Introduction
Gastrointestinal helminths are responsible for significant production losses in field breeding systems. Anti-helminthic drugs represent the main form used for the control of helminths; however, the results in the reduction in the parasitic load of the animals are not always satisfactory and these drugs can leave residues in products for human consumption. The reports of the development of anthelmintic resistance are very frequent and thus, it is necessary to search for complementary methods in the control of helminths (Fazzio et al., Reference Fazzio, Sánchez, Streitenberger, Galvan, Giudici and Gimeno2014; Gasbarre, Reference Gasbarre2014).
The biological control of parasitic helminths of animals through the use of nematophagous fungi presents satisfactory results in several studies (Silva et al., Reference Silva, Braga, Araújo, Benjamim, Souza and Carvalho2011; Tavela et al., Reference Tavela, Araújo, Braga, Araujo, Magalhães, Silveira and Borges2012; Braga et al., Reference Braga, Araújo, Tavela, Vilela, Soares, Araujo and Queiroz2013; Oliveira et al., Reference Oliveira, Carvalho, Vieira, Campos, Freitas, Araujo, Braga and Araújo2018a, Reference Oliveira, Vieira, Carvalho, Campos, Freitas, Araujo, Braga and Araújo2018b). The ovicidal fungus Pochonia chlamydosporia parasites eggs of helminths through structures known as apressory, developed from undifferentiated hyphae, allow the colonization of the egg surface and the penetration by a mechanical and enzymatic action (Braga et al., Reference Braga, Araújo, Campos, Silva, Araujo, Carvalho, Corrêa and Pereira2008). There are reports in the literature that P. chlamydosporia, in addition to the ovicidal action, parasite females of nematodes are colonized and fully digested (Podestá et al., Reference Podestá, Dallemole-Giaretta, Freitas, Lopes, Ferraz and Zooca2009; Zouhar et al., Reference Zouhar, Douda, Novotny, Novakova and Mazakova2010). The predatory fungus Arthrobotrys cladodes produces tridimensional traps that promote adhesion, immobilization, penetration and consequently, the destruction of helminth larvae (Oliveira et al., Reference Oliveira, Carvalho, Vieira, Campos, Freitas, Araujo, Braga and Araújo2018a).
Ovicidal and predator nematophagous fungi have distinct mechanisms of action, thus, when used together, they may present a complementary and synergistic action in the biological control of helminths. However, the joint growth of distinct nematophagous fungi may not be feasible, so it is necessary to know the interaction between them through compatibility studies (Ayupe et al., Reference Ayupe, Monteiro, Braga, Soares, Mello, Araujo, Freitas and Araújo2016). The objective of this work was to evaluate the growth compatibility of fungi P. chlamydosporia and A. cladodes, as well as to evaluate the predation capacity of these fungi associated and separately.
Material and methods
The fungi P. chlamydosporia (VC4 isolate) and A. cladodes var macroides (CG719 isolate) used in the assays were maintained in the dark at 4 °C, in test tubes containing 2% corn-meal-agar, in the Laboratory of Parasitology of the Department of Veterinary Medicine of the Federal University of Viçosa, Minas Gerais, Brazil.
Test of antagonism in direct confrontation
5 mm diameter discs containing P. chlamydosporia mycelium were placed at a distance of 1 cm from the border of the Petri dishes (9 cm in diameter) containing 2% potato-dextrose-agar medium (2% PDA). The plates were stored in the dark at 26 °C, for 10 days. After this period, A. cladodes mycelial discs were placed on the plates opposite the P. chlamydosporia colony. For the control group, colonies of the same fungus were confronted. The colonies were incubated in the dark at 26 °C, for 8 days. For the evaluations, the adapted scale of notes, proposed by Bell et al. (Reference Bell, Wells, Markhabell and Wells1982) was used: 1 – complete colonization of the plaque by P. chlamydosporia; 2 – colonization of 2/3 of the plaque by P. chlamydosporia; 3 – colonization of 50% of the plaque per fungus; 4 – colonization of 2/3 of the plaque by A. cladodes; 5 – complete colonization of the plaque by A. cladodes. Ten replicates were performed per treatment.
