Introduction
Gastrointestinal Nematodes (GIN) are an important cause of disease in small ruminant production worldwide, especially in tropical and subtropical countries. These parasites are responsible for substantial losses in productivity and can lead to mortalities and clinicopathological changes (Cantacessi et al., Reference Cantacessi, Campbell and Gasser2012; Roeber et al., Reference Roeber, Jex and Gasser2013).
Goats and sheep are infected with the same GIN species, mainly Haemonchus contortus, Trichostrongylus axei, Teladorsagia circumcincta (abomasum), T. colubriformis, Strongyloides papillosus, Nematodirus spp. (small intestine), Oesophagostomum columbianum and O. venulosum (large intestine) (Hoste et al., Reference Hoste, Sotiraki, Landau, Jackson and Beveridge2010; Bentounsi et al., Reference Bentounsi, Meradi and Cabaret2012; Moreno-Gonzalo et al., Reference Moreno-Gonzalo, Manolaraki, Frutos, Hervás, Celaya, Osoro, Ortega-Mora, Hoste and Ferre2013a). The nematode of major concern is H. contortus, a highly pathogenic parasite, because its blood-sucking feeding habits cause anemia, submandibular edema and lethargy. These infections promoted decrease in milk production, reduction of growth, weight loss, diarrhea, reproductive disorders and changes in carcass quality. In cases of massive infections, high mortality rates are observed (Molento et al., Reference Molento, Fortes, Pondelek, Borges, Chagas, Torres-Acosta and Geldhof2011; Cantacessi et al., Reference Cantacessi, Campbell and Gasser2012).
Goats are more susceptible to GIN probably due to a deficiency in the immune mechanism of parasite expulsion. Moreover, they metabolize anthelmintic drugs faster than do sheep, reducing the efficacy of anthelmintics when they are treated with the same dose recommended for sheep (Hoste et al., Reference Hoste, Sotiraki, Landau, Jackson and Beveridge2010).
The most common method used to control GIN is the repeated use of synthetic anthelmintics. The main anthelmintic drugs used belong to the groups of benzimidazoles, imidazothiazoles, macrocyclic lactones (Hoste and Torres-Acosta, Reference Hoste and Torres-Acosta2011), monepantel and derquantel (Kaminsky et al., Reference Kaminsky, Bapst, Stein, Strehlau, Allan, Hosking, Rolfe and Sager2011). There are some disadvantages, such as the development of resistant populations, high cost, risk of environmental pollution and reduction of animal production due to low effectiveness (Adamu et al., Reference Adamu, Naidoo and Eloff2013). Drug inefficacy can be associated with many factors, such as incorrectly or badly managed/inadequately stored drug, dose level, inaccurately calculated weight and differences among animal species in pharmacokinetics (Torres-Acosta and Hoste, Reference Torres-Acosta and Hoste2008).
Anthelminthic treatments are usually less effective in goats and could contribute to the nematode resistance. The goats are more susceptible than are sheep to gastrointestinal nematode infections with a higher production of worm eggs and elevated number of adult parasites in the gastrointestinal tract. These factors show that goats can be responsible for the dissemination of worm eggs from resistant nematodes in populations of small ruminants (Torina et al., Reference Torina, Dara, Marino, Sparagano, Vitale, Reale and Caracappa2004).
In this context, the investigation of the antiparasitic activity of natural bioproducts can contribute to the development of alternative treatments and the reduction of the dependence on conventional chemotherapy (Santos et al., Reference Santos, Lima, Santos, Serra, Uzeda, Reis, Botura, Branco and Batatinha2017). The interest in their anti-parasitic properties is increasing as illustrated by several reviews concerning ethnoveterinary medicine in different continents (Hounzangbe-Adote et al., Reference Hounzangbe-Adote, Paolini, Fouraste, Moutairou and Hoste2005; De Medeiros et al., Reference De Medeiros, de Moura, Napoleão, Paiva, Coelho, Bezerra and da Silva2018).
The scientific validation of plants with antiparasitic activity requires the conduction of in vitro and in vivo studies to determine its effectiveness. Several in vitro tests were used to screen plants with anthelmintic activity of plants at different stages of the parasite, such as egg hatch assay (EHA), larval development assay (LDA), larval feeding inhibition assay (LFIA), larval migration inhibition assay (LMIA), larval exsheathment inhibition assay (LEIA) and adult motility inhibition assay (AMIA). Some assays were developed for in vitro drug screening or drug resistance testing of parasitic stages (Borges and Borges, Reference Borges and Borges2016).
The adult motility inhibition assay (AMIA) is the only in vitro test to evaluate the anthelmintic effects on the parasitic life stage of the nematode (adult worm), which is the target of synthetic anthelmintic drugs. However, this test presents some limitations as the need to euthanize an animal infected with GIN and the maintenance of nematodes in a CO2 incubator (short time of parasite viability) (Hounzangbe-Adote et al., Reference Hounzangbe-Adote, Paolini, Fouraste, Moutairou and Hoste2005; Andre et al., Reference Andre, Ribeiro, Cavalcante, Santos, Macedo, de Paula, Freitas, Morais, Melo and Bevilaqua2016).
The in vitro tests are characterized by low cost, rapid collection, good sensitivity, repeatability and their use of free-living stages (eggs, first- and third-stage larvae) and adult nematodes species. However, they are not able to evaluate host factors since the substances are applied directly on the parasite (O'Grady and Kotze, Reference O'Grady and Kotze2004; Borges and Borges, Reference Borges and Borges2016).
The in vivo evaluation of anthelmintic efficacy is performed by the fecal egg count reduction test (FECRT) or controlled test. The FECRT provides an estimate of the reduction in the excretion of eggs after treatment, while in the controlled test the effectiveness is assessed by comparing the parasite burdens in the treated groups compared to the control group. The controlled test is the most reliable method, but also the most expensive analysis in terms of labor requirements and animal usage (Taylor et al., Reference Taylor, Hunti and Goodyear2002).
