Published online by Cambridge University Press: 28 November 2001
The initial attachment of Toxoplasma tachyzoites to the target host cell is an important event in the life-cycle of the parasite and a critical stage in infection. Previous studies have shown that polyclonal antibodies directed against the major surface antigen of Toxoplasma gondii (SAG1) inhibit the infection of enterocyte cell lines. Here, we demonstrate that antibodies raised against a central peptide (V41T) of SAG1 and the SAG1 protein itself are able to inhibit the infection of various cell lines by the tachyzoites. Antibodies directed against SAG1 peptides were used to define a site on the SAG1 antigen that interacts with the host cell. The epitope carried by V41T was identified on the tachyzoite surface by immunofluorescence. The peptide sequence seems to be conserved in all the members of the SAG1 Related Sequence family (SRS). Using undifferentiated and differentiated Caco-2 cells, we found that tachyzoites enter preferentially via the basolateral side of the cell. These findings highlight the role of the SRS family members in the mediation of host cell invasion.
Toxoplasma gondii is a widespread protozoan parasite of warm-blooded vertebrates. In adults infection is usually benign, but it can cause congenital disease with severe consequences for foetuses. T. gondii is also an important opportunistic pathogen in immunocompromized hosts, such as AIDS sufferers (Luft & Remington, 1992).
T. gondii is an obligatory intracellular parasite. Cell invasion initially involves the initial attachment of the parasite to the host cell which probably has to be followed by the active penetration through the plasma membrane. Protein kinase C and mitogen activated protein (MAP) kinases (Robert-Gangneux et al. 2000) and calcium mobilization (Bonhomme et al. 1999; Vieira & Moreno, 2000) are probably required. In the host cell, the parasite occupies a specialized vacuole throughout the whole intracellular phase. The entry of T. gondii involves several steps, the penetration step takes about 5–10 sec (Dubremetz, 1998) and the entire process is completed within 25–40 sec (Morisaki, Heuser & Sibley, 1995). The precise molecular events that mediate the initial interaction between the parasite and its host cell, are poorly understood.
The ability of T. gondii to invade a wide range of hosts and cell types suggests that it may recognize very common molecule(s) during cell attachment (Silverman & Joiner, 1997) and that the parasite–host membrane interaction also involves commonly occurring specific ligands. Extracellular matrix proteins such as the glycoprotein laminin, and the host cell laminin receptor may be important in this process, since parasite penetration is increased in the presence of laminin (Furtado et al. 1992). It has been shown that this interaction is complex, and involves both parasitic and cellular laminins, and that laminin receptors form a bridge between the host cell and the parasite. Several laminin receptors have been identified, such as integrins α6/β1 or LBP (laminin binding protein) depending on cell type (Furtado, Cao & Joiner, 1992). Toxoplasma invasion of host cells is also closely coupled to the release of proteins stored within apical secretory granules known as micronemes. Protein MIC3 presents several domains with adhesin properties (Garcia-Reguet et al. 2000) and MIC2 also seems to be involved in the invasion process (Carruthers, Giddings & Sibley, 1999).
Carbohydrates are also involved in pathogen-host cell interactions (Jacobson & Doyle, 1996). Although monosaccharides are not able to inhibit the penetration of T. gondii into host cells, parasite lectin-like components related to sulphated sugars have been identified on the surface of the host cell and may be involved in parasite invasion (Ortega-Barria & Boothroyd, 1999). The inhibition of the invasion of fibroblasts induced by glucosamide–bovine serum albumin has been shown to be greater for a wild-type strain than for the SAG1 null-mutant strain that expresses less surface SAG1 (Kasper, 1987), and this suggests that SAG1 probably binds to a glycosylated host cell receptor (Mineo & Kasper, 1994). The main T. gondii surface protein, SAG1, is one of the most studied proteins of this parasite, but its precise function remains unclear. Previous studies have shown that SAG1-specific antibodies are able to inhibit the invasion of host cells by the parasite, (Grimwood & Smith, 1992; Mineo & Kasper, 1994; Mineo et al. 1993). However, the existence of a SAG1 null-mutant (Kasper, 1987), capable of invading host cells makes it necessary to define the precise contribution of SAG1 to this process. Other parasite surface proteins belonging to the SAG1 Related Sequence family (SRS), SAG2 and SAG3, are present on the virulent tachyzoite form and are also involved in the invasion process (Grimwood & Smith, 1996; Gross, 1996). SRS proteins have shown a sequential identity that could indicate conserved tri-dimensional conformation (Cesbron-Delauw et al. 1994; Manger, Hehl & Boothroyd, 1998). Other members of this family are being discovered, but their role in the attachment step is unclear.
