Introduction
Schistosomiasis is a parasitic disease caused by helminths of the genus Schistosoma. Currently, an estimated 200 million people in tropical and subtropical regions are infected, with up to 700 million people living in endemic areas at risk of infection and 200 000 deaths occurring annually (Steinmann et al., Reference Steinmann, Keiser, Bos, Tanner and Utzinger2006; WHO, 2018; Verjee, Reference Verjee2019). Hence, there is an urgent need to develop new treatment strategies to treat such a large number of infected individuals. The only drug indicated for the regular treatment of schistosomiasis is Praziquantel (PZQ), which has been used since the 1970s (Cioli et al., Reference Cioli, Pica-Mattoccia, Basso and Guidi2014; Neves et al., Reference Neves, Andrade and Cravo2015; Bergquist et al., Reference Bergquist, Utzinger and Keiser2017). It was the first drug to demonstrate good activity when administered orally or intramuscularly, against all Schistosoma species that infect humans (Cioli et al., Reference Cioli, Pica-Mattoccia and Archer1995, Reference Cioli, Pica-Mattoccia, Basso and Guidi2014; Melman et al., Reference Melman, Steinauer, Cunningham, Kubatko, Mwangi, Wynn, Mutuku, Karanja, Colley, Black, Secor, Mkoji and Loker2009). However, PZQ has low efficacy against immature forms of the parasite (Pica-Mattocci and Cioli, Reference Pica-Mattoccia and Cioli2004; Oliveira et al., Reference Oliveira, Rehder, Oliveira, Jeraldo, Linhares and Allegretti2014) and several reports, mainly of resistance of S. mansoni strains to the drug have already been confirmed (Fallon et al., Reference Fallon, Sturrock, Niang and Doenhoff1995; Ismail et al., Reference Ismail, Metwally, Farghaly, Bruce, Tao and Bennett1996; Picquet et al., Reference Picquet, Vercruysse, Shaw and Diop1998; Crellen et al., Reference Crellen, Walker, Lamberton, Kabatereine, Tukahebwa, Cotton and Webster2016). In addition to the treatment of infected individuals, blocking contact of people with infectious larvae (cercariae), which are shed on a large scale in freshwater bodies by schistosome vector snails (e.g. Biomphalaria, Bulinus, Oncomelania) (Steinmann et al., Reference Steinmann, Keiser, Bos, Tanner and Utzinger2006), could help to avoid infection. However, to date, no chemical compounds have been approved or are currently available for this purpose.
Secondary metabolites (such as crude extracts and essential oils) are present in different parts of plant species; these natural products can be used as prototypes for the development of new antiparasitic drugs (Kaiser et al., Reference Kaiser, Kiderlen and Croft2003; Silva Filho et al., Reference Silva Filho, Resende, Fukui, Santos, Pauletti, Silva, Gregório, Bastos and Nanayakkara2009; Newman and Cragg, Reference Newman and Cragg2012; Castro et al., Reference Castro, Dias, Rezende, Magalhães, Silva Filho, Rai and Kon2013). Plant extracts are, in general, cheaper and easy to obtain (Kaur et al., Reference Kaur, Michael, Aropa, Harkonen and Kumar2005; Varanda, Reference Varanda2006). In Brazil, there is great vegetal diversity, corresponding to 30% of the world flora, which represents an ideal ground for the search of novel natural compounds for new treatments (Nodari and Guerra, Reference Nodari and Guerra2001). Jatropha gossypiifolia and Piper arboreum are plants commonly used in Brazilian folk medicine. J. gossypiifolia is popularly known as ‘peão roxo’ and belongs to the Euphorbiaceae family (Abreu et al., Reference Abreu, Marinho, Paes, Freire, Olea, Borges and Borges2003). The main biological activities experimentally demonstrated for J. gossypiifolia are microbicidal (Nair et al., Reference Nair, Kalariya and Chanda2007), larvicidal (Rahuman et al., Reference Rahuman, Gopalakrishnan, Venkatesan and Geetha2008), molluscicidal (Pereira-Filho et al., Reference Pereira-Filho, França, Oliveira, Mendes, Gonçalves and Rosa2014) and anti-Leishmania activities (Martins et al., Reference Martins, Alves, Viera-Araújo, Rondon, Braz-Filho and Morais2018). P. arboreum belongs to the Piperaceae family and is popularly known as ‘pimenta de macaco’ (Kato and Furlan, Reference Kato and Furlan2007; Andrade et al., Reference Andrade, Guimarães and Maia2009; Regasini et al., Reference Regasini, Cotinguiba, Passerini, Bolzani, Cicarelli, Kato and Furlan2009; Ferreira et al., Reference Ferreira, Kayano, Silva-Jardim, Silva, Zuliani, Facundo, Calderón, Almeida-e-Silva, Ciancaglini and Stábeli2010; Paes-Gonçalves et al., Reference Paes-Gonçalves, Facundo, Santos, Silva, Ballico, Lima, Stábeli and Silva-Jardim2012). Previous studies have already demonstrated some important biological activities of this plant species, such as antifungal (Silva Costa et al., Reference Silva Costa, Navickiene, Kato, Bolzani, Méda, Young and Furlan2002), trypanosomicidal (Figueiredo et al., Reference Figueiredo, Tintino, Brito, Braga, Leite, Lucena, Sobral-Souza, Gomez and Coutinho2014), leishmanicidal (Figueiredo et al., Reference Figueiredo, Tintino, Brito, Braga, Leite, Lucena, Sobral-Souza, Gomez and Coutinho2014) and microbicidal activities (Regasini et al., Reference Regasini, Cotinguiba, Siqueira, Bolzani, Silva, Furlan and Kato2008).
Despite the biological potential displayed against several organisms, antiparasitic activities of the extracts of J. gossypiifolia and P. arboreum leaves on S. mansoni have not yet been evaluated. Thus, we aimed to evaluate the cercaricidal and schistosomicidal effects of ethanolic extracts of J. gossypiifolia and P. arboreum leaves in vitro and to describe preliminary classes of the main chemical compounds present in these extracts.
Material and methods
Collection of plants and preparation of ethanolic extracts
The leaves used for the production of crude extracts were collected in the morning at two collection points – J. gossypiifolia in the municipality of São Bento, State of Maranhão, Brazil (S02°42′06.4″ and W044°49′55.0″) and P. arboreum in the municipality of São Luís, Maranhão, Brazil (S02°33′38.5″ and W044°14′19.6″).
The plants were identified at the Rosa Mochel Herbarium of the State University of Maranhão (UEMA), with specimen voucher number ‘4929’ for P. arboreum and ‘4464’ for J. gossypiifolia. The plant material was collected, cleaned, dried and powdered. The powder was macerated with a 70% hydroethanolic solution, a traditional extraction used in Brazilian folk medicine (Rodrigues et al., Reference Rodrigues, Albuquerque, Nascimento, Campos, Godinho, Araújo, Brito, Jesus, Miranda, Rezende, Negrão-Corrêa, Rocha, Silva, Guerra and Nascimento2020) and mixed every 12 h for a total of 48 h at a ratio of 1:5 (w/v). The extract was first filtered five times and then concentrated under reduced pressure to obtain a dry extract (Neiva et al., Reference Neiva, Ribeiro, Nascimento, Cartágenes, Coutinho-Moraes and Amaral2014). The final yield was 16% of the weight of the crushed dry leaves for P. arboreum and 48% for J. gossypiifolia.
