Introduction
Virulence, the severity and harmfulness of a disease, comprises the myriad mechanisms by which a parasite can reduce its host's fitness (Clayton and Moore, Reference Clayton and Moore1997). The reduction in fitness, reflected in the diminution of the host's reproductive success, can occur through indirect means such as death, debilitation, or behavioural changes and directly through diminished reproductive performance. Macroparasites such as the diphyllobothridean cestode Schistocephalus solidus usually have more chronic, hence progressive, effects on their hosts than do microparasites (Clayton and Moore, Reference Clayton and Moore1997). Moreover, severe pathologies can occur in intermediate hosts infected by parasites whose complex life cycles involve transmission through predation (trophic transmission; Ewald, Reference Ewald1994), such as the threespine stickleback fish, Gasterosteus aculeatus, infected by S. solidus. The life cycle of S. solidus begins with a free-living coracidium larva when it is eaten by cyclopoid copepod (first intermediate host) which is consumed by a threespine stickleback (second intermediate host); the stickleback, in turn, is ingested by a piscivorous bird (definitive host) in which the parasite reproduces (Smyth, Reference Smyth1962).
The completion of complex parasitic life cycles depends upon exploitation of one or more intermediate hosts. Two recent investigations have presented data suggesting that single infections of S. solidus exploiting host fish are more harmful to their hosts than are multiple infections. In a laboratory experiment on selfing and outcrossing in S. solidus, Christen and Milinski (Reference Christen and Milinski2003) found that body condition of stickleback infected with one parasite was significantly lower than in multiply infected hosts. During the experiment, fish with single infections experienced a significant decrease in condition while those with multiple infections did not show a significant decrease (Christen and Milinski, Reference Christen and Milinski2003). In a field-based study, Nordeide and Matos (Reference Nordeide and Matos2016) found that solo infections of S. solidus in stickleback resulted in lower host body condition than multiple infections. Thus, host exploitation at different parasite intensities potentially can lead to differential effects on hosts.
Host exploitation by S. solidus also has consequences for parasite growth and fitness (Wedekind et al., Reference Wedekind, Strahm and Schärer1998; Heins et al., Reference Heins, Baker and Martin2002; Barber, Reference Barber2005). Strategies of parasite growth have received attention in recent theoretical and empirical investigations. Parker et al. (Reference Parker, Chubb, Roberts, Michaud and Milinski2003) provided predictions for two theoretical models of larval growth in helminths: resource constraints hypothesis (RC) and life history strategy hypothesis (LHS). These models attempt to inform us about the influence of intensity on parasite growth. They predict the maximum volume of individual parasites and the combined total volume of all parasites within one host (Parker et al., Reference Parker, Chubb, Roberts, Michaud and Milinski2003; Michaud et al., Reference Michaud, Milinski, Parker and Chubb2006). The two models differ importantly on assumptions about the ability of parasites within one host to detect conspecifics and to alter their own growth with respect the presence of other individuals in the same host. Parasites might use available resources for growth without influence of the presence and number of conspecifics as modelled by the RC hypothesis (Parker et al., Reference Parker, Chubb, Roberts, Michaud and Milinski2003; Michaud et al., Reference Michaud, Milinski, Parker and Chubb2006). Alternatively, individual parasites could detect the presence of one or more conspecifics and adjust their maximal growth in relationship to other parasites in the host, which is modelled by the LHS hypothesis (Parker et al., Reference Parker, Chubb, Roberts, Michaud and Milinski2003; Michaud et al., Reference Michaud, Milinski, Parker and Chubb2006). Both hypotheses predict that the maximal volume of individual parasites decreases with intensity. Under the RC hypothesis, the total volume of all parasites does not vary with intensity; however, LHS model predicts that the total volume increases with intensity (Parker et al., Reference Parker, Chubb, Roberts, Michaud and Milinski2003; Michaud et al., Reference Michaud, Milinski, Parker and Chubb2006). Michaud et al. (Reference Michaud, Milinski, Parker and Chubb2006) performed experimental tests of these hypotheses using S. solidus in copepods and found their results were consistent with the LHS hypothesis.
Herein, we present the results of a natural experiment on body condition and clutch size (CS) (egg number) of threespine stickleback infected with single vs multiple larval parasites (plerocercoids) of S. solidus. Our goal is to ask whether intensity has an effect on host fitness, using a large dataset from samples taken over a number of years from one lake. Body condition should have indirect effects on host fitness through mortality and reproductive performance. CS serves as a direct measure of the reproductive output (fecundity) of female fish. Additionally, we ask whether our results for individual parasite mass and total parasite mass are consistent with either the RC or LHS hypothesis for parasite growth in stickleback.
