Published online by Cambridge University Press: 19 April 2005
Tick fever or cattle fever (babesiosis) is economically the most important arthropod-borne disease of cattle worldwide with vast areas of Australia, Africa, South and Central America and the United States continuously under threat. Tick fever was the first disease for which transmission by an arthropod to a mammal was implicated at the turn of the twentieth century and is the first disease to be eradicated from a continent (North America). This review describes the biology of Babesia spp. in the host and the tick, the scale of the problem to the cattle industry, the various components of control programmes, epidemiology, pathogenesis, immunity, vaccination and future research. The emphasis is on Babesia bovis and Babesia bigemina.
Babesiosis is caused by intraerythrocytic protozoan parasites of the genus Babesia that infect a wide range of domestic and wild animals and occasionally man. The disease is tick transmitted and distributed worldwide. The major economic impact of babesiosis is on the cattle industry and the two most important species in cattle, Babesia bovis and B. bigemina, will form the focus of this review.
Babes (1888) investigated disease outbreaks causing haemoglobinuria in cattle in Romania and was the first to describe piroplasms in the blood of cattle. He believed it to be a bacterium and named it Haematococcus bovis although the name was later changed to Babesia bovis (Angus, 1996). Shortly afterwards investigations by Smith and Kilborne (1893) in the United States of America demonstrated the causative organism of ‘Texas Fever’ (babesiosis) which they called Pyrosoma bigeminum (=Babesia bigemina). They were the first to demonstrate transmission of a disease organism from an arthropod to a mammalian host when they showed the organism was transmitted by Boophilus annulatus to cattle (Smith & Kilborne, 1893). After the publication of this work, babesiosis was discovered in various parts of the world. Dr Sidney Hunt confirmed that bovine ‘redwater’ in Australia was identical in aetiology to Texas fever (Angus, 1996). In Argentina, Lignières (1903) described two forms of ‘Tristeza’ (babesiosis) ‘forme A’ and ‘forme C’. These were later known as Babesia bigemina and B. argentina (=B. bovis) and from descriptions of parasites, from cases of babesiosis described in Australia and USA in the late 1890s and early 1900s it is now believed that both B. bigemina and B. bovis were present in these also (Angus, 1996).
The confusing nomenclature of the bovine Babesia was thoroughly reviewed by Hoyte in the late 1960s and the four species of bovine Babesia which are now recognised are Babesia bovis (=B. argentina; B. berbera; B. colchica), B. bigemina, B. divergens (=B. caucasica; B. occidentalis; B. karelica) and B. major (Angus, 1996).
The genus Babesia belongs to the phylum Apicomplexa, class Sporozoasida, order Eucoccidiorida, suborder Piroplasmorina and family Babesiidae (Levine, 1971, 1985; Allsopp et al. 1994). Criado-Fornelio et al. (2003) used the 18s rRNA gene for phylogenetic analysis and divided piroplasmids into five proposed clades: (1) a group composed mainly by Babesia species from ungulates: B. caballi, B. bigemina, B. ovis, B. bovis and Babesia sp. from cattle (proposed name for the group, without taxonomic value: Ungulibabesids); (2) a second group of Babesia species including B. canis and B. gibsoni from canids together with B. divergens and B. odocoilei (proposed name: Babesids); (3) the B. microti group with B. rodhaini, B. felis, B. leo, B. microti and Theileria annae (proposed name: Archaeopiroplasmids); (4) Western USA Theilerid-like group (proposed name: Prototheilerids); (5) Theileria group, containing all Theileria species from Bovinae (proposed name: Theilerids).
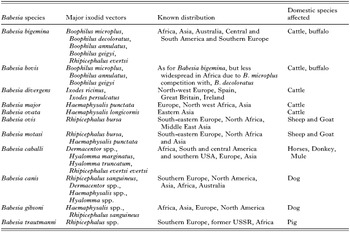
The major Babesia spp. known to infect domestic animals, and their proven vectors, are listed in Table 1. B. bovis and B. bigemina are present in many countries between 40°N and 32°S (McCosker, 1981). The main vectors of Babesia are Boophilus ticks. The reader should note that recently Boophilus species have been reclassified as Rhipicephalus species. However, it is desirable that the name Boophilus remains available since there is a wealth of literature on Boophilus species and a large part of the tick, babesia and livestock community use these names on a regular basis. Throughout this review we have retained the Boophilus name. A detailed account of Rhipicephalus and Boophilus being synonymised is given in the chapter by Barker & Murrell in this Supplement. Boophilus microplus is the most important and widespread vector, but in southern Africa, a closely related tick, Boophilus decoloratus, interferes with its spread in drier and colder areas. Interbreeding between the two species produces sterile progeny which creates a zone through which B. microplus has difficulty passing (Sutherst, 1987). Generally both parasites have the same distribution, but in Africa B. bigemina is more widespread than B. bovis because of the ability of B. decoloratus and Rhipicephalus evertsi to act as vectors (Friedhoff, 1988). Boophilus annulatus is the principal vector of B. bovis and B. bigemina in Northern Africa (Ndi et al. 1991; Sahibi et al. 1998; Bouattour, Darghouth & Daoud, 1999), the Middle East (Pipano, 1997), Turkey (Sayin et al. 1996), some areas of southern Europe (Caeiro, 1999) and is mentioned as the vector in southern areas of the former USSR.
Babesia divergens is transmitted almost exclusively by Ixodes ricinus in northern Europe (Friedhoff, 1988) and this probably explains its limited distribution. As this review concentrates on B. bovis and B. bigemina, a recent review by Zintl et al. (2003) should be referred to for a comprehensive summary of B. divergens biology, including its life cycle, host specificity and morphology and the current state of knowledge about both human and bovine infections.
Most of the 1·2 billion cattle in the world are exposed to babesiosis but this figure is not a true reflection of the number at risk to disease (McCosker, 1981). Breeds of cattle that are indigenous to Babesia-endemic regions often have a certain degree of natural resistance to these diseases and the consequences of infection are not as serious as when exotic Bos taurus breeds are involved. In addition, in tropical areas with a high vector population, natural exposure usually occurs at an early age and cattle are therefore immune to subsequent challenge as adults (see endemic stability later).
Costs due to babesiosis are incurred not only from mortality, ill-thrift, abortions, loss of milk/meat production and draft power and from control measures (such as acaricide treatments, purchase of vaccines and therapeutics), but also through its impact on international cattle trade. McLeod & Kristjanson (1999) developed a spreadsheet model (Tick Cost) to assess the overall impact of ticks and tick-borne diseases. They calculated that losses and control of babesiosis and anaplasmosis alone cost the Australian cattle industry US$16·9m per annum with ‘tick worry’ adding US$6·4m to annual losses. The model further estimated that losses and control of babesiosis and anaplasmosis in Kenya, Zimbabwe, Tanzania, South Africa, China, India, Indonesia and Philippines cost 5·1, 5·4, 6·8, 21·6, 19·4, 57·2, 3·1 and 0·6 million US dollars annually, respectively.
The development of Babesia spp. in ticks was reviewed by Friedhoff (1988). Despite many detailed studies, our understanding of the life cycles of Babesia spp. is still incomplete. In electron microscope studies, Rudzinska et al. (1983) showed sexual reproduction in B. microti. More recently, DNA measurements showed that sexual reproduction does occur for B. divergens (Mackenstedt et al. 1990), B. bigemina and B. canis (Mackenstedt et al. 1995) and is therefore likely in the other species. Mackenstedt et al. (1995) also revealed important differences in the life cycle of the Babesia species they studied, indicating they are not characterized by a life cycle that is specific for the genus. Therefore, the emphasis here will be on B. bovis and B. bigemina with particular reference to the latter as more information is available on this species. The development of B. bovis and B. bigemina follow similar patterns in adult Boophilus spp. (Potgieter & Els, 1976; Potgieter, 1977; Mehlhorn & Shein, 1984; Friedhoff, 1988). The development life cycle (Fig. 1) as currently understood is outlined below. To prevent confusion about the terminology used in other literature, alternatives are given in parentheses.
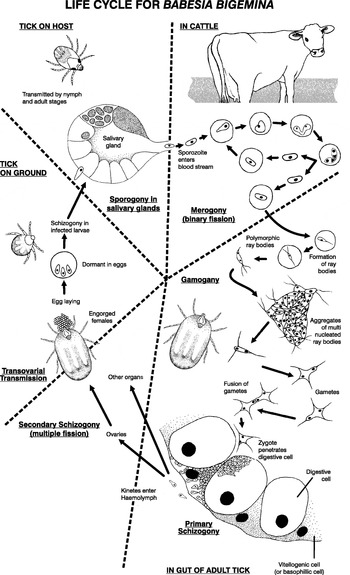
Fig. 1. The development life cycle of Babesia bigemina in cattle and the ixodid tick vector Boophilus microplus as currently understood (adapted from Mehlhorn & Shein, 1984; Mackenstedt et al. 1995; Gough et al. 1998).