Parasite
Biomphalaria glabrata snails infected with the LE (Luís Evangelista) strain of S. mansoni were exposed to light and heat for 4 h (Brasil, 2008) to obtain cercariae for performing experimental infections and bioassays. Subsequently, the cercariae were washed, concentrated, counted and a total of 100 cercariae added to 500 μL of saline (0.9% g NaCl/100 mL dechlorinated water) were percutaneously inoculated in male Swiss mice (3–4 months old, weighing ~40 g) (n = 12), to obtain adult worms (Pellegrino and Macedo, Reference Pellegrino and Macedo1955). The care of and experiments with mice were approved by the Ethics Committee for the Use of Animals (CEUA) of UEMA, under approval number 03/2018.
The infected animals were kept in ventilated racks and food and water were provided ad libitum. After 60 days of infection, the mice were euthanized with an anaesthetic overdose (150 mg kg−1 ketamine hydrochloride, Vetanarcol® and 120 mg kg−1 xylazine hydrochloride, Rompum®). Next, the circulating blood was perfused with sterile saline solution to recover adult worms (Pellegrino and Siqueira, Reference Pellegrino and Siqueira1956), which were separated into males, females and couples. The adult worms were immediately placed in RPMI 1640 medium, supplemented with 100 μg mL−1 of penicillin, 100 μg mL−1 of streptomycin, 10% fetal bovine serum and washed twice (Soares et al., Reference Soares, Dias, Vieira, Souza, Cruz, Badoco, Nicolella, Cunha, Groppo, Martins, Tavares, Magalhães and Crotti2017).
Toxicity assay against cercariae
Toxicity tests were performed according to Rodrigues et al. (Reference Rodrigues, Albuquerque, Nascimento, Campos, Godinho, Araújo, Brito, Jesus, Miranda, Rezende, Negrão-Corrêa, Rocha, Silva, Guerra and Nascimento2020), with some modifications. Briefly, 24-well culture plates (Costar, Corning, NY, USA) were used with 10 active cercariae per well. Each well was filled with incomplete RPMI culture medium containing RPMI-1640 (Sigma-Aldrich) supplemented with 10 mm HEPES, 11 mm sodium bicarbonate, 100 U mL−1 penicillin, 100 mg mL−1 streptomycin, 2 mm L-glutamine, 23 mm L-asparagine and 0.1 mm pyruvic acid. Subsequently, the ethanolic extracts of P. arboreum and J. gossypiifolia leaves were added at final concentrations of 250, 500, 1000, 5000 and 10 000 μg mL−1 (test groups) in a final volume of 500 μL (RPMI). For the negative control (C-), 10 cercariae were added to 500 μL of incomplete RPMI medium. The plates with the cercariae were kept in an oven at 30°C and 5% CO2. The assessment of cercaricidal activity was based on the visualization and counting of larvae with preserved mobility at 15, 30, 60, 90, 120, 150 and 180 min after adding the extract, using a stereomicroscope. The tests were performed in triplicate (biological replicates) with three replications (technical replicates).
Toxicity assay against adult worms
Two worms (male or female) were distributed into wells in 24-well culture plates containing 2 mL of RPMI complete medium and incubated in an oven (under 5% CO2 at 37°C). After 1 h of incubation, extracts of P. arboreum and J. gossypiifolia were added at concentrations of 50, 150 and 250 μg mL−1 (test groups). As a negative control group (C-), the worms were incubated only in complete RPMI medium. The positive control group was composed of two worms incubated with 10 μg mL−1 of PZQ (Farmanguinhos, Rio de Janeiro, Brazil). An inverted microscope (Olympus CK40) was used to assess the worms' survival at 3, 6, 12, 18, 24, 48 and 72 h of culture. The treatment was considered effective when the worms did not show any movement after 2 min of observation (for analysis time). All procedures were performed according to Rodrigues et al. (Reference Rodrigues, Albuquerque, Nascimento, Campos, Godinho, Araújo, Brito, Jesus, Miranda, Rezende, Negrão-Corrêa, Rocha, Silva, Guerra and Nascimento2020), with some modifications. The tests were performed in triplicate (biological replicates) with three replications (technical replicates).
Egg-laying assay
For this assay, one worm couple was added per well along with 50 μg mL−1 of extract for 30 h (test groups). In the positive control group, a sub-lethal dose of PZQ (0.5 μg mL−1) was used, as described by Mendonça et al. (Reference Mendonça, Feitosa, Veras, Matos-Rocha, Cavalcanti, Barbosa, Brayner and Alves2016). In the negative control group (C-), only complete RPMI medium was used. An inverted microscope was used to count the eggs in each well containing viable couples. Worm couples that eventually died after 30 h were not considered for this analysis. The tests were performed in triplicate (biological replicates) with two repetitions (technical replicates) employing a culture protocol as described by Soares et al. (Reference Soares, Dias, Vieira, Souza, Cruz, Badoco, Nicolella, Cunha, Groppo, Martins, Tavares, Magalhães and Crotti2017) and Rodrigues et al. (Reference Rodrigues, Albuquerque, Nascimento, Campos, Godinho, Araújo, Brito, Jesus, Miranda, Rezende, Negrão-Corrêa, Rocha, Silva, Guerra and Nascimento2020).
Assessment of morphological changes in adult worms
The parasites were carefully removed from the wells and fixed in AFA (70% ethanol, 3% formaldehyde and 2% glacial acetic acid) (Torres et al., Reference Torres, Nascimento, Menezes, Garcia, Santos, Maldonado, Miranda, Lanfredi and Souza2011). The fixed worms were stained in hydrochloric carmine for 30 min and the excess dye was removed with 70% ethanol. Next, the worms underwent gradual dehydration in an alcoholic series (70%, 90% and absolute alcohol, 3 min at each stage) and clarification in methyl salicylate with Canada Balsam (1:2) for preparing permanent slides (Neves et al., Reference Neves, Alencar, Costa-Silva, Águila, Mandarim-De-Lacerda, Machado-Silva and Gomes2007). The worms were subjected to morphological analysis according to Neves et al. (Reference Neves, Pereira, Oliveira, Gomes and Machado-da-Silva1998) using an optical microscope (ZEISS, Axio Imager).
Preliminary phytochemical screening
High-Performance Liquid Chromatography (HPLC) was used to identify the presence of phytocompounds in ethanolic leaf extracts of J. gossypiifolia and P. arboreum. For screening with HPLC-PAD (HPLC coupled with Photodiode Array Detection), 1 mg aliquot of each extract was solubilized in ACN (acetonitrile): H2O (95:5) and subjected to a clean-up process using Sep-pak cartridge RP18 (previously activated with methanol [MeOH]) and filtered through a 0.22 μm membrane. The extracts were injected individually in a Shimadzu analytical liquid chromatography system, model LC-20 A. A CBM-20 A controller with UV-PAD detector was used in gradient mode using 5–95% of B mixed in A (B, ACN; A, H2O) for 45 min at a flow of 1.0 mL min−1. A LUNA 5μm HPLC C18 (250 × 4.6 mm2) column was used. All solvents used were HPLC grade.