We focused on one member of this family, SAG1, which is the best-known tachyzoite antigen. Previous studies have shown that peptides of SAG1, such as V41T, carry the B and T epitopes (Velge-Roussel et al. 1994). The aim of this study was to provide a detailed definition of the SAG1 sequence involved in the attachment phase to the host cell. We report here the inhibitory effect of specific anti-peptide antibodies on the infection of enterocytes by T. gondii tachyzoites.
RH strain T. gondii were maintained by the serial passage of infected peritoneal exudate in Swiss OF1 mice. The SAG1 null-mutant T. gondii strain was maintained by passage in Mode-K cells.
The cell lines used were the CBA/J mouse epithelial Mode-K (Vidal et al. 1993), the human enterocyte Caco-2 (Zweibaum et al. 1991), and the rat IEC-6 line (Quaroni et al. 1979). All the cells were maintained in culture in 25 cm3 flasks containing RPMI-1640, 5% fetal calf serum (FCS), 10 mM HEPES, 10 mM glutamine, 1000 U/ml penicillin/streptomycin, at 37 °C, 5% CO2, and 95% humidity. For the inhibition experiment, the cells were placed in 96-well plates (Falcon) at a concentration of 2.5×104 per well in 200 μl of culture medium on the previous day. The Caco-2 cells were maintained in culture for 7 or 20 days in the same 96-well plates, with the medium changed every 2 days, and they are referred to as 20D-Caco-2 and 7D-Caco-2 respectively.
SAG1 was purified using an anti-SAG1 mAb (1E5) affinity column as previously described (Debard, Buzoni-Gatel & Bout, 1996). The purity of the protein was determined by 12% SDS–PAGE, followed by silver staining. Two SAG1-peptides were synthesized by the solid-phase method and purified by HPLC. The V41T peptide (VTVTVQARASSVVNNVARCSYGADSTLGPVKLSAEGPTTMT) and the N17V peptide (NNVARCSYGADSTLGPV), correspond to residues 125–165, and 138–154 of the SAG1 protein (Velge-Roussel et al. 1994).
Specific polyclonal antisera against the above peptides were prepared by subcutaneous immunization of CBA/J mice with 50 μg of peptide (V41T- or N17V) in Complete Freund's Adjuvant (Gibco, France). The mice were boosted 1 month later with 25 μg of peptide in Incomplete Freund's Adjuvant (Gibco) and bled every 2 weeks for 2 months.
Cells were cultured the previous day in 96-well plates (Falcon) at a concentration of 2.5×104 per well in 200 μl of culture medium. The plates were incubated at 37 °C in 5% CO2, at 95% humidity. For inhibition experiments in the presence of SAG1, the cells were previously incubated for 30 min at room temperature with various concentrations of SAG1 protein. Parasites were then added to each well, at a ratio of 5 parasites/cell. For inhibition experiments in the presence of sera, the parasites were previously incubated under the same conditions with normal mouse serum as control and with the specific anti-V16V or anti-V41T sera at the dilutions indicated on the figures. Parasites were then added to each well. Intracellular parasite multiplication was evaluated from the [3H]uracil incorporation by the parasite as follows. Two hours after infection and after washing twice, 2.5 μCi of [5,6-3H]uracil (specific activity, 50 μCi/mmol) was added to each well, and the cultures were incubated for a further 16 h before being harvested (Pfefferkorn & Pfefferkorn, 1977; Woodman, Dimier & Bout, 1991). Data were reported after subtracting the cpm reading obtained for the cells alone.
The percentage of infected cells, the number of parasites per cell and the parasite stages present were determined after treating the tachyzoites with specific antibodies as follows. Mode-K cells (4×104) were cultured the previous day in 24-well plates. Tachyzoites of T. gondii were incubated for 30 min with specific sera in 300 μl of RPMI, then added to the cells. After 2 h in contact, the cells were washed with warm RPMI-1640 and maintained in culture medium for a further 16–18 h. The plates were then washed with PBS and stained with Giemsa stain (Merck, Darmstadt, Germany). The number of parasites in the vacuoles and the number of infected cells were counted microscopically by 2 independent examiners.