Most studies on the anthelmintic activity of plants in small ruminants were performed in sheep. However, the results obtained from studies with this species cannot be extrapolated to goats because of immunological, physiological and behavioral differences between sheep and goats (Torres-Acosta et al., Reference Torres-Acosta, Jacobs, Aguilar-Caballero, Sandoval-Castro, May-Martinez and Cob-Galera2004). The aim of this study is to provide an overview of the anthelmintic properties of the plants against GIN of goats, as well as to identify bioactive constituents related to this effect.
Methods
For the article collection, we used the databases PubMed (database of the National Library of Medicine of the United States of America) and Science Direct, the Elsevier scientific literature platform belonging to the RELX group. We considered all original articles published between 2008 and 2018 and that investigated the activity of plants and their derivatives in the treatment against GIN of goats. The search strategy was based on three descriptors: plants, gastrointestinal nematodes and goats.
The abstracts of the articles were analyzed and after the initial screening, all relevant studies were recovered in full-text and evaluated. Only studies investigating plant extracts, its fractions and isolated compounds against GIN of goats were considered for potential inclusion in the review. In vitro and in vivo studies were included. The methods of exclusion were based on the following criteria: studies in small ruminants without specifying the animal species, studies testing synthetic or commercial substances and studies that were not research articles (i.e. review articles, encyclopedia, correspondence and book chapters).
Results
337 articles were recovered in the two databases. After screening, 56 studies that presented the research object were found, out of which 17 were referred on the PubMed platform, 26 on the Science Direct platform and 13 on both platforms. These duplicate studies were compared through authors, title, year and journal of publication. Figure 1 shows the Flowchart of the general characteristics in the selection process to recover these articles.
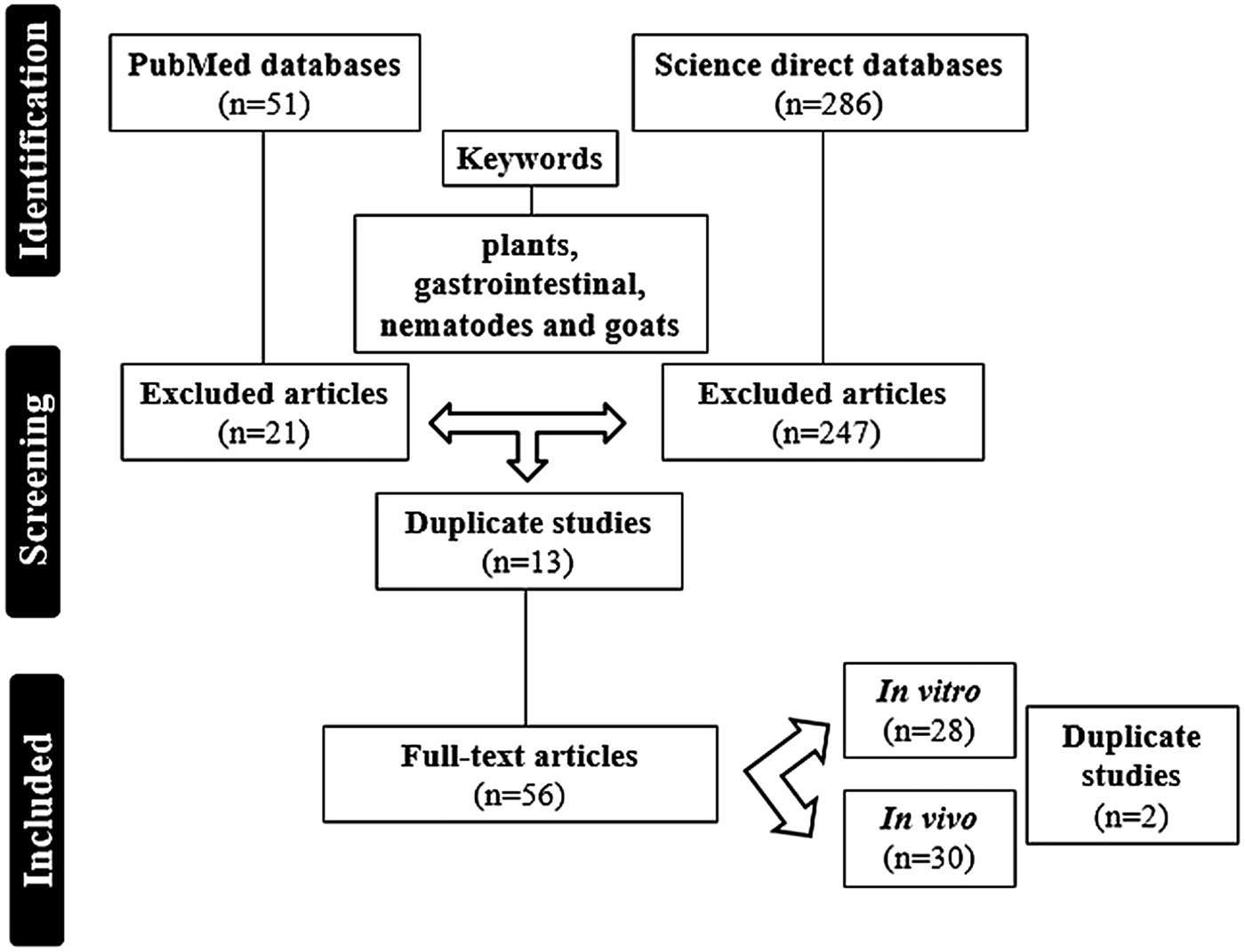
Fig. 1. Flowchart of the general characteristics for selection process of the articles.
The years from 2012 and 2013 presented most scientific productions, with 8 publications each. As for the countries responsible for the publications, Brazil was the country with the largest number (n = 14), followed by the United States of America (n = 10) and Spain (n = 6).