Antibiosis test
The antibiosis test followed the methodology described by Martins-Corder and Melo (Reference Martins-Corder and Melo1998), modified by the use of 2% PDA medium (Ferreira et al., Reference Ferreira, Ferraz, Lopes and Freitas2008). On the surface of the 2% PDA culture medium, in 9 cm diameter Petri dishes, dialysis membrane discs (SIGMA®) were placed. Subsequently, disks of 5 mm in diameter of mycelium of P. chlamydosporia and A. cladodes were placed separately in the centre of distinct plates. Colonies were incubated in the dark at 26 °C, for 96 h. The growth area of the colonies was demarcated externally at the bottom of the plates and the dialysis membrane, along with the respective colony, was removed. Then, the plates were inverted and 1 mL of chloroform was added to the lower part in order to eliminate the possible structures of the fungus. After the evaporation of the product, the plates were left for 30 min under direct irradiation of ultraviolet light in a laminar flow chamber. Then, an aqueous suspension containing P. chlamydosporia mycelium was added on the surface of the culture medium on the plates where the fungus A. cladodes was grown and a suspension of A. cladodes where P. chlamydosporia was grown. These suspensions were obtained from colonies previously grown in 2% PDA culture medium in the dark at 26 °C, for 10 days. The control group consisted of cultivation of P. chlamydosporia and a subsequent suspension culture containing mycelium of P. chlamydosporia, being equal for the control group of A. cladodes. The plates were kept in the dark at 26 °C, for 10 days and after this period it was observed if there was the formation of an inhibition halo in the growth of P. chlamydosporia formed by A. cladodes, or vice versa. Ten replicates were performed per treatment.
Effect of volatile compounds
Petri dish covers of 9 cm in diameter, containing 2% PDA culture medium, were positioned one above the other according to the technique described by Bharat et al. (Reference Bharat, Singh and Singh1980), modified by the use of 2% PDA medium (Ferreira et al., Reference Ferreira, Ferraz, Lopes and Freitas2008). A 5 mm diameter disc containing A. cladodes mycelium was added to the lower plate and a 5 mm diameter disc containing P. chlamydosporia mycelium was added to the top plate. In another treatment, a 5 mm diameter disc containing P. chlamydosporia mycelium was added to the lower plate and a 5 mm diameter disc containing A. cladodes mycelium was added to the top plate. For the control group, P. chlamydosporia was grown in the lower and upper plaques, the same procedure was used to control A. cladodes. The plates were laterally sealed with a plastic membrane (Parafilm M®) and held in the dark at 26 °C, for 15 days. For the evaluations, a measurement of the area of the colonies and comparison with the control was performed. Ten replicates were performed per treatment and the areas of the colonies were compared statistically by the Student's t-test at a significance level of 5%.
Predatory capacity of the association of nematophagous fungi on infective larvae
Gastrointestinal nematode larvae were obtained from naturally contaminated bovine feces collected at a farm, with no history of antihelmintic resistance, in the city of Abre Campo, state of Minas Gerais, southeastern Brazil, latitude 20°18′04″S, longitude 42°28′39″W. Coprocultures were made with 20 g of feces from the animals mixed with vermiculite and kept in an oxygen demand chamber for 12 days. After this period, the infective larvae (L3) were recovered using the Baermann funnel technique, with water at 42 °C for 6 h and identified according to the criteria of Keith (Reference Keith1953). The percentages of the L3 recovered were 70.05, 19.23 and 10.72%, respectively, for the genera Haemonchus, Cooperia and Oesophagostomum.
Forty Petri dishes with 9 cm in diameter containing 2% water-agar (WA 2%) were divided into four groups: ‘CG719’ (A. cladodes), ‘VC4’ (P. chlamydosporia), ‘CG719 + VC4’ (A. cladodes and P. chlamydosporia, grown in association) and control (plaques containing only 2% WA). Each treated group consisted of 10 plaques, with the previously grown fungal isolates. 1000 L3 was poured into each plate. The control group consisted of 10 other plaques with 2% agar-water and 1000 L3. The plates were kept in an oxygen demand chamber oven in the dark at 25 °C. On the 7th day, the L3 were recovered with the aid of the Baermann funnel, with water at 42–45 °C and for 12 h waiting for decantation. The L3 were counted and identified, following the criteria of Keith (Reference Keith1953), obtaining the average of the recovered larvae of each group.