Babesia spp. do not parasitise any vertebrate host cell other than erythrocytes (Friedhoff, 1988). Each sporozoite (merozoite) penetrates the cell membrane of an erythrocyte with the aid of a specialised apical complex (Potgieter & Els, 1977a; Potgieter & Els, 1979). Once inside, it transforms into a trophozoite from which two merozoites develop by a process of merogony (binary fission) (Potgieter & Els, 1977a, 1979; Friedhoff, 1988). In B. bigemina, Mackenstedt et al. (1995) identified an ovoid type of merozoite they called a gamont precursor which unlike other piroplasms studied had diploid DNA levels. These gamont precursors do not develop further until they are taken up by the tick.
Changes experienced in the passage from host blood to the midgut of the tick vector stimulate the development of two populations of ray bodies (strahlenkörper) from the gamonts (gametocytes) (Gough, Jorgensen & Kemp, 1998). The ray bodies undergo further multiplication within the erythrocyte that continues after they have emerged. Large aggregations of multinucleated ray bodies form, but once division is complete, single-nucleated ray bodies that are now haploid and assumed to be gametes (Mackenstedt et al. 1995) emerge from the aggregates and then fuse together in pairs (syngamy) (Gough et al. 1998) to form a spherical cell (zygote) (Friedhoff, 1988). The zygote selectively infects the digestive cell of the tick gut where they probably multiply and then the basophilic cells (vitellogenin synthesising cells) where further multiplication occurs with development to kinetes (vermicules) that escape into the tick haemolymph (Agbede, Kemp & Hoyte, 1986). B. bigemina undergoes a one-step meiosis to form a haploid zygote at some stage in development in the gut (Mackenstedt et al. 1995). In the gut cells, schizogony (multiple fission, sporogony) occurs with the formation of polyploid kinetes (vermicules, sporokinetes, large merozoites) (Mackenstedt et al. 1995). These motile club-shaped kinetes then escape into the haemolymph and infect a variety of cell types and tissues, including the oocytes where successive cycles of secondary schizogony take place. Thus, transovarial transmission occurs with further development taking place in the larval stage. This is an important life cycle adaptation as the Boophilus vectors are one-host ticks (Fig. 2). Kinetes enter the salivary glands and are transformed into multinucleated stages (sporogony) and these then break up to form sporozoites (small haploid merozoites) (Mackenstedt et al. 1995). In all species, sporozoite development usually only begins when the infected tick attaches to the vertebrate host. In B. bigemina, some development takes place in the feeding larvae, but infective sporozoites take about 9 days to appear and therefore only occur in the nymphal and adult stages of the tick (Hoyte, 1961; Potgieter & Els, 1977b). Transmission can occur throughout the rest of the nymphal stage and by adult females and males (Callow & Hoyte, 1961; Riek, 1964; Dalgliesh, Stewart & Callow, 1978). In the case of B. bovis, the formation of infective sporozoites usually occurs within 2 to 3 days of larval tick attachment (Riek, 1966).

Fig. 2. Dorsal and ventral view of adult female Boophilus microplus ticks and their egg mass. Babesia parasites develop in oocytes of Boophilus microplus resulting in transovarial transmission an essential component in babesiosis epidemiology as Boophilus spp. are one-host ticks.
Despite its classification as a one-host tick, Boophilus microplus, particularly males, can transfer amongst cattle in close proximity and this can lead to a much-shortened prepatent period (6–12 days) for B. bigemina (Callow & Hoyte, 1961; Callow, 1979), but it is usually 12–18 days after tick attachment (Callow, 1984). As B. bovis does not persist in an infective form in the ticks beyond the larval stage (Mahoney & Mirre, 1979), the prepatent period for B. bovis is generally 6–12 days and peak parasitaemias are reached about 3–5 days after that (Callow, 1984). However, unlike B. bigemina, heat stimulation of larval ticks prior to attachment (37 °C for 3 days and 30 °C for 8 days) enables transmission of B. bovis immediately the larvae attach and this can lead to shortened prepatent periods particularly in summer (Dalgliesh & Stewart, 1982).
Endemic stability is defined as the state where the relationship between host, agent, vector and environment is such that clinical disease occurs rarely or not at all (Perry, 1996). Passively acquired resistance from colostrum lasts about 2 months but is followed by innate immunity (as defined under immunity) to babesiosis from 3 to 9 months of age (Mahoney & Ross, 1972; Mahoney, 1974). Therefore calves exposed to babesiosis during the first 6 to 9 months rarely show clinical symptoms and develop a solid long-lasting immunity (Dalgliesh, 1993). Under conditions of endemic instability, some animals will fail to become infected for a considerable period after birth and may therefore develop severe, life threatening disease if they are exposed later in life, depending on their breed (Callow, 1984).
Mahoney (1974) estimated that if at least 75% of calves were exposed to B. bovis infection by 6 to 9 months of age the disease incidence would be very low and a state of natural endemic stability would exist. One infected tick is sufficient to transmit B. bovis, but tick infection rates can be low and the rate of transmission to cattle is therefore usually slow. In an Australian field study, only 0·04% of larval ticks were infected with B. bovis if Bos taurus cattle were involved and even less in paddocks stocking Bos indicus cattle (Mahoney & Mirre, 1971; Mahoney et al. 1981). Tick infection rates with B. bigemina are usually higher (0·23% in the Mahoney & Mirre (1971) study). Therefore, transmission rates in this species are higher than B. bovis and endemic stability is more likely to develop to B. bigemina than to B. bovis in regions where both are present.
A simple mathematical model has been used to predict the level of stability in a herd (Mahoney & Ross, 1972; Mahoney, 1974). It utilises the rate at which infection occurs in calves (determined serologically at a specific age), thereby providing a means of estimating the status of B. bovis infections (Mahoney & Ross, 1972; de Vos & Potgieter, 1983). It has proved useful in predicting stability levels in European breeds but less so in other breeds. More recently, a disease prediction spreadsheet model was developed to more accurately calculate incidence risk from age-specific seroprevalence (Ramsay, 1997). The model predicts the proportion of animals in each age and sex class that would be affected by different severities of the disease, and the information is then converted into a herd model to estimate the number of animals in each severity class. This model has been used to predict the potential impact of Babesia spp. on some large properties in Australia and the cost/benefit of control measures (unpublished observations).
Bos indicus breeds almost invariably experience milder clinical symptoms to primary B. bovis infections than Bos taurus breeds (Lohr, 1973; Callow, 1984; Bock et al. 1997a; Bock, Kingston & de Vos, 1999a). This phenomenon is thought to be a result of the evolutionary relationship between Bos indicus cattle, Boophilus spp. and Babesia (Dalgliesh, 1993).
Mahoney et al. (1981) compared transmission rates of B. bovis in Bos taurus and in three-eighths to half of Bos indicus crossbred cattle in Australia and concluded that, in an environment unfavourable for tick survival, stocking with Bos indicus or Bos indicus crossbred cattle will, over several seasons, almost lead to the disappearance of the ticks. However, Bock et al. (1999a) showed that if naïve Bos indicus cattle are moved to a paddock with a high resident Boophilus microplus infestation, Babesia transmission rates can initially be very high.
In Australia, B. bigemina is usually of lower pathogenicity than B. bovis (Callow, 1979) and there is some ambiguity in the literature concerning the relative susceptibility of Bos indicus and Bos taurus breeds to this parasite, but most studies conclude that Bos indicus are much more resistant (Lohr, 1973; Parker et al. 1985; Bock et al. 1997a, 1999b).
The immune response of cattle to infection with B. bovis or B. bigemina involves both innate and acquired immune mechanisms.
Innate immunity is non-specific and includes factors such as host–parasite specificity, genetic factors, age of the host and the response of host cells (such as the mononuclear phagocyte system and polymorphonuclear leukocytes).
Most Babesia spp. are highly host specific and often splenectomy is needed to establish an infection in an unnatural host (Mahoney, 1972). Cattle with long standing infections of Babesia can relapse to the infections if splenectomised (Callow, 1977). Susceptible cattle that have been splenectomised develop much higher parasitaemias to primary Babesia infections than do intact cohorts. These observations imply that the spleen plays a major role in the immune response to Babesia spp. However the use of splenectomy to develop experimental laboratory models for infection has not always been successful (Frerichs, Johnson & Holbrook, 1969). Different breeds of cattle are known to have different susceptibilities to infection with B. bovis and B. bigemina (see Epidemiology – Breed Resistance). The genetically determined factors that might control the susceptibility of different cattle breeds have not been identified.
