Introduction
Gastrointestinal nematodes (GINs) are one of the major health problems of ruminants and horses throughout the world. It has been documented that GINs are responsible for significant economic losses in livestock farming systems. Strongyles (mainly cyathastomins and Strongylus vulgaris) and Parascaris equorum are the major parasites capable of causing clinical diseases in naturally infected horses (Reinemeyer and Nielsen, Reference Reinemeyer and Nielsen2009). Effective control of parasites is essential to achieve optimum equine health, productivity and efficient breeding herd performance. Apart from clinical disease, GIN infection in many livestock species negatively affects the utilization of nutrients that can result in protein deficiency and increased amino acid demands (Coop and Holmes, Reference Coop and Holmes1996; Fox et al., Reference Fox, Uche, Vaillant, Ganabadi and Calam2002). As observed in other animals, parasitism in horses causes poor body condition, distended abdomen, retarded growth, weakness and poor digestion and malabsorption especially in young horses and immunocompromised foals (Owen and Slocombe, Reference Owen and Slocombe1985).
Control of GINs in an extensive grazing system is one of the most significant challenges in veterinary medicine (Craig, Reference Craig2006). Since the discovery of anthelmintics, parasite control has relied heavily on frequent use of anthelmintics often applied round the year. The intensive use of anthelmintics has resulted in the development of resistance in benzimidazoles (BZ)s as well as macrocyclic lactones (MLs) and tetrahydropyrimidines (Peregrine et al., Reference Peregrine, Molento, Kaplan and Nielsen2014; Scott et al., Reference Scott, Bishop and Pomroy2015). Anthelmintic resistance (AR) is generally defined as ‘when a previously effective drug is unable to kill the parasite population while exposed to therapeutic doses (Jabbar et al., Reference Jabbar, Iqbal, Kerboeuf, Muhammad, Khan and Afaq2006) or loss of sensitivity to a drug in parasitic population that was sensitive to the same drug which is thought to be genetically transmitted (Kohler, Reference Kohler2001)’. AR has been documented in parasites of different animal species including cattle (Lifschitz et al., Reference Lifschitz, Suarez, Sallovitz, Cristel, Imperiale, Ahoussou, Schiavi and Lanusse2010; Geurden et al., Reference Geurden, Chartier, Fanke, di Regalbono, Traversa, von Samson-Himmelstjerna, Demeler, Vanimisetti, Bartram and Denwood2015), sheep and goats (Coles, Reference Coles2005; Domke et al., Reference Domke, Chartier, Gjerde, Hoglund, Leine, Vatn and Stuen2012) and horses (Geurden et al., Reference Geurden, Betsch, Maillard, Vanimisetti, D'Espois and Besognet2013; Wolf et al., Reference Wolf, Hermosilla and Taubert2014; Saes et al., Reference Saes, Vera, Fachiolli, Yamada, Dellaqua, Saes, Amarante and Soutello2016).
The emerging significance of AR demands an urgent need for the development of reliable, reproducible and standard methods/assays for its detection (Coles et al., Reference Coles, Jackson, Pomroy, Prichard, von Samson-Himmelstjerna, Silvestre, Taylor and Vercruysse2006). Accurate and timely detection of AR and the knowledge of the mechanism(s) involved in its development might aid to adopt the measures to slow the development of resistance. In addition, this will also help in developing new anthelmintic drugs as the control of GINs will remain at least partly dependent on anthelmintics in the foreseeable future, although adoption of complementary approaches such as bioactive diets may also play an increasing role (Taylor et al., Reference Taylor, Hunt and Goodyear2002). Our knowledge of the mechanisms associated with the development of AR in ruminants is much more advanced than those of horses. In addition, a number of in vitro AR detection tests have been successfully used in sheep nematodes, whereas, very few of these tests are reported in horse nematodes and the results are not satisfactory, indicating that these tests require further refinement. The purpose of this article is to comprehensively review the history and current status of AR in equine GINs, to discuss the scientific aspects of development and detection of resistance and control strategies that are recommended to counter the development of AR. This article also provides further insights into key future research areas that may be considered by the equine industry and parasitology researchers for achieving sustainable parasite control in equines.
Historical hierarchy of AR in horses
Despite significant advances in the discovery of anthelmintic agents, AR has arisen as a major economic issue in animal production throughout the world, currently being most severe in parasitic nematodes of small ruminants (Kaplan et al., Reference Kaplan, Burke, Terrill, Miller, Getz, Mobini, Valencia, Williams, Williamson, Larsen and Vatta2004b). For example, in Australia, the prevalence and magnitude of resistance to all major classes of anthelmintics threatens the profitability of sheep farming (Besier and Love, Reference Besier and Love2003). This problem was initially highlighted in the mid-20th century when resistance to phenothiazine was reported in small strongyles in horses (Gibson, Reference Gibson1960). Thiabendazole was approved for use in horses in 1962 as a broad-spectrum anthelmintic with low toxicity; however, resistance to thiabendazole was reported in cyathostomins within few years of its discovery (Drudge et al., Reference Drudge, Szanto, Wyant and Elam1964). Pyrantel (an imidazothiazole-tetrahydropyrimidine) pamoate resistance was suspected when treatment failure to equine cyathostome population occurred in 1996 (Chapman et al., Reference Chapman, French, Monahan and Klei1996). Subsequently, suspected ivermectin (IVM)-resistant populations of P. equorum were reported in 2002 (Boersema et al., Reference Boersema, Eysker and Nas2002). Currently, MLs treatment failure in cyathostomin nematodes has been observed and it is suggested that resistance to MLs is emerging primarily detected as reduced egg reappearance period (ERP) following treatment (Geurden et al., Reference Geurden, van Doorn, Claerebout, Kooyman, De Keersmaecker, Vercruysse, Besognet, Vanimisetti, di Regalbono, Beraldo, Di Cesare and Traversa2014; Kooyman et al., Reference Kooyman, van Doorn, Geurden, Mughini-Gras, Ploeger and Wagenaar2016). These patterns of resistance development highlight the need of either adopting strategies to slow down the development of resistance or hasten the discovery of new anthelmintics. Therefore, control of horse nematodes should rely on a combination of anthelmintic therapy and other management strategies to minimize the environmental contamination and reducing the exposure of animals to infection.
Prevalence of AR in equine nematodes
There is a great deal of literature available on the prevalence of AR in livestock, horses and companion animal parasitic nematodes throughout the world. Resistance to all three broad-spectrum anthelmintics, including BZs, imidothiazoles-tetrahydropyrimidines and MLs has been reported in ruminants and horses (Kaplan, Reference Kaplan2002; Traversa et al., Reference Traversa, Iorio, Otranto, Giangaspero, Milillo and Klei2009a, Reference Traversa, von Samson-Himmelstjerna, Demeler, Milillo, Schurmann, Barnes, Otranto, Perrucci, di Regalbono, Beraldo, Boeckh and Cobb2009b; Peregrine et al., Reference Peregrine, Molento, Kaplan and Nielsen2014). Generally, a single dose of anthelmintic drug should eliminate more than 95% of the parasitic nematodes and efficacy below this, certainly <90% is taken as evidence of drug resistance. However, in equine medicine, different available anthelmintic classes show different efficacy levels against cyathostomins; therefore, an arithmetic mean of <95% in fecal egg count reduction (FECR) for MLs and a cut-of value <90% for BZ and tetrahydropyrimidine anthelmintic classes is recognized as resistance to these drugs (Relf et al., Reference Relf, Lester, Morgan, Hodgkinson and Matthews2014; Stratford et al., Reference Stratford, Lester, Pickles, McGorum and Matthews2014).
