INTRODUCTION
The group of equine strongyle parasites consists of about 60 different species described (Lichtenfels et al. Reference Lichtenfels, Kharchenko and Dvojnos2008). They are widely occurring across the world and are practically ubiquitous in grazing horses. Traditionally the group is subdivided into the large (strongylinae) and the small (cyathostominae) strongyles. The small strongyles consist of 50 different species distributed over 14 different genera. During the 1980s, the emphasis was shifted from the pathogenic large strongyles that were declining in prevalence (Herd, Reference Herd1990) to the small strongyles that were developing resistance to commonly used anthelmintics (Kaplan, Reference Kaplan2004), and now also recognized as potentially significant pathogens (Love et al. 1999). The large strongyles comprise the three Strongylus species Strongylus vulgaris, Strongylus equinus and Strongylus edentatus, but also Triodontophorus spp. as well as Craterostomum acuticaudatum, Oesophagodontus robustus and Bidentostomum ivaschkini (Lichtenfels et al. Reference Lichtenfels, Kharchenko and Dvojnos2008). Common to the large strongyles is their large buccal capsules, but only larvae of Strongylus species migrate outside the intestinal tract.
The pathogenic impact of Strongylus spp. is well described. They all have prepatent periods of 6 months and above (Round, Reference Round1969) and spend several months migrating through various tissues and organs of the horse. The life cycle of S. vulgaris is classical in veterinary parasitology, with the L3s migrating towards their predilection site at the root of the cranial mesenteric artery. Here, they dwell for several months before they are transported by the bloodstream back to the large intestinal walls (Duncan and Pirie, Reference Duncan and Pirie1972). Pathological lesions caused by the larvae have been associated with a painful and sometimes fatal colic syndrome named ‘thromboembolic colic’ (Enigk, Reference Enigk1951). The larvae of S. edentatus migrate via the portal system to the liver, from where they continue to the adipose tissue in the ventral abdominal walls and eventually back to the intestinal walls (McCraw and Slocombe, Reference McCraw and Slocombe1978). The prepatent period of S. edentatus has been reported to be around 11 months (Wetzel, Reference Wetzel1952). Strongylus equinus has become very rare in managed horse populations, but has been described to migrate through the peritoneal cavity, passing through the pancreas on the way to the liver and eventually back to the intestine. Larvae of S. edentatus and S. equinus are described to cause significant lesions, but unlike S. vulgaris they have not been associated with any specific clinical syndromes. The life cycles of the remaining large strongyle species strongly resemble those of the cyathostomins, without a pronounced phase of tissue migration and with immature stages undergoing encystment in the large intestinal walls. The prepatent period of Triodontophorus spp. has been reported to be 2–3 months (Round, Reference Round1969). There are no specific clinical syndromes ascribed to Triodontophorus spp. infection. It is worth noting, however, that Triodontophorus tenuicollis causes characteristic lesions in the dorsal colon consisting of deep ulcers, in which whole colonies of adult parasites of this species are residing (Drudge, Reference Drudge1972). The clinical significance of these lesions is unknown.
Compared with the cyathostomins, large strongyle species are generally rare in occurrence and less abundant, when present (Lyons et al. Reference Lyons and Tolliver2006). To date, there have been no convincing reports of anthelmintic resistance in any large strongyle species, and this appears to largely explain the prevalences of these observed in managed horse populations (Lyons et al. Reference Lyons, Swerczek, Tolliver, Bair, Drudge and Ennis2000). We recently published a study illustrating that on Danish farms adopting the widely recommended selective therapy principle, where horses are treated based on their fecal egg count level, S. vulgaris had a significantly higher occurrence compared with farms that did not use selective therapy (Nielsen et al. Reference Nielsen, Vidyashankar, Olsen, Monrad and Thamsborg2012). This prompts for implementing routine surveillance of S. vulgaris on farms using similar parasite control programmes, and one survey documented that larval cultures are widely used by Danish equine practitioners (Nielsen et al. Reference Nielsen, Monrad and Olsen2006). However, the negative predictive value for diagnosing S. vulgaris infection in individual horses has been found to be 0·37, which allows for false negative results (Nielsen et al. Reference Nielsen, Baptiste, Tolliver, Collins and Lyons2010a). Other large strongyle species can be identified in the larval cultures as well, but it remains unknown if these can be interpreted as indicative of S. vulgaris being potentially also present. In the dataset generated in our recent publication (Nielsen et al. Reference Nielsen, Vidyashankar, Olsen, Monrad and Thamsborg2012), large strongyle parasites besides S. vulgaris were identified in the larval cultures, but they were sparse in occurrence. While it can be hypothesized that there is an association between the occurrences of these large strongyle species, it remains a statistical challenge to analyse such data given the relatively rare occurrence of these species.
