INTRODUCTION
Ectoparasites are of growing significance in contemporary veterinary medicine and human healthcare, therefore an understanding of their biology and biochemistry is fundamental. One of the most abundant, worldwide ecoparasitic fly species is Calliphora vomitoria, commonly found around houses and livestock facilities. Adults of C. vomitoria are attracted to feces and decomposing organic matter. Eggs are normally laid in batches in carrion or other waste material, but sometimes also on purulent wounds and excreta which may result in myiasis (Wall and Shearer, Reference Wall and Shearer2001). The high abundance of C. vomitoria in cadavers indicates the ecological significance of this necrophagous species in decomposition of human and animal vestiges and its usefulness in forensic cases including the sophisticated detection of morphine accumulation and metabolism (Bourel et al. Reference Bourel, Fleurisse, Hedouin, Cailliez, Creusy, Gosset and Goff2001; Grassberger and Frank, Reference Grassberger and Frank2004). The larvae of C. vomitoria are also used in the treatment of gangrene and wounds (maggot therapy), although less frequently than the larvae of Lucilia sericata or the closely related Calliphora vicina. On the other hand, an unhygienic habit of C. vomitoria gives rise to the mechanical transport of microorganisms that are potentially dangerous to humans and animals (Förster et al. Reference Förster, Klimpel, Mehlhorn, Sievert, Messler and Pfeffer2007).
One method of reducing noxious populations is the use of entomopathogenic microorganisms as insecticides (Oliveira et al. Reference Oliveira, Pereira, Lino-Neto, Bento and Baptista2012). Bacteria, such as Serratia sp. and Bacillus thuringiensis or microsporidium Octosporea muscaedomesticae, can induce lethal effects in insect victims (Smallbridge et al. Reference Smallbridge, Cooper and Pinnock1995; O'Callagan et al. Reference O'Callagan, Garnham, Nelson, Baird and Jackson1996). Entomopathogenic fungi cause lethal infections of insects and can regulate their populations in nature by epizootics. Currently about 35 genera with more than 400 species of entomopathogenic fungi are known. Approximately 1800 associations between fungi and different insects have been recorded (Jankevica, Reference Jankevica2004). Most pathogenic fungi have a broad host range, while fungi belonging to Entomophthorales are characterized by high selectivity (Bałazy, Reference Bałazy2004). As a contact insecticide, entomopathogenic fungi invade their host through the cuticle, covered by an outermost lipid layer mainly composed of highly stable, very long chain structures (Pedrini et al. Reference Pedrini, Crespo and Juárez2007).
There are a few natural insect defence mechanisms, including innate immunity and cuticular lipids, to counteract fungal infections. The cuticular surface of insects plays a primary role in biochemical and physiological functions, such as preventing insect desiccation (Yoder and Denlinger, Reference Yoder and Denlinger1990; Gibbs et al. Reference Gibbs, Chippindale and Rose1997, Reference Gibbs, Louie and Ayala1998; Benoit and Denlinger, Reference Benoit and Denlinger2007). In many species, cuticular lipids also have communicative functions (Vásquez et al. Reference Vásquez, Schal and Silverman2008; Kühbandner et al. Reference Kühbandner, Sperling, Mori and Ruther2012). Antimicrobial activity of insects’ cuticular lipids is frequently described (Kerwin, Reference Kerwin1982; Gołębiowski et al. Reference Gołębiowski, Maliński, Boguś, Kumirska and Stepnowski2008a, Reference Gołębiowski, Dawgul, Kamysz, Boguś, Wieloch, Włóka, Paszkiewicz, Przybysz and Stepnowski2012c, Urbanek et al. Reference Urbanek, Szadziewski, Stepnowski, Boros-Majewska, Gabriel, Dawgul, Kamysz, Sosnowska and Gołębiowski2012). Susceptibility or resistance of various insect species to fungal invasion may result from several factors, including composition of the cuticular lipids. In particular, free fatty acids are responsible for resistance to fungal infection (Gołębiowski et al. Reference Gołębiowski, Maliński, Boguś, Kumirska and Stepnowski2008a). Cuticular fatty acids are toxic and fungistatic but also may be stimulatory. For example, palmitoleic acid enhances mycelial growth, but is toxic to conidia of Erynia variabilis (Kerwin, Reference Kerwin1984). The toxic effects of palmitoleic acid can be mitigated by the presence of a sufficient concentration of oleic acid.
