europe (e.g. UK, Denmark, Germany, Austria, Czech Republic, Serbia; see Behnke, Reference Behnke1975; Sage et al. Reference Sage, Heyneman, Kee-Chong and Wilson1986; Moulia et al. Reference Moulia, Aussel, Bonhomme, Boursot, Nielsen and Renaud1991; Kataranovski et al. Reference Kataranovski, Vukićević-Radić, Kataranovski, Radović and Mirkov2008; Baird et al. Reference Baird, Ribas, Macholan, Albrecht, Pialek and Gouy de Bellocq2012) and throughout the world (Hugot, Reference Hugot1980; Tattersall et al. Reference Tattersall, Nowell and Smith1994; de Bellocq et al. Reference De Bellocq, Ribas and Baird2012).
Aspiculuris tetraptera has also been recorded from a range of other rodent species, including wood mice (Apodemus sylvaticus Linnaeus, 1758), but infrequently in this host and usually only as a few worms, suggesting incidental infections (e.g. Bernard, Reference Bernard1987; Ryan and Holland, Reference Ryan and Holland1996; Behnke et al. Reference Behnke, Barnard, Bajer, Bray, Dinmore, Frake, Osmond, Race and Sinski2001; de Bellocq et al. Reference De Bellocq, Sara, Casanova, Feliu and Morand2003). However, several authors, including ourselves, have reported the worm from European bank voles [Myodes (= Clethrionomys) glareolus (Schrieber, 1780)] (Sharpe, Reference Sharpe1964; Lewis, Reference Lewis1987; Behnke et al. Reference Behnke, Bajer, Harris, Newington, Pidgeon, Rowlands, Sheriff, Kuliś-Malkowska, Siński, Gilbert and Barnard2008; Bjelic-Cabrilo et al. Reference Bjelic-Cabrilo, Kostic, Popovic, Cirkovic, Aleksic and Lujic2011), not just as incidental infections, but showing high prevalences and with heavy worm burdens, indicating frequent transmission among individuals of this host species and a capacity to mature and survive to, and beyond, patency (Thomas, Reference Thomas1953; Sharpe, Reference Sharpe1964; Lewis, Reference Lewis1987). For some time we have suspected that the worms from bank voles and house mice may represent different species, despite their superficial morphological similarity. The ecology of their host species is quite different, with little overlap of frequented territories. In most parts of the world, including Europe, house mice are mainly anthropophilic, living in close proximity to villages, farms and other human habitation, although in Australia they have adapted to colonize agricultural land on which cereals are grown and eruptions of plague proportion occur at regular intervals (Singleton et al. Reference Singleton, Brown, Pech, Jacob, Mutze and Krebs2005). On the other hand, red-backed voles of the genus Myodes Pallas, 1811 are predominantly woodland animals and while they may occasionally be trapped in cultivated fields and may even venture into buildings, these species do not overlap extensively in their territories with house mice, their diets differ and they are rarely likely to encounter one another in the wild (Flowerdew et al., Reference Flowerdew, Gurnell and Gipps1985; Bujalska and Hansson, Reference Bujalska and Hansson2000). Although some degree of gene flow cannot be ruled out, nevertheless, it is not easy to see how a species such as A. tetraptera could remain panmictic as a major parasite of rodents in both Mus and Myodes, a key requirement if these taxa are not to diverge and eventually speciate. Evolutionary theory predicts that ecological separation should eventually lead to genetic separation and separate species status (Coyne and Orr, Reference Coyne and Orr2004).
It may also be pertinent that nematodes of the order Oxyurida Weinland, 1858, are renowned for their host specificity and are well recognized as having undergone co-evolution with their hosts. Some of the best evidence for co-evolution of parasites with their hosts is derived from the tightly congruent host–parasite phylogenetic trees that have been generated using both morphological and genetic criteria (Enterobiinae; Hugot, Reference Hugot1999). Based on data such as these, it would be remarkable therefore if the worms from M. glareolus and M. musculus were conspecific. Evidence that the species of Aspiculuris Schultz, Reference Schulz1927 from bank voles may be different from that infecting house/laboratory mice is also provided by an attempt to infect wild-caught bank voles with fully embryonated and infective eggs isolated from worms recovered from laboratory mice: the eggs given to bank voles failed to survive to maturity with larvae persisting for fewer than 10 days, while those given to laboratory mice developed normally (Behnke, Reference Behnke1974).
While records of species of Aspiculuris from Apodemus appear incidental, two other species of the genus have been described from voles; Aspiculuris dinniki Schultz, Reference Schulz1927, from the snow vole Chionomys nivalis (Martins, 1842) (see Schulz, Reference Schulz1927) from the northern Caucasus in Russia, and Aspiculuris tianjinensis Liu et al. Reference Liu, Bu and Zhang2012, recently reported as parasitizing the grey-sided vole Clethrionomys rufocanus, now known as the grey red-backed vole Myodes rufocanus (Sundevall, 1846) in China (Liu et al. Reference Liu, Bu and Zhang2012). Neither of these two Aspiculuris spp. is well known, and neither has been subjected to molecular genetic analysis. In this paper, we tested the null hypothesis that A. tetraptera from bank voles and from house/laboratory mice are the same species. First, we compared specimens of Aspiculuris recovered from bank voles (M. glareolus) and from house and laboratory mice (M. m. musculus and M. m. domesticus), using conventional light and scanning electron microscopy, with a focus on the key morphological features that are known to vary between species within the genus. We also compared our material to descriptions of A. tetraptera and A. tianjinensis in the literature. We then amplified and sequenced a fragment of the nuclear ribosomal RNA gene spanning the 5·8S rDNA molecule, the Internal Transcribed Spacer 2 region (ITS2) and the mitochondrial Cytochrome Oxidase 1 (CO1) gene, generating a phylogeny of Aspiculuris for the first time to demonstrate the relationship between isolates from house and laboratory mice, bank voles and other rodent hosts that were available to us (Table 1).