Statistical analysis
All data were initially analyzed for normality (Kolmogorov–Smirnov test). To compare the averages of parametric data between the groups, the one-way ANOVA test was used, followed by Tukey test (Mishra et al., Reference Mishra, Singh, Pandey, Mishra and Pandey2019). Viability rates between groups were comparatively analyzed using the log-rank test (Hazra and Gogtay, Reference Hazra and Gogtay2017). Values of P < 0.05 were considered significant. To perform these tests, the GraphPad Prism version 8 software was used.
Results
In the cercariae assay, at the concentrations of 10 000 and 5,000 μg mL−1 of J. gossypiifolia extract, all the cercariae were killed after 120 and 150 min, respectively. At lower concentrations (1000–250 μg mL−1), the larvae showed a 100% mortality rate only after 180 min. These mortality rates were statistically different when compared to the control group. However, there were no differences found between viability rates of the different concentrations of the extract (Fig. 1A).

Fig. 1. Survival rate of Schistosoma mansoni cercariae exposure to ethanolic extracts of Jatropha gossypiifolia (A) and Piper arboreum (B) leaves over a time period of 180 min. Ten cercariae per well were used in triplicate at different concentrations (μg mL−1) of the extracts. In the negative control group (C-), only incomplete RPMI was used. # Statistically different from the negative control group. Statistical results were obtained using the log-rank test.
For P. arboreum extract, it was observed that after exposure to 10 000 μg mL−1 for 60 min, all the cercariae were dead. At 5000 and 1000 μg mL−1 concentrations, the cercariae viability was totally reduced in 120 and 150 min, respectively. Moreover, at the lowest concentrations (500 and 250 μg mL−1), there was a 100% reduction in viability only after 180 min. These mortality rates were statistically different when compared to the control group. However, the viability rates for different concentrations of the extract were not different from each other (Fig. 1B).
Regarding the adult worm assay, P. arboreum was able to reduce 100% of viability of males and females only after 72 h at the highest concentration (250 μg mL−1), being significantly different from the negative control (P = 0.003). At the lowest concentrations (150 and 50 μg mL−1), viable parasites (50% for male and 80% for female worms) were observed even after 72 h (Fig. 2A and B).

Fig. 2. Viability of adult worms (male and female) of Schistosoma mansoni after 72 h of exposure to ethanolic extracts of Piper arboreum (A and B) and Jatropha gossypiifolia (C and D) leaves. Two adult male or female worms were used per well in triplicate at different concentrations (μg mL−1) of the extracts. In the negative control group (C-), only complete RPMI medium was used, whereas, in the positive control group, PZQ (10 μg mL−1) was used. *Viability of worms in the positive control group was significantly different from all the concentrations of P. arboreum and from 50 μg mL−1 concentration of J. gossypiifolia. #Statistically different compared to the negative control group. Statistical results obtained by the log-rank test.
Regarding J. gossypiifolia, the concentration of 250 μg mL−1 was able to kill all male worms after 12 h and all females after 24 h. Even at the lowest concentration (50 μg mL−1), the reduction in viability of the worms (males and females) occurred in less time compared to P. arboreum (Fig. 2C and D). All the mortality rates for male and female worms obtained at different concentrations of J. gossypiifolia extract were higher than those of the negative control group (P < 0.02) (Fig. 2C and D).
In the positive control group (PQZ), there was a 50% reduction in the viability of worms (males and females) in 6 h and after 18 h, all the worms were dead. Treatment with PQZ was more effective than with all the concentrations of P. arboreum extract (P < 0.05) (Fig. 2A and B) but was similar to the two highest concentrations (150 and 250 μg mL−1) of J. gossypiifolia extract (Fig. 2C and D).
The extracts of J. gossypiifolia and P. arboreum leaves were also able to reduce the number of eggs laid by the paired females of S. mansoni by approximately 93% in the test groups than in the negative control group (P < 0.0003) (Fig. 3). Also, no eggs were found in the positive control group (P < 0.0002). There was no difference in the number of eggs laid between the test and positive control groups (Fig. 3).

Fig. 3. Number of eggs laid by female Schistosoma mansoni worms after 30 h of culture with 50 μg mL−1 of ethanolic extracts of Jatropha gossypiifolia and Piper arboreum leaves. In the negative control group (C-), only complete RPMI medium was used; in the positive control group, PZQ (0.5 μg mL−1) was used. One couple of worms per well was used in triplicate. #Statistically significant compared to the negative control. Statistical results obtained using one-way ANOVA test.
Regarding morphology of the worms, the negative control group showed external and internal morphological integrity (Fig. 4). However, the worms from the positive control group (PZQ) exhibited severe muscle/body contractions, loss of tubercles, damage to the integument and changes in the intestines (Fig. 5).
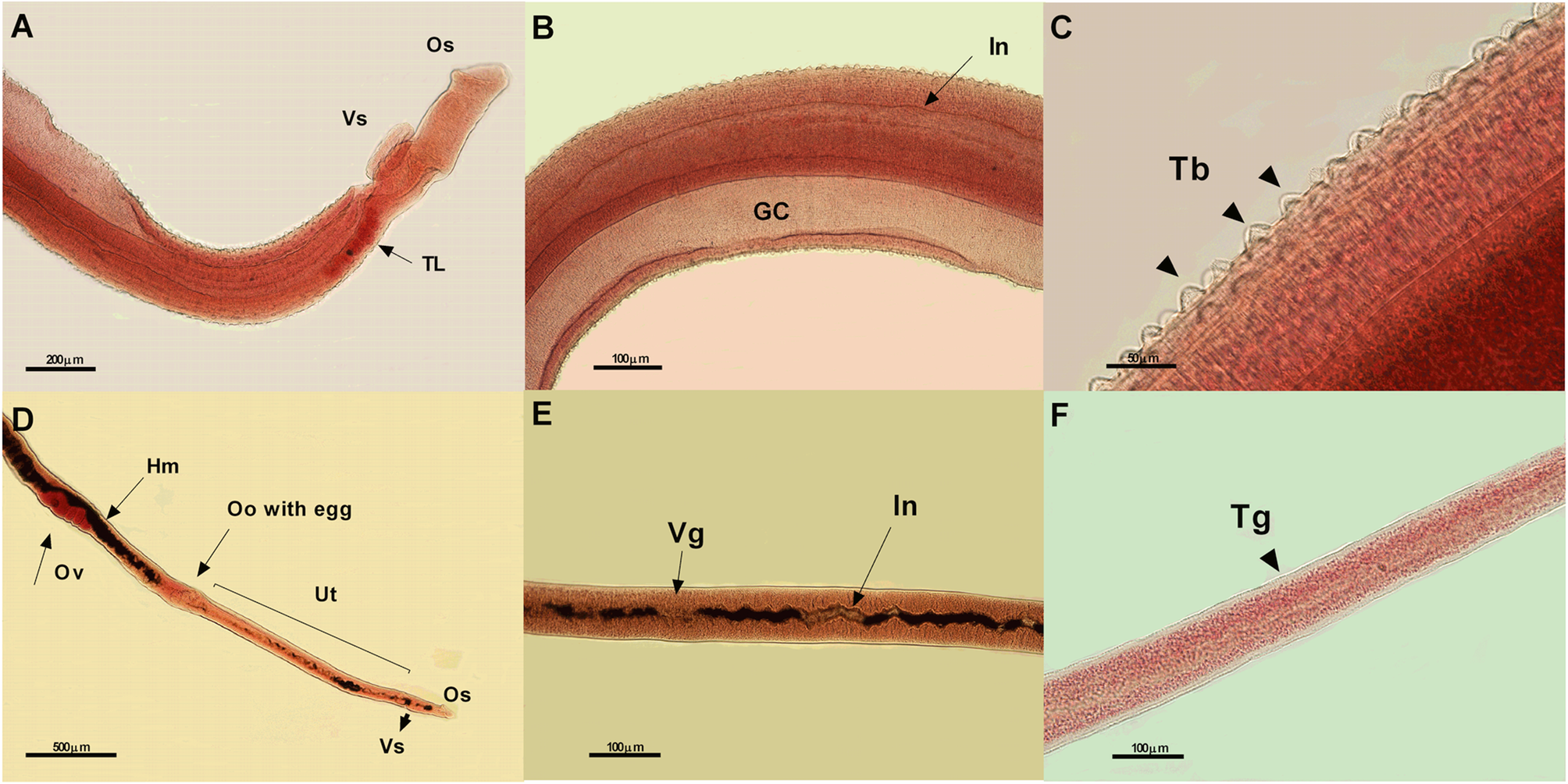
Fig. 4. Photomicrographs of male (A – 10x, B – 20x and C – 40x) and female (D – 5x, E – 20x, and F – 20x) worms of Schistosoma mansoni from the negative control group (treated with only complete RPMI) showing normal morphological characteristics. Os, oral sucker; Vs, ventral sucker; TL, testicular lobes; In, intestine; GC, ginecophore canal; Tb, tubercles; Hm, hemozoin; Ov, ovary; Oo, ootipus; Ut, uterus; Vg, vitelogenus glands; and Tg, tegument; μm, micrometers.