According to previous studies in horse nematodes, BZ-resistant cyathostomins are prevalent on most of the farms in majority of the developed countries (Pook et al., Reference Pook, Power, Sangster, Hodgson and Hodgson2002; Kaplan et al., Reference Kaplan, Klei, Lyons, Lester, Courtney, French, Tolliver, Vidyashankar and Zhao2004a; Wirtherle et al., Reference Wirtherle, Schnieder and von Samson-Himmelstjerna2004; Meier and Hertzberg, Reference Meier and Hertzberg2005; Lind et al., Reference Lind, Kuzmina, Uggla, Waller and Höglund2007; Stratford et al., Reference Stratford, Lester, Pickles, McGorum and Matthews2014). Pyrantel-resistant cyathostomins have also been reported in a large number of horse farms (Kaplan et al., Reference Kaplan, Klei, Lyons, Lester, Courtney, French, Tolliver, Vidyashankar and Zhao2004a; Traversa et al., Reference Traversa, Iorio, Otranto, Giangaspero, Milillo and Klei2009a, Reference Traversa, von Samson-Himmelstjerna, Demeler, Milillo, Schurmann, Barnes, Otranto, Perrucci, di Regalbono, Beraldo, Boeckh and Cobb2009b). Recently, Lester et al. (Reference Lester, Spanton, Stratford, Bartley, Morgan, Hodgkinson, Coumbe, Mair, Swan, Lemon, Cookson and Matthews2013) reported resistance to pyrantel in South of England with approximately 87% FECR on two horse farms. In contrast, MLs demonstrate higher efficacies on almost all the farms examined (Lind et al., Reference Lind, Kuzmina, Uggla, Waller and Höglund2007; Lester et al., Reference Lester, Spanton, Stratford, Bartley, Morgan, Hodgkinson, Coumbe, Mair, Swan, Lemon, Cookson and Matthews2013; Stratford et al., Reference Stratford, Lester, Pickles, McGorum and Matthews2014). However, there have been few reports describing the various incidences of reduced efficacy of IVM in cyathostomin nematodes (Edward and Hoffmann, Reference Edward and Hoffmann2008; Lyons et al., Reference Lyons, Tolliver, Ionita, Lewellen and Collins2008b; Traversa et al., Reference Traversa, von Samson-Himmelstjerna, Demeler, Milillo, Schurmann, Barnes, Otranto, Perrucci, di Regalbono, Beraldo, Boeckh and Cobb2009b). It has been suggested that reduced ERP following IVM and moxidectin treatments is an early indication of the emerging resistance to this class of anthelmintics (Geurden et al., Reference Geurden, van Doorn, Claerebout, Kooyman, De Keersmaecker, Vercruysse, Besognet, Vanimisetti, di Regalbono, Beraldo, Di Cesare and Traversa2014; Kooyman et al., Reference Kooyman, van Doorn, Geurden, Mughini-Gras, Ploeger and Wagenaar2016). The shortened ERPs following IVM and moxidectin treatments have been associated with emerging ML resistance in the fourth stage larvae (Lyons et al., Reference Lyons, Tolliver and Collins2009, Reference Lyons, Tolliver, Kuzmina and Collins2010). Similarly, there are increasing number of reports describing the reduced efficacy of MLs treatment against P. equorum (Stoneham and Coles, Reference Stoneham and Coles2006; Schougaard and Nielsen, Reference Schougaard and Nielsen2007; von Samson-Himmelstjerna et al., Reference von Samson-Himmelstjerna, Fritzen, Demeler, Schürmann, Rohn, Schnieder and Epe2007; Lind and Christensson, Reference Lind and Christensson2009). Some selected studies reporting overt AR and shortened eggs reappearance periods in small strongyles (cyathostomins) and ascarid species (P. equorum) in horses are summarized in Table 1.
Table 1. Selected studies reporting anthelmintic resistance and/or reduced egg reappearance period in equine gastrointestinal nematodes (cyathostomins and Parascaris equorum) from different parts of the world over the last two decades
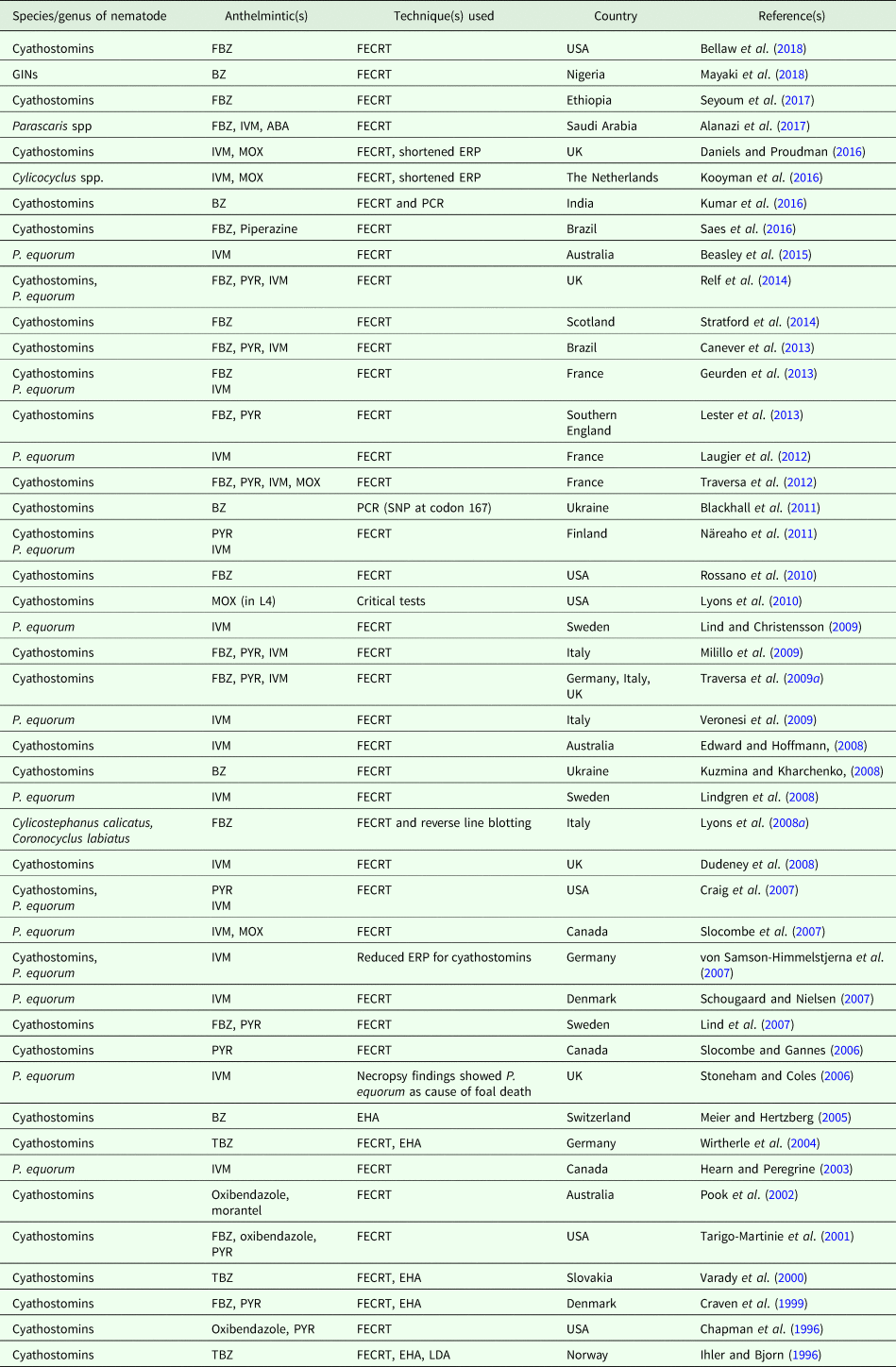
FECRT, fecal egg count reduction test; FBZ, febendazole; PYR, pyrantel; TBZ, thiabendazole; MOX, moxidectin; BZ, benzimidazole; EHA, egg hatch assay; P., Parascaris; LDA, larval development assay; ERP, eggs reappearance period.