There is an age-related immunity to primary infection of cattle with B. bovis and B. bigemina (Riek, 1963). Young calves exhibit a strong innate immunity compared to adult cattle (Trueman & Blight, 1978; Goff et al. 2001). Initially this innate immunity was thought to be due to the passive transfer of protective antibodies in the colostrum of immune dams (Mahoney, 1967c). However, calves from non-immune mothers exhibit resistance to B. bovis and B. bigemina (Riek, 1963). Recently, it has been shown that the innate immune response of young calves to infection with B. bovis involves the early induction of interleukin (IL)-12 and interferon (IFN)-γ and the presence of inducible nitric oxide synthase (iNOS) mRNA expression in the spleen (Goff et al. 2001). In contrast, IL-12 and IFN-γ mRNA were induced later in the infection and iNOS was not induced in adult cattle.
Activated monocytes, macrophages and neutrophils provide the first line of defence during an infection with Babesia spp., employing antimicrobial agents, including reactive nitrogen intermediates (RNI), reactive oxygen intermediates (ROI) and phagocytosis. In addition, these cells secrete cytokines that regulate the inflammatory response. Macrophages stimulated by B. bovis produce IL-1β, IL-12, tumour necrosis factor alpha (TNF-α) and nitric oxide (NO) (Shoda et al. 2000).
Increased phagocytic activity of macrophages has been proposed as a mechanism for the elimination of B. bovis in cattle (Mahoney, 1972; Jacobson et al. 1993) with specific antibodies playing an important role as opsonins (Jacobson et al. 1993). During a primary B. bovis infection in cattle, peripheral blood monocytes display a suppressed phagocytic ability and neutrophils exhibit an increased phagocytic ability that coincides with peak parasitaemia (Court, Jackson & Lee, 2001).
Nitric oxide is an RNI produced by iNOS in macrophages, monocytes, neutrophils and endothelial cells during acute infections. In vitro experiments have suggested that NO can reduce the viability of B. bovis (Johnson et al. 1996) and that B. bovis merozoites (intact and fractionated) can induce the production of NO by monocytes/macrophages in the presence of IFN-γ and TNF-α (Stich et al. 1998; Goff et al. 2002). Indirect in vivo evidence suggests that NO may have a role in the pathology caused by B. bovis (Gale et al. 1998). Gale et al. (1998) administered aminoguanidine, an inhibitor of iNOS, to cattle during a virulent B. bovis infection and caused a reduction in parasitaemia and amelioration of anaemia and pyrexia.
Phagocytosis is closely linked with an oxidative burst and the release of ROI (superoxide anion, hydrogen peroxide and hydroxyl radicals) within the phagocyte (Auger & Ross, 1992). The oxidative burst reaction can be stimulated by cross-linking of receptors on the phagocyte surface, such as Fc receptors for IgG, and ROI are released within the phagosome and into the extracellular environment. In vitro experiments have shown that ROI (superoxide anion and hydroxyl radicals but not hydrogen peroxide) produced by activated macrophages are babesiacidal (Johnson et al. 1996). Furthermore, ex vivo-derived monocytes exhibit increased, and neutrophils display decreased, oxidative activity during a primary B. bovis infection (Court et al. 2001).
Hyperimmune serum (from cattle infected with B. bovis many times) or a mixture of IgG1 and IgG2 prepared from hyperimmune serum of cattle can be used to immunise naïve calves passively against B. bovis infection (Mahoney et al. 1979a). This protection is strain specific. Splenectomised calves given hyperimmune serum and challenged with B. bovis recovered as effectively as intact calves. Serum collected after a primary infection was less effective than hyperimmune serum for transferring immunity to naïve calves and it was thought that this could be related to the antibody isotype present in the serum. Following B. bovis infection, antibodies directed against protective and non-protective parasite antigens and host antigens are produced (Goodger, Wright & Waltisbuhl, 1985). Antibodies probably act as opsonins for increased phagocytosis rather than having a direct effect on the parasite's viability (Mahoney et al. 1979a) and antibodies are thought to be important effectors during a secondary infection (Mahoney, 1986). Immune complexes of babesial antigen, bovine immunoglobulin and C3 form following B. bovis infection (Goodger, Wright & Mahoney, 1981). The major immunoglobulin in the complexes was IgM but some IgG1 and IgG2 in lower concentrations was present. Both bovine IgG1 and IgG2 are capable of fixing complement, and bovine IgG2 is the superior opsonising antibody subclass (McGuire, Musoke & Kurtti, 1979). Following B. bovis infection, IgG1 and IgM, but not IgG2, complement-fixing antibodies have been detected (Goff et al. 1982). Antibody-dependent cell-mediated cytotoxicity may be involved in the resolution of B. bovis infection in cattle (Goff, Wagner & Craig, 1984).
Adoptive transfer studies have not yet been performed with cattle to investigate the definitive role of T cells in the immune response to B. bovis and B. bigemina. However, in vitro experiments performed with T cell lines and clones from immune cattle have demonstrated a role for T cells. Peripheral blood mononuclear leukocytes and helper T cell clones from immune cattle proliferate in response to B. bovis antigens (Brown et al. 1991; Brown & Logan, 1992). The cytokine response by these CD4+ helper T cell clones was either a Th1 (IL-2, IFN-γ and TNF-α) or Th0 (IL-2, IL-4, IFN-γ and TNF-α) response with no Th2 clones detected (Brown et al. 1993). Abundant helper T cell clones were isolated from cattle immunised with rhoptry-associated protein-1 (RAP-1) and in vitro these clones produced a Th1 cytokine profile with plentiful IFN-γ (Rodriguez et al. 1996). These Th1 clones promoted IgG2 production by autologous B cells in the presence of antigen. Supernatants from helper T cell lines contain IFN-γ and TNF-α that induce macrophages to produce NO (Stich et al. 1998).
The model of acquired immunity to B. bovis and B. bigemina focuses upon the pivotal role of CD4+ helper T cells in the immune response (Brown et al. 2001). These helper T cells produce cytokines, of which IFN-γ seems to be of major importance, which activate phagocytic cells and enhance antibody production by B cells.
Protective cross species immunity against infection cannot be induced with B. bovis and B. bigemina (Smith et al. 1980). This is in agreement with Legg (1935) who found that B. bovis infection does not protect against B. bigemina challenge, but B. bigemina immunity does give some protection against B. bovis, the latter being further confirmed by Wright et al. (1987). The observation was exploited in Australia for protection against B. bovis for a short period in the 1930s, but was found to be unreliable (Callow, 1984).
Using a complement fixation test (CFT), (Mahoney, 1964) detected antibodies to B. bovis and B. bigemina for 7 and 4 months, respectively, following infection. Homologous antibodies were detected by indirect fluorescent antibody test (IFAT) for a much longer period of time following B. bovis infection than infection with B. bigemina (Smith et al. 1980). Smith et al. (1980) found that heterologous antibody cross-reactivity as measured by the IFAT following B. bovis or B. bigemina infection was restricted to the period during, and shortly after, recovery. Wright et al. (1987) also found that B. bovis antisera reacts with B. bovis and B. bigemina parasites in an IFAT but that B. bigemina antisera only reacts with B. bigemina parasites and not with B. bovis parasites. However, in the latter study it was unclear at what time after infection the sera were collected. In the IFAT, parasites and not the infected erythrocyte are stained (Wright et al. 1987).
In contrast to the IFAT, immunoblotting experiments demonstrated two-way cross-reactivity between B. bovis and B. bigemina (Wright et al. 1987). Antisera to B. bigemina and to B. bovis reacted strongly with homologous and heterologous antigens demonstrating that many antigens are common to both parasites (Wright et al. 1987). B. bigemina antisera recognised the same proteins in B. bigemina and B. bovis antigen extracts (Wright et al. 1987). However, B. bovis antisera recognised more proteins in the B. bovis than in the B. bigemina antigen extracts. Two way serological cross-reactivity between B. bovis and B. bigemina was demonstrated by (Mahoney, 1967a) using a CFT.
