Published online by Cambridge University Press: 17 October 2003
We tested the chemotactic responses of dauer juvenile stages (DJs) of the insect parasitic nematode Heterorhabditis bacteriophora to a variety of compounds that are known to be highly attractive or highly repellent to Caenorhabditis elegans. While H. bacteriophora DJs respond to alcohols and some aromatic compounds as well as to host metabolites such as uric acid and CO2, the most notable difference in the responses of these two nematodes is that H. bacteriophora DJs are unresponsive to a large number of compounds which C. elegans finds highly attractive. The latter compounds are typical by-products of bacterial metabolism and include aldehydes, esters, ketones and short-chain alcohols. While C. elegans finds long-chain alcohols (e.g. 1-heptanol and 1-octanol) repellent and short-chain alcohols highly attractive, H. bacteriophora DJs are strongly attracted to 1-heptanol, 1-octanol and 1-nonanol and find short-chain alcohols to be only slightly attractive. Parasitic-stage H. bacteriophora nematodes show a very weak chemotactic response to volatile molecules that DJs find highly attractive. Our results suggest that, associated with the adoption of a parasitic mode of life by Heterorhabditis, there was an adaptive change in chemotactic behaviour of the infective stages, resulting in a decreased sensitivity to volatile by-products of bacterial metabolism and an increased sensitivity towards long-chain alcohols and other insect-specific volatiles and possibly also to herbivore-induced plant volatiles.
Chemosensation and chemotaxis are essential processes in the survival of both free-living and parasitic animals. Animals rely on chemical signals in their environment to detect food sources, potential hosts, noxious compounds, reproductive partners and sometimes to enable them to choose between alternative developmental states (see reviews by Krieger & Breer, 1999; Prasad & Reed, 1999). The chemotactic responses of the free-living soil nematode Caenorhabditis elegans have been extensively investigated. C. elegans responds to a wide spectrum of water-soluble and volatile chemicals. Na+, Li+, Cl−, and OH− ions are attractive to C. elegans, as are the water-soluble molecules cAMP, cGMP, lysine, histidine, cysteine and biotin (Ward, 1973; Dusenbery, 1974; Bargmann & Horvitz, 1991). Water-soluble chemicals tend to diffuse slowly in the soil and may provide short-range chemosensory cues whereas volatile compounds diffuse more rapidly and thus can be used for long-range chemotaxis to distant food sources (Bargmann & Mori, 1997). In the soil C. elegans feeds on a large variety of bacteria associated with decaying organic matter (Andrew & Nicholas, 1976). The by-products of bacterial metabolism include various carboxylic acids, alcohols, aldehydes, esters, ketones and hydrocarbons (Zechman & Labows, 1985; Schöller, Molin & Wilkins, 1997) and several of these compounds are highly attractive to C. elegans (Bargmann, Hartweig & Horvitz, 1993).
When conditions are unfavourable for growth, C. elegans reproduction ceases and a long-lived, non-feeding, stress-tolerant dauer juvenile (DJ) stage is produced at the second juvenile moult (Cassada & Russell, 1975; Golden & Riddle, 1984). C. elegans DJs can survive for months in the absence of food and when they encounter food they rapidly resume development to become self-fertile hermaphrodite females.
The infective stage of the insect-parasitic genus Heterorhabditis is also a modified 3rd-stage juvenile. It is non-feeding, occurs free in the soil and is adapted for dispersal, long-term survival, host finding and infection. Heterorhabditid DJs respond chemotactically to insect hosts in the soil. They enter insect larvae through natural openings (mouth, anus, spiracles) or by penetrating the intersegmental membranes. Once in the insect haemocoel the DJ releases cells of its bacterial symbiont (Boemare, Akhurst & Mourant, 1993), which replicate rapidly and secrete insecticidal toxins and lytic enzymes. These secretions are lethal to the insect, which normally dies within 48 h (Forst et al. 1997). The bacterial cells and digested host tissues provide a rich medium for nematode growth and reproduction. The DJ resumes development and feeding and matures to become an adult hermaphrodite female. Nematode reproduction is prolific and continues over 2–3 generations at which point adult development is suppressed, DJs accumulate and begin to emerge into the soil from the insect cadaver.