Development of AR
Drug resistance in parasites generally results from the selection of a sub-population of parasites that can withstand the toxic effects of drugs which were previously lethal to them. The parasite population select specific genes under drug pressure that allow them to survive. These alleles are responsible for the development of resistance as a result of mutation. When the worms are treated with drugs for which resistant alleles are present, it provides them a chance to survive, leading to increased frequency of resistant worm population in the environment. The rate of resistance development is defined by the frequency of alleles coding for resistance when the worms are exposed to the drug (Gilleard and Beech, Reference Gilleard and Beech2007; Ihler, Reference Ihler2010). AR is a multi-component phenomenon that involves more than single genetic change and quite often non-receptor-based mechanisms also contribute to resistance (Beech et al., Reference Beech, Skuce, Bartley, Martin, Prichard and Gilleard2011). The quantity of anthelmintic drug used and frequency of drug exposure also impact the development of AR. Frequent use of anthelmintics exposes more generations of nematode parasites to the drug especially when pre-patent periods are shorter as compared with the parasites with longer pre-patent periods. This phenomenon is more likely associated with the development of AR (Ihler, Reference Ihler2010).
It has been previously suggested that many horses are being treated unnecessarily, which may expose the parasites to selection pressure (Matthews, Reference Matthews2014; Nielsen et al., Reference Nielsen, Pfister and von Samson-Himmelstjerna2014a; Peregrine et al., Reference Peregrine, Molento, Kaplan and Nielsen2014). Selection pressure can be reduced if treatment of selected animals with only a higher FEC is practised (Nielsen et al., Reference Nielsen, Reist, Kaplan, Pfister, van Doorn and Becher2014b). Refugia is a term used for the parasite population not exposed to the drug and it has been suggested that refugia-based parasite control approaches are important for the effective management of AR (Cornelius et al., Reference Cornelius, Jacobson, Dobson and Besier2016), as it lowers the selection pressure on the whole population. The reversal or delaying the development of resistance to anthelmintics has been shown by maintaining the worm population in refugia in nematode parasites of small ruminants (Sissay et al., Reference Sissay, Asefa, Uggla and Waller2006), and using combination therapies in preference to an annual rotation (Bartram et al., Reference Bartram, Leathwick, Taylor, Geurden and Maeder2012; Leathwick et al., Reference Leathwick, Ganesh and Waghorn2015). Therefore, treatment of selected animals and adoption of alternate parasite control strategies would help to slow down the development of drug resistance. In horse nematodes, this concept of restoring drug efficacy has been reported in limited number of studies. Although the concept of refugia has been recommended as valuable to equine parasite control (Matthews, Reference Matthews2008; Kaplan and Nielsen, Reference Kaplan and Nielsen2010), there is no published evidence demonstrating the effectiveness of this strategy in controlling equine parasites. However, it remains a useful working hypothesis to consider refugia as an important tool in delaying the development of resistance in equine nematodes.
Detection of AR
Apart from developing the new anthelmintics, early diagnosis of resistance is also very important to maintain the efficacy of available drugs by adopting suitable measures, for example, reduced treatment intensity and promoting the refugia, as in the future, control of helminths will remain dependent on anthelmintic chemotherapy. A range of in vitro and in vivo tests are used for measuring reduced anthelmintic efficacy in nematode populations (Coles et al., Reference Coles, Jackson, Pomroy, Prichard, von Samson-Himmelstjerna, Silvestre, Taylor and Vercruysse2006). These tests were generally developed for detecting AR in ruminant nematodes; however, some of these tests have been modified to use for detecting the emerging AR in equine nematodes. The benefits and drawbacks of the tests that have been utilized in equine parasitology are discussed below.
FECR test
FECR test (FECRT) is the only reliable and suitable test in detecting the reduced efficacy of currently available anthelmintics in horses. In this in vivo test, FECR is determined based on FEC in the same horse before and after the administration of anthelmintic, or comparison of the reduced FEC in treated with an untreated group of horses (Coles et al., Reference Coles, Bauer, Borgsteede, Geerts, Klei, Taylor and Waller1992). This test is only reliable if resistance level is higher than 25% of the total worm population (Martin et al., Reference Martin, Anderson and Jarrett1989). According to the World Association for the Advancement of Veterinary Parasitology (WAAVP), a single dose of anthelmintic drug should eliminate more than 95% of the parasitic nematodes and efficacy below this, certainly <90% is taken as evidence of drug resistance. This threshold cut-off limit does not seem applicable for determining anthelmintic efficacy in horses, hence, this limit has not been applied in some other studies (Ihler, Reference Ihler1995). The currently available anthelmintic classes show different efficacy levels against cyathostomins (Saes et al., Reference Saes, Vera, Fachiolli, Yamada, Dellaqua, Saes, Amarante and Soutello2016). Therefore, it was suggested to review these cut-off values particularly for some anthelmintic classes (Coles et al., Reference Coles, Jackson, Pomroy, Prichard, von Samson-Himmelstjerna, Silvestre, Taylor and Vercruysse2006). For example, pyrantel often reduced 95–100% FECR in susceptible worm populations (Valdez et al., Reference Valdez, DiPietro, Paul, Lock, Hungerford and Todd1995), similarly BZ-treatment usually shows more than 95% reduction in FEC (DiPietro and Todd, Reference DiPietro and Todd1987). Whereas MLs have been reported to reduce fecal egg count by 99% or higher (Lind et al., Reference Lind, Kuzmina, Uggla, Waller and Höglund2007; von Samson-Himmelstjerna et al., Reference von Samson-Himmelstjerna, Fritzen, Demeler, Schürmann, Rohn, Schnieder and Epe2007). Some of the recent studies have described more precise methods for measuring anthelmintic sensitivity in horses (Lester et al., Reference Lester, Spanton, Stratford, Bartley, Morgan, Hodgkinson, Coumbe, Mair, Swan, Lemon, Cookson and Matthews2013; Relf et al., Reference Relf, Lester, Morgan, Hodgkinson and Matthews2014; Stratford et al., Reference Stratford, Lester, Pickles, McGorum and Matthews2014). These authors have used an arithmetic mean FECR of >95% for MLs and a cut-of value >90% for BZ and tetrahydropyrimidine anthelmintic classes.