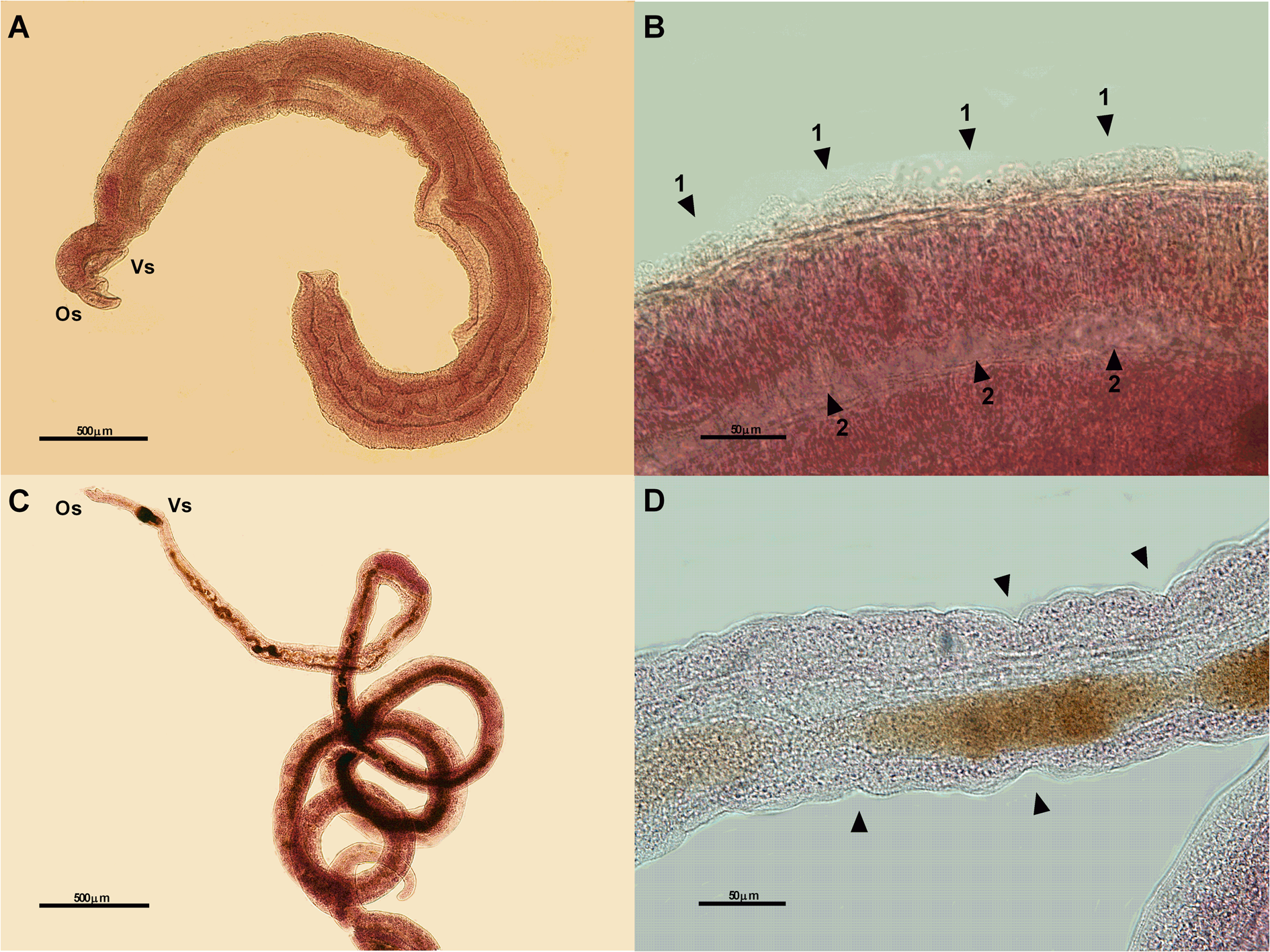
Fig. 5. Photomicrographs of male (A – 5x and B – 40x) and female (C – 5x and D – 40x) worms of Schistosoma mansoni from the positive control group (exposed to 10 μg of PZQ) showing some changes: atypical worm body contractions in male (A), destruction of the tubercles (arrows 1) and dilation in the anterior intestine (arrows 2) (B), atypical contractions of the female worm body (C), destruction of the integument (arrows) (D). Os, oral sucker; Vs, ventral sucker; μm, micrometers.
The worms exposed to the extracts of J. gossypiifolia and P. arboreum presented atypical body contractions and external microstructural damage. Similar damage of internal structures was observed for all the concentrations. However, an evident worsening of morphological changes was observed at the highest concentration (250 μg mL−1).
Piper arboreum extract induced destruction of tubercles and dilatation of the oesophagus and anterior intestine in male worms. Morphological changes such as destruction of sensorial papillae and tegument layers, as well as the reduction in the vitelline glands, were observed in female worms (Fig. 6).