Table 1 shows 28 in vitro studies on the effects of plants against the GIN of goats. 32 families and 78 plant species were evaluated in in vitro studies. While Table 2 shows 30 in vivo studies, we identified the citation of 41 plants belonging to 18 families. Only two papers presented in vitro and in vivo tests in the same study. Considering the number of articles analyzed, the most frequently investigated family was Fabaceae (46.42%), followed by Ericaceae (14.29%), Asteraceae (7.14%) and Rhamnaceae (5.35%). In this review, the Lespedeza cuneata (Fabaceae) and Calluna vulgaris (Ericaceae) were the most studied species (6 articles for each species).
Table 1. In vitro studies on the effects of plants against gastrointestinal nematodes
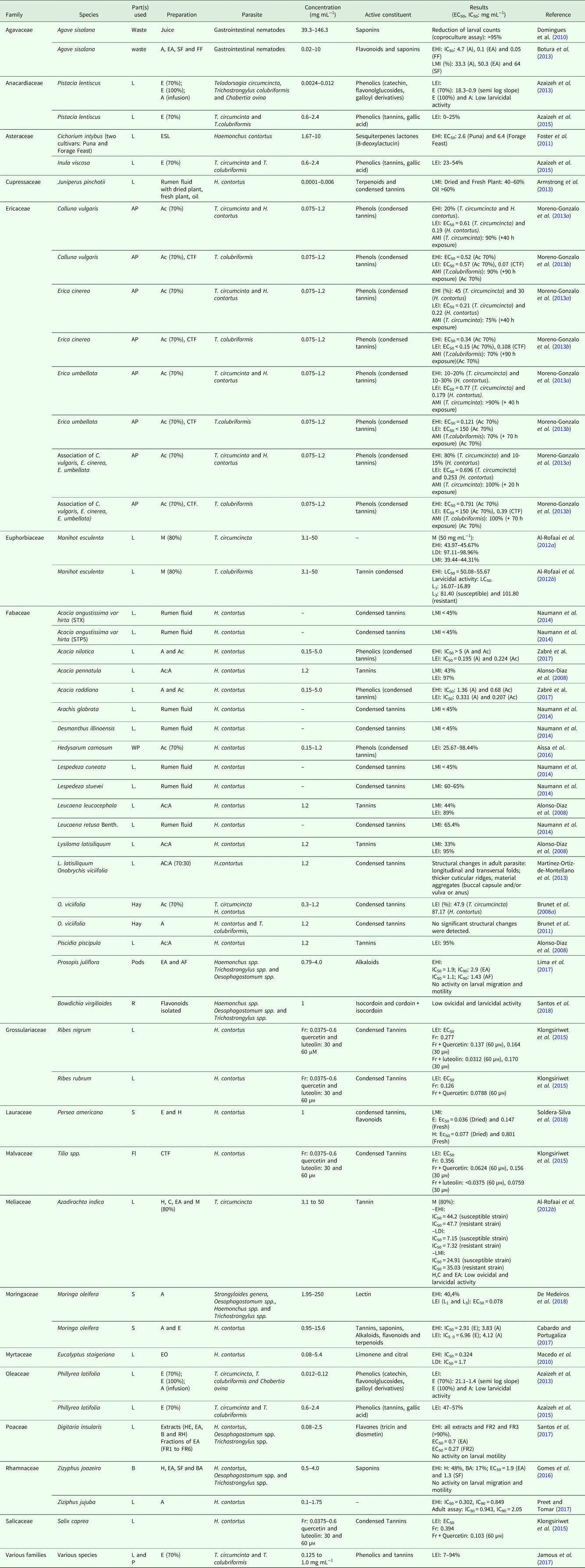
Parts used: AP, Aerial Part; B, bark; L, leaf; Fl, Flower; Fr, Fruit; R, Root; S, Seed; ST, Stem; WP, Whole Plant.
Extracts: A, Aqueous; Ac, Acetone; EA, Ethyl Acetate; B, Butanol; C, Chloroform; D, Dichloromethane; E, Ethanol; HE, Hydroethanol; RH, Residual Hydroethanol; M, Methanol; H, hexane; EO, Essential Oil; ESL, Extract Rich in Sesquiterpene Lactones; CTF, Condensed Tannins Fraction; FGF, Flavonol Glycosides Fraction; SF, saponins fraction; FF, flavonoids fraction; AF, alkaloid-rich fraction; BA, betulinic acid; SA, Succinic acid.