With Bos taurus cattle, Mahoney, Wright & Mirre (1973) found tick-transmitted B. bovis infection lasts for at least four years but with B. bigemina it is usually less than 6 months (Mahoney et al. 1973). Immunity to both parasites however, remained for at least four years. Tjornehoj et al. (1996) placed a great deal of importance on the correlation between the persistence of antibodies to B. bovis and the duration of immunity at the herd level. However, there is ample evidence in the literature suggesting the presence of antibodies is not necessarily an indication of immunity nor is the absence of detectable antibodies necessarily an indication of a lack of immunity. Mahoney et al. (1979a) clearly showed that while antibodies alone provide good homologous protection, the same did not apply in cross-protection between strains. They concluded that the protective mechanism of cross-immunity relied on priming of the host's immune system by the protective antigen(s) of the initial strain so that a secondary response against the heterologous strain occurred after challenge.
Johnston, Leatch & Jones (1978) showed that immunity of Bos indicus cross cattle to B. bovis lasted at least 3 years despite the fact that most of the trial cattle eliminated the infection during that time. Unfortunately, Johnston and co-workers did not monitor antibody levels in the cattle. In a different study Callow et al. (1974a,b) showed that indirect fluorescent antibody tests (IFAT) on sera from vaccinated cattle sterilised with Imidocarb were generally negative within six months of treatment, but this decrease in IFAT titre was not associated with a loss of immunity. In a long-term study of immunity in Bos taurus cattle to B. bovis, Mahoney, Wright & Goodger (1979b) found that 30% to 50% of the vaccinated or infected trial cattle became sero-negative in an indirect haemaglutination test during the trial period. Nevertheless, these cattle were still immune 4 years after initial vaccination or exposure. Therefore, it appears that a persistent, detectable antibody titre is not a prerequisite for immunity. However, it is a very effective indicator of recent infection either naturally or by vaccination.
Isolates and selected strains of B. bovis and B. bigemina differ antigenically (Dalgliesh, 1993) and cross-immunity experiments have shown that recovered cattle are more resistant to challenge with the same (homologous) isolates than with different (heterologous) ones (de Vos, Dalgliesh & Callow, 1987; Dalgliesh, 1993; Shkap et al. 1994).
Antigenic variation is also known to occur during B. bovis and B. bigemina infections with recovered cattle retaining a latent infection varying from six months to several years with detectable recrudescence of parasitaemia occurring at irregular intervals during the latent phase of the infection (Mahoney & Goodger, 1969). Antigenic variation within the vertebrate host is thought to allow variant Babesia parasite populations to continue to adhere to endothelial cells thus avoiding splenic clearance and persist in the face of apparent immunity (Allred, 1995, 2001). Many species of Babesia establish infections of long duration in immune hosts. Allred (2003) suggested that antigenic variation, cytoadhesion/sequestration, host–protein binding, and induction of immunosuppression probably facilitate persistence in the individual immune host. He also suggested that monoallelic expression of different members of a multigene family might facilitate multiple infections of immune hosts, and population dispersal in endemic areas (Allred, 2003).
Cytokines and other pharmacologically active agents have an important function in the immune response to Babesia. The outcome is related to the timing and quantity produced, but their overproduction contributes to disease progress causing vasodilation, hypotension, increased capillary permeability, oedema, vascular collapse, coagulation disorders, endothelial damage and circulatory stasis (Wright et al. 1989; Ahmed, 2002). Although stasis is induced in the microcirculation by aggregation of infected erythrocytes in capillary beds, probably the most deleterious pathophysiological lesions occur in the brain and lung. This can result in cerebral babesiosis and a respiratory distress syndrome associated with infiltration of neutrophils, vascular permeability and oedema (Wright & Goodger, 1988; Brown & Palmer, 1999). Progressive haemolytic anaemia develops during the course of B. bovis infections. While this is not a major factor during the acute phase of the disease, it will contribute to the disease process in more protracted cases.
The acute disease generally runs a course of 3 to 7 days and fever (>40 °C) is usually present for several days before other signs become obvious. This is followed by inappetence, depression, increased respiratory rate, weakness and a reluctance to move. Haemoglobinuria is often present; hence, the disease is known as redwater in some countries. Anaemia and jaundice develop especially in more protracted cases. Muscle wasting, tremors and recumbency develop in advanced cases followed terminally by coma (de Vos & Potgieter, 1994). The fever during infections may cause pregnant cattle to abort (Callow, 1984) and bulls to show reduced fertility lasting six to eight weeks (Singleton, 1974). Cerebral babesiosis is manifested by a variety of signs of central nervous system involvement and the outcome is almost invariably fatal (de Vos & Potgieter, 1994).
Lesions include an enlarged soft and pulpy spleen, a swollen liver, a gall bladder distended with thick granular bile, congested dark-coloured kidneys and generalised anaemia and jaundice. Other organs may show congestion or petechial haemorrhages and occasionally there will be pulmonary oedema. The grey matter surface of the brain can appear pink. Acute cases will show haemoglobinuria, but this may be absent in subacute or chronic cases. Clinical pathology centres on a haemolytic anaemia, which is characteristically macrocytic and hypochromic. Haematological, biochemical and histopathological changes are described by deVos & Potgieter (1994).
Non-fatal cases may take several weeks to regain condition but recovery is usually complete. In subacute infections, clinical signs are less pronounced and sometimes difficult to detect. Calves that become infected before they attain nine months of age often develop subclinical infections only (Callow, 1984). Recovered cases remain symptomless carriers for a number of years with the duration of infection being breed dependent (Mahoney, 1969; Johnston et al. 1978).
Pathogenesis is almost entirely related to rapid, sometimes massive, intravascular haemolysis (Callow, 1984). Coagulation disorders, cytoadherence and the hypotensive state seen in acute B. bovis infections are not features of B. bigemina infections (Wright & Goodger, 1988; Dalgliesh et al. 1995). With most strains of B. bigemina, the pathogenic effects relate more directly to erythrocyte destruction. Haemoglobinuria is present earlier and more consistently than in B. bovis infections and fever is less of a feature. Acutely affected cattle are usually not as severely affected as those with B. bovis infections. There is no cerebral involvement and recovery in non-fatal cases is usually rapid and complete. However, in some cases the disease can develop very rapidly with sudden and severe anaemia, jaundice and death, which may occur with little warning (Callow, Rogers & de Vos, 1993). Animals that recover from B. bigemina remain infective for ticks for 4 to 7 weeks and carriers for only a few months (Mahoney, 1969; Johnston et al. 1978).
Babesia bovis is classically known as a ‘small’ Babesia measuring up to 2 μm in diameter, while B. bigemina is larger and can extend to the full diameter of an erythrocyte (Potgieter, 1977). The two species both show considerable morphological variation, making it difficult to identify one from the other on morphological grounds alone (Callow, 1984; de Vos & Potgieter, 1994).
Diagnoses of babesiosis are made by examination of blood and/or organ smears stained with Giemsa (Callow et al. 1993; Böse et al. 1995). For the best results, blood films should be prepared from capillary blood collected, for instance, after pricking the tip of the tail or margin of an ear. The temptation to use blood of the general circulation should be resisted as these specimens may contain up to 20 times fewer B. bovis than capillary blood (Callow et al. 1993). In B. bigemina infections, parasitised cells are evenly distributed throughout the blood circulation. Thick blood films are 10 times more sensitive and are therefore very useful for the detection of low level B. bovis infections (Böse et al. 1995). These films differ from thin ones in that the blood is not spread over a large area and is not fixed before staining, thus allowing lysis of the red blood cells and concentration of the parasites (Böse et al. 1995). Diagnoses are sometimes not confirmed at the laboratory because poorly prepared or unsuitable specimens are submitted.
Serological tests are reviewed by Böse et al. (1995) and de Vos, Jorgensen & Molloy (2000). These tests are not of value in the clinical stage of the disease but are used for the purposes of research, epidemiological studies, export certification or where vaccine breakdowns are suspected.
Labelling of parasites with fluorescein or horse-radish peroxidase conjugated anti-B. bovis and anti-B. bigemina IgG is a sensitive, specific laboratory tool to identify parasites in blood and organ preparations, provided adequate numbers of parasites are present (Johnston, Trueman & Pearson, 1977). Böse et al. (1995) also reviewed the relative sensitivity of DNA probes, PCR assays, in vitro cultures and subinoculation into susceptible, usually splenectomised, calves to provide a diagnosis. Despite the added sensitivity of these methods, stained blood and/or organ smears offer considerable advantages in cost and speed for clinical cases, if experienced microscopists are available.