The percentage reduction of larvae of the treated groups in relation to the control was calculated according to the formula:
 $${\rm \%\ reduction}={\rm } \displaystyle{{\matrix{({\rm mean\ control\ group\ larvae\ -\ }\cr {\rm mean\ group\ treated\ larvae})} \over {{\rm mean\ of\ control\ group\ larvae}}}}\, \times \,100$$
$${\rm \%\ reduction}={\rm } \displaystyle{{\matrix{({\rm mean\ control\ group\ larvae\ -\ }\cr {\rm mean\ group\ treated\ larvae})} \over {{\rm mean\ of\ control\ group\ larvae}}}}\, \times \,100$$The L3 means recovered, the percentages of each L3 genus and the reduction percentages were transformed into log (x + 1) and compared by the Tukey's test at a significance level of 5%.
Results
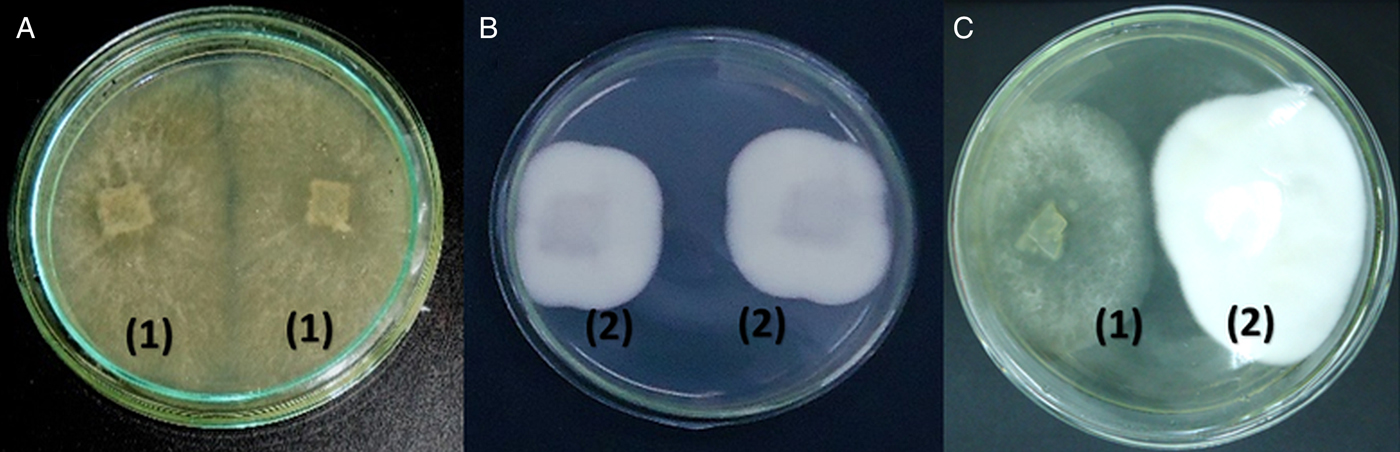
Figure 1 shows the results of the direct confrontation test performed with the tested fungi. Pochonia chlamydosporia and A. cladodes presented homogeneous growth in the direct confrontation tests, with no overlap of the colonies and none of the fungi confronted inhibited the growth of the other (Fig. 1c). In the scale of notes adapted, the test presented note ‘3’ (colonization of 50% of the plate for each fungus).

Fig. 1. Direct confrontation test: (a) Arthrobotrys cladodes (1) vs. A. cladodes (1); (b) Pochonia chlamydosporia (2) vs. P. chlamydosporia (2); (c) A. cladodes (1) vs. P. chlamydosporia (2).
In the antibiosis tests, there was no inhibition of halo formation among the nematophagous fungi, so P. chlamydosporia and A. cladodes did not produce any compounds and did not compete directly for nutrients to the point of inhibiting their growth together.
In Table 1, the mean values of the mycelial growth area in the upper parts of the plaques of the P. chlamydosporia and A. cladodes fungi submitted to the volatile metabolite effect test are presented. There was no difference in the mycelial growth area of any of the tested isolates (P ⩽ 0.05).