Similarly, there are no well-defined principles for calculating the ERP for strongyles. ERP is generally calculated in two ways: firstly, the period between anthelmintic administration to the week of first positive fecal egg count (Dudeney et al., Reference Dudeney, Campbell and Coles2008; Lyons et al., Reference Lyons, Tolliver, Ionita, Lewellen and Collins2008b); secondly, it comprises the time period when the group mean FEC surpasses 10–20% of the group mean FEC at day 0 (von Samson-Himmelstjerna et al., Reference von Samson-Himmelstjerna, Fritzen, Demeler, Schürmann, Rohn, Schnieder and Epe2007; Larsen et al., Reference Larsen, Ritz, Petersen and Nielsen2011). The later approach provides more conventional estimation of egg reappearance in relation to the level and spread of the egg count data sampled before treatment, and hence provides a precise measure of a population's susceptibility to anthelmintics (reviewed by Matthews, Reference Matthews2014). The first positive egg count approach seems imprecise, because the results then depend heavily on the pre-treatment FEC and the sensitivity of detection. So, the second approach is reasonably better, as it uses relative measures that remain useful no matter the pre-treatment FEC or the method used for FEC. For example, for ML drugs, since we expect 99.9% FECR, then a return to a group mean of 10% of pre-treatment levels seems a good definition for ERP. For other non-ML drugs, 10% can also be used, but 20% might be a better choice, since these are not nearly as effective as ML and efficacy >99% is rarely achieved. In these cases, if FECR at 14 days is only around 90%, then the relevance of ERP is questionable, as egg re-appearance cannot occur if the eggs do not disappear in the first place. In addition, waiting for the first horse to shed eggs may be biased by pre-treatment egg count levels or individual animal variability. Individual horses may be extremely higher egg shedders and may continue to shed eggs even after treatment with fully effective ML. A reduced ERP represents an early indication of changing patterns of population's susceptibility to anthelmintics, providing a warning for the possible emergence of resistance particularly to the long-term effects of MLs in horses; therefore, further research is required to measure and standardize the ERP parameters so that analysis can be made between studies.
As stated above, FECRT, currently used as ‘gold standard’ test is reliable only when >25% of the nematode worms in a given population are resistant (Martin et al., Reference Martin, Anderson and Jarrett1989). Thus, this test may likely misdiagnose the relatively low proportion of genotypically resistant individuals in a population; therefore, in vitro tests or molecular techniques are urgently required for measuring AR in horses.
In vitro tests
Various in vitro tests including the egg hatch assay (EHA), larval development assay (LDA), larval migration inhibition assay (LMIA), larval motility assay, larval feeding inhibition assay and larval paralysis test have been described to detect AR in ruminant nematodes (Coles et al., Reference Coles, Jackson, Pomroy, Prichard, von Samson-Himmelstjerna, Silvestre, Taylor and Vercruysse2006). In horses, few in vitro tests have been reported for detecting the relative drug sensitivity of cyathostomin nematodes in addition to FECRT. These assays include the EHA, LDA and LMIA which determine the relative sensitivity of free-living stages (eggs and larvae) to a series of drug concentrations. The protocols for these tests have been discussed previously to measure the relative sensitivity of cyathostomins to BZ, pyrantel and MLs (Ihler and Bjorn, Reference Ihler and Bjorn1996; Craven et al., Reference Craven, Bjorn, Barnes, Henriksen and Nansen1999; van Doorn et al., Reference van Doorn, Kooyman, Eysker, Hodgkinson, Wagenaar and Ploeger2010) but still there are discrepancies on reproducibility and reliability of these tests.
Egg hatch assay
This assay is used to measure inhibition of hatching of nematode eggs by an anthelmintic agent. The assay is not suitable for anthelmintics, which cannot penetrate the eggs, for example, IVM. It was first reported by Le Jambre (Reference Le Jambre1976) and later on modified by Coles et al. (Reference Coles, Bauer, Borgsteede, Geerts, Klei, Taylor and Waller1992). This assay has some limitations, for example, the sensitivity of eggs to thiabendazole decreases with age; therefore, eggs should be used soon after collection (usually within 3 h) or stored under anaerobic conditions. BZ-sensitivity also decreases as embryonation progresses, so unembryonated eggs are a prerequisite for this assay (Hunt and Taylor, Reference Hunt and Taylor1989). The EHA is capable of detecting resistance when at least 25% of the worms carry resistance genotype as has been showed previously by experimentally infecting the animals with mixtures of nematode populations with a known level of susceptibility (Martin et al., Reference Martin, Anderson and Jarrett1989). In horses, it is generally recommended that horses with a minimum individual egg count of 150 eggs per gram (EPG) should be included in the study (von Samson-Himmelstjerna et al., Reference von Samson-Himmelstjerna, von Witzendorff, Sievers and Schnieder2002). This test can be applied to detect BZ-resistance in cyathostomins (Coles et al., Reference Coles, Jackson, Pomroy, Prichard, von Samson-Himmelstjerna, Silvestre, Taylor and Vercruysse2006). Previous investigations have reported a positive correlation between FECRT and EHA (Craven et al., Reference Craven, Bjorn, Barnes, Henriksen and Nansen1999; Varady et al., Reference Varady, Konigova and Corba2000), but this correlation is not very strong and further research is required to validate the use of EHA in small strongyles. Standardization of fecal culture conditions for equine nematodes may further improve the suitability of this assay to detecting AR.
Larval development assay
The effects of anthelmintic drugs on the growth of parasites provide a chance to develop techniques useful for detection of resistance. In the LDA, the eggs or first-stage larvae are exposed to a range of anthelmintic concentrations incorporated into agar wells in 96-well plates containing growth medium. Methods based upon inhibition of larval development are more laborious and time consuming than for the EHA but are useful to detect resistance to all the major anthelmintic classes including MLs (Jabbar et al., Reference Jabbar, Iqbal, Kerboeuf, Muhammad, Khan and Afaq2006). The LDA is more sensitive than the FECRT as it identifies resistance when it is present in a worm population at levels down to 10% (Dobson et al., Reference Dobson, LeJambre and Gill1996). The suitability of the LDA for detection of resistance to pyrantel in livestock and horse nematodes has also been established (Ihler and Bjorn, Reference Ihler and Bjorn1996; Kotze et al., Reference Kotze, Stein and Dobson1999). Currently, this test is considered as reliable, inexpensive and suitable for use in the field investigations of AR in ruminants but still not in horses. The test can also utilize first-stage larvae; therefore, there is no prerequisite for undeveloped eggs or fresh fecal samples (Coles et al., Reference Coles, Tritschler, Giordano, Laste and Schmidt1988). In equine nematodes, higher levels of variability, poor reproducibility and narrow resistance-to-susceptible ratios along with lack of significant correlation with FECRT have been reported (Pook et al., Reference Pook, Power, Sangster, Hodgson and Hodgson2002; Tandon and Kaplan, Reference Tandon and Kaplan2004; Lind et al., Reference Lind, Uggla, Waller and Hoglund2005). Therefore, this test is not a reliable alternative to FECRT in horses, thus needs further improvement. There are certain limitations to using LDA with equine nematodes including lack of established cut-off values for susceptible and resistant populations and interpretation problems related to multi-species infections, so, different species of cyathostomins may show different native drug susceptibility and may require slightly different conditions for optimal development in vitro. In addition, the developing larvae may not show the phenotypic resistance in the LDA at equivalent levels to that of adult worms. In the future, the test can be improved by developing rapid species identification tools, in vitro propagating different cyathostomin species with known susceptibility profiles for use as reference strains and standardising optimal in vitro conditions for cyathostomin development. Given the difficulty in dealing with heterogeneous mixture of numerous species of cyathostomins, especially, lack of valid morphologic means to distinguish eggs of these species, this assay may not be as useful as it is with sheep nematodes.