Egg hatch inhibition (EHI), Larval development inhibition (LDI), Larval migration/motility inhibition (LMI), Larval exsheathment inhibition (LEI), Adult motility inhibition (AMI)
Table 2. In vivo studies on anthelmintic activity of plants in goats infected with gastrointestinal nematodes
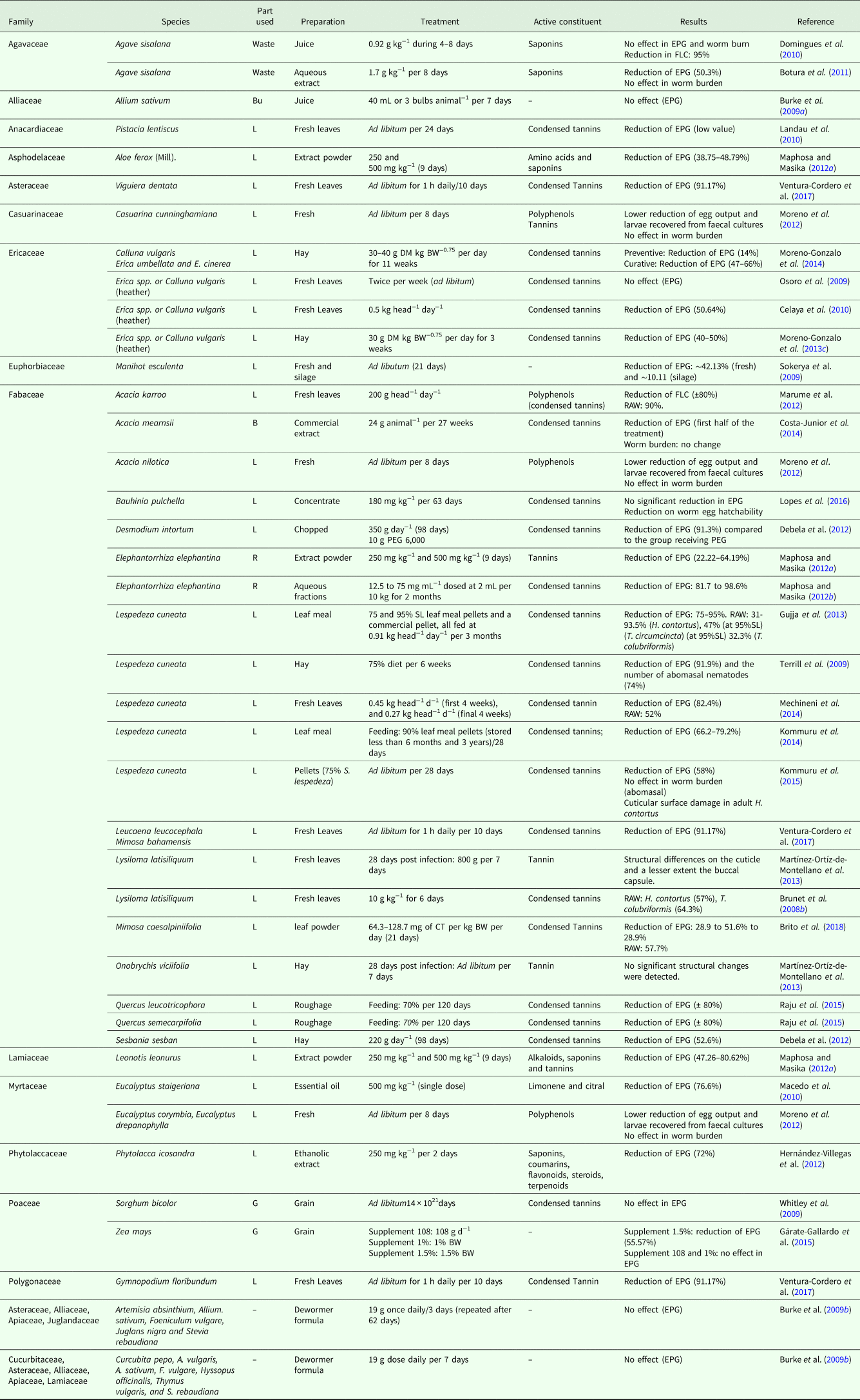
Par used: B, Bark; Bu, Bulb; G, Grain; L, Leaf; R, Root; EPG, Eggs per gram; FLC, Faecal Larval Counts; RAW, Reduction Adult Count; PEG, Polyethylene Glycol; DM, Dry Matter; BW, Body Weight; CT, Condensed Tannins
The in vitro studies were carried out mainly with stages of free-living Haemonchus contortus (64.2%) and Thrichostrongylus (35.7%) spp. Many plants exhibited in vitro anthelmintic activity at a lower concentration (EC50 <1.0 mg mL−1), such as Calluna vulgaris, Erica cinerea, E. umbellata (Ericaceae), Acacia nilotica, A. raddiana (Fabaceae), Persea Americana (Lauraceae) and Digitaria insularis (Poaceae). The ethyl acetate extracts of C. vulgaris and E. cinerea were active on different stages of GIN (egg, larvae L3 and adult parasite) (Table 1).
The in vivo studies were characterized by the oral administration of the leaves (fresh, hay, meal), aqueous extract and oil of plants to goats infected naturally or experimentally with GIN. The duration of treatment ranged from 01 to 189 days. The efficacy parameter frequently evaluated was the reduction in EPG (90%), followed by recovery of adult parasites (16.7%).
The main plant secondary metabolites associated with anthelmintic effect are condensed tannins (68%), saponins (12.5%) and flavonoids (8.9%). This review showed that the evaluation of anthelmintic activity of isolated compounds on goat's parasites is scarce. Only two studies in vitro (7.4%) used commercial substances that are found in plants: the flavonoids (quercetin, rutin and epicatechin) and the saponins (escin and digitonin).
Discussion
In vitro studies of the anthelmintic activity of plants
Plants stand out as an important source of new candidates for anthelmintic drugs. Several in vitro tests, as egg hatch and larval migration, motility and exsheathment assays, have been used to identify plants with effect against the GIN of goats.
Studies show that many plants evaluated bear anthelmintic properties against different abomasal and small intestine nematodes. In this review, antiparasitic effects of plants vary depending on the plant species, stage, the nematode species and the parasitic stage. Erica cinera was more active against eggs and L3 of T. columbriformis than to inhibit the egg hatching and larvae exsheathment of abomasal parasites (H. contortus and T. circumcinta) (Moreno-Gonzalo et al., Reference Moreno-Gonzalo, Manolaraki, Frutos, Hervás, Celaya, Osoro, Ortega-Mora, Hoste and Ferre2013a, Reference Moreno-Gonzalo, Manolaraki, Frutos, Hervás, Celaya, Osoro, Ortega-Mora, Hoste and Ferre2013b). The Agave sisalana and Moringa oleifera has greater ovicidal effect while Acacia nilotica and A. raddiana are more active against L3 of GIN (Botura et al., Reference Botura, Santos, Silva, Lima, Oliveira, Almeida, Batatinha and Branco2013; Zabré et al., Reference Zabré, Kaboré, Bayala, Katiki, Costa-Júnior, Tamboura, Belem, Abdalla, Niderkorn, Hoste and Louvandini2017; De Medeiros et al., Reference De Medeiros, de Moura, Napoleão, Paiva, Coelho, Bezerra and da Silva2018). These variations can be related with differences in various enzymatic constituents and membrane structures of the species and nematodes' life stages. Plant secondary metabolites can act by means of different mechanisms such as: inhibition of egg hatching enzymes, competition with membrane receptors and binding to proteins in the membrane (Chan-Pérez et al., Reference Chan-Pérez, Torres-Acosta, Sandoval-Castro, Hoste, Castañeda-Ramírez, Vilarem and Mathieu2016).