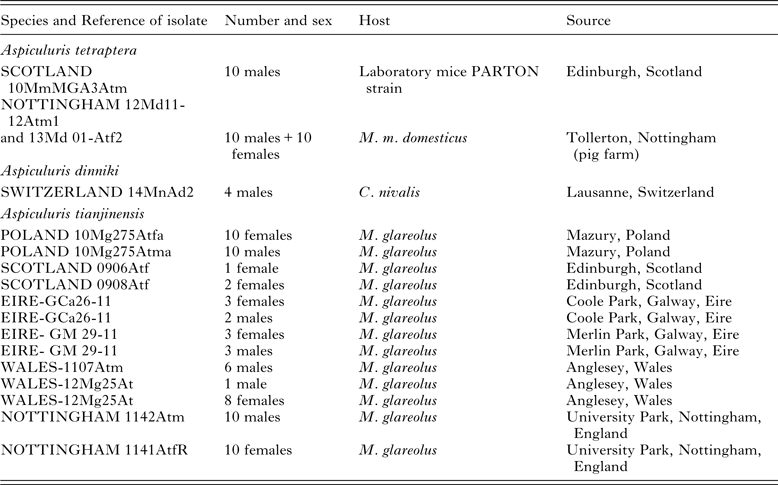
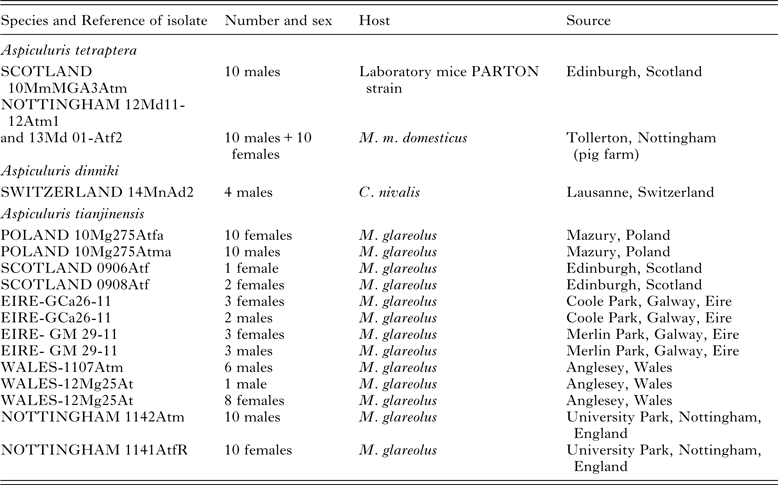
Table 1. Details of the worms, A. tetraptera, A. tianjinensis and A. dinnicki used in the current study for morphometrics
MATERIALS AND METHODS
Nomenclature and terminology
In this paper, nomenclature for bank voles (the genus Myodes) follows Carleton et al. (Reference Carleton, Musser and Pavlinov2003, Reference Carleton, Gardner, Pavlinov and Musser2014) and not Tesakov et al. (Reference Tesakov, Lebedev, Bannikova and Abramson2010) and for all other rodents Musser and Carleton (Reference Musser, Carleton, Wilson and Reeder2005). Terminology used to describe the cephalic end and cervical alae of species of Aspiculuris follows Inglis et al. (Reference Inglis, Harris and Lewis1990) and Hugot (Reference Hugot1980).
Sources of Aspiculuris
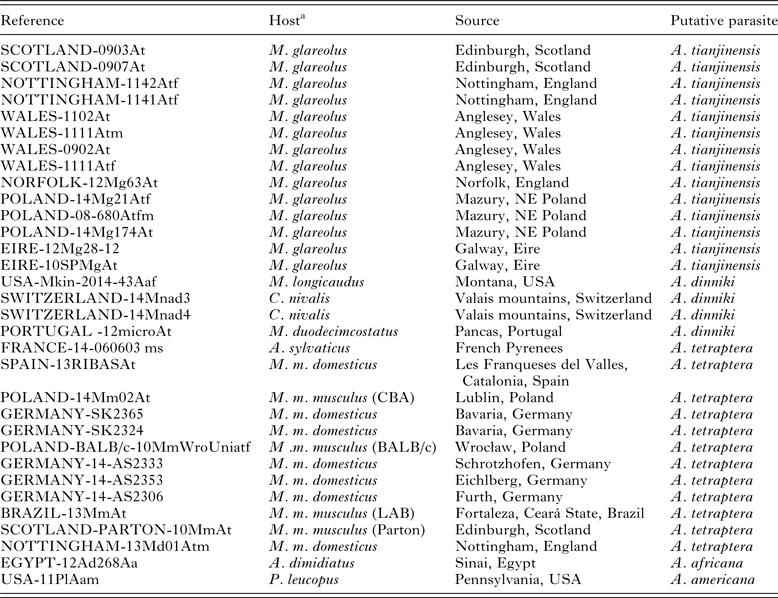
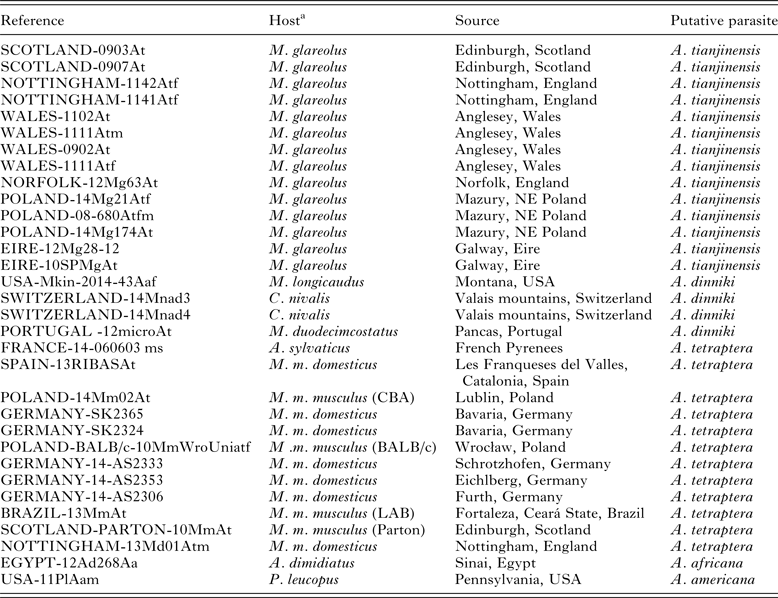
Specimens of Aspiculuris were collected from M. musculus (both laboratory cultures and from wild mice) and from the arvicolids Microtus duodecimcostatus (de Selys-Longchamp, 1839), C. nivalis and M. glareolus from various locations in Europe (Tables 1 and 2). As outgroup material for the molecular analysis we included Aspiculuris africana Quentin, Reference Quentin1966 from the spiny mouse Acomys dimidiatus (Cretzschmar, 1826) from the Sinai in Egypt (Behnke et al. Reference Behnke, Harris, Bajer, Barnard, Sherif, Cliffe, Hurst, Lamb, Rhodes, James, Clifford, Gilbert and Zalat2004) and Aspiculuris americana Erickson, Reference Erickson1938 from Peromyscus leucopus (Rafinesque, 1818) (Erickson Reference Erickson1938) from Pennsylvania, USA (Table 2). We also included worms collected from Microtus longicaudus (Merriam, 1888) from Montana, USA. All pertinent details of hosts, parasites and collection localities are given in Tables 1 and 2, which include also the specific reference codes employed by ourselves and our collaborators for worms in their collections. Rodents were collected according to the legal and ethical guidelines current in the countries where they were sampled.