Heterorhabditis belongs to the same zoological family (the Rhabditidae) as C. elegans, an important technology development platform for nematode research (Grant & Viney, 2001) and Heterorhabditis was also was the most closely related parasitic nematode to C. elegans in the phylogeny of Blaxter et al. (1998). Sudhaus (1993) has postulated that Heterorhabditis evolved from a free-living bacterial feeding rhabditid nematode which developed a symbiotic association with an entomopathogenic bacterium. Because they are closely related phylogenetically to C. elegans and can also complete their life-cycle in vitro (in contrast to the vast majority of animal- or plant-parasitic nematodes), heterorhabditids have the potential to be important model organisms for parasitic research. Here we describe our study of the chemotactic behaviour of H. bacteriophora towards a variety of volatile and water-soluble molecules. In the aroma-rich soil environment, the infective stages of animal- and plant-parasitic nematodes need to be able to detect diagnostic host-specific odours to enable them to locate and infect appropriate hosts. Unlike free-living nematodes such as C. elegans which feed on a wide range of bacterial species (Andrew & Nicholas, 1976; Balan, 1985) and probably also feed on filaments of fungal mycelium, fungal spores and yeast (Balanova & Balan, 1991), insect-parasitic nematodes must fine tune their chemosensory repertoire to respond more precisely to host-specific cues. We have investigated the chemotactic responses of H. bacteriophora to a variety of volatile and water-soluble chemicals, among them many compounds that are known to be highly attractive or highly repellent to C. elegans. Our results show that H. bacteriophora DJs respond strongly to a restricted range of volatile and water-soluble molecules. We also demonstrate that the chemotactic repertoire of H. bacteriophora differs substantially from that of the free-living nematode C. elegans.
H. bacteriophora (strain HP88) were cultured in vivo at 25 °C in Galleria mellonella (wax moth) larvae using standard protocols (Woodring & Kaya, 1988). Parasitized larvae were placed on water traps (White, 1927) to collect the infective-stage nematodes. The water traps were constructed and nematodes harvested as described by O'Leary et al. (1998). To collect a mixture of parasitic juvenile stages of H. bacteriophora, G. mellonella cadavers 6–8 days post-infection were cut open in a Petri dish containing M9 buffer (Brenner, 1974) using a sterile scalpel. The nematodes which emerged from the cadaver into the M9 buffer were washed twice with distilled water and allowed to move through a nylon mesh (70 μm) to isolate living worms. To isolate young female adult nematodes, G. mellonella cadavers 5–7 days post-infection were dissected in a Petri dish containing M9 buffer and the young females were picked out using an aspirator and washed twice with distilled water.
G. mellonella were infected in a 9 cm diameter Petri dish with 1 ml of water containing H. bacteriophora DJs (1000/ml) as described by Dolan, Jones & Burnell (2002). Fifteen G. mellonella cadavers were transferred to water traps at room temperature, 10 days post-infection. The water traps were monitored daily by microscopic examination for emergence of DJs into the water. Normally, DJs were detected in the water traps 12–13 days post-infection. DJs were collected on each day of emergence and washed twice with distilled water and allowed to move through a nylon mesh (40 μm) to isolate living worms. The DJs were then allowed to settle in distilled water in a 50 ml Greiner tube. The DJs were assayed on their day of emergence from G. mellonella cadavers for their ability to chemotax to 1-heptanol. Chemotaxis assay plates were set up using 1-heptanol and M9 buffer as described below. This procedure was continued for 8 days, by which time very few DJs were observed emerging from the cadavers. Six replicate chemotaxis assays were carried out each day of DJ emergence from their hosts, and the experiment was repeated with a second batch of nematodes to analyse batch variation.