The use of standardized in vitro methods is essential for the assessment of the efficacy of plant products, particularly the determination of EC50 and EC90 (effective concentration 50% and 90%), which allows the comparison of activities of different plants (Borges and Borges, Reference Borges and Borges2016). Adamu et al. (Reference Adamu, Naidoo and Eloff2013) established that a plant extract with EC50 below 6 mg mL−1 shows great anthelmintic potential. Considering this parameter, many plants recorded in this review have a promising antiparasitic effect. However, we found variations in the indices of efficacy for the same plant species, such as Agave sisalana, Pistacia lentiscus, Calluna vulgaris and Erica umbellata. This finding may be related to the type of preparation of the plant, stage and species of the parasite, contact and the time of exposure of the parasite to the vegetal product (Hernández-Villegas et al., Reference Hernández-Vilegas, Borges-Argáez, Rodrúguez-Vivas, Torres-Acosta, Méndez-González and Cáceres-Farfán2011). Some factors should be observed to avoid false positive or false negative results such as operator experience, choice of solvents, packaging and solubilization of plant products, quality of water and pH of the solutions used in the tests (Borges and Borges, Reference Borges and Borges2016).
Variations in collection and preparation procedures of plant materials can interfere in the reproducibility of assays. Hence, chemical characterization of herbal products may be useful for the scientific validation of their antiparasitic activity. Phytochemical analyses using methods of mass spectrometry (liquid-chromatography mass spectrometry and gas-chromatography mass spectrometry) can aid in identifying the bioactive compounds found in plants (Hoste et al., Reference Hoste, Athanasiadou, Thamsborg and Hoskin2006).
In this review, it can be seen that anthelmintic activity of plants was evaluated mainly against stages of free-living nematodes (egg and larvae L3). Although the synthetic anthelmintic drugs act on the parasitic stages, the action on the stages of free-living nematodes can be useful for the control of helminths. Ovicidal action of plants can prevent parasitic development of the infective stage and reduce the pasture contamination (Silveira et al., Reference Silveira, Chagas, Botura, Batatinha, Katiki, Carvalho, Bevilaqua, Branco, Machado, Borges and Almeida2012). The evaluation of the activity of plant products against different stages of parasites is necessary, since the action of phytocompounds can be different depending on the phases of the parasite's development (Borges and Borges, Reference Borges and Borges2016).
The anthelmintic activity of medicinal plants has been related to secondary metabolites. The major classes of bioactive metabolites are flavonoids, alkaloids, coumarins, lignoids, triterpenes, saponins, polyphenols and tannins. The tannins are the most studied class of natural products for nematode control in small ruminants (Hoste and Torres-Acosta, Reference Hoste and Torres-Acosta2011). The anthelmintic effect of tannins is attributed to its ability to bind to the proteins present in the cuticle, oral cavity, esophagus, cloaca and vulva of the nematodes, changing its physical and chemical properties. Another possibility is related to an indirect action of these compounds, which can enhance the host immune response due to its binding with proteins of the diet, protecting these substances from ruminal degradation and thereby increasing protein availability in the small intestine (Hoste et al., Reference Hoste, Athanasiadou, Thamsborg and Hoskin2006).
Several studies have showed the anthelmintic potential of the plants rich in condensed tannins. In vitro evaluations demonstrated effects of tanniferous plant extracts on the larval migration, larval artificial exsheathment, adult motility inhibition and egg hatching of H. contortus (Alonso-Díaz et al., Reference Alonso-Díaz, Torres-Acosta, Sandoval-Castro, Aguilar-Caballero and Hoste2008; Moreno-Gonzalo et al., Reference Moreno-Gonzalo, Manolaraki, Frutos, Hervás, Celaya, Osoro, Ortega-Mora, Hoste and Ferre2013a; Naumann et al., Reference Naumann, Armstrong, Lambert, Muir, Tedeschi and Kothmann2014). The use of polyvinylpolypyrrolidone (PVPP), an inhibitor of tannins, confirmed that this metabolite is responsible for the anthelmintic activity of the tanniferous plants (Alonso-Díaz et al., Reference Alonso-Díaz, Torres-Acosta, Sandoval-Castro, Aguilar-Caballero and Hoste2008). The effect of larval exsheathment may be related to the presence of proline and hydroxiproline-rich proteins in the nematode larval sheath, cuticle and exsheathing fluid and these substances have high affinity for tannins. The exsheathment process is an important step, which is the transition from the free stage to the parasitic stage, allowing the larvae infection of the host (Alonso-Díaz et al., Reference Alonso-Díaz, Torres-Acosta, Sandoval-Castro and Hoste2011).
In vitro study with Lysiloma latisiliquum and Onobrychis viciifolia, rich in tannins, showed that the exposure of H. contortus (adult stage) to the extract acetone: water (70:30) of these plants led to changes in parasite structure: longitudinal and transversal folds and thicker cuticular ridges, aggregate material in the regions of the buccal capsule and anus or vulva. Alterations in cuticular structure can interfere with the movement of nematodes and changes in the anterior part of the digestive tract may interfere with parasite nutrition and consequently lead to malnutrition, reduced fertility and mortality (Martínez-Ortíz-de-Montellano et al., Reference Martínez-Ortíz-de-Montellano, Arroyo-López, Fourquaux, Torres-Acosta, Sandoval-Castro and Hoste2013).