Materials and methods
Sampling and study site
Collections of threespine stickleback were obtained from Walby Lake (61.6198 N, 149.2118 W) in the Matanuska–Susitna (Mat–Su) Valley of southcentral Alaska in 8 years for which we have samples suitable for this study: 1993, 1996, 1998 and 1999–2003. The datasets were drawn from those used in prior studies, including but not necessarily limited to Heins et al. (Reference Heins, Singer and Baker1999), Heins and Baker (Reference Heins and Baker2003), Heins et al. (Reference Heins, Baker, Toups and Birden2010a) and Heins (Reference Heins2012). The dates of annual sampling varied between 22 and 31 May, which was at the height of the annual breeding season not long after it had begun (Heins et al., Reference Heins, Singer and Baker1999).
Gee-type metal traps (3- or 6-mm mesh) with funnels at both ends were set near the shore at approximate intervals of 5–10 m. Captured fish taken for study were anaesthetised until quiescent in MS222 before fixation and subsequent storage in 10% formalin until examination. Fish not taken as specimens were returned to the lake. Females examined in this study typically were 2-year-old fish that had been infected during their first year of life (Heins et al., Reference Heins, Singer and Baker1999, Reference Heins, Eidam and Baker2016).
The Mat–Su Valley encompasses the Matanuska and Susitna river valleys and the intervening area north of the Cook Inlet. Walby Lake is one of many lakes and ponds dotting the glacial moraine in the Valley. Lakes in the Mat–Su Valley usually are covered with ice from October to May (Woods, Reference Woods1985).
Data gathering
Specimens of stickleback were measured to the nearest 0.1 mm standard length (SL) and then dissected to determine sex and reproductive condition and to remove any S. solidus plerocercoids. Female threespine stickleback, the experimental organisms used in this study, were classified into reproductive stages following Baker et al. (Reference Baker, Foster, Heins, Bell and King1998) and Heins et al. (Reference Heins, Singer and Baker1999): latent (LA), early maturing (EM), late maturing (LM), mature (MA), ripening (MR) and ripe (RE). Additionally, we were able to divide the MA stage in to early and late stages based on observable changes the developing oocytes; the early stages were classified MA and the late stages LMA. During the breeding season, females produce multiple clutches. The classification scheme incorporates the ‘clutch-production cycle’ (Heins and Baker, Reference Heins and Baker1993; Brown-Peterson and Heins, Reference Brown-Peterson and Heins2009) as sexually mature females cycle repeatedly among LM, MA, LMA, MR and RE stages during the spawning season. Females with ovaries classified as LA are sexually immature. Females with ovaries that are EM and LM are sexually mature because they were apparently going to produce one or more clutches that season, but when the sample was taken they did not possess a discernable clutch. Females with MA, LMA, MR and RE ovaries were both sexually and reproductively mature.
CS was determined by direct counts after separating out all enlarged oocytes or eggs, which were discernable in the ovaries of MA, LMA, MR and RE females (Heins and Baker, Reference Heins and Baker1993). During each spawning bout, females ovulate all ripening oocytes in each clutch and then oviposit all of the ripe eggs (Wootton, Reference Wootton1976; Bakker and Mundwiler, Reference Bakker and Mundwiler1994; Brown-Peterson and Heins, Reference Brown-Peterson and Heins2009). Thus, the count of the number of eggs in each clutch represents the actual CS. A small number of fish with extreme pathologically small ‘clutches’ in their follicles were not used in subsequent analyses of clutch characteristics, but they were used in analyses of body condition. These females might not have been able to spawn the few developing oocytes in the ovaries and might not have been able to produce another ‘clutch’. Although we classified them as ‘LM’ given the overall condition of the ovaries, they could have been classified as ‘non-reproductive’ because they were clearly debilitated in comparison to all other parasitised females with clutches. We describe them here, however, in the interest of repeatability.
Following dissection, carcasses of eviscerated fish (all contents of body cavity removed, excepting kidneys) were weighed to the nearest 0.001 g after they were blotted with a paper towel to measure somatic body mass (BM). Plerocercoids from each host were counted directly in a watch glass using a binocular microscope. The weighable parasites (greater than ca. 1 mg) were removed from the watch glass and weighed together to the nearest milligram after they were blotted individually. We estimated the mass of each un-weighable parasite (less than ca. 1 mg) to be 0.5 mg, based on individual measurements of mass for a number of small parasites on a more sensitive, precise balance. For each infected fish, the total estimated mass of the unweighable parasites was added to the mass of those that were weighed. The combined parasite:host biomass ratio (PI, parasite index; expressed as a percentage) for each host was calculated using the formula PI = PM/BM, where PM is the total weight of all parasites and BM is the mass of the eviscerated carcass (Arme and Owen, Reference Arme and Owen1967; LoBue and Bell, Reference LoBue and Bell1993; Tierney et al., Reference Tierney, Huntingford and Crompton1996). PI was used as a metric for severity of infection because parasite biomass should be related to nutrient theft, and the ratio should be related to pathology arising from nutrient loss (Hurd, Reference Hurd2001). Moreover, trophically transmitted parasites such as S. solidus should show intensity-independent effects on the host, with the full extent of pathology expected in single infections as well as in multiple ones (Lafferty and Kuris, Reference Lafferty and Kuris2002; Kuris, Reference Kuris2003; Fogelman et al., Reference Fogelman, Kuris and Grutter2009).