Table 2. Details of the worms used in the current study for genetic analysis
a For Mus hosts, the strain of mouse is given in parenthesis if the parasites were derived from laboratory mice or (LAB) is given if uncertain. All other records from Mus hosts are from wild-caught animals
Methods utilized for morphological comparison of species
Individuals of Aspiculuris were recovered from the large intestines of voles and mice, the intestines having been preserved in 70% ethanol prior to dissection. The recovered worms were then frozen at −80 °C in 80% ethanol. Prior to microscopic examination, specimens were cleared in lactophenol for study as wet mounts. En face and transverse sections were prepared by hand cutting with a cataract scalpel and mounted in polyvinyl lactophenol. Figures were prepared with the aid of a drawing tube and measurements in micrometres, unless otherwise stated, were taken using an eyepiece micrometer and light micrographs were taken using an Olympus photomicrographic system. Representative specimens were fixed in glutaraldehyde and dehydrated in ethanol, followed by critical point drying and gold coating (Sputter Coater SCD-030, Balzers Union, FL9496), for viewing under scanning electron microscopy (Jeol JSH-840 Scanning Electron Microscope).
All specimens examined morphologically for this study, except those used for scanning electron microscopy, were deposited in the South Australian Museum, Adelaide with registration numbers SAM AHC47104–47108 and 47543–47546.
Molecular genetic comparison of species
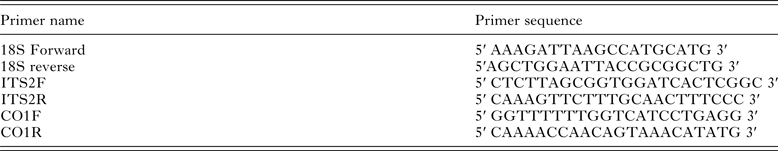
DNA was isolated from individual worms using DirectPCR Lysis Reagent (Viagen Biotech), according to the manufacturer's instructions. DNA primers (Table 3) were used in 25 µL PCR reactions containing 12·5 µL of BioMix Red (Bioline), 0·5 µ m forward primer, 0·5 µ m reverse primer, <250 ng template DNA and nuclease-free water to a final volume of 25 µL. Thermal cycler conditions for the 18S ribosomal locus amplification were as described by Fontanilla and Wade (Reference Fontanilla and Wade2008; 94 °C for 2 min, then 38 cycles of 94 °C for 30 s, 45 °C for 30 s and 65 °C for 1 min). For ITS2 primer reactions were: 98 °C for 2 min, 35 cycles of (98 °C for 15 s, 61 °C for 15 s, 72 °C for 15 s) and 72 °C for 5 min. Thermal cycle and times for CO1 primer reactions were: 98 °C for 2 min, 35 cycles of (98 °C for 15 s, 60 °C for 20 s, 72 °C for 20 s) and 72 °C for 5 min. All PCR reactions were conducted in a Biorad PTC-200 DNA Engine Cycler. PCR products were visualized on 1·5% agarose gels, incorporating ethidium bromide to a final concentration of 0·5 µg mL−1, then purified (ExoSAP, Affymetrix), and DNA concentration estimated (Nanodrop), before dilution with nuclease-free distilled water. Sequencing primers (identical to amplification primers) were added to the appropriate concentration prior to Sanger sequencing (Source Bioscience) and chromatograms inspected visually to resolve ambiguities.
Table 3. Primers for the amplification of specific products as used in the phylogenetic analysis
Alignments were produced using ClustalX within the Mega 6.0 package (Tamura et al. Reference Tamura, Peterson, Peterson, Stecher, Nei and Kumar2011) followed by visual inspection. Phylogenetic analysis was conducted using a maximum-likelihood algorithm implemented in RaxML version 8.0 (Stamatakis, Reference Stamatakis2014) via the CIPRES Science Gateway portal (Miller et al. Reference Miller, Pfeiffer and Schwartz2010). Analysis of the ribosomal sequences was undertaken using the full sequences (including indels) with A. africana and A. americana as outgroups; using an alignment omitting the outgroups (minimizing indels); and excluding all indels and ambiguous regions. None of these changes made any difference to the final tree, which is presented based on the full sequences plus outgroups. Reference sequences for A. tetraptera from GenBank were included in the alignment, as were sequences from A. tetraptera collected from Chinese mice (details below in Results section). The CO1 sequences were checked as inferred amino acid sequences to ensure that there were no frame shifts or unlikely amino acid substitutions in the fragment. Voucher sequences have been deposited in GenBank (CO1 – KT175702 to KT175724; ITS2 – KT175725 to KT175737).
RESULTS
Morphological examination of Aspiculuris
The worms from laboratory mice (Parton strain) from Edinburgh, Scotland (Reference = SCOTLAND 10MmMGA3Atm, 10 males and 10 females) and those from wild mice in Nottinghamshire, England (Reference = NOTTINGHAM 12Md11-12Atm1, 10 males and 10 females), all conformed to the accepted descriptions of A. tetraptera (see Hugot, Reference Hugot1980).
Examination of four males of Aspiculuris from snow voles from Switzerland showed that morphologically they conformed to the description of A. dinniki (see Schulz, Reference Schulz1927), for which the type host is C. nivalis. They could be easily distinguished from A. tetraptera in having 10 and not 12 caudal papillae and a single pair of caudal alae that do not reach the tail tip, rather than 3 pairs of alae, the third pair reaching the tip of the tail, as is typical of A. tetraptera.
Examination of 32 male and 37 female Aspiculuris from bank voles from England, Poland, Scotland, Ireland and Wales (Table 1) showed that morphologically they conformed to the description of A. tianjinensis as reported by Liu et al. (Reference Liu, Bu and Zhang2012).
Morphological comparison of Aspiculuris spp. from bank voles and house/laboratory mice
Aspiculuris tetraptera and A. tianjinensis differ morphologically in the details of the distinct, elaborate inflated region formed by an anterior cephalic cap bearing lateral amphids, dorso-ventral cephalic papillae and lobed posterior extensions of the dorsal and ventral surfaces of the body, that together make up the cephalic vesicle (Inglis et al. Reference Inglis, Harris and Lewis1990). This complex of structures is difficult to interpret and describe but can be more clearly illustrated (Figs 1–3). Both species have the typical small hexagonal mouth opening without lips (Figs 1.1, 1.3 and 3) surrounded by 6 small sessile labial papillae. The differences are seen in the proportions of the four dorso-ventral cephalic papillae and the 2 lateral epaulettes with amphids on the anterior-most level (Figs 1–3). These are larger and more robust in A. tianjinensis than A. tetraptera. Moreover, the proportions of the lateral cap are relatively smaller in A. tianjinensis, such that the lobes of the cephalic papillae extend over the edges of the cap, whereas in A. tetraptera the lobes of the cephalic papillae do not extend over the edges of the cephalic cap (Figs 1 and 3). The cervical alae of A. tianjinensis appear to begin more posteriorly to the cephalic cap than those of A. tetraptera although this small difference may be due to the specimens examined having not been processed using identical protocols. The males of both species have 12 caudal papillae, 5 pairs and 2 median; however, the median papillae are double in A. tetraptera and single in A. tianjinensis, the post-cloacal papilla being relatively larger in A. tetraptera (see Hugot, Reference Hugot1980; Liu et al. Reference Liu, Bu and Zhang2012). The cervical alae terminate more abruptly in A. tetraptera (ending about the mid-oesophageal bulb) than A. tianjinensis (ending nearer to the oesophageal junction) although the actual lengths of the alae overlap. Accurate determination of the relative positions of alae and mid-oesophageal bulb is likely to be influenced by fixation of the worms and whilst efforts were made to keep this consistent between the two species, some variation was inevitable when processing material collected and fixed in the field, and in examining material supplied by collaborators for this project (see Acknowledgements for list).