The second most found metabolite with anthelmintic activity in goats was the flavonoid. Flavones of the Turnera ulmifolia reduced egg hatch, larval development and larval motility of H. contortus (Oliveira et al., Reference Oliveira, Junior, Lima, Silva, Ribeiro, Mesquista, Rocha, Tangerina and Vilegas2017). Santos et al. (Reference Santos, Lima, Santos, Serra, Uzeda, Reis, Botura, Branco and Batatinha2017) have also suggested that flavones (tricin and diosmetin) are related to the anthelmintic activity of Digitaria insularis. The extracts of the waste of Agave sisalana showed in vitro activity on two different stages of the GIN of goats, and their possible active ingredients are flavonoids and saponins (Botura et al., Reference Botura, Santos, Silva, Lima, Oliveira, Almeida, Batatinha and Branco2013). Thus, saponin is the third class of metabolites most found in plants with anthelmintic activity. Gomes et al. (Reference Gomes, Lima, Vaz, Santos, Santos, Dias, Botura, Branco and Batatinha2016) reported this activity of Zizyphus joazeiro on eggs of H. contortus.
De Medeiros et al. (Reference De Medeiros, de Moura, Napoleão, Paiva, Coelho, Bezerra and da Silva2018) evaluated the in vitro effect of water-soluble Moringa oleifera lectin (WSMoL) on hatching of eggs and on the development of early-stage larvae of gastrointestinal nematodes from naturally infected goats. The mechanism of action of this substance is attributed to the interference of WSMoL on the activity of proteases and the affinity of the lectin for glycosylated, interacting with intestinal glycoconjugate receptors in the embryo, as well as in cuticle of the larvae.
The extracts rich in sesquiterpene lactones of two forage chicory (Cichorium intybus) showed inhibition of hatching of a predominantly H. contortus egg population. The most active cultivar presented higher concentration of the 8-deoxylactucin. This action is associated to the presence of α-methylene-γ-lactone functional group capable of reacting with sulfhydryl proteins (Foster et al., Reference Foster, Cassida and Turner2011).
Two studies have demonstrated the anthelmintic activity of essential oils in goats, obtained from the species Eucalyptus staigeriana (Macedo et al., Reference Macedo, Bevilaqua, Oliveira, Camurça-Vasconcelos, Vieira, Oliveira, Queiroz-Junior, Tomé and Nascimento2010) and Juniperus pinchotii (Armstrong et al., Reference Armstrong, Klein, Whitney, Scott, Muird, Lambert and Craig2013). The antiparasitic effect of these plants has been related to the presence of terpenoids (lemonene, eugenol, carvacrol and citral). The chemical substances of this class can act by inhibiting the growth, reducing the reproductive capacity or causing damage during parasite maturation process (Zhu et al., Reference Zhu, Dai, Yang and Qiu2013).
Only two studies that used isolated substances of plants were found in this review. Santos et al. (Reference Santos, Santos, Lima, Da Silva, Uzêda, Dias, Branco, Cardoso, David, Botura, Costa and Batatinha2018) used two isolated saponins and presented effect above 90% for egg hatch inhibition (aescin) and larval motility inhibition (digitonin). These authors attributed the anthelmintic effect of saponin to their ability to form complexes with cellular membrane components leading to a pore formation and consequent increase in membrane permeability. The second work was performed by Soldera-Silva et al. (Reference Soldera-Silva, Seyfried, Campestrini, Zawadzki-Baggio, Minho, Molento and Maurer2018), who tested quercetin, rutin and epicatechin against H. contortus larval. They observed high efficacy with low EC50 of 7.8, 30 and 10 µg mL−1, respectively.
The therapeutic properties of plants can be attributed to one substance or combination of compounds produced by the secondary metabolism of the plant. Klongsiriwet et al. (Reference Klongsiriwet, Quijada, Williams, Mueller-Harvey, Williamson and Hoste2015) reported the synergistic effects between the fraction of condensed tannins and two flavonoids, quercetin and luteolin, in terms of inhibiting the in vitro exsheathment of H. concortus L3 larvae obtained from small ruminants. The complexity and diversity of structures of the phytocompounds could enable their interaction with multiple molecular targets on the parasite and may consequently hinder the appearance of populations resistant to these substances (Chan-Pérez et al., Reference Chan-Pérez, Torres-Acosta, Sandoval-Castro, Hoste, Castañeda-Ramírez, Vilarem and Mathieu2016).
The in vitro studies are indicated as screening tests, which must be performed prior to the in vivo evaluation. The in vitro tests cannot be enough to confirm anthelmintic efficacy of plants, since in vitro conditions are different from in vivo experiments, particularly due to the gastrointestinal tract of ruminants. Therefore, pharmacokinetic studies should be carried out for the determination of the natural product bioavailability in small ruminants (Githiori et al., Reference Githiori, Athanasiadou and Thamsborg2006).
In vivo studies of the anthelmintic activity of plants in goats
The in vivo evaluation of the activity of plant against the GIN of goats has been performed mainly by means of FECRT and controlled tests (Githiori et al., Reference Githiori, Athanasiadou and Thamsborg2006). The results obtained in the FECRT cannot estimate anthelmintic efficacy accurately because egg count does not always correlate well with worm numbers. The controlled test is considered as more reliable because the efficacy was measured by the count of adult parasite in the gastrointestinal tract and the evaluation of egg output (Taylor et al., Reference Taylor, Hunti and Goodyear2002).