The aim of the present study was to adopt a rare-event Poisson regression to evaluate whether the presence of S. vulgaris in individual horses was associated with presence of S. edentatus and Triodontophorus spp. also recorded in the dataset mentioned above.
MATERIALS AND METHODS
The study design has previously been described (Nielsen et al. Reference Nielsen, Vidyashankar, Olsen, Monrad and Thamsborg2012), but will be briefly outlined below.
Farms
Veterinary practitioners were contacted in all regions of Denmark, and asked to enrol farms in the study in the spring months of 2010 (March–May). Inclusion criteria were as follows: farm sizes should be of 6 horses or more, anthelmintic treatments should not have been prescribed on the farm for at least 2 months prior to the onset of the study, and anthelmintic strategies should fall into one of two broad categories: (1) anthelmintic treatments were based on routine fecal egg counts performed from all horses, or (2) anthelmintic treatments were applied to all horses without performing fecal egg counts. As a guideline, each veterinarian was asked to identify one farm in each of these categories for the study. For each farm, information about the time of the most recent anthelmintic treatment was recorded for every horse.
A total of 663 horses from 42 different farms entered the study. For a detailed description of the participating farms and horses, the readers are referred to our recent publication (Nielsen et al. Reference Nielsen, Vidyashankar, Olsen, Monrad and Thamsborg2012). The principles defined in the European Convention for the Protection of Vertebrate Animals used for Experimental and Other Scientific Purposes were followed, and the study was designed to collect data from the minimum number of horses needed to produce experimentally reproducible results.
Parasitological procedures
Fecal samples
Samples were collected by the participating veterinarians following published recommendations (Nielsen et al. Reference Nielsen, Vidyashankar, Andersen, DeLisi, Pilegaard and Kaplan2010b). Samples were collected fresh, packed airtight in plastic bags and shipped overnight to the parasitology laboratory at the Department of Large Animal Sciences, University of Copenhagen. Here, samples were kept refrigerated and cultures were set up within 5 days.
Larval cultures
Larval cultures for detecting large strongyle parasites were performed as follows: Ten grams of feces were weighed and mixed with an equal volume of vermiculite. Tap water was added to yield a moist texture of the feces. Samples were cultured in individual humidity chambers created as described by Henriksen and Korsholm (Reference Henriksen and Korsholm1983). Incubation occurred at room temperature (20–24 °C) for 14 days, during which time samples were regularly checked for desiccation, and more water added if necessary. Upon incubation, third-stage larvae were harvested after 24 h of sedimentation in a Baermann glass. All larvae were examined and identified under the microscope using morphological criteria (Russell, Reference Russell1948). All cultures were examined by the first author. Results were recorded as total number of larvae harvested, and total number of S. vulgaris, S. edentatus and Triodontophorus spp. larvae identified.
Statistical analyses
Development of statistical framework for rare-event analysis
To develop the statistical frame work, we express the data as a 2×2 table involving two levels of two factors: Factor 1 concerns occurrence or non-occurrence of S. vulgaris in each horse; and factor 2 concerns occurrence or non-occurrence of S. edentatus (or Triodontophorus spp.) in each horse. When relative risk is our primary interest in the 2×2 table, binomial regression is usually recommended since the data in each cell of the table represent counts. However, if the probability of some cells are very small (referred to as a rare event), convergence problems arise with binomial regression models; in this case, they may fail to provide an accurate estimate of the relative risk. Under these circumstances, Poisson regression has been known to be a better choice (Wallenstein and Bodian, Reference Wallenstein and Bodian1987; Zou, Reference Zou2004).