We used blotted wet weight instead of dry weight (Nordeide and Matos, Reference Nordeide and Matos2016) in our analyses of body condition, as well as for analyses of reproductive traits. Nordeide and Matos (Reference Nordeide and Matos2016) found that using wet weights gave similar results to analyses using dry weights. Moreover, we were unable to duplicate the results of Nordeide and Matos (Reference Nordeide and Matos2016) who found increased water content in the muscle mass of infected fish; however, our methods for assessing water content differed from theirs. Given concerns about using derived variables such as ratios (Sokal and Rohlf, Reference Sokal and Rohlf1995), we determined the water loss of a subset of eviscerated female specimens by subtracting dry weight from blotted wet weight. High drying temperatures can cause the loss of lipids (Baker and Heins, Reference Baker and Heins1994). Thus, we dried carcasses to constant weight at 40 °C (Baker and Heins, Reference Baker and Heins1994). Using analysis of covariance (ANCOVA) (covariate, log10SL), we tested whether the water lost in infected females was greater than in uninfected females. Our model accounted for 96% of the variance (r 2 = 0.958, n = 147). The effect of S. solidus was non-significant (F = 0.140; df = 1, 144; P = 0.709), the water loss being 0.214 g (n = 75) among infected females and 0.216 g among uninfected females.
Data analysis
The effect of parasite presence or absence on BM was quantified using an ANCOVA on the natural log-transformed BM with the natural log-transformed SL3 [model: ln(BM) ~ ln(SL3) + Infected(Y/N)]. Fish body condition was standardised for fish size in our analyses because fish BM is dependent upon SL. We used the residuals from a linear regression of the natural log-transformed eviscerated BM against the natural log-transformed SL3 as a measure of body condition.
A Pearson's χ 2 test was used to determine if non-mature (LA, EM, LM) or mature (MA, LMA, MR, RE) female fish were more likely to have parasites. Maturation stages that influenced the differences between non-mature and mature fish were identified by conducting a Kruskal–Wallis χ 2 test and a post hoc Dunn's test for multiple comparisons with Bonferonni corrections.
Redundancy analysis (RDA) – a multiple linear regression ordination method (Rao, Reference Rao1964) – was used to determine the relative influence of PI, intensity, date of collection and stage of sexual maturity on host body condition and CS in hosts separately. RDAs were performed in the vegan package for R (Oksanen et al., Reference Oksanen, Blanchet, Kindt, Legendre, Minchin, O'Hara, Simpson, Solymos, Stevens and Wagner2016) and estimated the adjusted coefficient of determination (R adj2) for each explanatory variable. We used forward stepwise model selection with AIC to improve the fit of each model and to reduce the likelihood of type I errors. Statistical significance of each predictor was determined using permutation tests to compare observed and randomised model R adj2. Since PI and intensity were correlated (Pearson's correlation: 0.40, P < 0.0001), we conducted variance partitioning with partial RDAs to estimate the variance in host body condition that is independently explained by each variable in the best-fit RDA model (Legendre, Reference Legendre2008; Peres-Neto and Legendre, Reference Peres-Neto and Legendre2010).
To test predictions of RC and LHS, we used mean parasite mass (total mass/intensity) as a proxy for individual parasite volume and total parasite mass as a proxy for maximum parasite volume (Parker et al., Reference Parker, Chubb, Roberts, Michaud and Milinski2003). Parasite mean mass and total mass were standardised for fish size (SL) as the residuals from a linear regression of natural log-transformed mean parasite mass or total parasite mass against the natural log-transformed fish SL3.
Results
Parasite infections
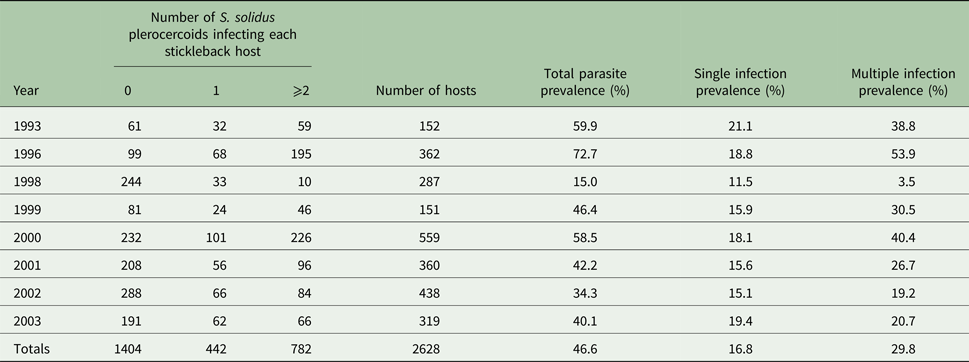
A total of 2628 stickleback were examined for this study (Table 1). The prevalence of plerocercoids in stickleback hosts (n = 1224) varied from 15 to 73% among years. Overall, prevalence averaged 46.6% while the prevalence of single infections averaged 16.8% and the prevalence of multiple infections averaged 29.8%. The intensity of infections ranged from 1 to 99 and averaged 5.08 plerocercoids. PI of infected females ranged from 0.013 to 112% and averaged 13.30%.