Larvae of C. vicina, closely related to C. vomitoria, are highly resistant to the entomophthoralean cosmopolitan soil fungus Conidiobolus coronatus (Gołębiowski et al. Reference Gołębiowski, Maliński, Boguś, Kumirska and Stepnowski2008a), known to be a potent entomopathogen (Boguś and Scheller, Reference Boguś and Scheller2002; Domsch et al. Reference Domsch, Gams and Anderson2007). Histological examination of C. vicina larvae exposed to sporulating C. coronatus colonies proved that conidia were unable to germinate on the fly cuticle, thus suggesting the presence of compounds inhibiting spore germination (Boguś et al. Reference Boguś, Kędra, Bania, Szczepanik, Czygier, Jabłoński, Pasztaleniec, Samborski, Mazgajska and Polanowski2007). In fact, the cuticular fatty acid profile of C. vicina larvae significantly differs from the profiles of Dendrolimus pini and Galleria mellonella, which are highly susceptible to fungal infection. The major difference is the presence of C14:0, C16:1 and C20:0 in the cuticle of C. vicina while these 3 fatty acids are absent in the cuticle of D. pini or present in trace amounts in G. mellonella cuticle (Gołębiowski et al. Reference Gołębiowski, Maliński, Boguś, Kumirska and Stepnowski2008a). In vitro cultivation of C. coronatus in the presence of these 3 fatty acids resulted in reduced sporulation, biomass of hyphae, ability to infect G. mellonella larvae and toxicity of metabolites released by the fungus into the culture medium (Boguś et al. Reference Boguś, Czygier, Gołębiowski, Kędra, Kucińska, Mazgajska, Samborski, Wieloch and Włóka2010), proving a contribution of these fatty acids to the resistance of C. vicina larvae to fungal assault. Recent studies on C. vomitoria showed that crude extracts containing both cuticular and internal lipids showed no antifungal activity against C. coronatus efficiently killing C. vomitoria adults, but not larvae and pupae.
The aim of the present work was to reveal whether cuticular and internal alcohols as well as fatty acid methyl esters (FAMEs) of C. vomitoria larvae, pupae and adults demonstrate antimicrobial activity against 6 entomopathogenic fungal strains. This work presents qualitative and quantitative analyses of the cuticular compounds of larvae, pupae, males and females of C. vomitoria. Hydrocarbons of C. vomitoria have already been identified (Trabalon et al. Reference Trabalon, Campan, Clement, Lange and Miquel1992), so we focused on FAMEs, alcohols, glycerol and cholesterol profiles.
MATERIALS AND METHODS
Insects
Calliphora vomitoria (Diptera: Calliphoridae) were reared from eggs laid on beef by adult flies at 25 °C, 70% relative humidity and a 12:12 h photoperiod. The maternal generation was maintained under the same conditions. The insects were fed on beef. Approximately 7 days elapsed between hatching and puparium formation, and it took another 7 days for the adults to emerge. Cuticular and internal lipids were extracted from post-feeding third-instar larvae, freshly formed pupae and 6-day-old sexually mature adults.
Extraction of cuticular and internal lipids
Two solvents of different polarity – petroleum ether (Chempur Piekary Śląskie, Poland) and dichloromethane (Eurochem BGD Tarnów, Poland) – were used for the lipid extractions. Three extracts were obtained from each developmental stage. The insects were immersed in 50 mL of petroleum ether for 10 sec (extract I). Then the insects were placed in dichloromethane (50 mL) and left there for 5 min (extract II). The same insects then were transferred to dichloromethane for 10 days (extract III). All extracts were concentrated using a roto-evaporator. Analytical samples were concentrated under a stream of nitrogen. For each sample, 19-methylarachidic acid was added as internal standard.
HPLC–LLSD
Extract lipids were separated into classes of compounds using high performance liquid chromatography with a laser light scattering detector (HPLC–LLSD) and a normal-phase 250×4·6 mm analytical column with Econosil Silica (Alltech, particle size 5 μm). The mobile phase consisted of n-hexane (Solvent A) and dichloromethane containing 15% acetone (Solvent B). The gradient was programmed linearly from A to B within 30 min.
Derivatization
To transform a chemical compound into a more volatile product, the samples were dried under a stream of nitrogen and then 50 mL of a mixture of 99% bis(trimethylsilyl)acetamide and 1% chlorotrimethylsilane (Supelco) was added to 1 mg of each extract. The samples were heated in a heating block at 100 °C for 1 h and cooled prior to GC–MS analysis. Alcohols, glycerol and cholesterol were identified as trimethylsilyl (TMS) derivatives of these compounds, while FAMEs were analysed as native compounds.
Gas chromatography–mass spectrometry
GC–MS analysis was performed with a Hewlett -Packard 6890 gas chromatograph equipped with an Rtx-5 capillary column (J&W Scientific, 30 m×0.25 mm i.d.×0.15 μm film thickness) and SSQ 710 equipped with an HP 6890 GC. The program started at an initial temperature of 80 °C with an initial hold for 8 min, and was increased gradually to 310 °C at 4 °C min−1 with a final hold for 20 min. Helium was used as a carried gas (column head pressure 82 kPa, flow rate of 1 mL min−1, operating at constant flow). Electron impact mode was performed at 70 eV. The temperature of the GC–MS interface was 310 °C. The ion source was maintained at 200 °C. Components were characterized by comparison of their individual mass spectra with standards.
Standards
In the HPLC analysis the following standards were used: n-tetracosane (Fluka AG), tricosanoic acid methyl ester (Applied Science Laboratories Inc.), eicosanoic acid (Fluka AG), hexadecanoic acid (Aldrich), 1-hexadecanol (Sigma) and cholesterol (Fluka AG). GC–MS analysis employed the following standards: 19-methylarachidic acid (Sigma), decanoic acid (Sigma), dodecanoic acid (Carl Roth RG), tetradecanoic acid (Fluka AG), hexadecanoic acid (Sigma), octadecenoic acid (Aldrich), octadecanoic acid (Sigma), eicosanoic acid (Fluka AG), tetracosanoic acid (Sigma) and n-alkane standards (Polyscience Corporation).