Fig. 1. Aspiculuris tetraptera from Mus musculus and Aspiculuris tianjinensis from Myodes glareolus. 1.1 – A. tetraptera female en face view; 1.2 – A. tetraptera male ventral view anterior end; 1.3 – A. tianjinensis male en face view; 1.4 – A. tetraptera male lateral view surface aspect; 1.5 – A. tetraptera female lateral view surface aspect; 1.6 – A. tetraptera male dorso-ventral view surface aspect; 1.7 – A. tianjinensis female lateral view surface aspect; 1.8 – A. tianjinensis female lateral aspect optical section; 1.9 – A. tianjinensis female lateral view optical section; 1.10 – A. tetraptera male dorso-ventral view optical section; 1.11 – A. tianjinensis male lateral view optical section; 1.12 – A. tianjinensis male lateral view surface aspect. Scale bars: 1, 4–12, 25 µm; 2, 100 µm; 3, 20 µm.

Fig. 2. Light microscope images of the cephalic ends of Aspiculuris tianjinensis from Myodes glareolus from Wales (A, C) and Aspiculuris tetraptera from Mus musculus from Scotland (B, D), showing dorso-ventral views (A, B) and lateral views (C, D). Arrows indicate cephalic alae. Scale bars: A, C – 35 µm; B, D – 50 µm.

Fig. 3. Scanning electron microscope images of the anterior ends of representative samples from bank voles and from house/laboratory mice. (A) En face view Aspiculuris tetraptera from wild house mouse from England. (B) En face view A. tetraptera from laboratory mouse from Poland. (C) En face view Aspiculuris tianjinensis from bank vole England. (D) Dorso-ventral view A. tianjinensis from bank vole England. The large lobes of the cephalic vesicle can be clearly seen. Bold black arrows point to cervical alae and their epaulettes as they connect with the cephalic vesicle; thin black arrows indicate the inflated regions on the dorsal and ventral surfaces of the worms as relevant; the white circles indicate each of the 4 cephalic papillae; white star indicates the dorsal surface in each image.
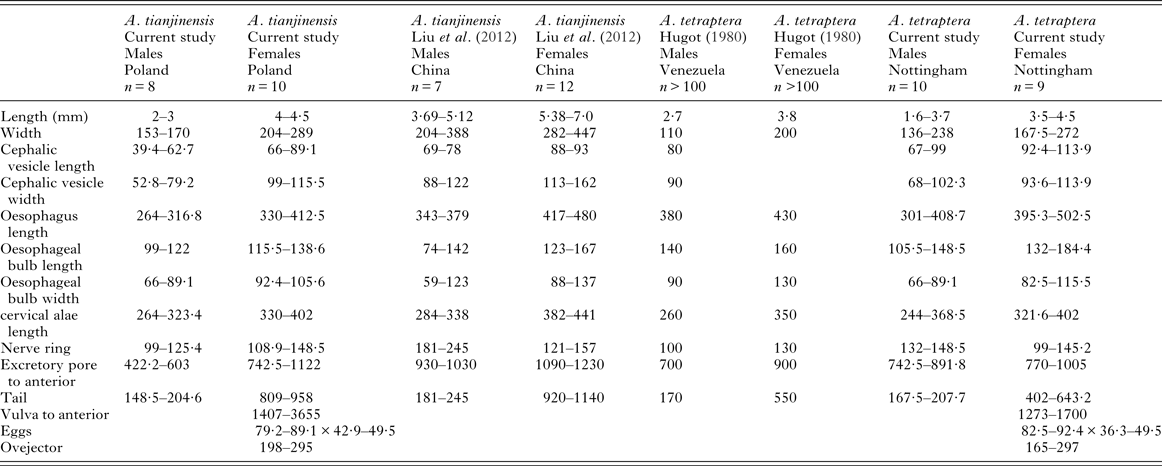
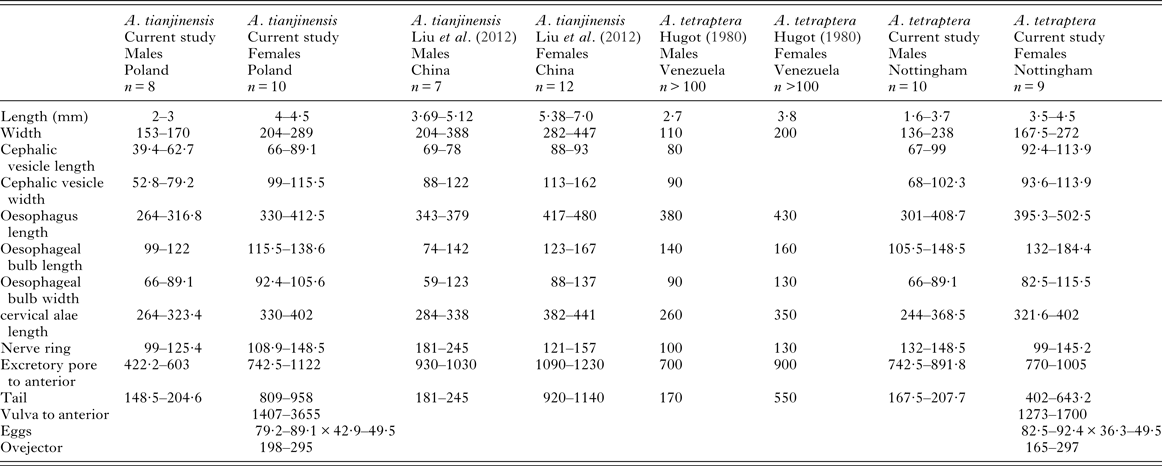
Based on Liu et al. (Reference Liu, Bu and Zhang2012) the Chinese specimens of A. tianjinensis are larger than the English, Irish, Scottish, Welsh or Polish specimens of A. tianjinensis collected from bank voles for this study and they are also larger than the English specimens of A. tetraptera studied. There are no clear differences in morphometrics between the two species (see Table 4 for comparative measurements).