Table 1. Categorical count (0 parasites, 1 parasite, 2 or more parasites) and percent prevalence of Schistocephalus solidus infections (overall prevalence regardless of single vs multiple infection, single infection only prevalence, multiple infection only prevalence) in threespine stickleback 1993–2003

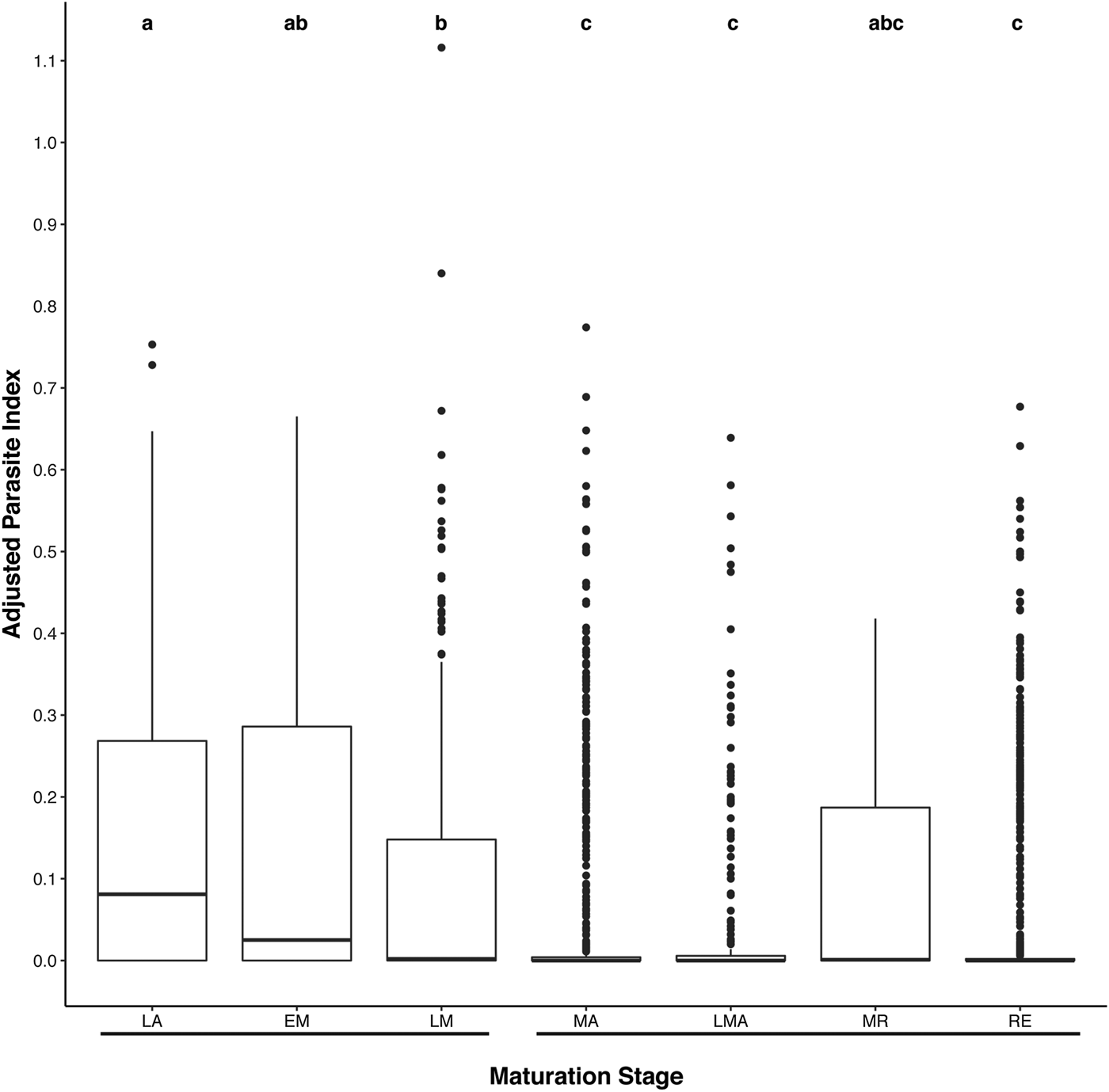
ANCOVA showed that uninfected fish weighed less for their lengths than infected ones (F 1, 2625 = 4.82, P = 0.028). The mean length-adjusted mass of uninfected fish was 0.802 g, whereas the mean length-adjusted mass of infected fish was 0.808 g. Pearson's χ 2 showed that non-mature fish were more likely to have parasites than mature fish (χ 2df=1 = 113.04, P < 0.0001). Kruskal–Wallis showed that there is a difference in the PI amongst maturation stages (χ2df=7 = 209.44, P < 0.0001). Dunn's post hoc test showed individuals in the LA stage having the highest PI and those in a MA, LMA and RE stages with the lowest PIs (Fig. 1).