Determination of antifungal activity
The minimum inhibitory concentration (MIC) was determined using the broth dilution method according to the procedures recommended by the CLSI (Clinical and Laboratory Standards Institute). The following fungal strains were tested: Beauveria bassiana (Tve-N39), B. bassiana (Dv-1/07), Lecanicillium lecanii, Metarhizium anisopliae, Paecilomyces fumosoroseus and Paecilomyces lilacinus. Entomopathogenic fungi were obtained from the Institute of Plant Protection (Poznań, Poland). Fungal strains were cultured in Sabouraud Glucose liquid medium (CARL ROTH GmbH) for 48 h at 25 °C with shaking (130 rpm). To determine the MICs, the suspensions of microorganisms in Sabouraud Glucose broth were adjusted with a spectrophotometer (Genesys 10uv, Thermo Electron Corporation) at λ=530 nm to obtain initial inocula of c. 5×103 cfu mL−1. Microorganisms placed on polystyrene 96-well plates were exposed to the investigated alcohols, FAMEs and their mixtures at appropriate concentrations (range: 2–1024 mg mL−1) for 48 h at 25 °C. Mixtures of alcohols and FAMEs were prepared according to their chemical composition found in the extracts obtained from different living forms of C. vomitoria. The MIC was taken to be the lowest concentration of the tested compound at which observable growth was inhibited. Experiments were performed in triplicate on 3 different days.
RESULTS
Total lipids and their identification
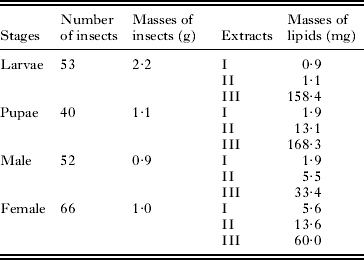
The respective total quantities of cuticular lipids of larvae, pupae, males and females extracted with petroleum ether and dichloromethane (extracts I and II) were 2·0, 15·0, 7·4 and 19·2 mg, which correspondingly made up 1·3, 8·9, 22·2 and 32·0% of the total lipids. Table 1 lists the total amounts of internal lipids (extract III). The 3 fractions obtained from the HPLC-LLSD separations of FAMEs, alcohols and sterols were then analysed by GC–MS. Alcohols were identified on the basis of the characteristic ions of their silyl derivatives. The characteristic alcohol ions were at m/z 73, 75, 103 and (M-15)+. The mass spectrum of the TMS ether of heptadecanol is illustrated by way of the example in Fig. 1. FAMEs were identified on the basis of the mass spectra obtained for native compounds. FAMEs have characteristic ions at m/z 74, 87 and (M)+. The mass spectrum of hexadecanoic acid methyl ester is shown in Fig. 2. The characteristic ions of the TMS ether derivatives of cholesterol were at m/z 129 (100%), 329 (87%), 368 (52%), 145 (38%), 121 (36%), 353 (32%) and 458 (M)+ (Fig. 3), while those of the TMS ether derivatives of glycerol were at m/z 73, 103, 133, 147, 205 and 218 (Fig. 4).

Fig. 1. Mass spectrum of the trimethylsilyl (TMS) ether of heptadecanol.

Fig. 2. Mass spectrum of hexadecanoic acid methyl ester.
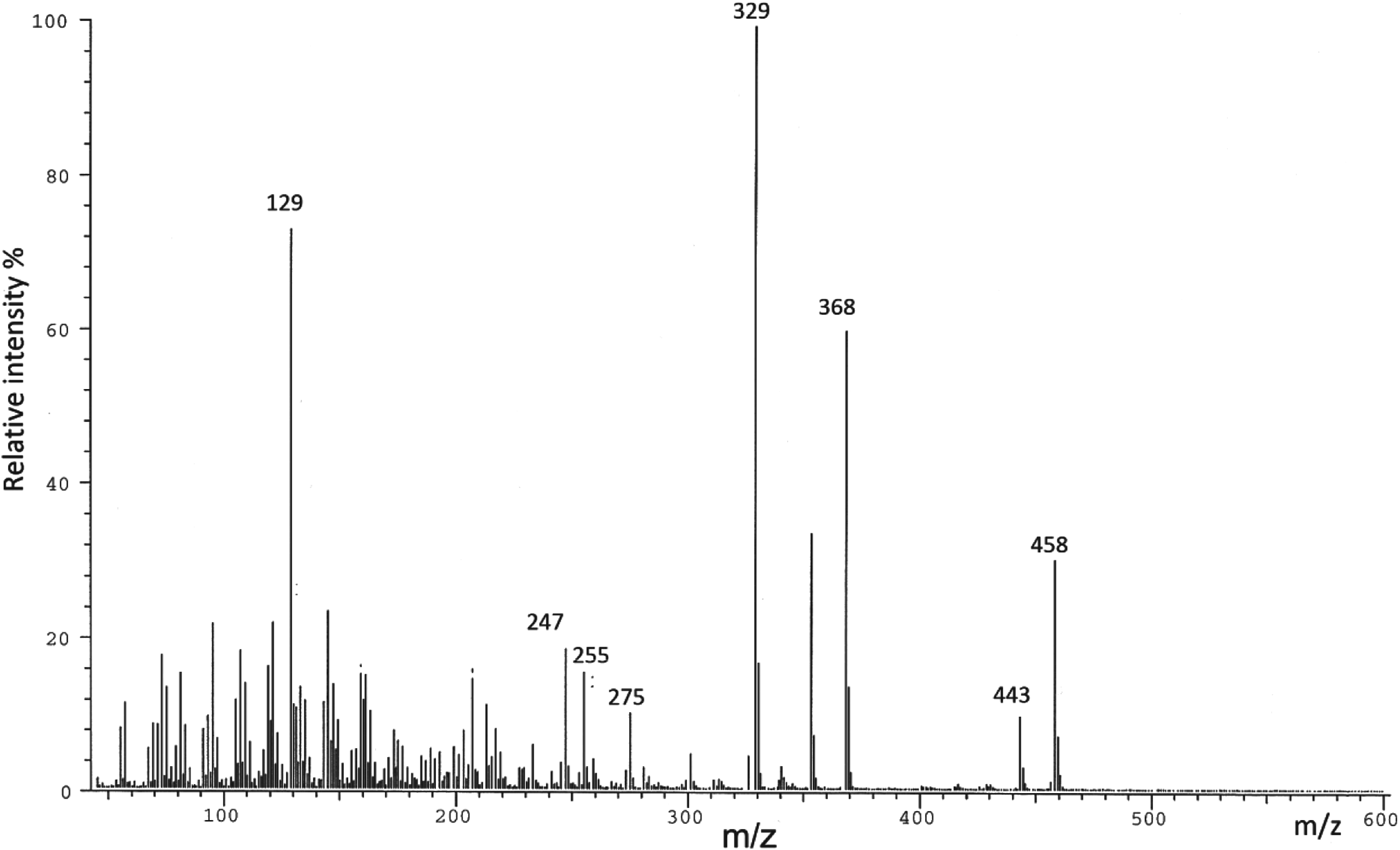