Larval migration inhibition assay
The LMIA was developed as a modification of the previously reported motility assay (Gill et al., Reference Gill, Redwin, van Wyk and Lacey1991) to detect the sheep nematodes resistant to IVM (Kotze et al., Reference Kotze, Le Jambre and O'Grady2006). Infective stage larvae (L3) are exposed to various dilutions of IVM for 48 h and then allowed to migrate through an agar/filter mesh system fitted over a receiver plate, for the next 24 h. The assay has also been standardized for detecting IVM resistance in cattle nematodes (Demeler et al., Reference Demeler, Küttler, El-Abdellati, Stafford, Rydzik, Varady, Kenyon, Coles, Höglund, Jackson, Vercruysse and von Samson-Himmelstjerna2010). There is limited information available on the suitability of this assay for use in cyathostomin nematodes. The LMIA has been evaluated for identifying the cyathostomin larvae suspected of being resistant to IVM and did not evaluate the diagnostic properties of the assay (van Doorn et al., Reference van Doorn, Kooyman, Eysker, Hodgkinson, Wagenaar and Ploeger2010). The authors concluded that LMIA may be used to study resistant cyathostomin populations. McArthur et al. (Reference McArthur, Handel, Robinson, Hodgkinson, Bronsvoort, Burden, Kaplan and Matthews2015) has reported the ability of LMIA in discriminating the IVM sensitivity in cyathostomin populations. The EC50 values for L3 larvae recovered from animals that showed <95% reduction in FEC were significantly higher than the EC50 values for L3 population from animals with >95% reduction in egg counts. In addition, recently, Beasley et al. (Reference Beasley, Coleman and Kotze2017) has also showed the ability of LMIA define the sensitivity of cyathostomin larvae to ML drugs. However, the authors suggested that the use of LMIA on known ML-resistant and -susceptible populations is required for further validation of its usefulness for diagnosis of AR.
In general, interpretation of all the in vitro tests is more complicated because of the cyathostomin species diversity present in field conditions and species can only be differentiated using molecular analysis. Furthermore, in identifying AR in field populations, it would be difficult to confirm that the different sensitivity patterns are due to resistance or the presence of different cyathostomin species, which show different susceptibility to IVM. These issues add further complications that must be considered when using in vitro bioassays to identify drug-resistant populations of cyathostomin nematodes. This could be tackled by coupling the FECRT data either with identifying species composition of the larval cultures obtained pre-and post-treatment (Kooyman et al., Reference Kooyman, van Doorn, Geurden, Mughini-Gras, Ploeger and Wagenaar2016) or molecular identification of cyathostome species using reverse line blotting (RLB) hybridization (Traversa et al., Reference Traversa, Iorio, Klei, Kharchenko, Gawor, Otranto and Sparagano2007). For example, Traversa et al. (Reference Traversa, Iorio, Otranto, Giangaspero, Milillo and Klei2009a) identified eight cyathostomin species in pre-treatment fecal samples using RLB method and showed that BZ resistance was present in Coronocyclus labiatus and C. goldi species. However, lack of target sequences for many species or even that GenBank® entries are unreliable regarding the cyathostomin species may make it difficult to identify cyathostomin species. In addition, proteome-based species identification of pathogens has already revolutionized diagnostic microbiology. Recently, Mayer-Scholl et al. (Reference Mayer-Scholl, Murugaiyan, Neumann, Bahn, Reckinger and Nockler2016) have recently applied proteome-based matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) for rapid species identification of Trichinella spp. This was achieved by adopting a simple formic acid/acetonitrile extraction from pooled larvae and compilation of a reference database. Such an approach could also be utilized for cyathostomin species identification, as it has been revealed by the preliminary data for cyathostomin which showed distinct patterns for adult individuals of different species (Bredtmann et al., Reference Bredtmann, Krücken, Murugaiyan, Kuzmina and von Samson-Himmelstjerna2017). However, this demands compilation of a reference database of master-spectra libraries which can only be generated with validated and correctly identified material.
Molecular techniques
Different molecular techniques have been developed for the detection of specific mutations that are associated with AR in trichostrongyloid nematodes, which include restriction enzyme digestion, direct sequencing, pyro-sequencing and diagnostic PCR. These techniques have been used to reveal a pattern of substitutions associated with BZ resistance, and the presence of specific single nucleotide polymorphisms (SNPs) in β-tubulin gene at codons 167, 198 and 200 have been reported in different trichostrongyloid species (Kwa et al., Reference Kwa, Veenstra and Roos1994; Prichard, Reference Prichard2001; Kotze et al., Reference Kotze, Cowling, Bagnall, Hines, Ruffell, Hunt and Coleman2012). In cyathostomin populations, similar SNPs at codons 167 and 200 within the β-tubulin isotype-1 have been associated with BZ-resistance (Hodgkinson et al., Reference Hodgkinson, Clark, Kaplan, Lake and Matthews2008; Lake et al., Reference Lake, Matthews, Kaplan and Hodgkinson2009; Blackhall et al., Reference Blackhall, Kuzmina and von Samson-Himmelstjerna2011); however, a polymorphism at codon 167 appears to be more common than the codon 200 polymorphism in equine cyathostomins. Currently, a few phenotypically well-defined cyathostomin populations have been studied for the mechanism of resistance at the molecular levels; thus, further research is required to assess the relative significance of these and other possible SNPs associated with BZ-resistance (von Samson-Himmelstjerna, Reference von Samson-Himmelstjerna2012). Reliable and precise quantification of resistance-associated SNPs from samples representing different worm numbers is generally a pre-requisite for developing a reliable molecular test for routine diagnosis of resistance (von Samson-Himmelstjerna, Reference von Samson-Himmelstjerna2006). In the case of cyathostomins, existence of more than 50 morphologically discrete species contributes considerable complications to a molecular approach for detecting AR (Lichtenfels et al., Reference Lichtenfels, Kharchenko and Dvojnos2008). In addition, association of non-specific mechanisms of AR including modified drug transporters [P-glycoprotein (P-gp)] (Raza et al., Reference Raza, Kopp, Bagnall, Jabbar and Kotze2016a), and altered drug metabolism (Matoušková et al., Reference Matoušková, Vokřál, Lamka and Skálová2016) that act irrespective of the drug class may further impede the development of molecular techniques. There has been very limited information available reporting the association of P-gps with AR in horse nematodes, Drogemuller et al. (Reference Drogemuller, Schnieder and von Samson-Himmelstjerna2004) described the existence of two P-gp genes in cyathostomins and, recently, Peachey et al. (Reference Peachey, Pinchbeck, Matthews, Burden, Lespine, von Samson-Himmelstjerna, Krücken and Hodgkinson2017) reported that P-gps play a role in resistance to IVM in cyathostomins. The authors also described that the P-gp-9 was transcribed at a significantly higher level in IVM-resistant larvae as compared with sensitive larvae. Janssen et al. (Reference Janssen, Krucken, Demeler, Basiaga, Kornas and von Samson-Himmelstjerna2013) studied the involvement of Pgp-11 in the level of IVM susceptibility in P. equorum and observed an increased pgp-11 mRNA expression in one putatively resistant population. This suggests that P-gps may play, at least a partial role in the observed AR in horse nematodes. Such non-specific mechanisms along with the widespread nature of IVM and BZ-resistance in horse cyathostomin populations should be considered in designing a molecular test detecting AR in cyathostomins. Furthermore, molecular markers for resistance to other anthelmintic classes in equine nematodes are still poorly understood; therefore, it has been suggested that currently FECRT may be the best available test for assessing the AR in horse nematodes (von Samson-Himmelstjerna, Reference von Samson-Himmelstjerna2012). However, FECRT lacks sensitivity and is able to detect resistance only when more than 25% of the worms in a population are resistant, the chances of detecting the resistant worms are less when the genotypically resistant worms are low in number. Therefore, highly sensitive molecular tests are urgently required to identify AR at as early a stage as possible in horse nematodes.