T. gondii were prepared from infected mice and, after washing 3 times, the parasites were placed on glass slides, dried at room temperature and fixed using acetone at −20 °C. The parasites were then stained either with normal mouse serum as control, or with anti-V41T or anti-N17V and antibody fixation was revealed by incubation with FITC conjugated anti-mouse IgG (Sigma, USA) for 30 min at 37 °C, after washing 3 times in PBS. The slides were mounted with Eukitt and examined using a Zeiss microscope (Zeiss, Germany).
The control and test groups were compared using means of a two tailed t-test.
Specific αV41T serum inhibited the infection of enterocyte cell lines (Mode-K and IEC6) in a dose-dependent manner (Fig. 1). For the IEC6 cell line, the inhibitory effect was up to 50% (P<0.001) at the 1/100 dilution; it was over 60% (P<0.001) for Mode-K. Control experiments were carried out using normal mouse serum (NMS), and displayed no inhibitory effect except at the highest concentration tested (Fig. 1). To check that the anti-V41T serum was specifically recognizing the T. gondii surface antigens, immunofluorescence studies of tachyzoites were performed (Fig. 2, bottom). Unlike normal mouse serum, anti-V41T serum and anti-N17V antibodies both recognized tachyzoite surface antigens (Fig. 2, bottom, left and right respectively).

Fig. 1. Inhibitory effect of polyclonal anti-V41T mouse serum on uracil uptake by Toxoplasma gondii tachyzoites within IEC6 and Mode-K cells. Tachyzoites (4×104) were incubated for 30 min in cell culture medium with decreasing concentrations of serum. The mixtures were then added to Mode-K or IEC-6 cells for 2 h and the cells were then expressed as the mean±S.D. of triplicate determinations minus the cpm obtained for cells alone. *Significant inhibition compared to the control with normal mouse serum (NMS), IEC6, 198725±10288 cpm, Mode-K, 43873±3482 cpm.

Fig. 2. Surface immunofluorescence of RH tachyzoites following staining by anti-V41T mouse serum. The specific binding was revealed using an FITC-labelled anti-IgG. Upper left, normal mouse serum diluted 1/100, upper right, anti-SAG1 1E5 mAb diluted 1/1000, bottom left, anti-V41T mouse serum diluted 1/100, bottom right, anti-N17V serum diluted 1/100.
To investigate further the parasite stage present during inhibition, experiments were done under the same conditions in 24-well plates, but counting the number of infected cells as well as the number of parasites per cell 16 h after parasite/cell contact. Treatment with anti-V41T and anti-N17V or with T. gondii 42 day-infected sera (42 day-inf. serum) dramatically reduced the number of infected Mode-K cells (Fig. 3) (P<0.001). Normal serum displayed no significant inhibitory effect. The parasite multiplication rate, as assessed by the percentage infection at each stage, in terms to the total infection level, was the same whatever the treatment. This suggests that the inhibitory effect of the anti-V41T serum is limited to the penetration process. In contrast, the 42 day-infected serum seemed both to limit entry and slow down intracellular multiplication, since no 16 parasites/cell or 32 parasites/cell stages were observed (Fig. 3).

Fig. 3. Parasite stage analysis of sera-treated RH tachyzoites after Mode-K infection. The number of parasites per cell and the division stage of the parasites were examined microscopically after Giemsa staining. The results were obtained from 3 independent experiments, (dilution of sera, Inf-D42 1/1000, normal mouse serum, anti-peptide serum 1/100; *P<0.001).
Anti-A41T antibodies recognized T. gondii tachyzoites. This suggests that the inhibitory effect of the serum could be linked to SAG1 recognition. The inhibition by anti-V41T serum of the infection of enterocytes was abolished in a dose-dependent manner by pre-incubating the anti-V41T serum with SAG1 before exposing the cells to the tachyzoites (Fig. 4). We can conclude that anti-V41T serum recognized an epitope that is present both in the soluble SAG1 and on the tachyzoite surface.

Fig. 4. Effect of adding SAG1 and anti-V41T serum on the infection of Mode-K cells by tachyzoites of Toxoplasma gondii. Various concentrations of SAG1 were first added to anti-V41T serum diluted 1/100 (about 60% inhibition). The tachyzoites were then incubated for 30 min with these mixtures and added to Mode-K cells for 2. After washing several times, parasite multiplication was evaluated from the [3H]uracil incorporation. The results were obtained in 3 independent experiments. *P<0.001, significantly different compared with SAG1 alone.