Table 4. Comparative morphometrics of A. tetraptera and A. tianjinensis, data from our study, Hugot (Reference Hugot1980) and Liu et al. (Reference Liu, Bu and Zhang2012).
All measurements are in micrometres, unless otherwise stated.
Molecular genetic comparison of Aspiculuris spp.
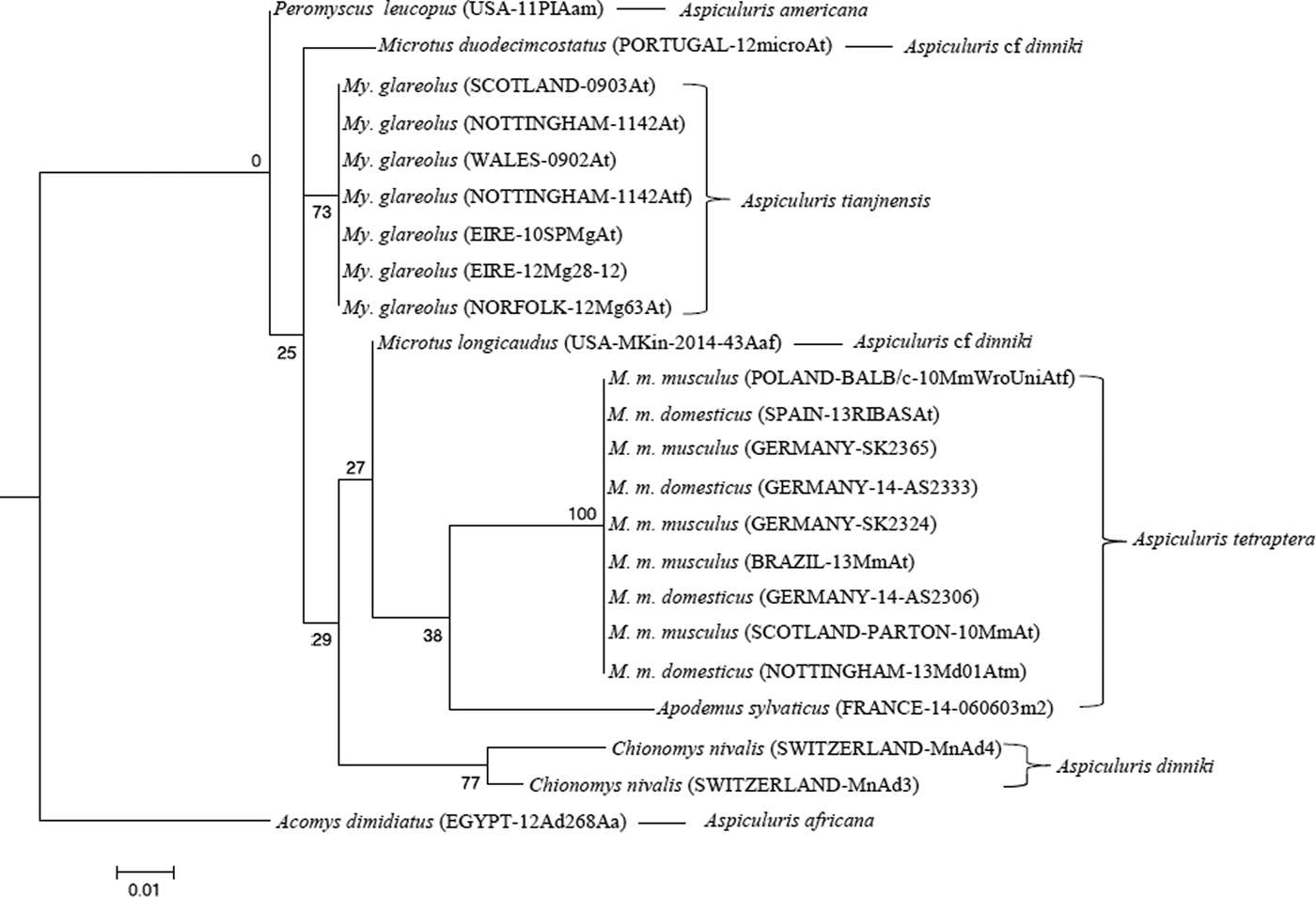
Molecular analyses of Aspiculuris from house mice were entirely consistent with previous analyses of A. tetraptera. The ribosomal primers amplified a fragment of 1024 bp from all isolates (laboratory or wild) extending through 5·8S rDNA and ITS-2 into 28S rDNA, which was identical to the reference sequences (NCBI accession EU263107 and EF464551) from laboratory mouse colonies deposited by Parel et al. (Reference Parel, Galula and Ooi2008) and Feldman (unpublished). Across a common stretch of 416 bp the consensus sequence differed by single nucleotide polymorphisms (SNPs) from four Chinese sequences in GenBank (NCBI accession KJ143618 A27G; KJ143617 T207C and G417A; KJ143616 T377C; KJ143615 G417A; Lou et al. Reference Lou, Zhang, Qiu, Gao, Wang, Xiao, Chang and Wang2015). The fragment amplified from Aspiculuris from voles varied in length between 1022 bp (M. longicaudus) and 1028 bp (M. glareolus), a difference primarily due to a TG microsatellite at the beginning of the 28S rDNA gene. The sequences from worms from P. leucopus (A. americana) and A. dimidiatus (A. africana) were highly divergent; the former was 1050 bp in length, the latter 1005 bp. A maximum-likelihood phylogeny showed a fundamental split between A. americana from P. leucopus and A. africana from A. dimidiatus, and the A. tetraptera-like forms from voles and mice (Fig. 4). The latter also showed a deep split between the forms from house mice (referable to A. tetraptera, with 100% bootstrap support) and the forms from voles (referable to A. tianjinenensis and A. dinniki based on morphology, which grouped with 87% bootstrap support). Maximum-likelihood analysis of aligned sequences from which indels and ambiguous regions had been deleted gave an identical phylogeny, with slightly improved bootstrap support for the major clades. The worms from M. duodecimcostatus, M. longicaudus and C. nivalis grouped together with strong bootstrap support (79%); forms from C. nivalis were morphologically identical to A. dinniki, and were collected from the type host. Tentatively we refer worms from Microtus Schrank, 1798 to A. cf dinniki, pending additional research on the variability of Aspiculuris from this vole genus. A single worm was available from A. sylvaticus collected from Spain, together with material from M. musculus in the same locality. Interestingly, at this ribosomal locus this worm represented a sister group to the A. tetraptera cluster infecting Mus: the ribosomal fragment was 1022 bp in length and differed at 3 SNPs, and by a total of 3 GT repeats at microsatellites. While this is within the range of variation for this locus observed in isolates from voles, it does exceed that seen within worms from Mus.