Returning to our problem, let n be the total number of horses that are positive for S. vulgaris and let X denote the number amongst these horses that are also positive for S. edentatus; let p denote the probability that a horse that is positive to S. vulgaris is also positive for S. edentatus. The probability distribution of number of horses that are positive for both S. vulgaris and S. edentatus (X) is given by the following binomial distribution:
If the event is rare, and p is very small, then the binomial distribution can be approximated by the Poisson distribution. Hence, if λ=np, the distribution for X is given by
This is the probability mass function for the Poisson λ distribution. However, when Poisson regression is applied to binomial data, the error for the estimated relative risk will be overestimated, leading to reduced statistical power (Zocchetti et al. Reference Zocchetti, Consonni and Bertazzi1995). This problem may be addressed by using a robust error variance procedure, referred to as modified Poisson regression (Zou, Reference Zou2004). Following the work in Zou (Reference Zou2004), one can show that
where a and c correspond to the number of events with and without occurrence, respectively, which refers to the classical positions in the 2×2 table. Further, n 0 is the total number of horses with larval cultures negative for S. vulgaris and n 1 represents the total number of horses with S. vulgaris infection detected. The estimated variance is given by:
And its robust version is given by:
The robust error estimation can be implemented by using the genmod procedure with repeated statements in SAS version 9.3 (SAS Institute Inc., Cary, NC, USA).
Similar analyses were performed for other occurrence variables in the dataset: (i) usage of selective therapy on the farm, and (ii) anthelmintic treatment within the last 6 months prior to the study.
Validation by computer simulation
To validate the significance of the robust error modification, simulations were performed comparing the percentage of confidence interval (CI) coverage with the modified vs the unmodified Poisson regression. The field dataset had 663 observations, which can only allow for relatively small sample sizes with resulting wide CIs. Therefore, for the simulations, total sample sizes N were considered as 500 and 1000, with relative risk values of 1, 2 and 5. In each of the 1000 simulated datasets, n subjects were randomly assigned to the S. vulgaris positive and negative groups with the same probability of 0·5. The probability of getting an event (i.e. infection with S. edentatus or Triodontophorus spp.) in the occurrence group was set to be 0·2, while it was 0·2, 0·1 and 0·04, respectively, in the non-occurrence group. These values were chosen to reflect a range of realistic probabilities as observed in the field dataset reported in this article.
Poisson regression analyses were performed both with and without the robust error modification described in the previous section, and 95% CIs are constructed using the original standard error (referred to unmodified in the tables) and the robust version (referred to as modified in the tables). The procedure was repeated 1000 times for each setting of N and RR, and the analysis yielding the empirical CI coverage closest to 95% was considered the more accurate approach.
Relative risks of S. edentatus and Triodontophorus spp.
Using the modified Poisson regression described above, the risks of occurrence of S. edentatus and Triodontophorus spp. were evaluated relative to the presence of S. vulgaris, recent anthelmintic treatment and use of selective therapy. When the 95% CI for the generated RR included 1, results were considered non-significant.
RESULTS
Of the participating horses, 211 (32%) had been treated within the last 6 months prior to the study. Of these, only 14 (2%) were treated within the last 2 months. As previously reported, the prevalence of S. vulgaris in this dataset was 12% on the individual level, and 64% on the farm level (Nielsen et al. Reference Nielsen, Vidyashankar, Olsen, Monrad and Thamsborg2012). The corresponding prevalences reported in this paper for S. edentatus were 3% and 14%, respectively, while the prevalences were 12% and 58% for Triodontophorus spp. Strongylus equinus was not found in this study.
Table 1 presents the results of the computer simulations evaluating the coverage of the 95% CIs generated with the modified compared with the unmodified Poisson regressions. It can be seen that the modified analysis consistently yielded coverage closer to the 95% compared with the unmodified Poisson regression. Tables 2 and 3 present the outcomes of the modified Poisson regressions analysing the risks of the occurrence of S. edentatus and Triodontophorus spp., respectively, relative to the presence of S. vulgaris, recent anthelmintic treatment and use of selective therapy in the study population.
Table 1. Empirical coverage of the 95% confidence intervals generated by the modified Poisson regression compared with the unmodified version of the same analysis

Table 2. Risk of occurrence of Strongylus edentatus relative to presence of Strongylus vulgaris in individual horses, recent anthelmintic treatment and use of selective therapy on the farm
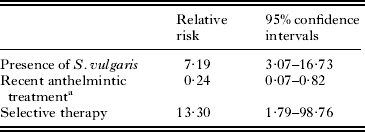
a Defined as treatment within 6 months prior to the study.
Table 3. Risk of occurrence of Triodontophorus spp. relative to presence of Strongylus vulgaris in individual horses, recent anthelmintic treatment and use of selective therapy on the farm

a Defined as treatment within 6 months prior to the study.
DISCUSSION
We have evaluated and illustrated the usefulness of a modified Poisson regression for analysing data for associations between sparsely occurring parasites in animal populations. Our data illustrate some of the possible associations between large strongyle species and the deworming regimens carried out on horse farms, and provide useful insight into the interpretation of larval culture results.