Preliminary incubation of Mode-K and IEC6 cell lines with immunopurified T. gondii SAG1 protein partially inhibited the infection of the enterocytes by T. gondii tachyzoites (Fig. 5A). The inhibition was over 40% for a range of SAG1 protein concentrations from 400 to 200 nM. Under the same conditions, analysis of the parasite stage showed a dramatic decrease in infected cells exposed to SAG1 at 330 nM, and the percentage inhibition was the same for all parasite stages (data not shown).

Fig. 5. Effect of SAG1 treatment on Toxoplasma gondii tachyzoite multiplication in Mode-K and IEC-6 cell lines. (A) Several concentrations of SAG1were added to the cells before adding the tachyzoites. The level of infection of IEC6 and Mode-K cells was evaluated from [3H]uracil incorporation. Results were expressed as the mean±S.D. of triplicate determinations minus the cpm obtained for cells alone. *Significantly inhibited compared to the control without SAG1. (B) Results show parasite multiplication in the presence of BSA (200 NM) and β octyl (0.1%) within Mode-K cells. They are expressed as in (A).
The SAG1 was purified using the n-octyl β-D-glucopyranoside (β octyl), which is cytotoxic, and various tests were carried out to rule out a possible toxic effect of the purified protein itself on cell viability and thus on tachyzoite penetration. The addition of n-octyl β-D-glucopyranoside (0.1%), a non-ionic detergent which promotes protein purification, bovine serum albumin (BSA) 200 nM or a combination of both reagents (β octyl+BSA) had no toxic effect and did not inhibit tachyzoite penetration (Fig. 5B). However, β octyl added alone actually increased parasite penetration, probably by disrupting the cell membrane (Fig. 5B).
The importance of SAG1 in the invasion process has been investigated by means of infection experiments with SAG1 null-mutant tachyzoites, which lack the SAG1 protein (Kasper, 1987). We demonstrated that anti-peptide sera and SAG1 both reduced the infection of Mode-K cells by a wild type and SAG1 KO T. gondii strain (Fig. 6).

Fig. 6. Effect of SAG1 or V41T sera on Mode-K infection by tachyzoites of wild type (wild type) or SAG1 null-mutant Toxoplasma gondii (SAG1 KO). Tachyzoites of both strains were pre-incubated with anti-V41T (1/200) or anti-N17V (1/200) sera and then added to Mode-K cells for 2 h. Cells were pre-incubated with SAG1 (170 nM), and the tachyzoites of each strain were then added. Parasite multiplication was evaluated from [3H]uracil incorporation. The results were obtained from 3 independent experiments. P<0.001, significantly different compared (*) to cells alone or (**) to normal mouse serum (NMS).
The stage of differentiation of the enterocyte Caco-2 cell line varies with time in culture. Seven days after the beginning of culture, the cells are still undifferentiated, whereas by day 20, the monolayer is fully differentiated and the cells are polarized and have tight junctions (Zweibaum et al. 1991). To investigate further the conditions of parasite penetration, we studied T. gondii infection of Caco-2 on D7 and D20 of culture. The incorporation of uracil by parasites was about twice as great in undifferentiated D7-Caco-2 in D20-Caco-2 (4100±804 for D20-Caco-2, 2254±712 cpm for D7-Caco-2). Parasites multiplied in both cell types, but the incorporation level was lower in D20-Caco-2 cells than in D7-Caco-2 cells, suggesting that the infection of Caco-2 cells varied with the culture time. This difference could be due either to a change in the vulnerability to penetration by the parasite or to a difference in parasite multiplication once within the cells. When cells were incubated in the presence of SAG1 (Table 1), a significant inhibition of parasite entry was observed in undifferentiated D7-Caco-2 cells, but not in differentiated D20-Caco-2 cells. In the presence of 170 nM of SAG1, a similar degree of inhibition was observed in all cell lines, suggesting that the tachyzoites entered Caco-2, IEC6 and Mode-K via the same way (Fig. 5A, Table 1). Using the same conditions as for IEC6 or Mode-K, an inhibitory effect was obtained for anti-V41T serum only with 7D-Caco-2 cells (range between 50 and 80%, P<0.001), and not with 20D-Caco-2 cells (Table 1).