Fig. 4. Molecular phylogenetic tree of Aspiculuris from mice and voles based on the nuclear 5·8S and ITS-2 following maximum-likelihood analysis with 100 bootstrap replicates implemented via the RaXML package. Scores at nodes represent bootstrap support for that node. Outgroups are Aspiculuris americana from Peromyscus leucopus and Aspiculuris africana from Acomys dimidiatus. Scale bar is proportional to the genetic distance in substitutions per site.
Amplification of the Small Subunit ribosomal RNA locus generated a fragment 515 bp long from worms from both Mus and Myodes. These fragments differed by a single SNP at position 198 in the alignment, which was a C in worms from Mus and a T in worms from Myodes. Lacking other SNPs, and lacking sequences from worms from C. nivalis, Microtus spp. or A. sylvaticus, this locus was not included in the alignment used for phylogenetic analysis.
Amplification at the CO1 locus was not as reliable as ITS-2, and a different set of isolates were used for this phylogeny. The 189 bp fragment contained 18 SNPS; three resulted in changes to the inferred amino acid sequence, and further variation was confined to synonymous substitutions. All amplicons from worms from Myodes had identical sequences, as did all PCR products from worms collected from Mus. The broad trends in the two phylogenies were similar with a clear cut difference between the sequences from voles and mice (Fig. 5). However, while the A. africana CO1 sequence differed by 3·5–5% from all other isolates, the A. americana sequence was only 1% different from the Aspiculuris sequences from voles. While the two principal groupings, worms from Mus (A. tetraptera) and worms from Myodes (A. tianjinensis) were both recreated in the CO1 phylogeny, only the clade (A. dinniki sensu stricto) containing worms from Chionomys (Satunin, 1909) was also supported (bootstrap support 77%), and at all higher levels bootstrap support was insufficient to imply relationship. The worms from Microtus spp. (A. cf dinniki) did not group with each other or with A. dinniki, but generally bootstrap support was so low that the significance of their failure to form a single clade cannot be assessed.
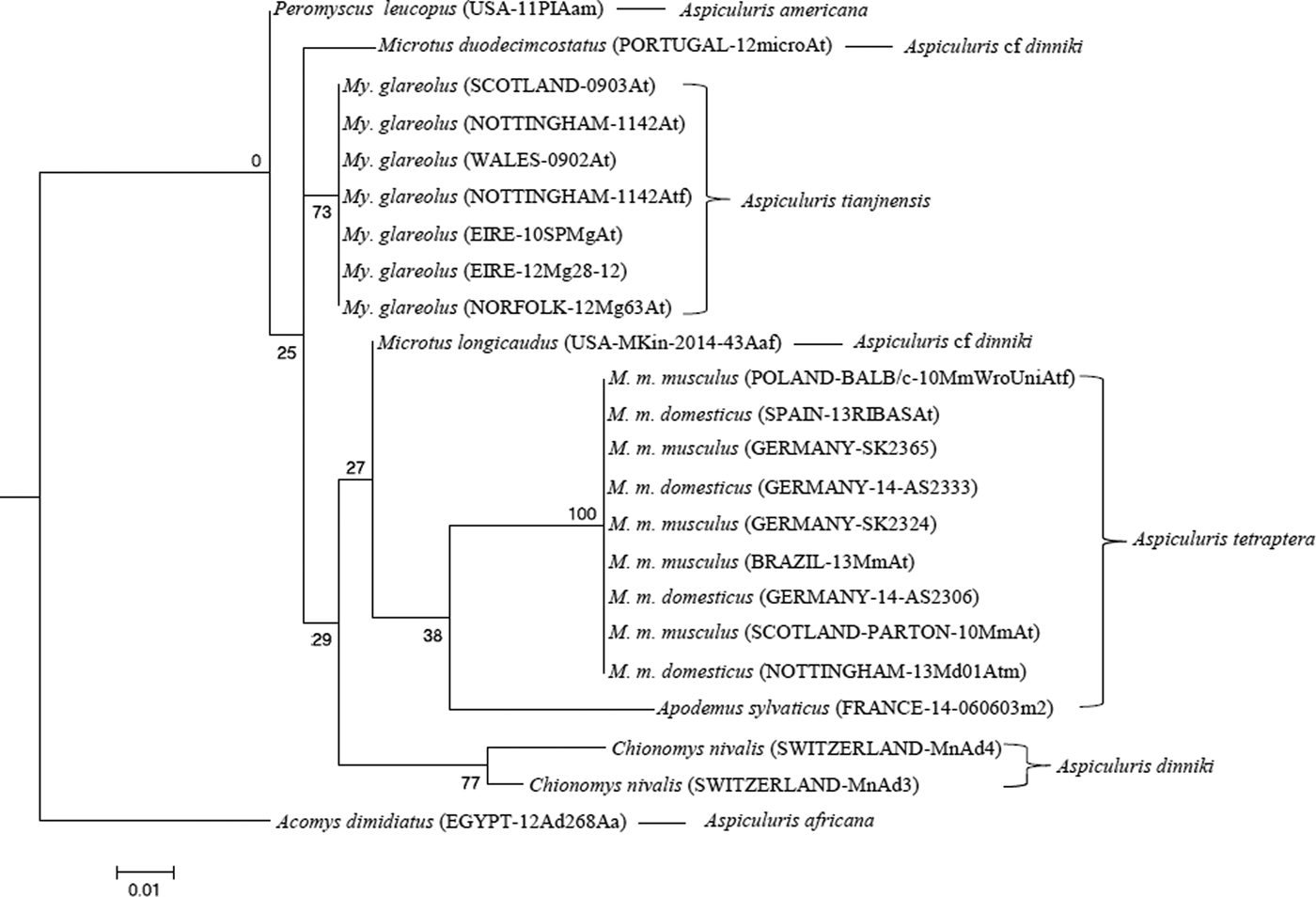
Fig. 5. Molecular phylogenetic tree of Aspiculuris from mice and voles based on the mitochondrial Cytochrome Oxidase 1 gene (CO1) following maximum-likelihood analysis with 100 bootstrap replicates implemented via the RaXML package. Scores at nodes represent bootstrap support for that node. Scale bar is proportional to the genetic distance in substitutions per site.
DISCUSSION
The molecular phylogenies based on available Aspiculuris material make it clear that the worms from the bank vole, M. glareolus, are distinct from both A. dinniki from the snow vole, C. nivalis, and from A. tetraptera from the house mouse. The molecular distance between the worms from voles and A. tetraptera is such that there can be no suspicion that these are recently diverged forms, due to isolation caused by the ecological differentiation of their hosts. Rather it is clear that Aspiculuris has radiated recently within Holarctic voles of the genera Microtus and Myodes, and that the greater diversity of the genus as currently understood, occurs within these hosts. We identify the material from M. glareolus with A. tianjinensis, based on morphological evidence as provided in the original description (Liu et al. Reference Liu, Bu and Zhang2012), because material of the latter taxon from eastern China was not available for either morphological or molecular study.