Genetics and functional genomics have been playing a vital role in discovering the possible molecular mechanisms of insecticide resistance, and the availability of annotated genomes of many parasitic nematodes such as Ascaris suum and H. contortus has the potential to accelerate drug discovery in these species In contrast, there is still lack of information about equine nematodes; for example, mitochondrial genome/transcriptome sequences have been published only for Cylicostephanus goldi (Cwiklinski et al., Reference Cwiklinski, Merga, Lake, Hartley, Matthews, Paterson and Hodgkinson2013), P. univalense (Jabbar et al., Reference Jabbar, Littlewood, Mohandas, Briscoe, Foster, Muller, von Samson-Himmelstjerna, Jex and Gasser2014), Triodontophorus brevicauda (Duan et al., Reference Duan, Gao, Hou, Zhang, Liu, Gao, Guo, Yue, Su, Fu and Wang2015), Strongylus equinus (Xu et al., Reference Xu, Qiu, Liu, Zhang, Liu, Duan, Yue, Chang, Wang and Zhao2015), Oxyuris equi (Zhang et al., Reference Zhang, Xu, Guo, Liu, Duan, Su, Fu, Yue, Gao and Wang2015) and P. equorum (Gao et al., Reference Gao, Zhang, Wang, Li, Li, Xu, Gao and Wang2018). However, there is still no complete genome sequence available for any of the equine nematode species, this should be a priority area for the future research on AR in equine nematodes. The major constraints to this lack of information on genome sequences for equine nematodes are the lack of funding as well as the greater diversity in the species present. Mitochondrial genome sequence information would be helpful in differentiating the species as mitochondrial genome has been widely used as a genetic marker in the identification and differentiation of closely related species. These genome sequences once fully available along with their transcriptomic data would provide major insights into the biology of parasitic nematodes, mechanisms involved in resistance and discovering specific AR markers; therefore, the equine industry may benefit by funding the genome studies.
AR management
Rotational deworming
Researchers have suggested the possible alternative use of different anthelmintics which can be classified as slow rotation and fast rotation. Rotational deworming is still controversial and there is discrepancy between the types of rotational strategies in horses. Although limited studies are available reporting the presence of multiple resistance in equines, the idea of increased multiple resistance as a result of frequent rotational use of anthelmintics is based on studies in sheep and goats (Dash et al., Reference Dash, Hall and Barger1988). A recent survey study showed that most of the horse owners tend to rely heavily on the IVM in parasite control programmes round the year, with the majority preferring to follow the same plan in subsequent years (Nielsen et al., Reference Nielsen, Branan, Wiedenheft, Digianantonio, Garber, Kopral, Phillippi-Taylor and Traub-Dargatz2018). The intensive use of IVM would increase the population of IVM-resistant nematodes, as has been observed for sheep parasites (Cezar et al., Reference Cezar, Toscan, Camillo, Sangioni, Ribas and Vogel2010). Therefore, slow rotation of different classes of anthelmintics may be suggested (Hearn and Peregrine, Reference Hearn and Peregrine2003). Fast rotation is more commonly used in horses than slow rotation, in which anthelmintic groups are rotated at intervals of three to six times a year (reviewed by Brady and Nichols, Reference Brady and Nichols2009). Fast rotation between anthelmintic classes minimizes the parasite exposure to a specific class. The only definitive study that supports this theory demonstrated that fenbendazole can be used again in a herd of horses infected with resistant fenbendazole worm population following rotations of various anthelmintic classes (Brady et al., Reference Brady, Nichols, Blanek and Hutchens2008). In contrast, Uhlinger and Johnstone (Reference Uhlinger and Johnstone1984) reported a lack of reversion to a susceptible state in parasite populations showing resistance to BZ despite of a 24–38 months withdrawal period.
In contrast, rotation of anthelmintics without monitoring anthelmintic efficacy by FECRT may lead to unchecked propagation of resistant worms, as resistant worms can dominate the population if drug does not kill 100% of the worms (especially in case of non-ML anthelmintics) (reviewed by Swiderski and French, Reference Swiderski and French2008). However, there is little experimental evidence available because such rotational experiments are difficult to carry out and are very prolonged; the majority of the work has been performed with models, and is thus predictive. Ideally, annual rotation should give the slowest rate of accumulation of resistance genes. Therefore, it seems a reasonable strategy to adopt in practice to treating horses with MLs in first year followed by treating with a different drug next year.
Combination therapy
In parasite control programmes, intensive and repetitive use of a single class of anthelmintic are generally the well-known causes of selection for drug resistance (Kaplan, Reference Kaplan2002; Kaplan and Nielsen, Reference Kaplan and Nielsen2010). As discussed above, there is widespread resistance to BZ and pyrantel in cyathostomin populations and less commonly in P. equorum throughout the world, which makes most of the horse owners more reliant on using MLs. Emerging reports of reduced efficacy of MLs in equine nematodes has further compounded the problem (Traversa et al., Reference Traversa, Castagna, von Samson-Himmelstjerna, Meloni, Bartolini, Geurden, Pearce, Woringer, Besognet, Milillo and D'Espois2012; Relf et al., Reference Relf, Lester, Morgan, Hodgkinson and Matthews2014). It has been suggested that combinations of anthelmintics, especially the drugs that target the same or a similar spectrum of parasite species, may play a potential role in parasite control programmes (Scott et al., Reference Scott, Bishop and Pomroy2015). Combination therapies allow the effective control of nematodes along with slowing down the development of AR (Bartram et al., Reference Bartram, Leathwick, Taylor, Geurden and Maeder2012; Kaplan et al., Reference Kaplan, West, Norat-Collazo and Vargas2014), and it may be quickly accepted for controlling the equine parasites due to higher efficacies. The different possible interactions following co-administration of two or more drugs include indifference, antagonistic, synergistic and additive/potentiative actions (Jia et al., Reference Jia, Zhu, Ma, Cao, Li and Chen2009). In case of anthelmintics, majority of evidence suggest that routinely used anthelmintic drugs show additive potentiative effect when co-administered (Entrocasso et al., Reference Entrocasso, Alvarez, Manazza, Lifschitz, Borda, Virkel, Mottier and Lanusse2008; Bartram et al., Reference Bartram, Leathwick, Taylor, Geurden and Maeder2012). This additive/potentiative effect results in a higher efficacy than would be obtained by either drug using as single entity, and can be determined by the following formula described by Bartram et al. (Reference Bartram, Leathwick, Taylor, Geurden and Maeder2012);
where the efficacy is expressed as proportion of the worms killed or reduction in FEC following the administration of either anthelmintic or a combination of A and B.