The species Quercus leucotricophora, Q. semecarpifolia and Desmodium intortum induced a reduction of EPG, whereas Bauhinia pulchella promoted a reduction of egg viability and pasture contamination (Debela et al., Reference Debela, Tolera, Eik and Salte2012; Raju et al., Reference Raju, Sahoo, Chandrakar, Sankar, Garg, Sharma and Pandey2015; Lopes et al., Reference Lopes, Barros, Louvandini, Abdalla and Junior2016). Other plants, such as A. karroo and Lespedeza cuneata, demonstrated effect against adult parasites (Marume et al., Reference Marume, Chimonyo and Dzama2012; Gujja et al., Reference Gujja, Terrill, Mosjidis, Miller, Mechineni, Kommuru, Shaik, Lambert, Cherry and Burke2013). In this review, we observed that some plants, as A. sisalana, A. mearnsii, A. nilotilica, were able to promote reduction in the egg count, but did not interfere in the worm burden. The reduction of EPG can be related to a reduction in the nematode burden or a lower fecundity of female worms (Githiori et al., Reference Githiori, Athanasiadou and Thamsborg2006).
There is a lack of specific guidelines for assessing the anthelmintic effect of plant-derived products. The present work identified variations in the protocols applied for in vivo studies, especially treatment time, dosage, number of animals, plant preparation and parameters evaluated.
Most of the plants evaluated in vivo contain tannin in their chemical composition, and the treatment consisted of the administration of the plant in animal feed. The treatment of goats with leaves of Bauhinia pulchella (180 mg kg−1 per 63 days), rich in condensed tannins, showed reduction in egg viability and pasture contamination of T. colubriformis (86%) (Lopes et al., Reference Lopes, Barros, Louvandini, Abdalla and Junior2016). The administration of the commercial extract of A. mearnsii (24 g animal−1 day−1), containing 16.7% of tannins, for an extended period (27 weeks) resulted in the reduction of EPG in the first half of the experimental period. However, there was no effect on the parasite count of adults recovered from goats (Costa-Júnior et al., Reference Costa-Júnior, Costa, Lôbo, Soares, Abdala, Chaves, Batista and Louvandini2014). The supplementation of goats with sorghum grains, containing high levels of condensed tannins, and the administration of commercial preparation of condensed tannins obtained from the bark of A. mearnsii did not significantly influence EPG (Whitley et al., Reference Whitley, Miller, Burke, Cazac, O´brien, Dykes and Muir2009; Max, Reference Max2010). According to Costa-Júnior et al. (Reference Costa-Júnior, Costa, Lôbo, Soares, Abdala, Chaves, Batista and Louvandini2014), the continuous consumption of tannin may cause an increase in the concentration of salivary proteins, especially in growing animals. These proteins have high affinity for tannin, which can lead to reducing the effect of this active compound.
The addition of Lespedeza cuneata hay in the goat diet, in the concentration of 50 to 75%, caused reduction of EPG 84.6 and 91.9%, respectively. Only the treatment with the highest concentration resulted in a significant decrease of the number of adult parasites (74%) (Terrill et al., Reference Terrill, Dykes, Shaik, Miller, Kouakou, Kannan, Burke and Mosjidis2009). In goats, fed pellets with the same species, a significant reduction in the EPG (58 to 70.9%), though there was no reduction in the number of H. contortus in the abomasum. The analysis of parasites by electron microscopy revealed changes in the cuticle (constricted folds and a disheveled cuticular surface appearance) (Kommuru et al., Reference Kommuru, Barker, Desai, Burke, Ramsay, Mueller-Harvey, Miller, Mosjidis, Kamisetti and Terril2014, Reference Kommuru, Whitley, Miller, Mosjidis, Burke, Gujja, Mechineni and Terril2015).
Most of the species compiled in this study belong to the family Fabaceae, which presents worldwide distribution. This family includes herbaceous plants, trees and shrubs (perennials or annuals). Some species are used as feed for small ruminants. These plants have agronomic characteristics that could provide beneficial effects to the nutrition and health of animals and environmental issues: palatability for ruminants and nutritive values; biological nitrogen fixation, which reduces the use of chemical fertilizers; carbon sequestration; provision of shade for livestock and flowers for pollinators; conservation of the biodiversity, reduction of methane emission and greenhouse gases (Fagbenro et al., Reference Fagbenro, Oshunsanya, Aluko and Oyeleye2015; Hoste et al., Reference Hoste, Torres-Acosta, Sandoval-Castro, Mueller-Harvey, Sotiraki, Louvandini, Thamsborg and Terrill2015). The legume species, Lespedeza cuneata, has been widely studied and has shown some advantages as the ability to produce seeds and to be cultivated efficiently, as well as the viability of large-scale production (Hoste et al., Reference Hoste, Torres-Acosta, Sandoval-Castro, Mueller-Harvey, Sotiraki, Louvandini, Thamsborg and Terrill2015). Puchala et al. (Reference Puchala, Min, Goetsch and Sahlu2005) observed lower methane emissions from goats fed on lespedeza (Lespedeza cuneata) and this effect was related to the presence of condensed tannins in the plant.
The use of forage legumes rich in condensed tannins as nutraceutical plants (species that present positive effects for animal nutrition and health) has been proposed to control GIN in small ruminants. Hoste et al. (Reference Hoste, Athanasiadou, Thamsborg and Hoskin2006) suggested that feeding small ruminants with tannin-rich plants (30–40 g of condensed tannins per kg dry matter) promotes antiparasitic effect. However, the excessive consumption of tannin can cause antinutritional effect in animals. The condensed tannins, at higher concentrations (7–8% DM), can depress feed intake, disturb digestive physiology and decrease nutrient digestibility and the production rate (Min et al., Reference Min, Barry, Attwood and McNabb2003).