Fig. 3. Mass spectrum of the trimethylsilyl (TMS) ether of cholesterol.

Fig. 4. Mass spectrum of the trimethylsilyl (TMS) ether of glycerol.
Table 1. Quantitative summary of the experiment: numbers and masses of insect; masses of lipids
Cuticular FAME composition of C. vomitoria
Table 2 lists the percentage contents of methyl esters in the cuticle of C. vomitoria as well as the methyl ester contents calculated per g of insect body. The total cuticular methyl ester content in C. vomitoria larvae was only 1·21 μg g−1 of the insect body. Only 6 methyl esters (from C15 to C19: 3 saturated and 3 unsaturated) were identified in the cuticular lipids of the larvae. The compounds present in the highest concentrations were C17:1 (0·67 μg g−1 of the insect body; relative content 55·4% of total methyl esters) and C19:1 (0·41 μg g−1 of the insect body; relative content 33·9% of total methyl esters). The remaining methyl esters, present in smaller quantities, were C17:0 (5·0%), C19:2 (3·3), C15:0 (2·5%) and C19:0 (traces).
Table 2. Chemical composition of the cuticular FAMEs found in Calliphora vomitoria

The data are presented as the mean±standard deviation of three separate analyses performed on different samples; ‘–’ not detected.
FAMEs were the second largest group of compounds detected in pupal cuticular lipids (Table 2). They consisted of 7 compounds, with C17:1 and C19:1 being the dominant components (42·5 and 42·0% respectively). Three other compounds were C15:0 (3·9%), C17:0 (6·2%) and C19:2 (5·5%). Traces of 2 other FAMEs were also detected (C16:0 and C19:0).
Five FAMEs were identified in the cuticular lipids of male adults: C19:1 (57·8%), C17:1 (30·1%), C17:0 (12·1%) and C15:0 and C19:0, traces of which were present (Table 2). The same FAMEs were identified in both cuticular extracts of males (extracts I and II).
C17:0 (14·1%) and C19:2 (10·4%) were present in relatively large amounts in addition to C17:1 (29·9%) and C19:1 (44·6%) in the lipids of females (Table 2). Three saturated FAMEs (C15:0, C16:0 and C19:0) were present in smaller amounts from traces to 0·5%.
Internal FAME composition of C. vomitoria
Nine FAMEs – from C15:1 to C19:0 – were found in the internal lipids of larvae (Table 3). The FAMEs present in the highest concentrations were C17:1 (1670·84 μg g−1 of the insect body; relative content 57·1% of total methyl esters) and C19:1 (907·12 μg g−1 of the insect body; relative content 31·0% of total methyl esters). The other methyl esters were present in smaller quantities, from trace amounts to 116·24 μg g−1 of the insect body: C15:1 (0·6%), C15:0 (2·9%), C16:0 (0·5%), C17:0 (3·8%), C18:0 (traces), C19:2 (4·0%) and C19:0 (0·1%). The internal lipids contained 2 even-numbered methyl esters (C16:0 and C18:0) and 1 unsaturated methyl ester (C15:1), which were absent in the cuticular lipids. Other FAMEs were present in both cuticular and internal lipids of larvae.