Reports in the literature refer to a number of effective babesiacides (de Vos & Potgieter, 1994) but few are now available commercially. Currently, diminazene aceturate and imidocarb dipropionate (imidocarb) are the most widely used. Diminazene works rapidly against B. bovis and B. bigemina at a dose of 3·5 mg/kg intramuscularly. It is well tolerated and will protect cattle from the two diseases for 2 and 4 weeks, respectively (de Vos, 1979). Imidocarb is used subcutaneously at a dose of 1·2 mg/kg for treatment while 3 mg/kg provides protection from B. bovis for 4 weeks and B. bigemina for at least 2 months (Taylor & McHardy, 1979). At the high dose, imidocarb also eliminates B. bovis and B. bigemina from carrier animals and at either dose can interfere with the development of immunity following live vaccination (de Vos, Dalgliesh & McGregor, 1986). Treatment with long-acting oxytetracycline following vaccination significantly reduces parasitaemia and red blood cell destruction without inhibiting the development of immunity (Pipano et al. 1987; Jorgensen et al. 1993). Oxytetracyclines are not usually able to control virulent field infections.
Cattle develop a durable, long-lasting immunity after a single infection with B. divergens, B. bovis or B. bigemina. This feature has been exploited in some countries to immunise cattle against babesiosis (Callow, 1984; Gray et al. 1989; de Vos & Jorgensen, 1992). Methods used to prepare live vaccines against bovine babesiosis have been described or reviewed in some detail (de Vos & Jorgensen, 1992; Pipano, 1995; Callow, Dalgliesh & de Vos, 1997). Most early attempts involved the use of blood from infected carriers (Callow, 1977, 1984; de Vos & Jorgensen, 1992) but, during the past 30 years, more sophisticated techniques have been developed to produce standardised live vaccines (Callow et al. 1997). The inherent disadvantages of these vaccines are well known, including the risk of reactions or contamination with pathogenic organisms, sensitisation against blood groups and the need for cold chain transportation (Wright & Riddles, 1989). Bock & de Vos (2001) reviewed the data available on the efficacy, degree and duration of immunity provided by live vaccines against B. bovis and B. bigemina infections in Australia. They found that, despite the disadvantages, live vaccines provided greater than 95% protection for the life of the animals.
The relative importance of different Babesia spp. in different countries dictates the composition of the vaccine. In parts of Africa B. bigemina predominantly causes disease whereas, in Australia, B. bovis causes approximately 20 times the economic loss caused by B. bigemina. As a result, protection against B. bovis has been the main aim in Australia for many years although demand for vaccine containing B. bigemina has rapidly increased and in 2002 over 65% of vaccine sold contained both species.
Most of the available live vaccines are produced in government-supported production facilities, notably in Australia, Argentina, South Africa, Israel and Uruguay. These vaccines include bovine erythrocytes infected with selected strains. An experimental B. divergens vaccine prepared from the blood of infected gerbils (Meriones unguiculatus) has also been used in Ireland (Gray & Gannon, 1992) but production ceased in 2002 because of licensing concerns about the safety of a vaccine based on whole blood (J. S. Gray, personal communication, 2003). The risk of contamination of blood-derived vaccine is real (Hugoson, Vennström & Henriksson, 1968; Rogers et al. 1988) and makes post-production quality control essential. Unfortunately, this puts production beyond the means of many countries in endemic regions (de Vos & Jorgensen, 1992). Techniques developed in Australia over many decades have formed the basis for production of live Babesia vaccines in most countries where they are used. The following section outlines procedures currently used in Australia.
Since 1990, three strains of B. bovis and one of B. bigemina (G strain) have been used to produce vaccines in Australia. Changes in the B. bovis vaccine strain were necessary due to periodic increases in the vaccine failure rate above an acceptable background level (Bock et al. 1992, 1995). Candidate low virulence B. bovis isolates were obtained from naturally infected, long-term carrier animals as described by (Callow, 1977). Contaminating haemoparasites such as Babesia, Anaplasma, Eperythrozoon and Theileria buffeli were eliminated using selective drug treatment combined with rapid passage in calves or culture (Dalgliesh & Stewart, 1983; Anonymous, 1984; Stewart et al. 1990; Jorgensen & Waldron, 1994).
Babesia bovis – The most reliable way of reducing the virulence of B. bovis involves rapid passage of the strain through susceptible splenectomised calves (Callow, Mellors & McGregor, 1979). The mechanism by which attenuation occurs is not fully understood, but may result from selective enrichment of less virulent parasite subpopulations or from down regulation of a virulence gene (Cowman, Timms & Kemp, 1984; Carson et al. 1990; Timms, Stewart & de Vos, 1990). Attenuation is seldom complete and reversion to virulence can occur following passage of parasites through ticks (Timms et al. 1990) or intact cattle (Callow et al. 1979). Attenuation is also not guaranteed, but usually follows after 8 to 20 calf passages (Callow, 1984).
Attenuation of Babesia spp. by irradiation has been attempted, but the results were variable (Purnell & Lewis, 1981; Wright et al. 1982). In vitro culture has also been used to attenuate B. bovis (Yunker, Kuttler & Johnson, 1987).
Babesia bigemina – Rapid passage in splenectomised calves is not a reliable means of attenuating B. bigemina (Anonymous, 1984) but the virulence of isolates decreases during prolonged residence in latently infected animals. This feature has been used to obtain avirulent strains by splenectomising latently infected calves and using the ensuing relapse parasites to repeat the procedure (Dalgliesh et al. 1981a). A single B. bigemina isolate (G strain) has been used in the Australian and South African vaccines since 1972 and the early 1980s, respectively.
The suitability of a strain for use in a vaccine can be determined by challenging vaccinated cattle and susceptible controls with a virulent, heterologous strain. Both safety and efficacy can be judged by monitoring fever, parasitaemia and depression of packed cell volume during the vaccine and challenge reactions (Timms et al. 1983a). Any candidate isolate must also be tested for freedom from potential contaminants.
After testing for virulence, immunogenicity and purity, suitable strains are preserved as master stabilates in liquid nitrogen. Polyvinyl pyrrolidone (MW 40000 Da) (Vega et al. 1985) is the preferred cryoprotectant as it is not toxic to the parasites at temperatures above 4 °C, allows intravenous inoculation and is safe to use (Standfast & Jorgensen, 1997). Dimethyl sulphoxide is a very effective cryoprotectant but its use was discontinued in Australia in 1991 because of the risk of toxicity to operators, recipient animals and parasites (Dalgliesh, Jorgensen & de Vos, 1990).
Susceptible splenectomised calves receive inocula from stabilate banks and parasitised blood is collected for production of vaccine when the parasitaemias exceed preset limits. Passaging of B. bigemina in splenectomised calves is not recommended but B. bovis usually requires passaging to produce a sufficiently high parasitaemias for vaccine. B. bovis vaccine strains are not passaged more than 30 times (including the attenuation passages) to safeguard against diminished immunogenicity (Callow & Dalgliesh, 1980).
A sufficient volume of blood can be collected from a 4–6 month old calf to provide up to 25000 doses. To do this the calf is sedated, the jugular vein catheterised and blood collected into a closed system using a peristaltic pump. Heparin is a suitable anticoagulant. After collection of the blood, the calf is treated with a babesiacide and given supportive therapy. Depending on the volume of blood collected, the calf is also transfused using blood from a suitable donor. Due to the high cost and limited availability of suitable, health-tested donors, calves previously infected with B. bovis can be used to provide B. bigemina organisms. In Australia, quinuronium sulphate (de Vos & Potgieter, 1994) is used to treat the primary B. bovis infection because it has no residual effect and will not suppress the development of a subsequent B. bigemina infection. The risk of red cell agglutination can be prevented by washing the B. bigemina-infected red cells by centrifugation to remove agglutinating antibodies. This does not appreciably affect parasite viability (Standfast et al. 2003).
Frozen vaccine has some very significant strengths over the chilled form, notably a long shelf life that allows thorough post-production testing of potency and safety before dispatch. Its production also allows for judicious use of suitable contaminant-free donor cattle. Frozen vaccine is the only product available in South Africa and Israel, and demand for it is growing in Australia reaching 8% of total demand in 2003.
Glycerol is used as cryoprotectant in Australia in preference to dimethyl sulphoxide because it allows post-thaw storage life of the vaccine for at least 8 hours (Jorgensen, de Vos & Dalgliesh, 1989b; Dalgliesh et al. 1990). Parasitised bovine blood is slowly mixed with an equal volume of phosphate buffered saline (PBS) solution containing 3 M glycerol, glucose and antibiotics, equilibrated at 37 °C for 30 minutes, dispensed into 5 ml cryovials and frozen at 10 °C/min. These vials of vaccine concentrate are then stored in liquid nitrogen. Vaccine is prepared by thawing a cryovial in water at 37 °C and then diluting the contents in 50 ml of PBS containing 1·5 M glycerol, glucose and antibiotics to make 25×2 ml doses of vaccine.