The most important finding of this study is the identification of a SAG1 sequence able to induce antibodies that inhibit the penetration of T. gondii. The V41T peptide is a very important epitope in anti-SAG1 immunity (Velge-Roussel et al. 1994), because it carries several B epitopes that appear to be exposed in the SAG1 sequence on the parasite surface (Velge-Roussel et al. 1997). Like polyclonal antibodies against SAG1, specific anti-V41T antibodies inhibit entry of the tachyzoite into 2 enterocyte cell lines in a dose-dependent manner. Not all anti-SAG1 antibodies were able to inhibit invasion, suggesting that only some specific epitopes are involved in the attachment phase (Grimwood & Smith, 1992; Mineo et al. 1993). Moreover, the anti-peptide inhibitory effect on parasite invasion is not attributable to the Fc binding activity of tachyzoites (Vercammen et al. 1999) or to steric hindrance, because normal mouse serum showed less inhibitory effect. Since anti-peptide antibodies recognized tachyzoites in immunofluorescence tests, the V41T epitopes in SAG1 must be accessible on the parasite surface. Although a direct interaction between SAG1 and the host cell surface antigens was not established, the inhibitory effect of anti-V41T serum is probably due to the blockade of the attachment of SAG1 to a specific host cell receptor that initiates the first step in parasite invasion. The data shown in Figs 2, 3 and 6 suggest that the epitope concerned is probably limited to the N17V peptide corresponding to the 138–154 sequence of SAG1.
The SAG1 protein itself inhibits the penetration of T. gondii. The fact that SAG1 inhibition is dose dependent could indicate the presence of a host cell receptor. Previous studies have suggested that SAG1 may have a host cell receptor, and this could be a glycoprotein (Mineo & Kasper, 1994), but it has not been identified. Other studies have shown that the tachyzoite attachment is up-regulated in the mid-S phase of the cell cycle (Dutta, Grimwood & Kasper, 2000; Grimwood, Mineo & Kasper, 1996).
Results obtained with the SAG1-mutant parasite could indicate that the V41T epitope is carried by surface proteins other than SAG1, in particular other members of the SRS family. Such an efficient inhibitory effect of the anti-V41T antibodies could be explained by the ubiquitous presence of the V41T sequence or a conformation of the SRS family involved in parasitic invasion. Comparison of the V41T sequence in the other members of the SRS family shows a high degree of conservation of this sequence in all the members tested by the MACAW program (SAG1, SAG3, SAG5, SAG5.1, SAG5.2, SRS2, SRS1, SRS3) (Manger et al. 1998) (data not shown). These proteins have a set of conserved cysteine residues that could involve the same steric conformation. Some SRS proteins other than SAG1 have also been implicated in the parasite attachment step prior to apical reorientation, these include SAG2 (Grimwood & Smith, 1996; Smith, 1995) and more recently SAG3 (Dzierszinski et al. 2000).
Finally, we have shown that T. gondii tachyzoites are not able to penetrate the epithelial barrier in differentiated enterocytes as they were not able to invade 20D-Caco-2 monolayers. In this study, we demonstrated that tachyzoite SAG1-linked penetration was dependent on the differentiation stage of the host cells. The lower parasite multiplication in D20-Caco-2 could be explained either by reduced entry of the parasite into the cells or reduced ability of the parasite to multiply within the cells. Studies with IEC-6 and Mode-K cell lines suggest than the anti-V41T effect on Caco-2 infection occurs at the parasite penetration step, but do not exclude the possibility that the lower multiplication rate of the parasite may also be lower in D20-Caco-2. This could mean that tachyzoites preferentially enter via the basolateral side of the host cell, which is no longer accessible once the cells have differentiated (Zweibaum et al. 1991). This entry route has been identified in numerous bacterial models such as Listeria monocytogenes and Shigella flexneri (Finlay & Falkow, 1988; Gaillard & Finlay, 1996; Mounier et al. 1992; Velge et al. 1997). T. gondii parasites are usually ingested by the oral route at the oocyst or bradyzoite stages (Dubey, 1997; Dubey et al. 1997; Speer & Dubey, 1998; Speer et al. 1997) which means that the tachyzoites are not the natural parasite stage to penetrate into the intestinal barrier. The presence of tachyzoites inside the intestine has been demonstrated and their dissemination begins after the bradyzoites or sporozoites have passed through enterocytes and have matured into tachyzoites (Carruthers & Sibley, 1997; Dubey, 1997; Speer & Dubey, 1998). Dissemination occurs in the lamina propria, before other cells in the epithelium such as enterocytes and intraepithelial lymphocytes are invaded (Speer & Dubey, 1998). SAG1, like many SRS proteins, is present only during the replication stage, the tachyzoite, and not in oocysts or bradyzoites. The only SRS proteins present on the bradyzoite surface are SAG3 and BSR4 (Manger et al. 1998). If host cell attachment is the main role of parasite SRS proteins, they could mediate attachment during the bradyzoite stage. At the sporozoite stage, mechanical penetration into the host cell could be assumed without the need for parasite–host cell contact (Speer et al. 1997).