Other classes of substances have been associated with anthelmintic activity in goats, such as saponin and essential oils. The treatment of goats naturally infected with gastrointestinal nematodes, with the aqueous extract obtained from the residue Agave sisalana (1.7 g kg−1 per 8 days), led to a reduction of EPG (50.3%) but did not affect the number of adult parasites. This anthelmintic effect was associated with the presence of saponins in this extract (Botura et al., Reference Botura, Silva, Lima, Oliveira, Souza, Santos, Branco, Moreira, Almeida and Batatinha2011). The oral administration of essential oil obtained from the leaves E. staigeriana (500 mg kg−1) resulted in a significant reduction of EPG (76.5%) in goats. The chemical constituents present in the oil associated with this activity were the citral and limonene (Macedo et al., Reference Macedo, Bevilaqua, Oliveira, Camurça-Vasconcelos, Vieira, Oliveira, Queiroz-Junior, Tomé and Nascimento2010).
Differences in anthelmintic efficacy of several plants were verified between the in vitro and in vivo studies, such as A. sisalana, A. nilotica, C. vulgaris and L. latisiliquum, which were more active in in vitro assays. The treatment in vitro is characterized by direct contact with the parasites and the concentrations of potentially active substances do not always correspond to their in vivo bioavailability (Githiori et al., Reference Githiori, Athanasiadou and Thamsborg2006). Furthermore, the possibility of biotransformation of these compounds within the gastrointestinal tract of the animal, modified by rumen microorganism, may lead to a reduction of biological activity (Athanasiadou and Kyriazakis, Reference Athanasiadou and Kyriazakis2004).
Most studies on potential in vivo anti-parasitic plants have showed lower percentage of efficacy to synthetic drugs. An anthelmintic product is considered as effective when it has a percentage reduction of over 90% of EPG and adult parasites (Vercruysse et al., Reference Vercruysse, Holdsworth, Letonja, Barth, Conder, Hamamoto and Okano2001). The treatments with Vigueira dentata, Lespedeza cuneate, Elephantorrhiza elephantina, Gymnopodium floribundum and association of Leucaena leucocephala and Mimosa bahamensis exhibited reduction of EPG over 90%, whereas only one species, A. karroo, induced 90% of reduction of adult nematodes (Marume et al., Reference Marume, Chimonyo and Dzama2012; Maphosa and Masika, Reference Maphosa and Masika2012b; Gujja et al., Reference Gujja, Terrill, Mosjidis, Miller, Mechineni, Kommuru, Shaik, Lambert, Cherry and Burke2013; Ventura-Cordero et al., Reference Ventura-Cordero, González-Pech, Jaimez-Rodriguez, Ortíz-Ocampo, Sandoval-Castro and Torres-Acosta2017). Githiori et al. (Reference Githiori, Athanasiadou and Thamsborg2006) suggest that a lower level of reduction should be established (70%) to the evaluation tests in vivo herbal preparations, since products with moderate anthelmintic activity may be part of an integrated program of parasite control in ruminant production systems. The integrated management is the combination of chemical and non-chemical methods of parasite control in order to maintain acceptable levels of production without total elimination of the parasites (Hoste and Torres-Acosta, Reference Hoste and Torres-Acosta2011).
The sustainable control of GIN infection must be guided by three principles: management of grazing systems; stimulation of host response and modulation of worm biology (Hoste et al., Reference Hoste, Torres-Acosta, Sandoval-Castro, Mueller-Harvey, Sotiraki, Louvandini, Thamsborg and Terrill2015). The reduction of contact with infective larvae (L3) can be achieved by strategies of grazing management such as pasture rotation system and mixed grazing. As the L3 survives on pastures for a limited period of time, the pasture rotation can cut down on the number of parasites ingested by the animal. It has better applicability in tropical climate. Mixed grazing among different animal species or by older animals may also be an alternative for the reduction of L3 because GIN presents a relative specificity for its hosts. However, mixed grazing between sheep and goats should be avoided due to the strong overlapping of nematodes infecting these two hosts (Torres-Acosta and Hoste, Reference Torres-Acosta and Hoste2008; Hoste and Torres-Acosta, Reference Hoste and Torres-Acosta2011). Another measure for parasite control is the use of organisms that aim at altering the biology of the free-living stages in the environment, decreasing the pasture infectivity. Amongst the various potential agents, one of the most extensively studied models is the use of nematophagous fungi, as Duddingtonia flagrans, which has the ability to invade and kill nematode larvae in the faeces (Sanyal et al., Reference Sanyal, Sarkar, Patel, Mandal and Pal2008).
The stimulation of immune response has been proposed to aid in the control of GIN infection in small ruminants, particularly the genetic selection of resistant animals and the manipulation of host nutrition. This strategy is important for goats since their immune response against GIN is less effective than that observed in sheep. Variability in host response is associated with several factors, such as herbivore behaviors (grazing and/or browsing), age, previous contact with parasites, gender, breed or individual genetic characteristics, nutrition status and the presence of other parasites (Torres-Acosta and Hoste, Reference Torres-Acosta and Hoste2008).
Conclusions/Future directions
Almost all the plants described in this review showed promising anthelmintic effects, especially in vitro studies. However, in vivo trials, generally, report lower plant efficacy, probably due to the interference of pharmacokinetic parameters of ruminants in the bioavailability of active compounds of plants. This work also noticed that there is a lack of studies on the effect of chemical constituents isolated from plants against GIN. Condensed tannins were the metabolite class most related with anthelmintic activity, followed by saponins and flavonoids. Studies on the mechanisms of action are required to improve our understanding of interactions between plant secondary metabolites and the different parasitic stages and species. Moreover, efforts should be performed to standardize the formulations of products derived from plants and the validation of their use as anthelmintic in goats. Plants are an important source of new bioactive molecules and may be useful as a part of integrated parasite control, which would lead to the reduction of the use of synthetic anthelmintic drugs.
Author ORCIDs
Mariana Borges Botura, 0000-0002-6404-0314
Acknowledgements
We thank teacher Abilio Borghi for the grammar review of the manuscript.
Financial support
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior–Brasil (CAPES) – Finance Code 001 and Programa de Pós-graduação em Biotecnologia of Universidade Estadual de Feira de Santana (UEFS).
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standards
Not applicable.