Table 3. Chemical composition of the internal FAMEs found in Calliphora vomitoria

The data are presented as the mean±standard deviations of three separate analyses performed on different samples; ‘–’ not detected.
Ten FAMEs were found in the internal lipids of the pupae (Table 3). Two of them (C17:1 and C19:1) were predominant, accounting for 35·8% and 43·3% of the total FAMEs. The remaining methyl esters were present in smaller quantities: C13:0 (0·1%), C15:1 (0·9%), C15:0 (4·1%), C16:0 (0·2%), C17:0 (8·4%), C18:0 (0·3%), C19:2 (6·7%) and C19:0 (0·3%).
The most abundant internal FAMEs in males and females were C17:1 (34·8 vs 34·1%) and C19:1 (54·3 vs 50·6%) (Table 3). Other FAMEs were present in smaller quantities, from trace amounts (C16:0 and C19:0) to 8·8% (C17:0) in males and from 0·1% (C15:0) to 10·3% (C19:2) in females.
Cuticular and internal alcohol composition of C. vomitoria
The cuticular lipids of larvae contained 7 saturated alcohols only, with even-numbered carbon chains from C12:0 to C24:0 (Table 4). The total cuticular alcohol content in C. vomitoria larvae was only 3·11 μg g−1 of the insect body. The alcohols occurring in the highest concentrations were C18:0 (25·7%), C20:0 (28·6%) and C22:0 (23·2%); those present in smaller quantities included C12:0 (4·8%), C14:0 (4·5%), C16:0 (7·4%) and C24:0 (5·8%). The internal lipids of the larvae contained only 1 saturated alcohol with an even-numbered carbon chain – C22:0 (1·14 μg g−1 of the insect body) (Table 5).
Table 4. Chemical composition of the cuticular alcohols found in larvae of Calliphora vomitoria

The data are presented as the mean±standard deviation of three separate analyses performed on different samples; ‘–’ not detected.
Table 5. Chemical composition of the internal alcohols found in Calliphora vomitoria

Data are presented as the mean±standard deviation of three separate analyses performed on different samples; ‘–’ not detected.
The cuticular and internal lipids of pupae contained 6 and 4 alcohols respectively (Tables 4 and 5): C18:0, C20:0 and C22:0 were the most abundant alcohols in these two developmental stages. Together, these compounds made up 88·0% of the alcohols present in cuticular lipids and 100% of those in the pupal internal lipids. Cuticular alcohols present in smaller quantities included C14:0 (6·3%), C16:0 (5·6%) and C24:0 (traces). All the cuticular and internal alcohols of pupae were saturated and had an even number of carbon atoms.
The cuticular lipids of males contained 5 alcohols, present in small amounts (Table 4). The most abundant alcohol was C22:0 (60·2%), but this made up only 0·80 μg g−1 of the insect body. The female cuticular lipids contained only trace amounts of 3 alcohols.
The internal lipids of males and females contained only 4 alcohols (Table 5); all of those in the males were present in trace amounts. The most abundant alcohol in the internal lipids of the females was C18:0 (53·4%; 7·32 μg g−1 of the insect body).
Cuticular and internal glycerol and cholesterol in C. vomitoria
The cuticular and internal lipids of larvae, pupae, males and females contained cholesterol and glycerol (Table 6). The respective quantities of cuticular cholesterol obtained from larvae and pupae of C. vomitoria were 0·68 and 6·48 μg g−1 of the insect body. There was a 10-fold higher concentration of cuticular cholesterol in the pupal lipids. However, a similar amount of internal cholesterol was identified in the larval and pupal lipids. The internal cholesterol content in larval and pupal lipids was 173·79 and 181·45 μg g−1 respectively. A similar amount of cuticular cholesterol was identified in the male and female lipids. However, the content of internal cholesterol in females was significantly higher than that of the internal lipids in males: 232·19 and 164·29 μg g−1 of the insect body respectively.
Table 6. Glycerol and cholesterol contents in larvae and pupae of Calliphora vomitoria

The data are presented as the mean±standard deviation of three separate analyses performed on different samples; ‘–’ not detected.
There was a 2- fold higher concentration of cuticular glycerol in larval lipids than in pupal lipids. On the other hand, the internal glycerol content in pupae was significantly higher than in larvae: 55·11 and 5·94 μg g−1 of the insect body respectively. A 10- fold greater concentration of cuticular glycerol was determined in male lipids than in female lipids, but the glycerol contents in male and female internal lipids were similar.
Antifungal activity of FAMEs
The individual FAMEs displayed only weak activity, if any, against the entomopathogenic fungi tested. Metarhizium anisopliae and P. fumosoroseus were susceptible to almost all the compounds when exposed to a concentration of 1024 mg L−1. The other fungal strains turned out to be resistant to the majority of FAMEs at the concentrations applied (2–1024 mg L−1), although some growth reduction was observed with most of them at the highest concentrations (data not shown). The mixtures of FAMEs found in living forms of C. vomitoria were somewhat more active than the individual compounds. Nearly all of the tested mixtures exhibited some activity against all the fungal isolates. The growth of P. fumosoroseus and B. bassiana (Tve-N39) was inhibited by the majority of extracts at a concentration of 1024 mg L−1, while B. bassiana strain DV-1/107, L. lecanii and M. anisopoliae demonstrated a higher susceptibility (Table 7).