Fig. 1. Box and whisker plots of Kruskal–Wallis χ 2 test for adjusted parasite index of non-clutch bearing and clutch bearing fish with Dunn's post hoc test; non-overlapping letters are significantly different from each other.
Body condition
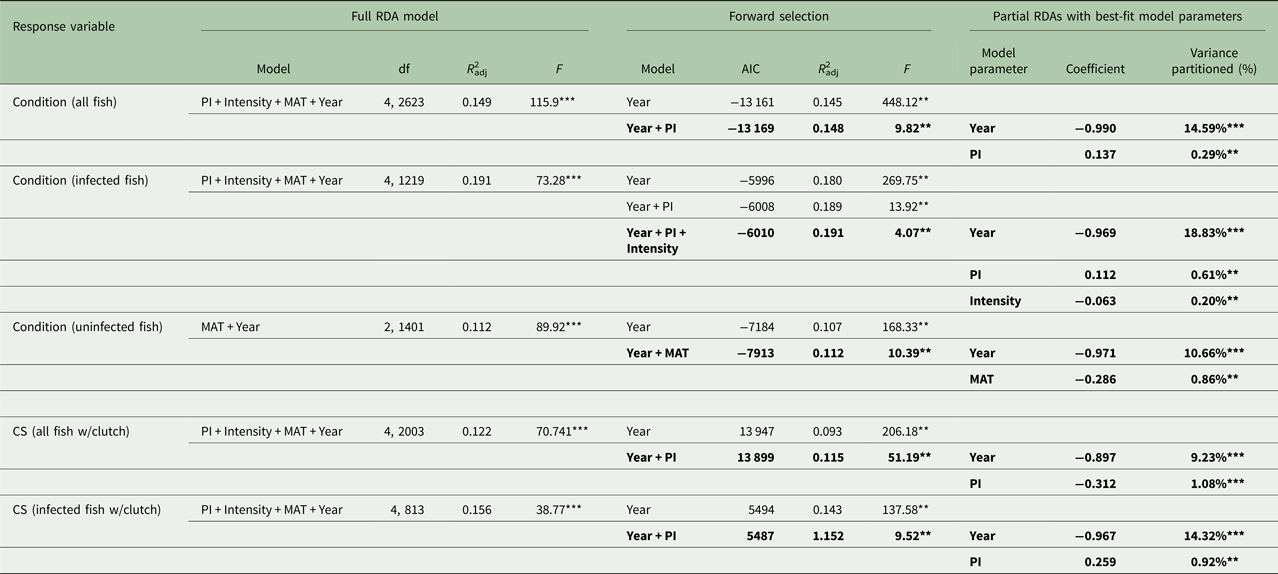
For RDAs of body condition and CS (Table 2), the year of sample was the largest contributing explanatory factor (9.2–18.8%). When year was held constant, PI had a statistically significant, positive relationship with host body condition when considering all fish and also when considering only infected fish. PI also had a significant, positive relationship with CS among infected fish alone. Thus, females in better condition had greater PIs and were able to produce larger clutches in the face of infection. When year and PI were held constant, however, intensity had a negative relationship with body condition among infected fish and with CS among all clutch-bearing fish. Thus, infected females with greater intensities were in poorer condition and produced smaller clutches.
Table 2. Redundancy analysis (RDA) models for each response variable, body condition and clutch size
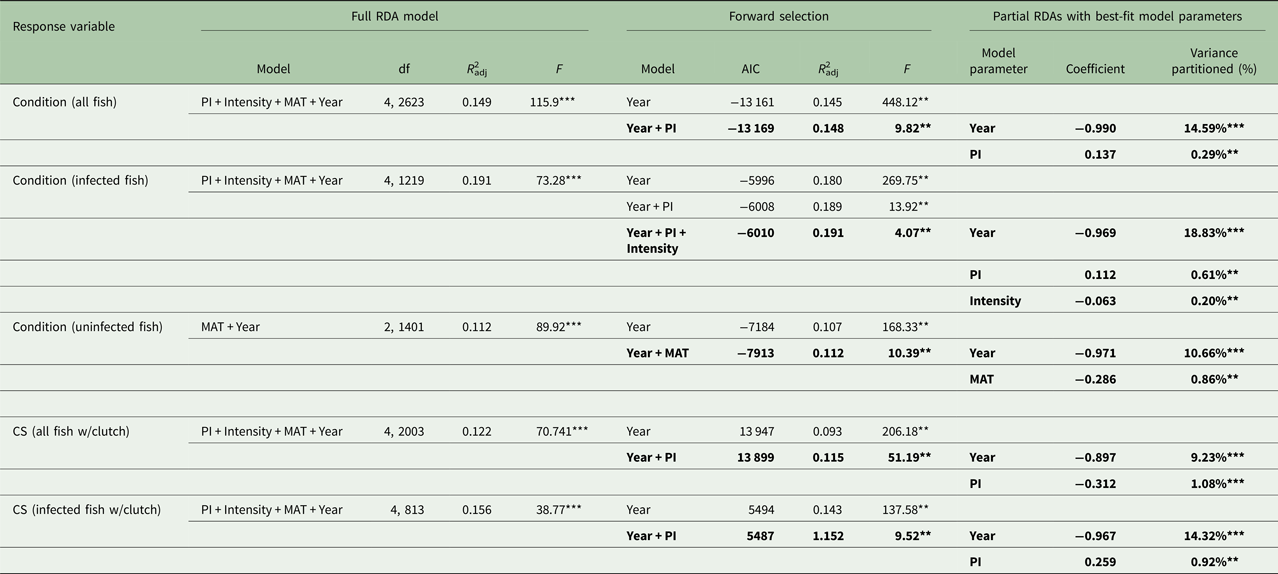
Results of the full-model RDAs, the RDAs with forward selection for best-fit model determination (shown in bold) and variance partitioning of each best-fit model parameter using parasite index (PI), intensity, maturity stage (MAT) and year as predictor variables (**P < 0.001, ***P < 0.0001).