The capability exists in Australia to produce monovalent B. bovis and B. bigemina vaccines but, since 2001, the only frozen vaccine marketed has been a vaccine concentrate registered as ‘Combavac 3 in 1’. It contains packed red cells infected with B. bovis, B. bigemina and Anaplasma centrale. Packed cells from 3 donors are concentrated using a blood concentration method recommended by the Kimron Veterinary Institute in Israel (V. Shkap, personal communication) and mixed to produce the trivalent concentrate.
Frozen vaccines are transported in suitably insulated containers with liquid N2 or solid CO2 as refrigerant and this limits the ability to supply vaccine to all destinations. To ensure infectivity, the prepared vaccine must be used within 8 hours of thawing and, once thawed, should not be refrozen. Vaccine prepared with glycerol must not be inoculated intravenously (Dalgliesh, 1972).
A frozen bivalent B. bovis and B. bigemina vaccine and frozen monovalent B. bovis and B. bigemina vaccines using dimethyl sulphoxide as the cryoprotectant are produced in South Africa (de Waal, 1996) and Israel (Pipano, 1997), respectively.
Most of the babesiosis vaccines produced to date have been provided in a chilled form. In Australia alone, 35 million doses were supplied between 1966 and 2003. Reasons for its popularity have been its ease of production even with limited resources, ease of transportation, ease of use and, in Australia at least, low cost. The chilled vaccines currently used in Australia contain 1×107 B. bovis, 2·5×106 B. bigemina and 1×107 Anaplasma centrale organisms per 2 ml dose (Standfast et al. 2003). Chilled vaccine has a very short shelf-life, which is currently 4 days in Australia. Therefore, rapid, reliable means of communication and transport are required to ensure viability of the distributed vaccine.
To reduce the risk of neonatal haemolytic disease in calves of vaccinated dams, users are advised not to vaccinate cattle repeatedly and most owners now vaccinate only young stock and seldom more than twice. Reduction of the dose rate from 5 ml to 2 ml and introduction of a cell free diluent (Callow, 1984) have also contributed to no case of acute neonatal haemolytic disease being confirmed in vaccinated cattle in Australia since 1976.
Australia is free of many of the infectious pathogens and arthropod-borne diseases that affect cattle in other countries (de Vos & Jorgensen, 1992). These potential contaminants therefore do not pose a serious risk to bovine blood-based vaccines produced in Australia but in other countries testing protocols may be required for such potential contaminants.
The calves to be used in vaccine production in Australia are bred on site using cattle sourced from herds in B. microplus-free regions. Breeder cattle are screened for, and maintained free, of B. bovis, B. bigemina, A. marginale, Bovine Leukaemia Virus (BLV), Bovine Immunodeficiency virus, Bovine Spumavirus (Bovine Syncytial virus), Bovine Pestivirus, Neospora caninum, Coxiella burnetii (Q Fever) and Boophilus microplus. The cattle are also routinely vaccinated with commercial bacterial and viral vaccines. Calves are produced throughout the year to ensure donors are available at regular intervals. The calves are brought into an insect-free quarantine environment at 7 to 14 days of age and undergo stringent quarantine and testing over a period of 8 weeks. During the quarantine period, each calf is also splenectomised. Only when all test results are available are calves cleared for use. Any calves showing evidence of infection other than Theileria buffeli are rejected. T. buffeli infections are eliminated using buparvaquone and primaquine phosphate (Stewart et al. 1990).
Potency (infectivity) is tested by thawing and diluting 5 vials of vaccine concentrate, storing the vaccine for 8 hours at 4 °C to 8 °C before inoculating susceptible groups of cattle. Each batch of vaccine is tested in a group of five cattle. The cattle are monitored for the presence of infection by examination of stained blood smears as well as retrospective serology (Molloy et al. 1998a,b). Postproduction monitoring and testing can be carried out on the product for specific disease agents if required for import/export purposes provided validated tests are available. Serum retention samples and DNA are collected from calves at the time of blood collection and again 2 weeks later and stored for this purpose.
As the chilled vaccines have a short shelf-life, undertaking quality assurance on the final product is not possible. Therefore, increased reliance has to be placed on pre-production quality control, especially for obtaining, testing and housing vaccine donors free of harmful infections. Despite precautions, one batch of vaccine produced in Australia during 1986 was later shown to be contaminated with bovine leucosis virus (BLV) (Rogers et al. 1988). The incident pointed to a deficiency in testing procedures that relied on serological assays. Major changes were made subsequently to minimise the risk of future contamination with BLV and have been progressively enhanced to include other disease agents.
Use of a vaccine in the face of an outbreak is common practice in Australia. Superimposing vaccination in this way on a natural infection will not exacerbate the condition, but will pre-empt the development of virulent infections in the proportion of the herd not yet exposed to field challenge. To prevent further exposure, the group should also be treated with an acaricide capable of preventing tick attachment from the time of diagnosis to 3 weeks after vaccination. Injectable or pour-on formulations of ivermectin and moxidectin (Waldron & Jorgensen, 1999) as well as fluazuron (Hosking et al. 2004) are highly effective acaricides but do not prevent transmission of Babesia.
Clinically affected cattle should be treated as soon as possible with a suitable babesiacide. In the case of a severe outbreak, it may be advisable to treat all the cattle with a prophylactic compound (e.g. imidocarb or diminazene) and to vaccinate them later when the drug residue will not affect vaccine parasite multiplication.
Any factor affecting the survival of the tick vectors will affect the risk of babesiosis occurring. Thus, increased tick numbers will increase the threat of disease until an endemically stable situation develops. Conversely, reduced tick numbers will increase the longer-term risk of babesiosis due to the reduced natural exposure of calves. For these reasons, cattle owners in endemic areas of Australia are advised to supplement natural exposure by vaccinating calves at weaning age. Vaccination is also recommended if cattle are being moved within the endemic area.
Large numbers of cattle, predominantly of Bos taurus breeds are being imported into tropical, developing countries to upgrade local livestock industries. This practice has, in the past, led to significant losses due to tick-borne diseases, including babesiosis (Callow, 1977) if preventative measures were not taken. Most of the countries involved did not have access to an effective vaccine. Vaccination of naïve cattle moving from ‘tick-free’ to endemic areas within Australia is usually very effective. This practice has played a crucial role in making the livestock industries in these parts more sustainable and competitive in meeting market demand with regard to breed type.
K strain B. bovis and G strain B. bigemina from Australia have been shown experimentally to be protective in South Africa (de Vos, Bessenger & Fourie, 1982; de Vos, Combrink & Bessenger, 1982); Sri Lanka (D. J. Weilgama, personal communication, 1986); Bolivia (Callow, Quiroga & McCosker, 1976) and Malawi (Lawrence et al. 1993). Vaccine containing these strains has also been used with beneficial results in countries in many parts of the world, including Zimbabwe and Swaziland in Africa, Venezuela and Ecuador in South America, Malaysia and the Philippines in Southeast Asia, and islands of the Caribbean. The feasibility of importing a vaccine strain that is known to be effective, of low virulence and free from contaminants to protect imported or local cattle should therefore be considered.
The likelihood of vaccine-induced reactions has been reduced with the development of attenuated strains but there is always a risk of reactions when highly susceptible, adult cattle are immunised. Calves 3 to 9 months of age have a high level of natural resistance and therefore a low risk of reactions. In some countries, such as Argentina, vaccination is only recommended for calves while in other countries such as Australia and South Africa, adult vaccination can be undertaken, provided proper precautions are taken. Cattle with a high Bos indicus content rarely show adverse reactions to vaccination. Bock et al. (2000) investigated reports of severe reactions to B. bovis vaccine and, using PCR assays on the DNA obtained from affected cattle, found that four of the five cases were due to concurrent field infections.
Except in late pregnancy, cows are no more likely to show severe vaccination reactions than any other class of adult stock. However, the consequences of severe reactions are more serious in pregnant cows as the accompanying fever may cause abortion. The risk to the cow and foetus from vaccine reactions is much less than from field infections, but close monitoring for reactions is nevertheless essential. Special care should also be taken with large (particularly fat) bulls, as a high fever can cause a temporary (6 to 8 weeks) loss of fertility (Singleton, 1974). In the case of valuable cows and bulls, it is advisable to take rectal temperatures during the reaction times and to treat any showing prolonged elevated temperatures or clinical evidence of disease with a suitable babesiacide. There is little field evidence that stress, including nutritional stress, has a significant effect on reaction rates or immunity following B. bovis vaccination (Callow & Dalgliesh, 1980).