In horses, there is limited information available on the use of combination therapy, anthelmintic combination therapy is less frequently adapted in regions other than Australia and New Zealand, where several anthelmintic combinations are commercially available for use in horses and ruminants (Geary et al., Reference Geary, Hosking, Skuce, von Samson-Himmelstjerna, Maeder, Holdsworth, Pomroy and Vercruysse2012). In the USA, experimental use of BZ anthelmintics combined with piperazine and other non-BZ anthelmintics has proved to be effective against BZ-resistant cyathostomins (Uhlinger and Johnstone, Reference Uhlinger and Johnstone1985). Kaplan et al. (Reference Kaplan, West, Norat-Collazo and Vargas2014) have recently reported that co-administration of oxibendazole and pyrantel shows a significantly higher efficacy in controlling cyathostomins in horses. The study showed that the reduction in FEC was significantly greater in horses given combination of both drugs (96.35%) compared with horses given either drug alone (90.03% with oxibendazole and 81.10% with pyrantel). The authors further suggested that anthelmintic combinations can considerably improve the effects of a given anthelmintic and there is clear indication that combination therapy substantially enhances the effectiveness of parasite control programmes by limiting the developing rate of AR. However, drug combinations may still lead to the development of cross-resistance to more than one anthelmintic, and may also result in selection for general mechanisms of resistance common to different drug classes, for example, drug transport proteins (P-gps and multidrug resistance proteins), as has been observed in vitro for H. contortus (Raza et al., Reference Raza, Bagnall, Jabbar, Kopp and Kotze2016b). Therefore, the anthelmintic combination therapy should be given thoughtful attention for controlling equine nematodes in future.
Selective therapy
Since AR has been established worldwide, a new pharmacological drug class has not been introduced for the equine industry since the introduction of IVM in the early 1980s and it remains uncertain when new drugs with different modes of action will be available for use in horses. Over the past two decades, veterinary parasitologists have recommended to adopt a reduced intensity of anthelmintic treatment to retard the development of AR. The recommended strategy of ‘selective therapy’ (targeted treatment) has been successfully applied for the control of trichostrongyle infection in small ruminants (Kaplan et al., Reference Kaplan, Burke, Terrill, Miller, Getz, Mobini, Valencia, Williams, Williamson, Larsen and Vatta2004b). Selective strategy is based on screening of the animals with a suitable parasite-related measure and then selection of the animals for anthelmintic treatment that exceed a predetermined threshold value.
In horses, the criterion of the targeted therapy is FECs from all horses in a given herd, treating only the horses with a higher FEC than the predetermined cut-off value (Nielsen et al., Reference Nielsen, Pfister and von Samson-Himmelstjerna2014a). Questionnaire-based survey studies in various countries showed that a large proportion of horse owners do not implement this recommendation (Matthee et al., Reference Matthee, Dreyer, Hoffmann and van Niekerk2002a; Relf et al., Reference Relf, Morgan, Hodgkinson and Matthews2012). However, Danish legislation of anthelmintics as prescription-only medicine disallowed random prophylactic treatments and appear to have strong effects on selective therapy (Nielsen et al., Reference Nielsen, Monrad and Olsen2006). The parasite populations are very unevenly distributed over a herd of hosts, and in horses, this pattern is quite obvious with strongyle FECs, where it has been shown that some horses are shedding the large majority of strongyle eggs within the population (Lester et al., Reference Lester, Morgan, Hodgkinson and Matthews2018). Based on this phenomenon, equine parasitologists have devised the 20/80 rule for horse strongyle egg counts which means in a given herd, approximately 20% of the horses shed about 80% of the total number of eggs within the population (Kaplan and Nielsen, Reference Kaplan and Nielsen2010; Relf et al., Reference Relf, Morgan, Hodgkinson and Matthews2013). Therefore, this raises a possibility that targeted treatment of the higher egg shedder horses and leaving the lower shedders untreated may result in satisfactory reduction in overall FECs despite using significantly fewer treatments. It has been suggested that in adult horses, treatment of horses with 200 EPG of feces using an anthelmintic drug with 99% efficacy will lead to an overall egg count reduction of 95% on herd level (Kaplan and Nielsen, Reference Kaplan and Nielsen2010). However, this predetermined cut-off value would vary with geography, season, breed and age of the horses; therefore, cut-off values should be predetermined for each herd accordingly.
Selective therapy may reduce the treatment intensity in addition to leaving a part of parasite population unexposed. The reduced intensity of anthelmintic drugs and maintenance of refugia decrease the selection pressure on parasite population which may slow the development of AR (Sissay et al., Reference Sissay, Asefa, Uggla and Waller2006). Although no such evidence is observed for equine parasites, findings of the sheep parasite studies may help to implement this strategy in equine parasites to counter the development of resistance. It has been previously documented that overall cyathostomin egg shedding can be controlled by treating half of the adult horse population. In addition, economical calculations suggested that the selective approach was cost-effective, when compared with treating all horses a fixed number of times in a year (Duncan and Love, Reference Duncan and Love1991). However, it remains unknown whether such an approach would also provide effective control over other important parasites such as ascarids, large strongyles and tapeworms (Nielsen et al., Reference Nielsen, Pfister and von Samson-Himmelstjerna2014a). A significant association has been found between prevalence of S. vulgaris and selective therapy which was particularly observed in parasite population of foals and young horses (Nielsen et al., Reference Nielsen, Olsen, Lyons, Monrad and Thamsborg2012). Strongylus vulgaris is more pathogenic compared with cyathostomins; therefore, control of this parasite should also be considered while implementing selective therapy as this may lead to potential health risks in untreated horses. In addition, processing large numbers of FECs is another drawback of the selective therapy. It may be even difficult to collect numerous fecal samples on large horse farms; in addition, it does not seem cost-effective when a single use of anthelmintic is cheaper than the fecal analysis (Nielsen, Reference Nielsen2012). Therefore, further work is required to evaluate the potential health risks accompanied with selective therapy, and to assess the usefulness of this technique in delaying the development of AR in equine GINs.
Pasture management strategies
Traditionally, free-living developmental stages of equine parasites were the major focus of parasite control programmes due to lack of safe and efficacious anthelmintic drugs, and pasture management was the major tool for modulating parasite burden. Later on, availability of broad-spectrum anthelmintics such as IVM precluded the importance of pasture management and free-living stages. However, emerging AR stresses the need to revisit the pasture management strategies including non-chemical-based approaches for controlling parasites in ruminants and equines. Fecal analysis should be performed routinely to monitor the status of parasites in the herd. Additionally, adapting good management strategies such as rotational grazing within and between species, avoidance of overstocking, avoid feeding on the ground, regular removal of feces and strict quarantine measures before introduction of new horses to the pastures are recommended (Brady and Nichols, Reference Brady and Nichols2009). Pasture hygiene has been recognized as one of the most effective methods to control horse parasites (Herd, Reference Herd1986; Matthee et al., Reference Matthee, Krecek, Milne, Boshoff and Guthrie2002b). Ideally, feces should be removed from the pasture regularly but practically this seems laborious, time consuming and unacceptable by most of the owners. In addition, multispecies grazing also results in reducing the parasite burden, but there is limited information available in equine parasitology and most of the guidelines are acquired from ruminant studies (Nielsen, Reference Nielsen2012). These practices should be employed in addition to the currently available therapies aiming to reduce the frequency of anthelmintic treatment for controlling equine parasites.