Chemotaxis assays were based on the assays developed by Ward (1973) and Bargmann, Hartweig & Horvitz (1993). The assay plates used were 9 cm diameter Petri dishes containing 25 ml of 1·2% technical agar (Oxoid), 5 mM potassium phosphate (pH 6·0), 1 mM CaCl2 and 1 mM MgSO4. Two circular marks (1 cm diameter) were made on the bottom of the plate 1·5 cm from the edge of the plate. Five μl of the chemical to be screened was placed on the agar surface over the centre of one circle and 5 μl of M9 buffer (Brenner, 1974) were placed on the agar over the centre of the second circle. Five μl of water containing 50 DJs were placed in the centre of the plate, equidistant from the M9 buffer control and chemical being screened. Petri dishes were sealed with PARAFILM®. DJs which emerged on day 3 of emergence from the insect cadavers were used for chemotaxis assays. These DJs displayed the highest chemotaxis indices when assayed against 1-heptanol, a strong attractant (Fig. 1). The chemotactic responses of parasitic-stage juveniles and adult hermaphrodites was investigated using 6 cm diameter Petri plates containing 10 ml of the agar mixture described above. Here, 2×1 cm circles were marked on the bottom of the Petri dish 1 cm from the sides. Fifty nematodes in 5 μl of water were applied to the centre of the plate equidistant from each circle. A specific chemotaxis index (Bargmann & Horvitz, 1991) was calculated as follows:
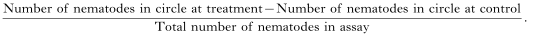
The chemotaxis index could vary from 1·0 (perfect attraction) to −1·0 (perfect repulsion). In the experiments reported here compounds with a chemotaxis index [ges ]0·2 are described as attractive, molecules with a chemotaxis index between 0 and 0·2 are considered weak attractants and molecules with a chemotaxis index [les ]−0·02 are considered to be repellent to H. bacteriophora. The optimum time-course required for nematodes to chemotax to the odorant source was observed to be ~180 min, calculated from 5 replicate experiments using 1-heptanol (unpublished data). The nematodes were allowed to move freely for 180 min at 25 °C in the dark, then the Petri dishes were placed in a freezer at −20 °C for 3 min to immobilize the nematodes. The numbers of nematodes at the treatment and control areas were counted using a binocular microscope at 25× magnification. Volatile compounds were applied to the agar plates immediately prior to the application of the nematodes (Bargmann et al. 1993). Water-soluble compounds were applied to the agar plates 120 min before the DJs were added so that a concentration gradient of the odorant could form. The assay was carried out and chemotaxis indices calculated, as described above, for the chemotaxis assay. Six replicates were set up for each treatment. All chemicals used were reagent grade from Sigma, Aldrich or Fluka.

Fig. 1. The effects of day of emergence from the host cadaver on the chemotactic behaviour of Heterorhabditis bacteriophora DJs to 1-heptanol. Each data point represents the mean±S.E. of 6 separate assays. The experiment was repeated with a second batch of nematodes to analyse batch variation (Mann–Whitney U test at 95% C.I., no significant difference between batches).
Chemotaxis towards a chemical cue could reflect either a non-specific response to organic molecules or a specific chemical recognition of the odorant molecule. To investigate these possibilities a saturation assay devised by Bargmann et al. (1993) was used. The response of DJs to 1-heptanol was saturated by evenly mixing 6 μl of 1-heptanol per 25 ml of agar mixture just prior to pouring the mixture into the plates. When 5 μl of 1-heptanol was spotted onto these plates we found that the DJs would not chemotax to a source point of 1-heptanol. However, chemotaxis to other attractants was normal on these 1-heptanol saturated plates. Similar saturation assays were repeated for the various categories of attractant identified. Petri dishes containing the attractant saturated agar were used for saturation assays immediately after the agarose had set. Fifty DJs which emerged on day 3 from the insect cadavers were used, on their day of emergence, for each assay. The plates were incubated at 25 °C in the dark and PARAFILM® used to seal the plates. The plates were examined after 3 h. Six replicates were set up for each treatment.