Parasite mass
A linear regression analysis of SL-corrected mean parasite mass against intensity revealed a significant negative relationship (β = 0.029, P < 0.001), whereas the linear regression of SL-corrected total parasite mass against intensity revealed a significant positive relationship (β = 0.075, P < 0.0001). Thus, maximum individual parasite volume appears to decrease with intensity while maximum total parasite volume increases with intensity.
Discussion
Host body condition
For parasites with complex life cycles, successful reproduction in the definitive host depends upon exploitation of intermediate hosts. Almost all the growth of S. solidus occurs in the stickleback host and results in a substantial energy drain on the host (Tierney et al., Reference Tierney, Huntingford and Crompton1996; Bagamian et al., Reference Bagamian, Heins and Baker2004; Schultz et al., Reference Schultz, Topper and Heins2006), notwithstanding the possibility that the parasite shows greater growth efficiency (Walkey and Meakins, Reference Walkey and Meakins1970). Our results show that the loss of body condition in stickleback females taken during the reproductive season increased with greater intensity, after adjusting for PI. Thus, the results of this investigation contrast with those of Nordeide and Matos (Reference Nordeide and Matos2016) and the observation of Christen and Milinski (Reference Christen and Milinski2003) that singly infected fish experience a greater reduction in condition than multiply infected fish. What, then, might explain the differences in results of the three studies?
Reports in the scientific literature provide variable data on the effect of S. solidus on body condition in stickleback, some of which might be attributed to the time of sampling – especially with respect to the reproductive season, which is energetically expensive. Bagamian et al. (Reference Bagamian, Heins and Baker2004) found that high levels of infection intensified the energy drain from reproductive activities, the latter of which was apparent late in the reproductive season. The presence of S. solidus alone did not reduce body condition among fish caught during the breeding season, but PI had a significant negative effect on condition (Bagamian et al., Reference Bagamian, Heins and Baker2004). Tierney et al. (Reference Tierney, Huntingford and Crompton1996) found similar results for fish sampled during autumn and spring, but not winter and summer when all fish were in poor condition; the timing of the spawning season was not given. Moreover, Bagamian et al. (Reference Bagamian, Heins and Baker2004) found that stickleback females in better condition were more likely to have a clutch; and their results suggested a threshold effect on reproductive capacity. Similarly, Heins (Reference Heins2012) found a threshold effect of condition on clutch production among female stickleback. Body condition decreased with increased PI; however, the simple presence of S. solidus also resulted in lower body condition among females sampled during the spawning season (Heins, Reference Heins2012). Pennycuick (Reference Pennycuick1971) found that fish dying in November had significantly lower body condition and significantly higher PIs than those that survived the wave of die offs. Threlfall (Reference Threlfall1968) reported upon the deaths of large numbers of fish infected with S. solidus in August.