Low doses of imidocarb or diminazene have been used in some countries to suppress potential vaccine reactions (de Waal, 1996) but this practice is not recommended because of the effect it may have on immunity (de Vos et al. 1986). Trials in Israel (Pipano et al. 1987) and Australia (Jorgensen et al. 1993) have shown that oxytetracycline can be used to ameliorate B. bovis and B. bigemina vaccine reactions without affecting subsequent immunity.
Concern is often expressed that natural spread of infection may occur from vaccinated to unvaccinated cattle. Australian evidence suggests that it is very unlikely that use of vaccine will introduce infection into a previously uninfected area. A 15 year study of the history of babesiosis outbreaks in Australia found no evidence suggesting that vaccination of cattle on a neighbouring property was the cause of an outbreak (Callow & Dalgliesh, 1980). On the other hand, inadequate tick control by a neighbour and spread of naturally-infected ticks was found to be a contributing cause. Similarly, there has been no evidence that use of a strain from one country resulted in the spread of infection in another. Tick numbers appear to be more relevant in transmission of Babesia than the presence of animal reservoirs of infection.
The current B. bovis strain (Dixie) used in Australia is known to be transmissible by Boophilus microplus under laboratory conditions and to increase in virulence following tick transmission (unpublished observations). Despite this, Bock et al. (1999a) offered circumstantial evidence that the presence of this strain in vaccinated cattle is unlikely to alter the dynamics of transmission of parasites under field conditions or constitute a significant risk to naïve cattle grazing with vaccinated cattle once vaccine induced parasitaemias have fallen to undetectable levels.
The G strain of B. bigemina used in Australia since 1972 (Dalgliesh et al. 1981a) is poorly, if at all, transmissible by Boophilus microplus (Dalgliesh, Stewart & Rodwell, 1981b; Mason, Potgieter & van Rensburg, 1986). Also, cattle infected with B. bigemina are reported to remain infective for ticks for only 4 to 7 weeks (Mahoney, 1969; Johnston et al. 1978) so if transmission to ticks occurs, it will be over a short period only.
Stored in liquid nitrogen, a frozen vaccine will remain viable for many years but it loses viability very rapidly after thawing. If glycerol is used as cryoprotectant, a thawed vaccine can remain viable for only 8 hours at temperatures ranging from 4–30 °C. If dimethyl sulphoxide is used, a vaccine should preferably be used within 30 minutes although work in South Africa has indicated that thawed dimethyl sulphoxide-based vaccine remains infective for 8 hours if kept on melting ice (D. T. de Waal, personal communication). Chilled vaccines can remain viable for up to a week if stored at 4 °C. At higher temperatures, viability is lost rapidly.
Since the introduction of a standardised method of production in Australia, live babesiosis vaccines have generally proved to be highly effective (Callow & Dalgliesh, 1980). In most cases, a single vaccination provided lasting, probably life-long immunity against field infections with antigenically different strains. However, troublesome failures of the Australian B. bovis vaccine occurred in 1966, 1976, and again in 1988–1990 (Bock et al. 1992), and were thought to be due to loss of immunogenicity brought about by frequent passaging of the vaccine strains in splenectomised calves (Callow & Dalgliesh, 1980). In each case, the problem was solved by replacing the vaccine strain. A similar loss of immunogenicity in a multi-passaged strain of B. bovis was reported in South Africa (de Vos, 1978). To prevent future recurrences of the problem, the number of passages of the vaccine strains of B. bovis is limited by frequently reverting to a master stabilate with a low passage number (Callow & Dalgliesh, 1980).
More recently (1992–1993), B. bovis vaccine failures were again reported in Australia despite restrictions on passage numbers and replacement of the vaccine strain (Bock et al. 1995). The occurrence of these failures did not correlate with time after vaccination. Bock et al. (1995) considered eight possible factors, and while the situation is complex and no simple cause is forthcoming, recent research emphasis has fallen on the immune responsiveness of the host and immunogenicity of vaccine parasite subpopulations (Dalrymple, 1993; Bock et al. 1995; Lew et al. 1997a,b). The B. bovis vaccine strain used in Australia since 1993 has been shown by PCR assay to contain two subpopulations. It was found to be more protective than higher passages of this strain that contained one subpopulation (Bock & de Vos, 2001).
Vector control was first used successfully to control and eventually eradicate Babesia from the USA (Pegram, Wilson & Hansen, 2000). Because, in Africa, babesiosis forms only part of very important complexes of ticks and tick-borne diseases, intensive, usually government-regulated tick control programmes have been used for many years. The situation in other continents is much less complex than in Africa but where babesiosis is endemic, disease control rather than eradication is generally the only realistic option. Eradication of the tick vectors (the so-called minimum disease situation) is a permanent solution to the problem but is rarely considered practical, environmentally sustainable or economically justifiable on either a national or a local basis.
Natural endemic stability can seldom be relied on as a disease control strategy. Firstly, in endemic areas, climatic effects, genetic make-up of hosts and management strategies, inevitably have a major effect on the rate of transmission and ultimately on the likelihood of endemic stability developing. Secondly, endemic stability is an economic concept that incorporates risk management and loss thresholds. The climatic, animal and management parameters that allow endemic stability can change on a seasonal, let alone on an annual, basis. For example, a dry season can drastically affect tick numbers and parasite transmission rates to produce a generation of susceptible cattle. Thirdly, the model for endemic stability was developed in Australia and the Americas where the disease/vector interactions are relatively simple. The African situation is more complex and less predictable with four main diseases, several vectors, presence of game reservoirs and a larger range of susceptibility of bovine breeds.
In a study of dairy farms in Boophilus microplus-endemic areas of Australia, Sserugga et al. (2003) found 74% of herds of farmers who allowed a ‘few’ ticks to persist, assuming their cattle would be protected from tick-borne disease, had insufficient exposure to confer herd immunity, and a high risk of tick fever outbreaks. Leaving a ‘few’ ticks, although it is likely to have some protective effect, therefore cannot be considered a satisfactory approach to controlling the disease. Presumably, because farmers underestimated the numbers of ticks needed or would not allow sufficient ticks on their cattle to achieve endemic stability.
A serological survey in 1996 of 7067 unvaccinated weaner cattle 6 to 12 months of age on 115 properties in officially Boophilus microplus-infested areas of Northern Australia indicated that the average percentage of animals seropositive for B. bovis and B. bigemina per herd was only 4% and 11%, respectively (Bock et al. 1997b). Given the generally high Bos indicus content and extensive management of these herds, the risk that this represents is reduced, but difficult to ascertain. However, in some areas, the risk appeared to have been very high and significant losses would be expected especially where the Bos taurus content of the herd was increased.
Certain breeds of cattle, notably Bos taurus breeds, are known to be more susceptible to primary B. bovis infection (Bock et al. 1997a). Bock et al. (1999a) showed pure-bred Bos indicus cattle had a high degree of resistance to babesiosis, but crossbred cattle were sufficiently susceptible to warrant the use of preventive measures such as vaccination. Similarly, genotype also plays a role in the development of protective immunity with Bos taurus breeds more likely to show a deficient immunity. An investigation of 62 reports of B. bovis vaccine failures in Australia showed that all were in cattle with no greater than 3/8 Bos indicus infusion and 85% were in pure Bos taurus herds (Bock et al. 1995). As discussed under breed susceptibility (see above), the use of cattle with more than 50% Bos indicus content will greatly reduce the impact of babesiosis.
Livestock industries need to take a pragmatic approach to management of the ticks and diseases associated with them. In the long term, an effective outcome can be achieved by integrating the strategic use of acaricides, exploitation of endemic stability, the application of vaccines in endemically unstable conditions and the use of tick-resistant breeds of cattle (Norval, Perry & Hargreaves, 1992; Perry et al. 1998).
Rapid advances in our understanding of mechanisms of immunity to many protozoa and the development of molecular tools for generating recombinant vaccines suggest that this is the future direction for protozoal vaccine development (see the chapter by Bishop et al. in this Supplement). However our lack of understanding of immune mechanisms to primary and secondary infection and the reality that many protozoa have developed elaborate mechanisms (e.g. antigen variation) for evading host immunity remain obstacles to developing effective vaccines using this technology (Jenkins, 2001).
Several attempts have been made to develop recombinant or subunit Babesia vaccines (Reduker et al. 1989; Wright et al. 1992; Dalgliesh, 1993; Brown & Palmer, 1999), to overcome the inherent deficiencies of vaccines based on blood or blood extracts. Wright et al. (1992) systematically tested biochemically fractionated merozoite antigens in immunisation and challenge trials using Quil A as an adjuvant and found 3 partially protective antigens (11C5, 12D3 and T21B4/RAP-1). These antigens were secreted proteins, not abundant and were not serologically immunodominant (Wright et al. 1992).