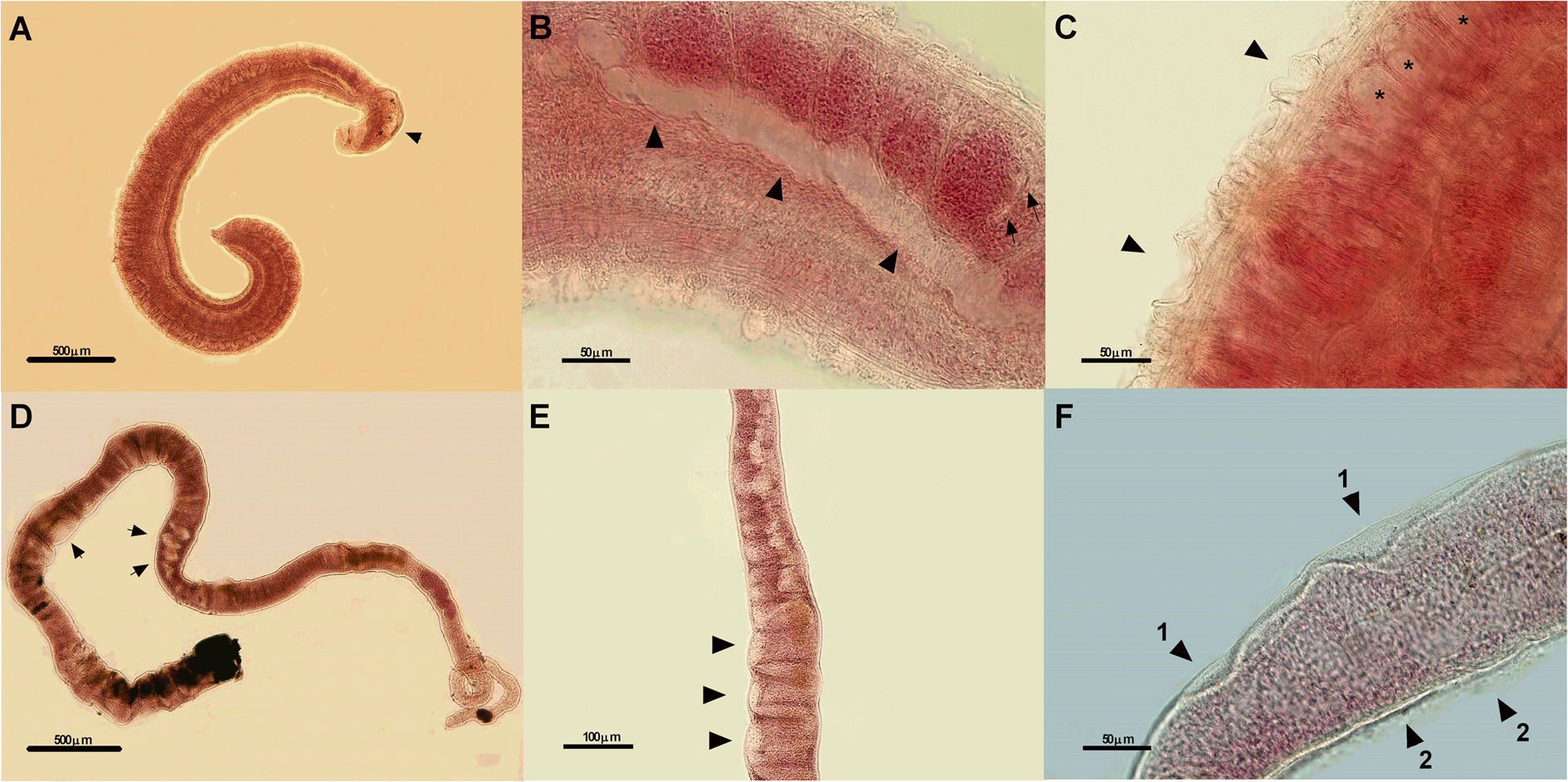
Fig. 6. Photomicrographs of male (A-5x, B-40x, and C-40x) and female (D-5x, E-20x, and F-40x) worms of Schistosoma mansoni exposed to 250 μg mL−1 of the ethanolic extract of Piper arboreum leaves for 72 h. Morphological changes observed are dilation of the oesophagus (arrow) (A), dilation of the intestine (big arrows) and cellular reduction of the testicular lobes (small arrows) (B), destruction of the tubercles (arrows), tegumentary dilation (asterisks) (C), atypical contraction of the female body and reduction of the vitelline glands (arrows) (D), dilation and reduction of the area of vitelline glands (arrows) (E), integument lift (arrows 1) and destruction of the sensorial papillae (arrows 2). μm, micrometers.
The extract of J. gossypiifolia caused atypical contractions of the body, dilatation of the oesophagus and destruction of tubercles in male worms. In female worms, changes also were observed, such as atypical contractions of the body; destruction of sensorial papillae; dilatation of the uterus, anterior intestine, viteloduct and oviduct; and damage to the eggs present in the ootype (Fig. 7).
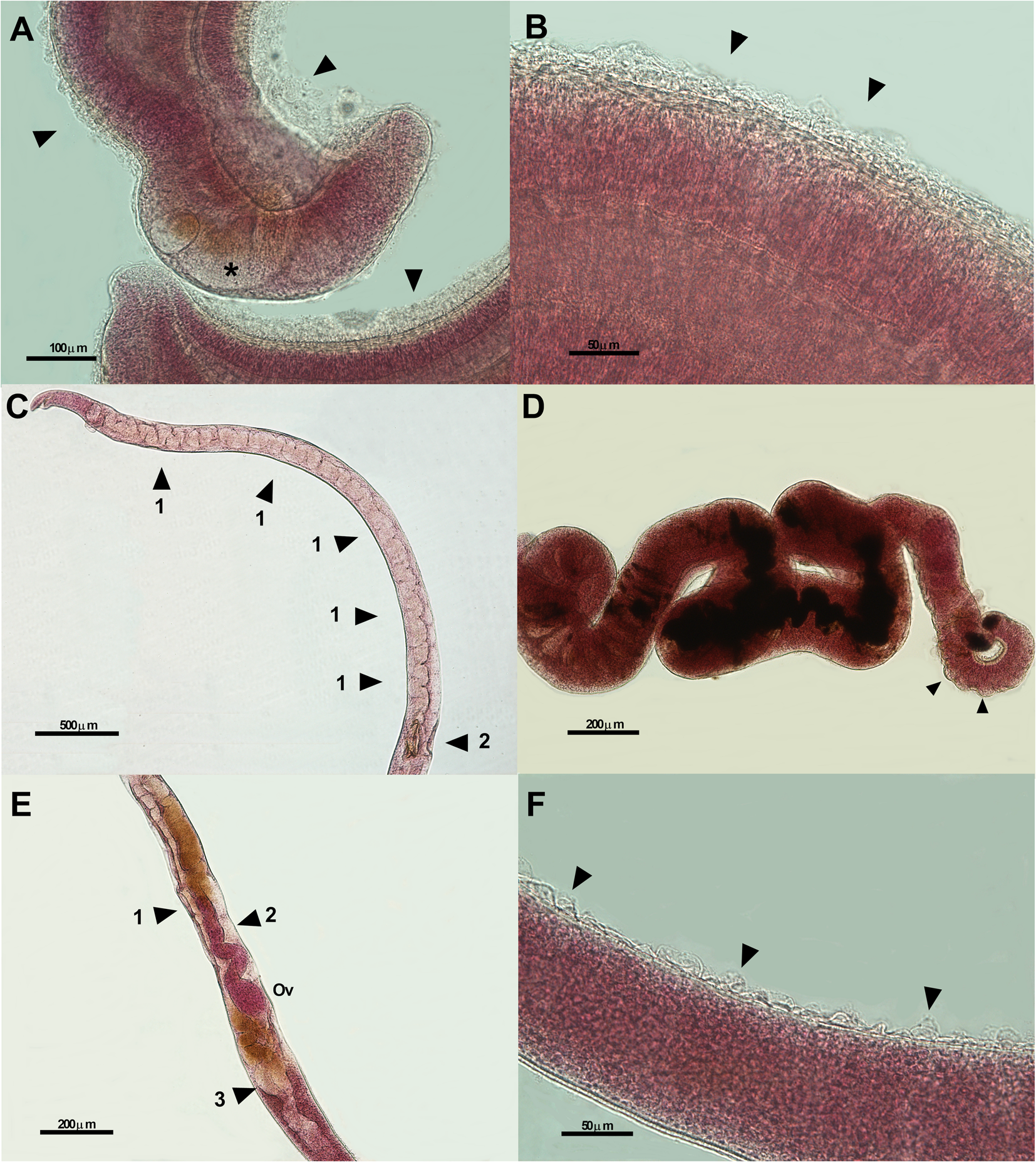