Table 1. The mean values and standard deviation of the mycelial growth area, in the upper Petri dishes, 9 cm in diameter, containing 2% potato-dextrose-agar (2% PDA), for 15 days, of the fungi Pochonia chlamydosporia (VC4 isolate) and Arthrobotrys cladodes (CG719 isolate) submitted to the test of the effect of volatile compounds

Different letters in the same column indicate the difference between the data (P ⩽ 0.05). CG719: Arthrobotrys cladodes; VC4: Pochonia chlamydosporia.
Table 2 shows the mean values of the number of infective larvae (L3) of bovine gastrointestinal parasitic nematodes recovered from plaques with 2% WA medium, containing the association of the fungi A. cladodes and P. chlamydosporia (CG719 + VC4), the fungus P. chlamydosporia (VC4) and fungus A. cladodes (CG719), the latter isolated, as well as the percentage distribution of the recovered L3 genera. The three groups that contained the nematophagous fungi showed lower values of recovered L3 compared to the control group without fungus (P ⩽ 0.05). The group that contained the associated A. cladodes and P. chlamydosporia had the lowest L3 recovery (30.17) among the groups. The group containing A. cladodes alone presented a lower L3 recovery (75.17) compared to the group containing P. chlamydosporia 130.67).
Table 2. The average values and standard deviations of the number of infective larvae (L3) recovered from plaques with 2% water-agar medium (WA2%), after 7 days of interaction, containing the association of the nematophagous fungi Arthrobotrys cladodes and Pochonia chlamydosporia (CG719 + VC4), P. chlamydosporia (VC4) and A. cladodes (CG719), as well as the percentage distribution of the recovered L3 genera

Different letters in the same column indicate the difference between the data (P ⩽ 0.05). CG719: Arthrobotrys cladodes; VC4: Pochonia chlamydosporia.
The percentage reduction in the L3 of the group containing the association of A. cladodes and P. chlamydosporia (92.67%) was higher than the percentage of the group containing A. cladodes (81.73%), which was greater than the percentage of the group containing P. chlamydosporia (68.25%), as shown in Fig. 2.

Fig. 2. The percentage reduction of the infective larvae (L3) of gastrointestinal parasitic nematodes recovered from plaques with 2% water-agar medium (2%WA), after 7 days of interaction, with an association of the fungi Arthrobotrys cladodes and Pochonia chlamydosporia (CG719 + VC4), P. chlamydosporia (VC4) and A. cladodes (CG719). Different letters indicate the difference between the values (P < 0.05).
The percentage of the L3 of the genus Haemonchus was lower in the groups treated with nematophagous fungi than in the control group without the fungus (Table 2). The group that contained the association between A. cladodes and P. chlamydosporia presented a higher percentage of the L3 of the genus Haemonchus (49.71%) than the group containing only A. cladodes (38.18%). However, the group containing only P. chlamydosporia showed no difference in the percentage of the L3 of the genus Haemonchus compared to the groups that contained the association of fungi nematófagos and the group containing only A. cladodes.
The percentages of the L3 of the genus Cooperia did not vary among the four groups. The percentage of the L3 of the genus Oesophagostomum was higher in the groups treated with nematophagous fungi than in the control group without the fungus (Table 2). The group that contained the fungi association had a lower percentage of the L3 of the genus Oesophagostomum (24.10%) than the group containing only A. cladodes (35.68%). However, the group containing only P. chlamydosporia showed no difference in the percentage of the L3 of the genus Oesophagostomum compared to the groups that contained the association of fungi and the group containing only A. cladodes.
Discussion
The nematicidal activity of fungi of the genera Arthrobotrys and Pochonia on populations of parasitic helminths is demonstrated in several studies. In view of the good results with fungal isolates of these genera, the present study evaluated the compatibility of joint growth between A. cladodes and P. chlamydosporia, as well as the joint and isolated action of these fungi on L3 of bovine parasitic helminths.
The direct confrontation tests performed did not demonstrate joint growth incompatibility between P. chlamydosporia and A. cladodes. Compatibility tests with other isolates of nematophagous fungi obtained conflicting results. Ferreira et al. (Reference Ferreira, Ferraz, Lopes and Freitas2008) reported the joint growth compatibility between P. chlamydosporia and Trichoderma spp. submitted to the direct confrontation test. However, Ayupe et al. (Reference Ayupe, Monteiro, Braga, Soares, Mello, Araujo, Freitas and Araújo2016), when evaluating the growth of Arthrobotrys robusta and Duddingtonia flagrans by the direct confrontation test, verified that A. robusta colonized approximately 2/3 of the plaque and D. flagrans colonized 1/3, suggesting competition and antagonism between them.