Our findings support the involvement of SAG1 in parasite penetration and provide some new indications about how the parasite enters the host cell. We may have the first indication of a SAG1 sequence that may be involved in host cell invasion. Understanding how the parasite penetrates cells has important practical implications for drug treatment and vaccination strategy.
The authors would like to thank J. F. Dubremetz for helpful advice. They also thank L. H. Kasper who provided the RH strain SAG1 null-mutant, and D. Guy-Grant, who provided the Mode-K cells. R. Magné and D. Tabareau kindly provided expert technical help in purifying SAG1.

Fig. 1. Inhibitory effect of polyclonal anti-V41T mouse serum on uracil uptake by Toxoplasma gondii tachyzoites within IEC6 and Mode-K cells. Tachyzoites (4×104) were incubated for 30 min in cell culture medium with decreasing concentrations of serum. The mixtures were then added to Mode-K or IEC-6 cells for 2 h and the cells were then expressed as the mean±S.D. of triplicate determinations minus the cpm obtained for cells alone. *Significant inhibition compared to the control with normal mouse serum (NMS), IEC6, 198725±10288 cpm, Mode-K, 43873±3482 cpm.
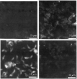
Fig. 2. Surface immunofluorescence of RH tachyzoites following staining by anti-V41T mouse serum. The specific binding was revealed using an FITC-labelled anti-IgG. Upper left, normal mouse serum diluted 1/100, upper right, anti-SAG1 1E5 mAb diluted 1/1000, bottom left, anti-V41T mouse serum diluted 1/100, bottom right, anti-N17V serum diluted 1/100.
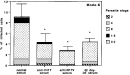
Fig. 3. Parasite stage analysis of sera-treated RH tachyzoites after Mode-K infection. The number of parasites per cell and the division stage of the parasites were examined microscopically after Giemsa staining. The results were obtained from 3 independent experiments, (dilution of sera, Inf-D42 1/1000, normal mouse serum, anti-peptide serum 1/100; *P<0.001).
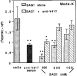
Fig. 4. Effect of adding SAG1 and anti-V41T serum on the infection of Mode-K cells by tachyzoites of Toxoplasma gondii. Various concentrations of SAG1 were first added to anti-V41T serum diluted 1/100 (about 60% inhibition). The tachyzoites were then incubated for 30 min with these mixtures and added to Mode-K cells for 2. After washing several times, parasite multiplication was evaluated from the [3H]uracil incorporation. The results were obtained in 3 independent experiments. *P<0.001, significantly different compared with SAG1 alone.
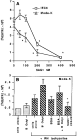
Fig. 5. Effect of SAG1 treatment on Toxoplasma gondii tachyzoite multiplication in Mode-K and IEC-6 cell lines. (A) Several concentrations of SAG1were added to the cells before adding the tachyzoites. The level of infection of IEC6 and Mode-K cells was evaluated from [3H]uracil incorporation. Results were expressed as the mean±S.D. of triplicate determinations minus the cpm obtained for cells alone. *Significantly inhibited compared to the control without SAG1. (B) Results show parasite multiplication in the presence of BSA (200 NM) and β octyl (0.1%) within Mode-K cells. They are expressed as in (A).

Fig. 6. Effect of SAG1 or V41T sera on Mode-K infection by tachyzoites of wild type (wild type) or SAG1 null-mutant Toxoplasma gondii (SAG1 KO). Tachyzoites of both strains were pre-incubated with anti-V41T (1/200) or anti-N17V (1/200) sera and then added to Mode-K cells for 2 h. Cells were pre-incubated with SAG1 (170 nM), and the tachyzoites of each strain were then added. Parasite multiplication was evaluated from [3H]uracil incorporation. The results were obtained from 3 independent experiments. P<0.001, significantly different compared (*) to cells alone or (**) to normal mouse serum (NMS).

Table 1. Percentage inhibition of the entry of Toxoplasma gondii tachyzoites into Caco2 cells by SAG1 protein or anti-V41T serum