The bioassay described by O'Leary et al. (1998) was used to compare the host-finding capabilities of DJs from the day 3 emergence group. Briefly, 2 G. mellonella larvae were placed inside a 1 ml disposable pipette tip containing steel wool near the top. At the opposite side of the Petri dish an empty pipette tip served as a control. Each tip was equidistant from the centre. A 1 cm diameter circle was marked on the bottom of the Petri dish directly under each pipette tip. Fifty DJs were spotted in the centre of the assay plate as described for the chemotaxis assays. Two types of host cue were assayed, the first contained uninfected G. mellonella cadavers, the second assay used G. mellonella cadavers which had been infected with H. bacteriophora DJs, as described above, 4 days prior to the assay. As per the chemotaxis assay, DJs which gathered inside each circle were used to calculate the chemotaxis index. Within the insect cadaver the parasitic stages feed on their symbiont bacteria and on digested host tissues. The ability of DJs and parasitic stages to chemotax to their normal food source was tested. Four-day-old G. mellonella cadavers infected with H. bacteriophora DJs, as described above, were dissected in sterile M9 buffer and a bacteriological loop was used to transfer approximately 10 μl of the symbiont bacteria and host tissue mixture into the test circle of a chemotaxis assay plate. This assay was carried out as described above for the water-soluble chemotaxis assay.
Carbon dioxide gas (CO2) is liberated upon addition of hydrochloric acid (HCl) to sodium carbonate (Na2CO3). Five grams of Na2CO3 powder were placed in the base of a 250 ml Buchner flask. The flask was plugged with a rubber bung through which was inserted a 100 ml burette containing 15 ml of hydrochloric acid (37% v/v). Two inches of rubber tubing connected the arm of the Buchner flask to a 150 mm disposable Pasteur pipette that was heat-treated to create a 90° angle bend in the middle of the pipette. The narrow end of the Pasteur pipette opened into a chemotaxis assay plate through a 1 mm hole previously bored into the Petri dish lid. Five μl of M9 buffer (control) was pipetted on to the agar surface of the assay plate and 50 DJs were spotted onto the centre, equidistant from the M9 buffer and the CO2 entry point. As the dilute HCl was added drop-wise to the sodium carbonate, CO2 gas was liberated, which travelled through the arm of the Buchner flask into the glass pipette and on to the assay plate. All connections were made airtight with Vaseline and adhesive plaster. Carbonated water (CO2 concentration between 3·8 and 4·2% (v/v), Ballygowan Ltd, Co. Limerick, Ireland, 5 μl per assay) and dry ice were also used as sources of CO2. Dry ice is the frozen form of CO2 and it sublimes directly to CO2 gas in normal atmospheric conditions without going through a liquid stage. A 0·5 g fragment of dry ice was used in the chemotaxis assay, and set up as described above.
The chemotaxis assays described above were carried out in sealed 6 or 9 cm diameter Petri dishes, containing respectively approximately 30 ml or 50 ml of head space above the agar surface as described by Bargmann et al. (1993). Under these conditions the head space would become saturated with volatile molecules within seconds. The velocity mean value for molecules of methanol, the lightest alcohol assayed, is 445·5 m/s at 300 K and for nonanol, the heaviest alcohol we assayed, the value is 200 m/s at 300 K, as calculated from the Maxwell–Boltzmann equation. To distinguish between volatile and water-soluble effects in the chemotaxis assays, we tested the ability of H. bacteriophora to respond to a volatile cue that was not allowed to mix directly with the agar surface. A heat-sealed plastic 10 μl pipette tip (0·1 cm3) was inserted into the agar and extended approximately 2 mm above the agar surface. Five μl of the chemical being screened was pipetted into the plastic tip and 5 μl of M9 buffer control was also applied onto the plate. As described for the chemotaxis assays, 2×1 cm circles were marked on the under-surface of the Petri dish beneath both control and chemical being tested. Fifty DJs were used in each assay. They were applied equidistant from control and the pipette tip containing the volatile chemical immediately after the application of the chemical being tested. The assay was allowed to proceed for 1 h, although DJ movement was observed every 10 min. The number of nematodes which were present inside the circles after 1 h were used to calculate the chemotaxis indices.