The observed differences between our study and that of Nordeide and Matos (Reference Nordeide and Matos2016) might be explained by the growth dynamics of individual parasites as well as the timing of sampling. For individual parasites to have a greater effect on host fitness than multiple parasites at all levels of PI (i.e. throughout the parasite's growth), the degree of energy theft, which is presumably related to the growth rate, must be greater than the combined energy theft of all parasites growing in the presence of conspecifics. Parasite growth cannot slow appreciably as the individual parasite becomes competent to infect the definitive host (50 mg; Tierney and Crompton, Reference Tierney and Crompton1992; but see Heins et al., Reference Heins, Baker and Martin2002) and then continues growing beyond that size threshold eventually to become much larger (153–264 mg, Heins and Baker, Reference Heins and Baker2002; Scharsack et al., Reference Scharsack, Koch and Hammerschmidt2007). But just the opposite appears to occur in single infections where the growth rate slows after an initial burst and is much reduced after the parasite becomes competent to infect the definitive host (Scharsack et al., Reference Scharsack, Koch and Hammerschmidt2007). The LHS model, however, predicts that individual parasite growth should increase with intensity while the total mass increases (Parker et al., Reference Parker, Chubb, Roberts, Michaud and Milinski2003; Michaud et al., Reference Michaud, Milinski, Parker and Chubb2006). Thus, on empirical and theoretical grounds, we would not expect that single infections would be more harmful than multiple infections. Indeed, multiply infected hosts should experience greater harm than singly infected ones, and the loss of host condition should occur at an increasing rate with increased intensity. If the LHS prediction of greater individual growth rates in multiple infections does not hold and parasites grow at the same rate at all intensities, then the impact of increasing intensity should be additive. From an empirical viewpoint, Nordeide and Matos (Reference Nordeide and Matos2016) used samples taken at the end of the reproductive season in one lake and three months after the season in another one. We can expect die offs of uninfected and infected fish as the reproductive season comes to a close and soon after spawning has ended. If multiply infected fish, especially those with heavier burdens, were in poorer condition and there were any differential mortality removing multiply infected hosts with greater burdens, sampling at and after the close of the spawning season might yield data suggesting that single infections had lower condition than multiple ones. Hosts having single infections with larger burdens than multiply infected fish might be able to live a little longer into the post-spawning period before dying themselves. The data from studies of body condition are essentially snapshots of a dynamic interplay between parasite and host in addition to or aside from the effects of host reproduction on condition. Thus, a series of samples taken over time would be necessary to test this suggested scenario.
The experiment of Christen and Milinski (Reference Christen and Milinski2003) focused on the genetic consequences of selfing and outcrossing in S. solidus. Christen and Milinski (Reference Christen and Milinski2003) observed that the total mass of single infections was significantly greater than the total mass of multiple infections 60 days post-infection when the experiment was concluded. Body condition was found to be lower in single infections than in multiple ones (Christen and Milinski, Reference Christen and Milinski2003), which would be expected if parasite:host BM ratios did not differ significantly and the parasite:host BM ratio reflects the level of host exploitation. The greater mass of single infections is unexplained but might have been influenced by the percentage of hosts with outcrossed and/or selfed parasites. Outcrossed parasites had greater average weights than selfed parasites (Christen and Milinski, Reference Christen and Milinski2003) and apparently grew faster while gaining greater mass. Moreover, intensity was significantly greater for outcrossed worms than for selfed parasites. The short duration of the experiment and differences in intensity and weight (hence growth) of outcrossed and selfed worms might have influenced their observation that body condition was lower in singly infected fish than in multiply infected ones. Statistical analysis was not conducted to parse out differences in host body condition with intensity and parasite mass among outcrossed and selfed parasites, and the relatively small sample sizes (n = 13 single infections, n = 27 multiple infections) would have prohibited such an analysis.
Other potential explanations for differences among the three investigations are the differences in virulence of S. solidus and stickleback host resistance among populations. Although these phenomena are not well understood, they have been considered in studies of host fish reproduction (Heins and Baker, Reference Heins and Baker2008; Heins et al., Reference Heins, Baker, Toups and Birden2010a, Reference Heins, Barry and Petrauskas2014), and evidence of differences among populations has been found (Kalbe et al., Reference Kalbe, Eizaguirre, Scharsack and Jakobsen2016). Given the observed population-level differences in virulence of S. solidus and stickleback host resistance, we would expect geographic variation in the dynamics of parasite growth resulting in variation in host exploitation and the virulence it engenders.
Clutch size
We found that CS decreased with intensity after correction for PI. Moreover, we found that intensity was a better predictor of CS than PI. Thus, females with single infections produced larger clutches than females with multiple infections – again, in contrast to the expectation that single infections should be more harmful than multiple infections. We were surprised to find that intensity was a better predictor of CS than PI notwithstanding the significant correlation between the two predictor variables. Our results suggest that future investigations of reproduction in infected fish should consider whether to use intensity rather than PI as a predictor variable, the latter of which has commonly been used as a proxy for the severity of infection. Our data also suggest that the effects on host fitness might be more closely linked to the growth rates of parasites in infections than the total mass.
Parasite growth strategies
We found that mean parasite mass decreased with intensity while total parasite mass increased as the number of parasites within a host rose. We used measures of mass as proxies for volume. Total parasite mass should be a good proxy for total parasite volume. We used mean parasite mass as a proxy for maximum parasite volume. As Parker et al. (Reference Parker, Chubb, Roberts, Michaud and Milinski2003) state, the LHS model assumes multiple infections occurred simultaneously. We know that infections of S. solidus occur asynchronously over several months (Heins et al., Reference Heins, Eidam and Baker2016). In such cases, the predictions of LHS should hold qualitatively using the average size of competing parasites (Parker et al., Reference Parker, Chubb, Roberts, Michaud and Milinski2003). Thus, we conclude that our results are consistent with predictions of the LHS hypothesis for the volume of individual parasites and for the total volume of all parasites within one host. Nordeide and Matos (Reference Nordeide and Matos2016) stated their results supporting the conclusion that virulence is less in multiply infected fish than in single infections were consistent with LHS.