The genus Aspiculuris was created by Schulz (Reference Schulz1924) to accommodate A. tetraptera, originally described as Ascaris tetraptera by Nitzsch (Reference Nitzsch1821) and transferred into Rudolphi's genus Oxyura by Diesing (Reference Diesing1861). Descriptions and drawings from the 1830s (e.g. Schmalz, Reference Schmalz1831) make it absolutely clear that this taxon described in 1821 as Asc. tetraptera was identical to that currently recognised as A. tetraptera. By the mid-1970s, when the genus had grown to include a number of heterogeneous species, Quentin (Reference Quentin1975) recognized the importance of the shape of the anterior cervical alae, either arrowhead-shaped or spear-shaped in species discrimination (Quentin, Reference Quentin1975; Inglis et al. Reference Inglis, Harris and Lewis1990). Four of the species in the present study A. dinniki, A. tianjinensis, A. tetraptera and A. americana all have arrowhead-shaped alae, while the fifth, A. africana has spear-shaped alae. Hugot (Reference Hugot1980) used developmental evidence to support the argument that species with spear-shaped outlines were more primitive. Our molecular ITS-2 phylogeny makes it clear that A. americana, from P. leucopus and A. africana from A. dimidiatus are the most divergent species included in our analysis, and the arrowhead alae do not group with a monophyletic clade in the genus. Therefore, the morphology of the cervical alae cannot be considered at this time as reliable evidence of relationship and consequently alternative evidence is required to determine whether Quentin's (Reference Quentin1975) division of the genus into four primitive species with continuous cervical and lateral alae (a spear-shaped anterior outline) and a larger group of species with discontinuous alae (an arrowhead-shaped anterior outline) is valid. Additional molecular analysis of a greater range of species, particularly those with a spear-shaped anterior outline, is required to fully resolve the relationship between these morphological features and evolutionary relationships between species.
In the past, the morphological identification of species of Aspiculuris has been based primarily on the relative length and shape of the cervical alae as for example in the key of Akhtar (Reference Akhtar1955) and the number and configuration of the caudal papillae of male worms. However, this has raised confusion because both of these character sets can be misinterpreted if the effects of fixation are not taken into account. In particular, not all caudal papillae are readily detectable when the ventral caudal area has become curled or creased during fixation. Furthermore, it may be difficult to see the median papillae and to decide whether these are single or double as described by Hugot (Reference Hugot1980). Consequently we believe that some authors have miscounted the number of caudal papillae for these species. As re-described by Hugot (Reference Hugot1980), A. tetraptera has 5 pairs of single papillae and 2 double papillae and we were able to confirm this with our specimens. These can be counted either as 12 (5 × 2 + 1 + 1) or as 14 (5 × 2 + 2 + 2) papillae. A. tianjinensis has 12 papillae, made up of 5 pairs and 2 single median papillae which do not appear to be double (Liu et al. Reference Liu, Bu and Zhang2012). Our specimens from bank voles also had 12 papillae, with the two median papillae not doubled, matching exactly the description given by Liu et al. (Reference Liu, Bu and Zhang2012). Comparisons of the en face and lateral aspects of the cephalic ends of specimens have proved to be more reliable characters for separating the species than either morphology of the alae or numbers of caudal papillae for these two species. Both Hugot (Reference Hugot1980) and Inglis et al. (Reference Inglis, Harris and Lewis1990) have provided useful descriptions of the complex of cephalic structures that are found within the genus, allowing species discrimination even where morphometrics are similar.
It is clear from the current work that A. tetraptera and A. tianjinensis are not sister taxa undergoing incipient speciation, but are only distantly related despite their superficial morphological resemblance. However, it does appear that A. tianjinensis and A. dinniki are more closely related, and that these are part of a wider radiation of Aspiculuris within the recently evolved Arvicolinae. While records of Aspiculuris from Apodemus appear incidental, several Aspiculuris spp. have been described from voles; two, A. dinniki, from the snow vole C. nivalis (see Schulz, Reference Schulz1927), and A. tianjinensis, recently reported as parasitizing M. rufocanus in China (Liu et al. Reference Liu, Bu and Zhang2012), and recorded here as widespread in M. glareolus in Central Europe, are fairly well documented. A. kazakstanica Nasarova and Sweschnikowa, 1930, a parasite of voles from central Asia, is known only from the secondary Soviet Russian literature, and is a species that requires re-assessment and detailed confirmation of the original description. It is especially interesting to note that whereas these species, or A. tetraptera s.l., are widely recorded from voles in the Soviet Russian host-parasite literature (e.g. Rishikov, Reference Rishikov1979), the only records of the genus from voles in North America are those of an ‘A. tetraptera’- like form from M. longicaudus and M. gapperi recorded by Kinsella (Reference Kinsella1967; and see also Doran, Reference Doran1955), although A. americana occurs in the neotomine rodent Peromyscus Gloger, 1841 in the Nearctic. We have been able to include worms from M. longicaudus in our molecular analysis, and can confirm these as related to A. dinniki, from Chionomys, forming a single clade at the ribosomal locus with A. dinniki sensu stricto and with worms from M. duodecimcostatus (Fig. 4). We identify these worms as A. cf dinniki, but a reappraisal of the taxonomy of Aspiculuris from Microtus and its allies is clearly necessary. It is likely that worms in this clade are Microtus and Chionomys specialists, these host genera being closely related. The colonization of Northern America by both Microtus and Myodes is very recent, and for example the North American M. gapperi (Vigors, 1830) has been suggested to be paraphyletic with respect to both the Eurasian M. glareolus and the holarctic M. rutilus Pallas, 1779 (see Cook et al. Reference Cook, Runck and Conroy2004), taxa which have probably diverged since the early Pleistocene, circa 2 million years ago (MYA). While multilocus sequencing with a larger dataset throws doubt on this paraphyly (Kohli et al. Reference Kohli, Speer, Kilpatrick, Batsaikhan, Damdinbaza and Cook2014), there is no doubt that the origin of the American Myodes is recent; Kohli et al. (Reference Kohli, Speer, Kilpatrick, Batsaikhan, Damdinbaza and Cook2014) estimate the date for the divergence of M. glareolus and M. gapperi (presumably a Eurasian vole) as 1·25 MYA ± 0·75 million years. The origin of the North American Microtus species, including M. longicaudus, is obscure, but is also unlikely to be as much as 2 MYA (Chaline et al. Reference Chaline, Brunet-Lecomte, Montuire, Viriot and Courant1999; Jaarola et al. Reference Jaarola, Martínková, Gündüz, Brunhoff, Zima, Nadachowski, Amori, Bulatova, Chondropoulos, Fraguedakis-Tsolis, González-Esteban, José López-Fuster, Kandaurov, Kefelioğlu, da Luz Mathias, Villate and Searle2004). It appears then that the Aspiculuris fauna of North America contains representatives of at least two radiations, the older in neotomine rodents while the second in voles is more recent.