Fig. 7. Photomicrographs of male (A-20x and B-40x) and female (C-5x, D-10x, E-10x, and F-40x) of Schistosoma mansoni worms exposed to 250 μg mL−1 of the ethanolic extract of Jatropha gossypiifolia leaves for 72 h. Morphological changes observed are esophageal dilation (asterisk) and destruction of the tubercles (arrows) (A), destruction of the tubercles (arrows) (B), dilation of the intestinal and uterus areas (arrows 1) and destruction of the egg inside the ootype (arrow 2) (C), atypical contraction of the female body and integument lift (arrows) (D), dilation of the viteloduct (arrow 1), oviduct (arrow 2) and the area of the vitellin glands (arrow 3) (E), destruction of the sensorial papillae (arrows) (F). Ov, ovary; μm, micrometers.
A HPLC-PAD analysis of the sample of ethanolic extract of J. gossypiifolia leaves was observed in the chromatogram at 330 nm; peaks of medium polarity in the retention times (Rt) in the range of 13.2–22.0 min with absorptions at 269, 348 nm; 267, 347 nm; 269, 337 nm; and 270, 337 nm characteristic of flavonoid compounds were observed (Fig. 8A) (Granados et al., Reference Granados, Balcázar, Guillén and Echeverri2015; Cavalcante et al., Reference Cavalcante, Santos and Almeida2020).
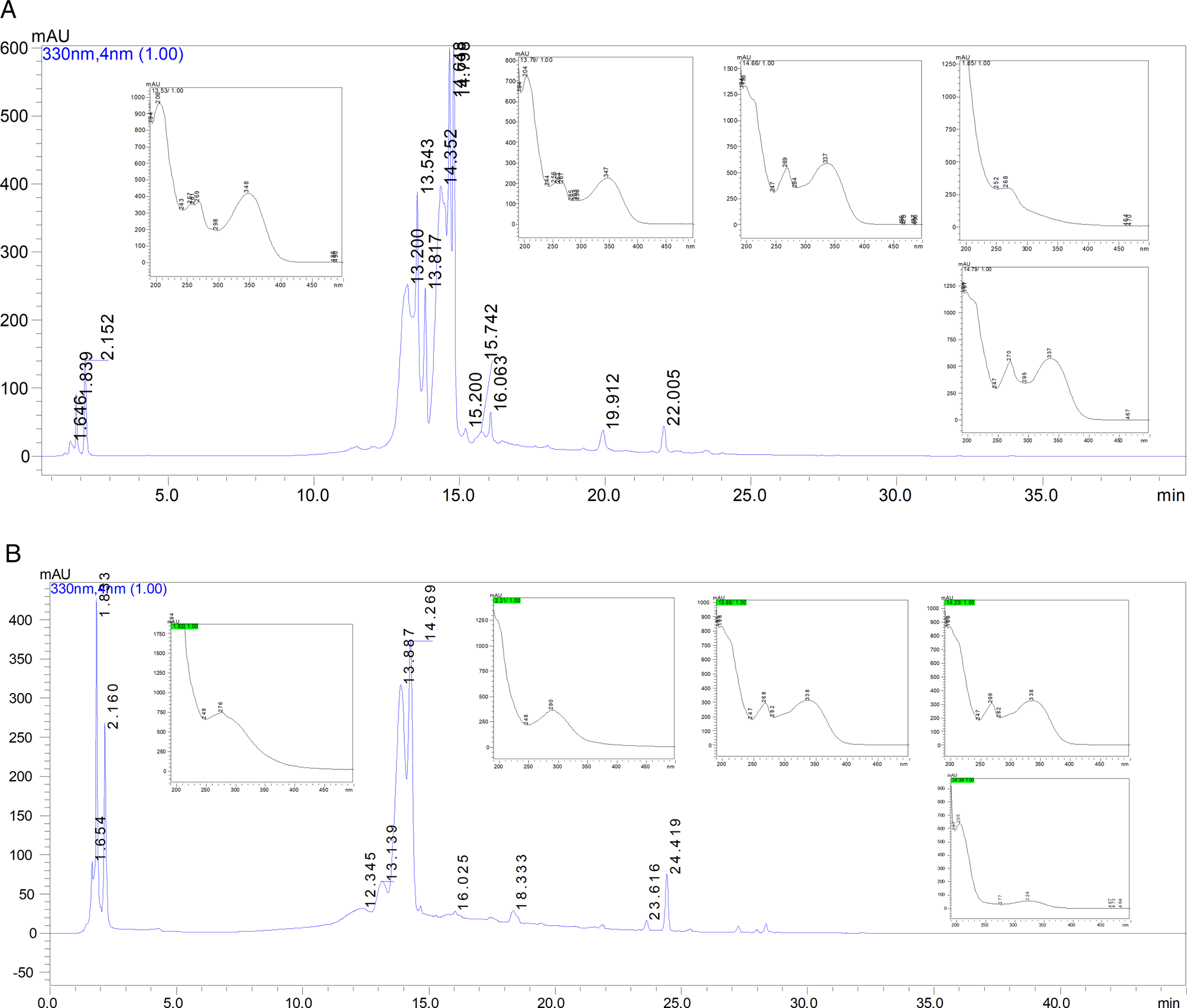
Fig. 8. Representative high-performance liquid chromatography (HPLC) profile and UV spectra of ethanolic extracts of Jatropha gossypiifolia (A) and Piper arboreum (B) leaves.