Table 7. Minimum inhibitory concentration (mg L−1) of the mixtures of FAMEs, mixtures of alcohols and individual alcohols found in Calliphora vomitoria

Antifungal activity of alcohols
The alcohols tested turned out to be very weak antifungal agents. They inhibited the growth of the test strains at a concentration of 1024 mg L−1 or were inactive at the concentrations applied (data not shown). Only C12:0 and C14:0 were more active against the entomopathogenic fungi in comparison with longer-chain alcohols (Table 7). Extracts containing alcohols also showed weak antimicrobial activity. The majority of the strains tested were resistant to the mixtures of internal alcohols extracted from larvae, pupae and females of C. vomitoria (data not shown). The activity of male internal alcohols and female cuticular alcohols were not tested as they were present only in trace amounts in the extracts. The remaining mixtures mostly suppressed fungal strains at a concentration of 1024 mg L−1. Growth of B. bassiana (DV-1/107) and M. anisopoliae was inhibited by cuticular alcohols obtained from larvae and pupae at 512 mg L−1, but only the cuticular alcohols obtained from larvae were effective towards P. lilacinus at this concentration (Table 7).
DISCUSSION
The composition of cuticular lipids typically includes various groups of compounds (Lockey, Reference Lockey1988; Buckner, Reference Buckner, Stanley-Samuelson and Nelson1993; Gołębiowski et al. Reference Gołębiowski, Boguś, Paszkiewicz and Stepnowski2011), often hydrocarbons (Lockey, Reference Lockey1980; Gołębiowski et al. Reference Gołębiowski, Maliński, Nawrot, Szafranek and Stepnowski2007, Reference Gołębiowski, Paszkiewicz, Grubba, Gąsiewska, Boguś, Włóka, Wieloch and Stepnowski2012b; Fan et al. Reference Fan, Eliyahu and Schal2008) and fatty acids (Gołębiowski et al. Reference Gołębiowski, Boguś, Paszkiewicz and Stepnowski2010; Gołębiowski, Reference Gołębiowski2012), but also aldehydes and ketones (Nelson et al. Reference Nelson, Guershon and Gerling1998), or wax esters, alcohols, FAMEs and sterols (Ikekawa et al. Reference Ikekawa, Morisaki and Fujimoto1993; Buckner et al. Reference Buckner, Nelson and Mardaus1994; Nelson et al. Reference Nelson, Fatland, Buckner and Freeman1999; Gołębiowski et al. Reference Gołębiowski, Boguś, Paszkiewicz, Wieloch, Włóka and Stepnowski2012a). The composition of lipids depends on various factors, primarily on the insect species, its developmental stage, environment and lifestyle (Mpuru et al. Reference Mpuru, Blomquist, Schal, Roux, Kuenzli, Dusticier, Clément and Bagnères2001). Each cuticular and internal compound of an insect has a specific role, governed by specific needs. The composition may also change with changing environmental pressures such as time of the year, or during ontogeny (Roux et al. Reference Roux, Gers and Legal2006). Cuticular lipids can be defensive or fungistatic substances, but some of them are active as pheromones. For example, unsaturated fatty acids and FAMEs can affect the settling behaviour of Liposcelis bostrychophila and have been found to be repellent to ants (Dani et al. Reference Dani, Cannoni, Turillazzi and Morgan1996; Green, Reference Green2009, Reference Green2011).
Although liquid chromatography has been used to analyse insect lipids (Kermasha et al. Reference Kermasha, Kubow and Goetghebeur1994; Jiann-Tsyh et al. Reference Jiann-Tsyh, McKeon and Stafford1995), gas chromatography, and gas chromatography hyphenated with mass spectrometry in particular, is the standard technique for this purpose (Nelson et al. Reference Nelson, Guershon and Gerling1998; Caputo et al. Reference Caputo, Dani, Horne, Fale, Diabate, Turillazzi, Coluzzi, Costantini, Priestman, Petrarca and della Torre2007; Jarrold et al. Reference Jarrold, Moore, Potter and Charnley2007; Ye et al. Reference Ye, Li, Zhu, Zhu and Hu2007; Buckner et al. Reference Buckner, Pitts-Singer, Guédot, Hagen, Fatland and Kemp2009). GC–MS has become so commonly used because mass spectra can be obtained, which in contentious situations (similar retention times, traces of compounds) permit the unequivocal identification of compounds, which are analysed by comparing the molecular and fragment ions and using library spectra. Obviously, therefore, the C. vomitoria lipids were analysed primarily using GC–MS.
The compounds identified in C. vomitoria were FAMEs, alcohols, glycerol and cholesterol. Comparison of the total ion currents indicates that the type of solvent used and the different extraction time affect the composition of the lipid extracts.