The search for vaccine candidate antigens has focused mainly on merozoite surface antigens that are functionally relevant and immunodominant in naturally immune cattle, as well as conserved among strains. Candidate antigens identified include B. bovis merozoite surface antigen 1 (MSA-1) (Hines et al. 1995), B. bovis merozoite surface antigen 2c (msa-2c) (Wilkowsky et al. 2003), and B. bovis and B. bigemina RAP-1 (Wright et al. 1992; Norimine et al. 2002). Immunization of cattle with recombinant MSA-1 induced antibodies that were capable of neutralizing merozoite invasion of erythrocytes, however the immunized cattle were not protected against virulent challenge (Hines et al. 1995). MSA-2c has been identified as highly conserved among B. bovis strains and bovine antibodies to recombinant MSA-2c were able to neutralize the invasion of erythrocytes by merozoites indicating a functional role for this antigen (Wilkowsky et al. 2003). However, this antigen has not yet been tested in cattle to determine its protective efficacy. RAP-1 is a 60 kDa antigen of Babesia that is recognised by antibodies and T cells from naturally immune cattle (Rodriguez et al. 1996; Norimine et al. 2002). Native and recombinant B. bovis RAP-1 confers partial protection against homologous challenge (Wright et al. 1992). Native B. bigemina RAP-1 also induces partial protection against challenge infection (McElwain et al. 1991).
Results to date suggest that vaccines based on single antigens or even several antigens in combination do not confer the level or duration of cross-protection provided by living vaccines. Most work has centred on merozoite antigens but sporozoite antigens need also to be evaluated (Brown & Palmer, 1999). Unfortunately, the majority of experimental immunization trials use homologous challenge infections that do not reflect the situation in the field where heterologous virulent challenge would occur. Even a multicomponent recombinant vaccine may not provide long-term protection against field strains of Babesia spp. which have been shown to be capable of considerable genetic variation (Dalrymple, 1993; Lew et al. 1997a). The difficulty is to identify antigens that are targeted by the host's protective immune response across all strains, induce an appropriate long-term memory response and deliver them to the animal in a way that is cost effective. A subunit vaccine that protects against clinical signs, but allows for limited parasite replication may be an ideal strategy for protecting susceptible individuals (Jenkins, 2001). Yet, no recombinant vaccine for bovine babesiosis has been registered for use in any country and it is unlikely that one will be available in the near future.
Another avenue of research for the development of an alternative to the use of a live vaccine is DNA vaccine adjuvants. Recognition of foreign DNA (in particular unmethylated CpG motifs in DNA) by the innate immune system is a relatively recent discovery. CpG motifs in DNA derived from B. bovis-stimulated B cells to proliferate and produce IgG (Brown et al. 1998) and activated macrophages to secrete IL-12, TNF-α and NO and may provide an important innate defence mechanism to control parasite replication and promote persistent infection (Shoda et al. 2001).
In vitro culture methods reviewed by Pudney (1992) are also used to provide B. bigemina and B. bovis parasites for vaccine (Jorgensen et al. 1992; Mangold et al. 1996). These techniques are not widely used for production of vaccines, but have proven to be valuable research tools. In countries where it is difficult to obtain sufficient numbers of disease-free, susceptible donor calves, and materials and facilities for tissue culture are available, in vitro production of vaccines may be a viable option. At the National Institute of Agricultural Technology in Argentina, over 50000 doses of B. bovis and B. bigemina vaccine are produced using in vitro culture and sold to cattle producers each year (A. A. Guglielmone personal communication). Some studies found neither virulence or immunogenicity of Babesia vaccine strains were appreciably modified by short-term (3 months) maintenance in culture (Jorgensen, de Vos & Dalgliesh, 1989a; Timms & Stewart, 1989). However, more recent observations using PCR of polymorphic genetic markers have shown that proportions of B. bovis sub-populations changed with long-term continuous cultivation (Lew et al. 1997b). These drifts may not be significant but indications are that protection provided by culture-modified B. bovis strains may be inferior to that of parasites not exposed to a culture environment (Bock et al. 1995). Until more information is available, use of long-term cultures in the production of vaccines is not recommended unless facilities are available to monitor changes in parasite populations and to test for immunogenicity.
Non-living vaccines would overcome many of the inherent difficulties in production, transport and use of live vaccines. One of the earliest attempts to induce protective immunity in cattle against B. bovis infection using a non-living vaccine was by Mahoney (1967b). The inoculation of cattle with killed parasites mixed with Freund's complete adjuvant partially protected against homologous challenge. Cell culture-derived exoantigens of B. bovis and B. bigemina have been extensively studied and proposed for use as vaccine in developing countries with reported promising results (Montenegro-James, 1989; Montenegro-James et al. 1992; Patarroyo et al. 1995). Other studies have shown the level and duration of protection conferred by these antigens against heterologous challenge was considerably less than those of live vaccines (Timms, Stewart & Dalgliesh, 1983b). Jorgensen et al. (1993) evaluated the use of pre-vaccination with homologous exoantigens as a method of reducing the risk of reactions following vaccination with live Babesia vaccine. This technique reduced the parasitaemia and development of anaemia but not the fever which sometimes follows the use of this vaccine.
Other approaches to the development of a non-living vaccine include fractionating merozoite antigens either by an empirical approach (Wright et al. 1992) or by continuous flow electrophoresis (Stich et al. 1999; Brown et al. 2001) the identification of antigens involved in the invasion process (Palmer & McElwain, 1995) and the identification of T cell and B cell epitopes of antigens that stimulate T helper cell clones (Wilkowsky et al. 2003).
A killed B. divergens vaccine has been prepared in Austria from the blood of infected calves (Edelhofer et al. 1998), but little information is available on the level and duration of the conferred immunity.
Laboratories producing frozen Babesia vaccines around the world still use the techniques reported by Dalgliesh et al. (1990) or the older technique reported by Mellors et al. (1982). Recent scientific and technological achievements offer great promise for the development of cryopreserved and user-friendly live vaccines. These include: new cryoprotectants and combinations of cryoprotectants in related organisms (reviewed by Hubalek (1996)); models to quantify cryopreservation efficiency (Pudney, 1992); mouse haemoprotozoan models (Wyatt, Goff & Davis, 1991); and a variety of programmable freezing machines that minimise the subjectivity of cryopreservation and deliver consistent, reproducible user-defined freezing conditions.
Live Babesia vaccines have an enviable level of efficacy (over 95% from a single vaccination); however, they have many disadvantages, notably shelf life, tick transmissibility and risk of transmission of adventitious disease agents. These disadvantages necessitate costly quality assurance and testing programmes, and even with the incorporation of these programmes it is extremely unlikely that live vaccines would be accepted in many countries (e.g. USA).
Vaccines based on recombinant antigens are potentially a solution to the draw-backs of live vaccines; however, Babesia have developed a variety of mechanisms such as antigenic variation, ‘smoke-screen’ antigens, and critical epitopes that are poorly immunogenic to evade the host immune response. Future recombinant antigens will need to contain several antigens to provide protection against challenge by heterologous strains for the majority of the target group. In addition, a novel delivery system may be required to minimise the need for repeat vaccination. Numerous options are being investigated and include viral and bacterial vectors, new generation adjuvants and antigen production in food plants.
Recombinant Babesia vaccines may be incorporated with other vaccines. For example, Willadsen & Kemp (2003) advocate combining recombinant tick and tick-borne disease vaccines to minimise mustering costs. Other options being considered for incorporation in such a multivalent vaccine are transmission-blocking vaccines derived from the Babesia stages residing in the tick vector. The target Babesia antigens have not undergone selection in response to the vertebrate host immune system and are consequentially relatively immunogenic and stable. Cattle can be immunised against a tick gut antigen (BM86) to provide effective tick control (Willadsen et al. 1995; and see chapter by Willadsen in this supplement). An approach similarly directed against antigens of Babesia developing within the tick vector may therefore be fruitful.
We are grateful to Cordelia Gosman from The Graphics Place for the original artwork used in the life cycle figure.

Table 1. Major Babesia species infective to domestic animals, their ixodid tick vectors and geographical distribution (Adapted from Uilenberg (1995).)

Fig. 1. The development life cycle of Babesia bigemina in cattle and the ixodid tick vector Boophilus microplus as currently understood (adapted from Mehlhorn & Shein, 1984; Mackenstedt et al. 1995; Gough et al. 1998).

Fig. 2. Dorsal and ventral view of adult female Boophilus microplus ticks and their egg mass. Babesia parasites develop in oocytes of Boophilus microplus resulting in transovarial transmission an essential component in babesiosis epidemiology as Boophilus spp. are one-host ticks.