The only murid which is regularly infected with Aspiculuris in Eurasia is M. musculus and although several species are known from M. musculus from other geographical regions (e.g. A. huascensis Falcon-Ordaz et al. Reference Falcón-Ordaz, Pulido-Flores and Monks2010 and A. lahorica Akhtar, Reference Akhtar1955), these appear closely related to A. tetraptera, if in fact they do indeed represent distinct species (Akhtar, Reference Akhtar1955; Falcon-Ordaz et al. Reference Falcón-Ordaz, Pulido-Flores and Monks2010). The commensal rat, Rattus rattus Linneaus, 1758, is also infected by A. pakistanica (Akhtar, Reference Akhtar1955), but this species is very similar to A. tetraptera in morphology (Akhtar, Reference Akhtar1955). Reports of A. tetraptera from rats usually indicate only low prevalence and intensity, and mostly from study sites frequented also by sympatric populations of house mice (Milazzo et al. Reference Milazzo, Cagnin, di Bella, Geraci and Ribas2010). A. tetraptera has been reported from Mus spretus Lataste, 1883 in Spain and Portugal, the latter in an environment where house mice also abounded, so these were probably also incidental infections from house mice (Behnke et al. Reference Behnke, Barnard, Hurst, McGregor, Gilbert and Lewis1993; Fuentes et al. Reference Fuentes, Cerezuela and Galan-Puchades2000; Sainz-Elipe et al. Reference Sainz-Elipe, Galan-Puchades and Fuentes2007). A. shikouleta, a species with alae continuous along the sides of the body, the spear-shaped profile, has been recorded from the distantly related African murid Micaelamys namaquensis (Smith, 1943) reported as Aethomys namaquensis by Inglis et al. (Reference Inglis, Harris and Lewis1990). However, the only other records of A. tetraptera-like worms from murids are the sporadic reports of this parasite from Apodemus. Our data show clearly on molecular genetic evidence that these worms from Apodemus are similar to A. tetraptera but not identical to it, being distinctly different from worms from Mus at both ITS-2 and CO1 loci. There are no records of A. tetraptera-like worms from any other murid, despite the popularity of these rodents for parasite taxonomic and faunistic surveys. There is certainly no evidence of close co-evolution between Aspiculuris and murid rodents in the way that has been described for species of Syphacia Seurat, 1916 (see Hugot, Reference Hugot1988; but see Okamoto et al. Reference Okamoto, Urushima, Iwasa and Hasegawa2007). The status of Aspiculuris from Apodemus should be reviewed further, with material collected from throughout the geographical and taxonomic range of wood mice in case these should prove to be distinct from A. tetraptera. The most likely explanation for the occurrence of A. tetraptera in Mus is a host switch into house mice at the time when the latter first became abundant commensals with the rise in agriculture some 10 000 years ago, an explanation similarly advanced to account for the infection of mice by Heligmosomoides bakeri (Durette-Desset et al. Reference Durette-Desset, Kinsella and Forrester1972; Nieberding et al. Reference Nieberding, Morand, Libois and Michaux2006) (see Behnke and Harris, Reference Behnke and Harris2010). The origin of the worms involved in this host switch is unknown, but several Aspiculuris species with arrowhead cervical alae are known from other cricetid rodents in similar habitats to those in which the host switch probably took place (Rishikov, Reference Rishikov1979).
The current work lends support to the experimental observations that A. tetraptera, from laboratory mice cannot mature in bank voles in experimental cross-infections (Behnke, Reference Behnke1974). We also predict that A. tianjinensis and A. tetraptera cannot interbreed, and they cannot be regarded as sibling or sister species. At first sight, the speciation of Aspiculuris in commensal murids is spectacular, if the timings suggested in this paper are correct; indeed, the divergence of a taxon such as A. huascensis must be very rapid since house mice did not reach Mexico until the relatively recent historical past, and there are no described examples of Aspiculuris with arrowhead alae from native Central or South American rodents. Indications of such a divergence may be simply the result of lack of sampling effort in the region. Alternatively, if genuine, divergence on the time scale proposed here may owe much to the unusual breeding biology of oxyuroid nematodes. Oxyuroids have a haplodiploid reproductive mechanism (Adamson, Reference Adamson1989, Reference Adamson1994), with haploid males developing from unfertilised eggs. This, coupled with auto-reinfection as a result of grooming could lead to a high frequency of back-crossing and fixation of minor morphological variants such as appear to be represented by A. huascensis and A. lahoricus. At the same time, this breeding biology makes Aspiculuris and Syphacia highly resistant to environmental change, and these two parasites are the most likely to be translocated successfully with their host mice under the most rigorous conditions (for example, in Iceland or on some sub-Antarctic islands; Skirnisson et al. Reference Skirnisson, Eydal, Gunnarsson and Hersteinsson1993; Pisanu et al. Reference Pisanu, Chapius and Périn2003). Clearly, aspiculurid nematodes could prove highly useful markers for the rapid evolution of both arvicoline and murine rodent evolution, when molecular markers from a sufficiently wide range of populations have been taken into consideration.
ACKNOWLEDGEMENTS
We thank Dr Mike Kinsella for his guidance and inspiration, and for provision of specimens from the USA, and Prof. Peter Vogel (who sadly died in January 2015 before the completion of our ms) and Charlotte Vogel, Switzerland for samples from C. nivalis from which we recovered A. dinniki. We are also grateful for the efforts of Dr. Keisuke Nakata, Japan for samples from M. rufocanus which unfortunately did not yield Aspiculuris and to Russian ecologists and parasitologists who also endeavoured to find for us A. tianjinensis from M. rufocanus. We thank the following colleagues for specimens sent to us at various times during our project: Sarah Perkins, Celia Holland and Karen Loxton for specimens from Eire, Claudia Bevilaqua for specimens from laboratory mice from Brazil, Grzegorz Zaleśny for specimens from Wrocław and Joelle Gouy de Bellocq for help in collecting samples from Germany. We also thank John Lewis and Celia Holland for their comments on earlier drafts of this paper. Technical assistance and expertise with scanning electron microscopy was provided by Denise McLean, in the Advanced Microscopy Unit (School of Life Sciences, University of Nottingham).
FINANCIAL SUPPORT
We are grateful to the Universities of Nottingham and Warsaw for financial support for travel and consumables. Our work was supported additionally by travel grants from the Royal Society (JMB), the British Ecological Society (JMB), the Grabowski Fund (JMB), the Polish State Committee for Scientific Research (KBN, AB), and by the KBN and British Council's Young Scientist Programme (AB). VKJ was supported in part by NIH (grant number R21-A11076631).