Ferreira et al. (Reference Ferreira, Ferraz, Lopes and Freitas2008) and Ayupe et al. (Reference Ayupe, Monteiro, Braga, Soares, Mello, Araujo, Freitas and Araújo2016) performed antibiosis tests, respectively, between P. chlamydosporia and Trichoderma spp., and between D. flagrans and A. robusta and they verified that the fungi did not produce metabolites capable of inhibiting the growth of them together. In the present work, we also did not verify the production of volatile metabolites by P. chlamydosporia and A. cladodes that inhibit the joint growth of these fungi.
In our study, the test of the effect of volatile metabolites did not demonstrate joint growth incompatibility between P. chlamydosporia and A. cladodes, however, Ferreira et al. (Reference Ferreira, Ferraz, Lopes and Freitas2008) and Ayupe et al. (Reference Ayupe, Monteiro, Braga, Soares, Mello, Araujo, Freitas and Araújo2016) reported some kind of incompatibility among the fungi studied by them. Ferreira et al. (Reference Ferreira, Ferraz, Lopes and Freitas2008) did not observe the production of volatile compounds of P. chlamydosporia against Trichoderma spp., but reported that Trichoderma spp. reduced the growth of P. chlamydosporia by producing volatile compounds. In the work of Ayupe et al. (Reference Ayupe, Monteiro, Braga, Soares, Mello, Araujo, Freitas and Araújo2016), the isolate of A. robusta reduced the growth of D. flagrans in the test of the effect of volatile metabolites but D. flagrans did not reduce the growth of A. robusta.
The results of the compatibility tests performed in this work, as well as the results of the other authors cited, show that it is important to evaluate the compatibility between specific isolates and that the extrapolation of the results to other isolates of the same species or associations would probably not be possible.
In the in vitro test of the efficacy of the L3 predation, the three groups treated with nematophagous fungi showed lower values of the recovered L3 compared to the control group (without fungus), which indicates the efficacy in the predation of bovine gastrointestinal parasitic nematodes by the fungi tested.
According to Oliveira et al. (Reference Oliveira, Carvalho, Vieira, Campos, Freitas, Araujo, Braga and Araújo2018a), A. cladodes presents a mechanism of nematicidal action based on the production of traps that promote adhesion, penetration and destruction of larvae, which justifies the predatory capacity on L3 of bovine parasitic helminths verified in our study.
Braga et al. (Reference Braga, Araújo, Campos, Silva, Araujo, Carvalho, Corrêa and Pereira2008) reported that P. chlamydosporia parasites eggs of helminths through the colonization of the egg surface and the penetration by a mechanical and enzymatic action, however, in our study we did not evaluate the ovicidal action of this fungus but its larvicidal activity. Our results indicate that P. chlamydosporia presents nematicidal action on larvae of parasitic helminths, as well as in the work of Podestá et al. (Reference Podestá, Dallemole-Giaretta, Freitas, Lopes, Ferraz and Zooca2009) and Zouhar et al. (Reference Zouhar, Douda, Novotny, Novakova and Mazakova2010). The mechanisms of the larvicidal action of P. chlamydosporia were not elucidated.
Ranjbar-Bahadori et al. (Reference Ranjbar-Bahadori, Rhazzagi-Abyaneh, Baya, Eslami, Pirali, Shams-Ghahfarokhi and Lotfollahzadeh2010) reported that A. cladodes was effective in reducing the infective larvae of Haemonchus contortus and the percentage reduction in the L3 was 78.8%. Braga et al. (Reference Braga, Araújo, Tavela, Vilela, Soares, Araujo and Queiroz2013) reported that A. cladodes was efficient in predating the L3 of Libyostrongylus douglassii (ostrich gastrointestinal parasite nematode) and presented a reduction percentage of 89.2% in relation to the control group without the fungus. Oliveira et al. (Reference Oliveira, Carvalho, Vieira, Campos, Freitas, Araujo, Braga and Araújo2018a) reported an in vitro percentage of 68.7% reduction of the L3 of bovine gastrointestinal parasitic nematodes due to the predatory action of A. cladodes. In the present study, the group containing only A. cladodes showed 81.73% reduction of the L3 parasites of bovines, being higher than the value found by Oliveira et al. (Reference Oliveira, Carvalho, Vieira, Campos, Freitas, Araujo, Braga and Araújo2018a) and similar to the values reported by Ranjbar-Bahadori et al. (Reference Ranjbar-Bahadori, Rhazzagi-Abyaneh, Baya, Eslami, Pirali, Shams-Ghahfarokhi and Lotfollahzadeh2010) and Braga et al. (Reference Braga, Araújo, Tavela, Vilela, Soares, Araujo and Queiroz2013).