For the ethanolic extract of P. arboreum leaves, the HPLC profile demonstrated in the chromatogram at 330 nm was as follows: (i) high polarity peaks were observed at Rt from 1.83 to 2.16 min with absorptions at 276 and 290 nm, respectively, which could be attributed to the presence of amides; these results are consistent with those of Nascimento et al. (Reference Nascimento, Araújo, Silva and Ramos2015) and Silva Costa (Reference Silva Costa, Navickiene, Kato, Bolzani, Méda, Young and Furlan2002); and (ii) medium polarity peaks at Rt of 13.1, 14.2 and 24.4 min with absorption bands at 268, 338, 269, 338 nm, suggestive of flavonoids (Tong et al., Reference Tong, Peng, Tong, Guo and Shi2018) and at 324 nm that may be characteristic of phenolic compounds (Fig. 8B) (Ferreres et al., Reference Ferreres, Grosso, Izquierdo, Valentão, Azevedo and Andrade2014).
Discussion
The search for new anti-Schistosoma drugs has intensified due to reports of resistance of some strains of S. mansoni to the currently used drugs (Fallon et al., Reference Fallon, Sturrock, Niang and Doenhoff1995; Ismail et al., Reference Ismail, Metwally, Farghaly, Bruce, Tao and Bennett1996; Picquet et al., Reference Picquet, Vercruysse, Shaw and Diop1998; Crellen et al., Reference Crellen, Walker, Lamberton, Kabatereine, Tukahebwa, Cotton and Webster2016). Thus, researches with the purpose to identify of biologically active compounds from plant origin against S. mansoni are the basis for the development of new therapies (Ndjonka et al., Reference Ndjonka, Rapado, Silber, Liebau and Wrenger2013; Seif El-Din et al., Reference Seif El-Din, EL-Lakkany, Mohamed, Hamed, Sterner and Botros2014).
Although several studies have demonstrated potential effects of plant products against S. mansoni (Yousif et al., Reference Yousif, Hifnawy, Soliman, Boulos, Labib, Mahmoud, Ramzy, Yousif, Hassan, Mahmoud, El-Hallouty, El-Gendy, Gohar, El-Manawaty, Fayyad and El-Menshawi2007; Braguine et al., Reference Braguine, Costa, Magalhães, Rodrigues, Silva Filho, Bastos, Silva, Cunha, Januário and Pauletti2010; Caixeta et al., Reference Caixeta, Magalhães, Melo, Wakabayashi, Aguiar, Aguiar, Mantovani, Alves, Oliveira, Tavares, Groppo, Rodrigues, Cunha, Veneziani, Silva Filho and Crotti2011; Koné et al., Reference Koné, Vargas and Keiser2011); to date, there are no reports on the cercaricidal and schistosomicidal activity of ethanolic extracts of J. gossypiifolia and P. arboreum leaves. Thus, antiparasitic effects against S. mansoni demonstrated in in vitro tests of this study are described for the first time, which may lead to alternative controls of schistosomiasis.
Regarding the cercaricidal assay, although both the extracts reduced 100% of larval viability, the best effect was observed with P. arboreum (when compared to J. gossypiifolia), which had the potential to induce the death of all cercariae in 60 min at the highest concentration. The antiparasitic effect of plant extracts on S. mansoni cercariae was also verified by Castro et al. (Reference Castro, Costa, Laktin, Carvalho, Geraldo, Moraes, Pinto, Couri, Pinto and Silva Filho2015), who demonstrated mortality of cercariae after 1 h of incubation with the substance 7-epiclusianone (derived from an ethanolic extract of Garcina brasiliensis); however, approximately 100% of the cercariae were killed only after 8 h. Al-Sayed et al. (Reference Al-Sayed, Hamid and Einin2014) tested the extract of Eucalyptus globules leaves at 20 ppm and observed a 100% mortality rate after 2 h of exposure. Thus, based on these studies, the extract of P. arboreum leaves presented modest results.
Although the extract of P. arboreum leaves exhibited the best cercaricidal effect in this study, the same efficacy was not observed in adult worms. However, we observed that the extract of J. gossypiifolia leaves displayed the best performance, with 100% mortality of males and females after 12 and 24 h, at a concentration of 250 μg mL−1.
Previous research using plant extracts in a schistosomicidal assay demonstrated that couples of S. mansoni worms subjected to the essential oil of Dysphania ambrosioides (L.) at 25 and 12.5 μg mL−1 concentrations were killed after 24 and 72 h, respectively (Soares et al., Reference Soares, Dias, Vieira, Souza, Cruz, Badoco, Nicolella, Cunha, Groppo, Martins, Tavares, Magalhães and Crotti2017). However, Matos-Rocha et al. (Reference Matos-Rocha, Cavalcanti, Veras, Feitosa, Gonçalves, Portela-Junior, Lúcio, Silva, Padilha, Marques, Barbosa-Filho, Alves and Brayner2016), using the essential oil of Mentha x villosa, demonstrated a mortality rate for all adult male worms of S. mansoni only at 500 μg mL−1 concentration after 24 h, while the lowest concentrations (5 and 10 μg mL−1) were not able to induce mortality even after 120 h of exposure.
Interestingly, we observed that male worms were more susceptible than females at all concentrations of both the J. gossypiifolia and P. arboreum leaf extracts. Previous studies have also found that male S. mansoni worms were more susceptible than females when exposed to PZQ, ginger extract, diamines, amino alcohols and some essential plant oils (Pica-Mattoccia and Ciolli, Reference Pica-Mattoccia and Cioli2004; Mostafa et al., Reference Mostafa, Eid and Adly2011; Tonuci et al., Reference Tonuci, Melo, Dias, Wakabayashi, Aguiar, Aguiar, Mantovani, Ramos, Groppo, Rodrigues, Veneziani, Cunha, Silva Filho, Magalhães and Crotti2011; Oliveira et al., Reference Oliveira, Rehder, Santos Oliveira, Montanari Júnior, Carvalho, Ruiz, Jeraldo, Linhares and Allegretti2012; Fernandes et al., Reference Fernandes, Rezende Júnior, Fernandes, Silveira, Rezende, Almeida, Paula, Rodrigues, Silva Filho and Couri2013). However, these are still controversial data, since Oliveira Penido et al. (Reference Oliveira-Penido, Zech-Coelho, Mello, Piló-Veloso, Oliveira, Kusel and Nelson2008) demonstrated that female worms are more susceptible than males in experiments with amino-alkane-thiosulfuric acid. Despite these reports, the mechanisms involved in the differences in susceptibility between male and female parasites of S. mansoni still remain unclear.
Although the viability of adult S. mansoni worms was differently affected by both the extracts, a similar efficacy was observed on inhibition in the oviposition assay because at 50 μg mL−1 concentration both the extracts were able to reduce the egg-laying capacity of S. mansoni worms.
The inhibition of oviposition in female worms of S. mansoni was also observed by Sanderson et al. (Reference Sanderson, Bartlett and Whitfield2002), who demonstrated that the extract of ginger ethyl acetate (Zingiber officinale) at a concentration of 50 mg mL−1 was able to significantly inhibit the cumulative production of eggs in females mated in vitro. Godinho et al. (Reference Godinho, Aleixo de Carvalho, Barbosa de Castro, Dias, Pinto, Crotti, Pinto, Moraes and Silva Filho2014) also showed that the essential oil of aerial parts of Tanacetum vulgare caused complete inhibition of oviposition, but only at 200 μg mL−1 after 120 h of incubation. The inhibition of S. mansoni oviposition is particularly of great interest since the pathology of schistosomiasis is caused by an inflammatory reaction around the eggs that are retained in the vertebrate host tissue, mainly in the liver and intestines, resulting in the formation of granulomas (Schwartz and Fallon, Reference Schwartz and Fallon2018).