FAMEs are minor constituents of cuticular lipids (Lockey, Reference Lockey1988). Methyl palmitate and methyl linolenate appear to act as pheromones for the honeybee and as a kairomone for the parasitic mite Varroa jacobsoni (Le Conte et al. Reference Le Conte, Arnold, Trouiller and Masson1990). FAMEs were detected in significant concentrations in the cuticle of Acyrthosiphon pisum and were primarily saturated (Brey et al. Reference Brey, Ohayon Lesourd, Castex, Roucache and Latge1985). The dominant components of A. pisum consisted of C19:2 (33%), C15:0 (23%), C19:1 (22%), C19:3 (13%) and C17:1 (9%). Other cuticular FAMEs were present in traces – C17:0 and C19:0. Only 3 FAMEs (C17:0, C19:1 and C19:0) were identified in the cuticular lipids of both males and females of Acanthoscelides obtectus (Gołębiowski et al. Reference Gołębiowski, Maliński, Nawrot and Stepnowski2008b).
The present study of the cuticular and internal lipids of C. vomitoria demonstrated a significant amount of C17:0, C17:1 and C19:1 FAMEs. In addition, females had a high content of the methyl ester of C19:2 (10·4% in cuticular lipids and 10·3% in internal lipids), whereas this compound was absent in males. Both saturated and unsaturated FAMEs were identified in the cuticular and internal lipids of larvae, pupae, males and females. Odd-numbered, saturated and unsaturated FAMEs are typically found in insect species. However, pupae and females of C. vomitoria contained 1 cuticular, even-numbered FAME (C16:0) in trace amounts. Larvae and pupae of C. vomitoria contained 2 internal, even-numbered FAMEs (C16:0 and C18:0), whereas the male internal lipids contained only traces of C16:0 methyl ester. Additionally, the presence of odd-numbered FAMEs (C15:1 (0·9%) and C13:0 (0·1%)) in the internal lipids of pupae is characteristic, although the C15:1 ester (0·6%) was also identified in the internal lipids of larvae.
Since the FAMEs found in insects act mainly as semiochemicals (Le Conte et al. Reference Le Conte, Arnold, Trouiller and Masson1990), it was not surprising that the compounds did not display a strong antifungal potential. The antimicrobial activity of cuticular and internal FAMEs found in C. vomitoria was not affected by either the source or the mode of isolation. Indeed, the composition of the isolated extracts was rather congruent. In all mixtures the most abundant FAMEs were C17:1 and C19:1.
The presence of alcohols in the cuticular lipids of some insects is well documented (Ohara and Lockey, Reference Ohara and Lockey1990; Buckner et al. Reference Buckner, Mardaus and Nelson1996, Reference Buckner, Hagen and Nelson1999; Jones et al. Reference Jones, Moran and Hurd1997). Short-chain unsaturated alcohols can be components of pheromones (Buckner, Reference Buckner, Stanley-Samuelson and Nelson1993). The composition of cuticular alcohols is very diverse. Some insect species contain only one or two cuticular alcohols (Buckner et al. Reference Buckner, Nelson and Mardaus1994; Jones et al. Reference Jones, Moran and Hurd1997), while others contain a large number of alcohols (Ohara and Lockey, Reference Ohara and Lockey1990).
In our study, we found the same cuticular alcohol level in pupae and males (1·42 vs 1·29%). However, 7, 6, 5 and 3 cuticular alcohols were respectively identified in the lipids of larvae, pupae, males and females. On the other hand, only 1 alcohol was identified in the internal lipids of larvae, and 4 alcohols were present in the internal lipids of pupae, males and females. Alcohols from C12:0 to C20:0 were present among the compounds identified in our study. The presence of alcohols in this range as major compounds was found only in lipids isolated from Locusta migratoria migratoides adults, Schistocerca gregaria adults (Ohara and Lockey, Reference Ohara and Lockey1990) and A. pisum (Brey et al. Reference Brey, Ohayon Lesourd, Castex, Roucache and Latge1985). However, even-numbered, saturated alcohols are compounds characteristic of insects. For example, only C28:0, C30:0, C32:0 and C34:0 alcohols were found in the lipids isolated from Bemisia argentifolii nymphs (Buckner et al. Reference Buckner, Hagen and Nelson1999), only 1 alcohol (C30:0) was identified in a Tenodera sinensis female, Tenodera angustipennis male and Stagmomantis carolina female (Jones et al. Reference Jones, Moran and Hurd1997), and 2 alcohols (C30:0 and C32:0) were found in Trialeurodes vaporariorum adults (Buckner et al. Reference Buckner, Nelson and Mardaus1994).
The antimicrobial activity of alcohols isolated from M. domestica has been reported (Gołębiowski et al. Reference Gołębiowski, Dawgul, Kamysz, Boguś, Wieloch, Włóka, Paszkiewicz, Przybysz and Stepnowski2012c). These compounds demonstrated antibacterial activity against Gram-positive bacteria and weak activity against Candida sp., whereas Gram-negative bacterial strains turned out to be resistant. In the present work most of the individual alcohols as well as the alcohol extracts isolated from C. vomitoria demonstrated weak antimicrobial activity towards entomopathogenic fungi. The effectiveness of extracts seems to depend on the strain tested rather than on the source or mode of isolation. However, we also found that the cuticular extracts obtained from larvae and pupae were slightly more active. These are the only mixtures containing short-chain alcohols that exhibited stronger antifungal activity in the MIC assay performed for individual compounds.