The group containing only P. chlamydosporia demonstrated a predatory capacity of 68.25% of the parasite L3 of cattle. Zouhar et al. (Reference Zouhar, Douda, Novotny, Novakova and Mazakova2010) evaluated the virulence of P. chlamydosporia in phytopathogenic species and the mortality rates due to the action of P. chlamydosporia on the nematodes Globodera rostochiensis and Meloidogyne hapla were, respectively, 20.0 and 39.0%, which were lower than the value found in our study. However, in the study by Silva et al. (Reference Silva, Braga, Araújo, Benjamim, Souza and Carvalho2011), there was no predation of the L3 of H. contortus by P. chlamydosporia fungus.
There are no reports in the literature of works that evaluated the capacity of the predation of the L3 by the association of fungi A. cladodes and P. chlamydosporia. However, Tavela et al. (Reference Tavela, Araújo, Braga, Araujo, Magalhães, Silveira and Borges2012) also associated predatory (D. flagrans, AC001 isolate and M. thaumasium, NF34 isolate) and ovicidal (P. chlamydosporia, VC1 isolate) nematophagous fungi and evaluated their effects against eggs and the L3 of cyathostomes. The following percentage reductions compared to the control group were observed by such authors: AC001 + VC1, 86.8%; NF 34 + VCl, 77.3%. Although the fungus and the L3 were different species, the percentage of reduction of the association between P. chlamydosporia and A. cladodes (92.67%) in the present study was higher than that of the associations made by Tavela et al. (Reference Tavela, Araújo, Braga, Araujo, Magalhães, Silveira and Borges2012).
There is variation between nematophagous fungus larvae predation values when comparing the results obtained and those cited by other authors. Mendoza-de-Gives et al. (Reference Mendoza-de-Gives, Davies, Clark and Behnke1999), Oliveira et al. (Reference Oliveira, Carvalho, Vieira, Campos, Freitas, Araujo, Braga and Araújo2018a) suggests that the differences in nematophagous fungi predation results are due to the particular structure and the composition characteristics of the nematode cuticle, or due to the antigenic variations in the different nematode species or to variations among nematode isolate species of fungus.
The lower percentages of L3 recovered from the genus Haemonchus in the fungus treated groups compared to the control group (without fungus), suggest a higher selectivity of the predation of larvae of the genus Haemonchus by the fungi used in the present experiment. However, Oliveira et al. (Reference Oliveira, Carvalho, Vieira, Campos, Freitas, Araujo, Braga and Araújo2018a), when evaluating the predatory capacity of the L3 parasites of cattle by the fungus A. cladodes, found no difference in the percentages of Haemonchus sp., Cooperia sp. and Oesophagostomum sp. recovered.
The results indicated that the growth of P. chlamydosporia and A. cladodes together is feasible, since none of the fungi caused inhibition or antagonism to the growth of the other. The association of nematophagous fungi A. cladodes (CG719) and P. chlamydosporia (VC4) showed a higher percentage of L3 reduction than fungi used alone and proved to be effective in the biological control of gastrointestinal nematodes of bovines. Thus, work evaluating the effectiveness of this association of fungi on the biological control of helminths under environmental conditions can be developed.
Author ORCIDs
Ítalo Stoupa Vieira, 0000-0002-3847-1882.
Acknowledgement
The authors thank ‘Coordenação de Aperfeiçoamento de Pessoal de Nível Superior’ (CAPES), ‘Conselho Nacional de Desenvolvimento Científico e Tecnológico’ (CNPq) and ‘Fundação de Amparo à Pesquisa do Estado de Minas Gerais’ (FAPEMIG) for support in this study, in the form of a doctoral scholarship.
Conflict of interest
None.
Ethical standards
Not applicable.