The LHS hypothesis predicts that S. solidus should overexploit but not kill the host. Why then do we see large die-offs of infected stickleback as reported in the literature? In cases where the fish are not already at the end of their normal lifespan, the likely cause is environmental stress resulting from exposure to changes whether they are within normal limits (e.g. onset of winter) or extreme (e.g. severe decline in food resources). That infected fish can be observed with burdens (total parasite mass) approaching or exceeding the mass of the host suggests that host senescence and environmental change are two major factors contributing to the death of overexploited hosts, not necessarily the infections themselves. Pennycuick (Reference Pennycuick1971) reported the deaths of heavily infected stickleback in November, about the time of the onset winter conditions. Heins et al. (Reference Heins, Birden and Baker2010b) reported the end of an epizootic over winter (between stickleback spawning seasons) in Walby Lake, Alaska, which apparently resulted from the deaths of adult fish that had reached the end of their normal lifespan or were in poor condition after having reproduced while also sustaining infections. A second (subsequent) enigmatic epizootic in the lake involved an increase in parasite intensity and prevalence without an increased PI or large host population size observed for the first epizootic. Another epizootic observed by Heins et al. (Reference Heins, Baker and Green2011) appeared to end with the deaths of heavily infected juvenile fish (1-year-olds) that did not survive the stress of winter under ice cover in Scout Lake, Alaska, to become adults the following spring.
Whether the RC or LHS models accurately represent the dynamics of parasite growth, especially for S. solidus, remains a salient question. The dynamics of the interplay among conspecifics and its effects on individual growth and total parasite mass could be very complex. Infections of threespine stickleback, for example, appear to occur asynchronously over a few months (Heins et al., Reference Heins, Eidam and Baker2016) and often include small, incompetent parasites along with larger ones competent to infect and reproduce in the definitive host (Heins et al., Reference Heins, Baker and Martin2002). Outcrossed parasites have been observed to have greater individual mean and total parasite mass than selfed parasites in stickleback (Christen and Milinski, Reference Christen and Milinski2003). And for S. solidus, at least, there might be different strategies in the first intermediate host as compared with the second one (Michaud et al., Reference Michaud, Milinski, Parker and Chubb2006).
Host BM
That uninfected females in our study had a lower length-adjusted BM than infected females might reflect the difference between the loss of energy from reproduction in comparison with the loss from the combined effects of reproduction and parasitism. Although the inter-spawning interval and the number of spawnings of infected and uninfected stickleback females does not appear to be significantly different (Heins and Brown-Peterson, Reference Heins and Brown-Peterson2010), the number and size of eggs, hence clutch mass, produced is reduced in host fish (Heins et al., Reference Heins, Baker, Toups and Birden2010a). Thus, overall reproductive effort appears to be reduced among infected female fish and might lead to less reduction of condition than from reproduction alone in uninfected fish.
Reproductive stage
Although stickleback females can reproduce despite the presence of S. solidus, the parasite has a deleterious effect on the ability of females to produce a clutch of eggs in females with heavy burdens (Heins et al., Reference Heins, Baker, Toups and Birden2010a). This phenomenon appears to be reflected in our data showing that infected females were more likely to be LA. Similar results were observed by Schultz et al. (Reference Schultz, Topper and Heins2006).
Concluding remarks
The virulence of S. solidus plerocercoids stemming from the dynamics of parasite growth in threespine stickleback fish has been investigated in two natural experiments (Nordeide and Matos, Reference Nordeide and Matos2016; present study), both of which built upon theoretical considerations of growth strategies in these parasites (Parker et al., Reference Parker, Chubb, Roberts, Michaud and Milinski2003; Michaud et al., Reference Michaud, Milinski, Parker and Chubb2006). The two empirical studies produced different results leading to opposite conclusions. Future research might aim to test the repeatability of the field studies and the potential reasons for differences between them. Additionally, extensive long-term laboratory experiments likely will have to be performed to learn more about the dynamics of growth of singly infected fish as opposed to those with multiple infections, some of which might need to include parasite intensities and size distributions similar to those observed in natural populations. Potential differences in parasitic virulence among populations of host fish should also be considered.
Acknowledgements
A number of Tulane University undergraduates assisted with field sampling, laboratory dissections and data processing, most notably Scarlet Singer and Emily Birden who co-authored earlier papers on this host–parasite system.
Financial support
Funding for this research was provided by grants from the Newcomb College Institute of Tulane University.
Conflict of interest
None.
Ethical standards
All collections were made under Fish Resource Permits issued by the Alaska Department of Fish and Game and in accordance with an approved animal use protocols from Tulane University.