Alternative measures for sustainable parasite control
Future control efforts for equine helminths may require a focus on discovering natural parasiticide drugs from plant origin, or other dietary additives such as probiotics. This has been a quite popular area of research in ruminants, but the use of plant (leaves, seed and/or other parts) extracts has gained little attention in equine parasitology research. There are limited studies available reporting the (mainly in vitro) efficacy of plant-based parasiticide agents in equine GINs. For example, Rakhshandehroo et al. (Reference Rakhshandehroo, Asadpour, Malekpour and Jafari2017) tested the anthelmintic activity of different plant extracts on P. equorum larval viability (inhibition of whip-like larval movement). The findings showed that all concentrations (50, 75, 100 and 125 mg mL−1) of A. dracunculus (tarragon) and M. pulegium (squaw mint/pudding grass) extracts were lethal against larvae while only higher concentrations of Z. multiflora (100 and 125 mg mL−1) showed toxic effects on larval motility. On the other hand, extracts from E. camadulensis (red river gum tree) and A. sativum (garlic) showed very little effects on larval viability. Similarly, Procyanidin A2, a bioactive compound from an Australian plant Alectryon oleifolius showed significant anthelmintic efficacy by completely inhibiting development of cyathostomin egg to third stage larvae at concentrations as low as 50 µg mL−1 and having an IC50 value of 12.6 µg mL−1 (Payne et al., Reference Payne, Kotze, Durmic and Vercoe2013; Payne et al., Reference Payne, Flematti, Reeder, Kotze, Durmic and Vercoe2018). Other novel anthelmintic candidates, such as Cry5B protein derived from Bacillus thuringiensis, have also recently been shown to have direct anthelmintic activity against cyathostomes (Hu et al., Reference Hu, Miller, Zhang, Nguyen, Nielsen and Aroian2018). Peachey et al. (Reference Peachey, Pinchbeck, Matthews, Burden, Mulugeta, Scantlebury and Hodgkinson2015) reported that hydro-alcoholic extracts of plants from Ethiopian (Acacia nilotica, Cucumis prophetarum and Rumex abyssinicus) and the UK [Allium sativum (garlic), Chenopodium album and Zingiber officinale (ginger)] showed significant anthelmintic activity in EHA and larval migration inhibition test in equine strongyle nematodes. The EC-50 values ranged from 0.18 to 2.3 mg mL−1, and the authors suggested that these plants have the potential as anthelmintic forages or feed supplements in equines. In addition, methanol extracts of Diospyros anisandra (bark and leaves) and Petiveria alliacea (stems and leaves) also showed potential anthelmintic effects by inhibiting the egg hatching in cyathostomins at much lower concentrations [>90% egg hatch inhibition at and above 37.5 µg mL−1 for D. anisandra (both bark and leaves) and at 75 µg mL−1 for P alliacea (both stems and leaves)] (Flota-Burgos et al., Reference Flota-Burgos, Rosado-Aguilar, Rodríguez-Vivas and Arjona-Cambranes2017). The effects of D. anisandra extracts were largely due to its ovicidal activity, whereas in the P. alliacea extracts, it was due to L1 larval hatch failure. These studies were conducted in vitro, and the effects of these plants remain to be confirmed through in vivo studies. Recently, Collas at el. (Reference Collas, Sallé, Dumont, Cabaret, Cortet, Martin-Rosset, Wimel and Fleurance2017) have investigated the efficacy of a short-term consumption of tannin-rich sainfoin (Onobrychis viciifolia) through in vitro and in vivo experiments in naturally infected horses. The in vivo experiments showed that a tannin-rich diet with 70% DM sainfoin pellets resulted in a lower rate of strongyle larval development. Similarly, addition of sainfoin pellets (29%) to feces reduced the strongyle egg development into infective larvae by 82%, suggesting that such bioactive forages may have the ability to disrupt the infection dynamics of strongyle nematodes. Moreover, other strategies may be applied to improve equine health in the face of drug-resistant nematodes. For example, given that helminth infection in horses has been shown to significantly disrupt the commensal gut microbiota (Clark et al., Reference Clark, Salle, Ballan, Reigner, Meynadier, Cortet, Koch, Riou, Blanchard and Mach2018; Peachey et al., Reference Peachey, Molena, Jenkins, Di Cesare, Traversa, Hodgkinson and Cantacessi2018), probiotic dietary additives that aim to restore microbiome homoeostasis may play a role in alleviating the negative effects of infection, as has been proposed for a variety of pathogens in different animal production systems (Markowiak and Śliżewska, Reference Markowiak and Śliżewska2018).
In vivo, controlled infection studies are inherently difficult to perform in horses due to expenses, ethical and logistical issues; therefore, a focus of the equine parasitology research community should be to develop effective models for in vivo testing of novel anthelmintic agents. In addition to measurement of fecal egg counts in naturally infected animals treated with novel plant extracts or grazed on bioactive forages, adoption of model laboratory systems such as rabbits to mimic the horse gastrointestinal environment may be a worthwhile alternative.
Furthermore, strategies for the biological control of parasites have been given considerable attention, but no such technology is available at commercial levels for use in most parts of the world. For example, the predacious fungus Duddingtonia flagrans has potential antiparasitic activity, and is able to survive passage through the herbivore digestive tract. After oral administration, D. falgrans has potential effects on growth and survival of strongyle larvae in the pasture environment (Larsen, Reference Larsen2000). The fungus would be more valuable in controlling resistant parasite populations by reducing the resistant larvae in pasture environment.
Conclusions and future directions
The widespread resistance to BZs and pyrantel in equine cyathostomins along with the emerging significance of ML resistance in P. equorum, suggests that our ability to control equine parasites with anthelmintics is being significantly compromised which emphasises the need to revisit the control strategies. In addition, many researchers find it difficult to define AR because there is no set standard for equine parasiticide drugs. Furthermore, no reliable tests other than the FECRT are available for diagnosing AR in equine parasites. There is limited information available on the usefulness of in vitro assays for detecting AR in cyathostomins. In addition, presence of multi-species populations of cyathostomin nematodes further complicates the interpretation of in vitro assays. This makes the detection of AR difficult since it may have an important impact on resistance ratio, as proportions of different species may affect the drug sensitivity patterns of the whole population (Matthews et al., Reference Matthews, McArthur, Robinson and Jackson2012). Some of the major reasons for lack of knowledge about AR in equine nematodes are few research groups researching equine nematodes globally as well as limited research funds as compared with ruminants which take the privilege of being the food animals and are considered important for food security. Therefore, in future equine nematode research, following points may be considered for reducing the AR and designing sustainable parasite control programme:
(1) Generating large-scale datasets about epidemiological patterns of AR and its impact on equine health from different geographical locations worldwide.
(2) Establishing a governing body to set anthelmintic standards and guidelines for standardizing FECRTs with cyathostomins and P. equorum, so the findings can be compared across the regions.
(3) Development and implementation of sensitive diagnostic tools capable of detecting resistance at an early stage, which is the pre-requisite of parasite control programmes. High-throughput molecular detection assays should be a future goal since these techniques could detect genotypic resistance beforehand.
(4) The equine industry may benefit by funding the research to investigate the resistance and discovering newer anthelmintics or dietary additives that restore a healthy gut in the face of helminth infection, especially from natural resources.
(5) One of the key priorities should be the development of genomic datasets and their accompanying transcriptomic data for cyathostomins and P. equorum which is essential to provide major insights into the biology of these nematodes.
In summary, a combination of sustainable approaches including maintenance of susceptible equine parasite populations and adoption of regular parasite surveillance by horse owners and the equine veterinarians as well as FEC-directed use of anthelmintics may be considered to prolong the efficacy of currently available anthelmintics until new drugs are available for treating horse parasites.
Acknowledgements
The authors acknowledge the efforts of Dr Anne Beasley, School of Veterinary Science, University of Queensland, Australia for her valuable inputs in refining the manuscript.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflict of interest
None.
Ethical standards
Not applicable.