Selective antibacterial and low antifungal activities of C. vomitoria cuticular alcohols and FAMEs indicate that these compounds may serve as additional factors supporting defence systems protecting this ectoparasite from microorganisms. Larvae of C. vomitoria and closely related C. vicina exposed to virulent colonies of C. coronatus remain unharmed (Boguś et al. Reference Boguś, Kędra, Bania, Szczepanik, Czygier, Jabłoński, Pasztaleniec, Samborski, Mazgajska and Polanowski2007; Gołębiowski et al. Reference Gołębiowski, Cerkowniak, Boguś, Włóka, Dawgul, Kamysz and Stepnowski2013). Callophora vicina is known to produce, after an experimental challenge with bacteria, a series of potent antimicrobial substances namely defensin, diptericins, cecropins, proline-rich peptides, as well as alloferons showing amino-acid stretches similar to those of the antifungal protein isolated from Sarcophaga peregrine (Chernysh et al. Reference Chernysh, Kim, Bekker, Pleskach, Filatova, Anikin, Platonov and Bulet2002). Contact with C. coronatus activates cecropins and lysozyme (Boguś et al. Reference Boguś, Kędra, Bania, Szczepanik, Czygier, Jabłoński, Pasztaleniec, Samborski, Mazgajska and Polanowski2007). Whether other elements of this weaponry are activated by contact with fungus still remains an open question.
In our study, glycerol was identified in the cuticle, as well as in the internal lipids of larvae, pupae, males and females. It occurred in the largest quantities in the internal lipids of pupae (55·11 μg g−1) and in the cuticular lipids of males (46·94 μg g−1 of the insect body). The physiological role of the sexual differences in cuticular glycerol concentration (female cuticles contain 10·3 times less than male cuticles) is unknown. The reason for the 16- and 7·8-fold lower concentrations of cuticular glycerol, respectively detected in pupae and larvae, compared with males, also remains obscure. Additional experiments are needed to explain the developmental and sexual differences in the internal glycerol concentrations and the mechanism underlying the divergences in the internal/cuticular glycerol concentration ratios, which range from 1:1 in larvae, 1·3:1 in males, 11·9:1 in females to 18·8:1 in pupae.
Glycerol was detected as the main compound in the haemolymph and gland secretions of Eudia pavonia, Saturnia pyri and Eupackardia calleta caterpillars (Deml and Dettner, Reference Deml and Dettner1993). This compound usually acts as an antifreezing agent in the haemolymph of a number of diapausing insects (Somme, Reference Somme1964, Reference Somme1965). The properties of glycerol as a factor making insects tolerant towards low temperatures have been studied. Larvae of the flesh fly Sarcophaga bullata, exposed to −10 °C immediately after administration of glycerol, survived at high rates. However, when glycerol-fed larvae were kept for 2 days at 25 °C before exposure to −10 °C, survival rates were low (Yoder et al. Reference Yoder, Benoit, Denlinger and Rivers2006), indicating the complex nature of the mechanism underlying glycerol-induced cold tolerance. No data are available concerning the role of glycerol in the overwintering of C. vomitoria.
The final compound identified in the cuticular and internal lipids of C. vomitoria was cholesterol. No other sterol was identified apart from cholesterol. The presence of cholesterol has an important impact on insect development, especially internal cholesterol, which is an ecdysteroid precursor (Svoboda and Weirich, Reference Svoboda and Weirich1995). For example, in Coleomegilla maculata, Pilorget et al. (Reference Pilorget, Buckner and Lundgren2010) examined whether and to what extent the type of food (corn pollen with a different sterol content – beta-sitosterol, cholesterol, ergosterol) would have an impact on the state of the insect. A strong correlation was found between the amount of administered sterols and population growth. The proportionately decreasing content of sterols in the diet over time and the growth of C. maculata larvae were also correlated (Pilorget et al. Reference Pilorget, Buckner and Lundgren2010). Although insects are unable to biosynthesize cholesterol (Ikekawa et al. Reference Ikekawa, Morisaki and Fujimoto1993), the cholesterol they contain may be derived from their food (Böröczky et al. Reference Böröczky, Park, Minard, Jones, Baker and Tumlinson2008), or they may convert phytosterols to cholesterol via the dealkylation of phytosterols. In our study, the high concentrations of cholesterol detected in the internal lipids of all developmental stages (with the highest values being in females) probably reflect the storage of lipids necessary for metamorphosis and the involvement of cholesterol in vitellogenesis. Note that internal cholesterol makes up 0·24–0·39% of the total extracted internal lipids in larvae, pupae and females, but is less than 0·04% in males. Assuming that lipids constitute a major metabolic store for the development of fly larvae to adults, large amounts of internal lipids in larvae and pupae cannot be surprising (Saunders, Reference Saunders2000). The physiological role of the observed developmental and sexual divergences in the concentrations of cuticular cholesterol remains to be discovered.
In conclusion, the methods applied enabled the cuticular and internal compounds of C. vomitoria to be identified and quantified. A total of 19 compounds were identified in the larvae, pupae and adult insects, including 10 FAMEs, 7 alcohols, cholesterol and glycerol. The alcohols and FAMEs displayed antimicrobial activity against entomopathogenic fungi.
FINANCIAL SUPPORT
Financial support was provided by the Polish Ministry of Science and Higher Education for 2010–2013 grant: N N303 504238 and DS 530-8110-D195-12.