Moreover, we also demonstrated the presence of internal and external morphological changes in adult S. mansoni worms, which could constitute one of the main mechanisms of action exerted by the extracts of P. arboreum and J. gossypiifolia, thereby explaining the mortality rates and inhibition of oviposition observed in this study.
The rigidity of the integument of S. mansoni performs essential functions for its survival, such as osmoregulation, protection, synthesis and secretion of metabolites, parasitic defence against the host's immune system and represents an important structure for the action of drugs (Xiao et al., Reference Xiao, Bingguim, Utzinger, Chollet and Tanner2002; Faghiri and Skelly, Reference Faghiri and Skelly2009; Yepes et al., Reference Yepes, Varela, Lopez-Aban, Dakir, Moliinedo and Muro2014; Pereira et al., Reference Pereira, Silva, Souza, Laurentiz, Rodrigues, Januário, Pauletti, Tavares, Silva Filho, Cunha, Bastos and Magalhães2015). Specifically in females, changes in the integument may result in decreased egg formation. Indeed, the main mechanism of action of PZQ is also to target the integument of this parasite, acting on the permeability of its membrane, increasing the calcium influx, thus resulting in abnormal contraction of the parasites, paralysis and death (Novaes et al., Reference Novaes, Souza and Araújo1999; Cioli and Pica-Mattoccia, Reference Cioli and Pica-Mattoccia2003; Cioli et al., Reference Cioli, Pica-Mattoccia, Basso and Guidi2014).
Microstructural changes in the integument, similar to those identified in our study, such as bubbles, erosion/flaking and body retraction, were also observed with the use of the antibiotic, doxycycline, especially at higher concentrations (Dias et al., Reference Dias, Castro, Campos, Souza-Silva, Goncalves, Souza and Novaes2019). In the same study, the disappearance, flattening and the collapse of tubercles were also observed. Damage to the tubercles of S. mansoni male worms was also caused by a hydroalcoholic extract of Arctium lappa fruits at the concentrations of 100 and 200 mg mL−1 (Dias et al., Reference Dias, Zuza, Riani, Faria Pinto, Pinto, Silva, Moraes, Ataíde, Silva, Cecílio and Silva Filho2017).
Internal changes in the digestive system were also demonstrated by Oliveira et al. (Reference Oliveira, Corrêa, Vieira, Mendes, Allegretti and Miguel2019), who described esophageal changes (bulb-shaped oesophagus) in female worms exposed to tamoxifen (anti-cancer drug). According to Matos-Rocha et al. (Reference Matos-Rocha, Cavalcanti, Veras, Feitosa, Gonçalves, Portela-Junior, Lúcio, Silva, Padilha, Marques, Barbosa-Filho, Alves and Brayner2016), internal changes were also verified by transmission electron microscopy; the presence of vacuoles was found in the region of the syncytial matrix and of glycogen granules close to muscle fibres in adult worms of S. mansoni treated with the essential oil of Mentha x villosa leaves (500 μg mL−1), similar to the tegumentary dilations found in our study. Simões et al. (Reference Simões, Kawano, Allegretti, Linhares, Magalhães and Zanotti-Magalhães2015) also demonstrated that the crude extract of P. tuberculatum is capable of acting internally in female worms, modifying some regions of the vitelline glands. However, the alterations in the female reproductive system are yet less explored; hence, the details mentioned in our study should be considered, as they may provide valuable information on new drug targets.
These external and internal morphological changes in the S. mansoni male and female adult worms may have been caused by the presence of secondary metabolites present in J. gossypiifolia and P. arboreum leaves. Chemical screening suggested the presence of flavonoid compounds in the ethanolic extracts of J. gossypiifolia and P. arboreum and phenolic compounds and amides only in the P. arboreum extract.
Among these compounds, flavonoids and amides seem to have an important anthelmintic effect (Silva et al., Reference Silva, Carvalho, Borba and Silva2008; Moraes et al., Reference Moraes, Nascimento, Lopes, Nakano, Yamaguchi, Kato and Kawano2011, Reference Moraes, Nacimento, Yamagushi, Kato and Nakano2012; Botura et al., Reference Botura, Santos, Silva, Lima, Oliveira, Almeida, Batatinha and Branco2013). Indeed, amides are one of the main compounds found in plants of the genus Piper and recent studies have shown that the main amide isolated from P. tuberculatum, named piplartine, was active against adult worms (Moraes et al., Reference Moraes, Nascimento, Lopes, Nakano, Yamaguchi, Kato and Kawano2011) and schistosomules of S. mansoni (Moraes et al., Reference Moraes, Nacimento, Yamagushi, Kato and Nakano2012). However, future work must be carried out to accurately identify the major compounds of P. arboreum and J. gossypiifolia that could explain the different effects observed with the use of these extracts on cercariae and adult worms of S. mansoni.
It is important to highlight that to continue exploring plant extracts that could be used as therapeutics in humans in the future, it is important to perform toxicity tests. Although these tests were not carried out in this study, previous research has already demonstrated reduced toxicity of P. arboreum and J. gossypiifolia extracts (Nagaharika et al., Reference Nagaharika, Kalyani, Rasheed and Ramadosskarthikeyan2013; Macedo et al., Reference Macedo, Silva, Moreira, Queiroz, Vasconcelos, Araujo, Kaplan, Pereira, Almeida, Valverde and Robbs2019). However, more tests are needed to be carried out to confirm the absence of toxicity of the extracts used in this study in non-target organisms and cells.
In summary, the ethanolic extracts of J. gossypiifolia and P. arboreum leaves are biologically active against cercariae and worms of S. mansoni in vitro. External and internal morphological damage is implicated to be involved in the mortality of adult worms. Moreover, flavonoids, phenolic compounds and amides may be responsible for this process. However, future in vivo studies, as well as isolation of the main substances present in these plant extracts, should be carried out to establish the true potential of J. gossypiifolia and P. arboreum as new therapeutic and prophylactic strategies against schistosomiasis.
Acknowledgements
We thank the Universidade Estadual do Maranhão and Universidade Federal do Maranhão, for providing the necessary infrastructure and carrying out the experiments.
Author contributions
GSM, and JGMR conceptualized the study. RRSA, JGMR, AT-R, RAN, ICLL, MGSL, RSA, TJASA and GSM performed experiments. RRSA, JGMR, AT-R, RAN, ICLL, MGSL, TJASA, NS-S and GSM analysed the data. GSM supervised the project administration. RRSA, JGMR, RAN, ICLL, MGSL, TJASA, NS-S and GSM drafted the manuscript. All the authors reviewed and approved the manuscript.
Financial support
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of interest
The authors declare there are no competing interests.
Ethical standards
The care of and experiments with mice were approved by the Ethics Committee for the Use of Animals (CEUA) of UEMA, under approval number 03/2018.