Both S. edentatus and Triodontophorus spp. were associated with presence of S. vulgaris, but only S. edentatus was statistically associated with selective therapy and anthelmintic treatment within the last 6 months. The reason for this is likely to be the large difference in prepatent periods between S. edentatus and Triodontophorus spp. The large majority of anthelmintic treatments carried out on the studied farms were ivermectin (Nielsen et al. Reference Nielsen, Vidyashankar, Olsen, Monrad and Thamsborg2012), which can be expected to have good larvicidal efficacy against migrating larvae of S. edentatus. With the very long prepatent period, just one yearly treatment would be enough to break the life cycle of S. edentatus, which can explain the much lower prevalence observed for this parasite. For Triodontophorus spp., however, the much shorter life cycle makes it unlikely to see any significant responses to the most recent treatment. This is simply because treatment intensities on the studied farms were too sparse to significantly impact the occurrence of this parasite. Thus, anthelmintic treatment within the past 6 months is unlikely to affect the occurrence of this parasite, while treatment within the past 2 months would be expected to have an effect. This study was carried out in the spring in Denmark after a winter period, where very few anthelmintic treatments are usually administered, which was illustrated by the fact that only 2% of horses had received anthelmintic treatment within the 2 months prior to the study.
These data illustrate that despite Triodontophorus spp. being a large strongyle, it epidemiologically behaves much like the cyathostomins. It is interesting, however, that it was associated with S. vulgaris without being related to the use of selective therapy. The scientific literature contains no reports of anthelmintic resistance in Triodontophorus spp., and there is no reason to speculate it playing a role in this dataset, as anthelmintic treatment intensities were very low. Of course, our findings could represent a chance association between the species, but there may be other explanations. For instance, horses harbouring S. vulgaris may exhibit a larger strongyle species diversity allowing for a higher abundance of Triodontophorus spp. The results may also represent a bias occurring when reading the larval cultures under the microscope. The identification of S. vulgaris larvae in a sample could have caused the operator to more critically scan the slide for other non-cyathostomin larvae leading to an apparent association between the species.
One practical implication of the results is that whenever S. vulgaris is identified on a farm, there is an increased likelihood to also find S. edentatus. As recently reported, the larval cultures for identifying either of these two species is characterized by high positive predictive values, but low to moderate negative predictive values (Nielsen et al. Reference Nielsen, Baptiste, Tolliver, Collins and Lyons2010a). This means that false negatives are likely to occur. However, if a finding of one Strongylus species can be interpreted as indicative of other Strongylus spp. being also present, the negative predictive value of the larval culture can be improved considerably for herd diagnosis.
As with the other large strongyle species, S. edentatus has not been convincingly reported resistant to anthelmintic treatment. One study illustrated an apparent loss of pyrantel efficacy (Coles et al. Reference Coles, Brown and Trembath1999), but it should be borne in mind that pyrantel efficacy always had significant variability (Lyons et al. Reference Lyons, Drudge and Tolliver1975) and that it has no activity against migrating stages. Therefore, treatment with a larvicidal drug should still be expected to effectively interrupt the life cycle of S. edentatus, and it can be argued that if a horse is positive for S. edentatus, it has been exposed to a deworming regimen that also allows for S. vulgaris to be present. Given the pathogenic potential and clinical implication of S. vulgaris, the primary reason for veterinary practitioners to perform larval cultures is to screen for presence of this parasite (Nielsen et al. Reference Nielsen, Monrad and Olsen2006), and a positive finding is likely to make the veterinarian adjust the deworming regimen on that particular farm. As other large strongyles have not been associated with specific severe parasitic disease complexes to the same extent as S. vulgaris, their mere finding in a larval culture is unlikely to lead to major changes in the parasite control programmes. Through the significant associations with S. vulgaris found in this study, there is now reason to consider findings of other large strongyles to be of potential clinical importance. Although similar larval culture-based surveys have not recently been performed in regions outside Denmark, results are likely to have relevance elsewhere as surveillance-based parasite control programmes are widely recommended with resulting lower anthelmintic treatment intensities (Kaplan and Nielsen, Reference Kaplan and Nielsen2010).
In summary, this study made use of a modified Poisson regression analysis for rare-event data to analyse the association between S. vulgaris and other large strongyle species in the field. The results illustrated a strong association between the occurrence of S. edentatus and S. vulgaris, which can be helpful for interpreting routine larval culture results on farms.
FINANCIAL SUPPORT
This study was funded by the Danish Research Council's Agency for Science, Technology and Production, grant number 274-08-0081.