Data are presented as the mean±S.E. A Mann–Whitney U test was used for data comparisons. Analysis of variance (ANOVA) was performed on data at a significance level of α[les ]0·05 and paired t-tests were carried out for pair-wise comparisons at 99% confidence intervals.
1-Heptanol, an attractive volatile to H. bacteriophora DJs (Table 1), was used to examine the effect of day of emergence of the DJs from the insect cadaver on their chemotactic responses. DJs which emerged from G. mellonella cadavers on the third day of emergence displayed the highest chemotaxis indices (Fig. 1). Thus, unless otherwise stated, freshly emerged DJs emerging on day 3 from G. mellonella cadavers were used in all further experiments. This experiment was repeated twice to analyse batch variation and the null hypothesis that the replicate batches were equal could not be rejected at α=0·05, Mann–Whitney U test (C.I.=95%).

The chemotactic responses of H. bacteriophora DJs to a variety of volatile and water-soluble compounds are presented in Table 1. Many of the selected compounds had previously been shown to be either highly attractive or highly repellent to adult C. elegans (Ward, 1973; Culotti & Russell, 1978; Bargmann et al. 1993; Bargmann & Mori, 1997). Our data show that H. bacteriophora DJs were unresponsive to several compounds which C. elegans finds highly attractive, namely the alcohols isobutanol and isoamyl alcohol, the ketones acetone, 2-butanone, 2-pentanone, 2-hexanone, 2-heptanone and diacetyl, the esters isoamyl acetate and ethyl acetate, the aldehydes benzaldehyde and valeraldehyde and also diethyl ether and L-cysteine. L-lysine and D-biotin, water-soluble compounds which are attractive to C. elegans, were repellent to H. bacteriophora DJs whereas the water-soluble compounds copper sulphate and zinc sulphate which are repellent to C. elegans were neutral to H. bacteripohora. We also found that, like C. elegans, H. bacteriophora DJs are attracted to a range of pyrazines and thiazoles. However, both nematode species find some pyrazines and thiazoles repellent.
A significant finding was that, whereas C. elegans finds long-chain alcohols (e.g. 1-heptanol, 1-octanol) repellent and short-chain acids attractive (Bargmann et al. 1993), the reverse is true for H. bacteriophora. The chemotaxis indices of H. bacteriophora DJs to a series of straight-chain alcohols are presented in Fig. 2, from which it can be seen that straight-chain alcohols containing 6–9 carbon atoms in a row are several-fold more attractive to H. bacteriophora DJs than are alcohols containing a chain of 2–5 carbons. Unlike C. elegans, whose positive chemotactic response was maintained over a broad range of concentrations for the straight-chain alcohols 1-butanol, 1-pentanol and 1-hexanol, the positive chemotactic response of H. bacteriophora DJs to the attractants 1-hexanol, 1-heptanol, 1-octanol and 1-nonanol was substantially reduced by a 1[ratio ]50 dilution, and in the case of 1-pentanol and 1-hexanol, dilute solutions are slightly repellent to H. bacteriophora DJs. An investigation of the chemotaxis indices of H. bacteriophora DJs to isomers of 1-nonanol and 1-heptanol indicated that as the position of the hydroxyl group moved further from the first carbon the chemotactic index decreased (Fig. 3A and B). Thus small changes in the length of the carbon chain (Fig. 2) and the positioning of the hydroxyl group can greatly affect the chemo-attractiveness of alcohols to H. bacteriophora DJs. The data obtained for the organic acids caproic (hexanoic) acid and caprylic (octanoic) acid also support these observations, where an increase from the chemotaxis index of 0·28 to 0·49 is observed in the lengthening of the carbon chain from 6 carbons (hexanoic acid) to 8 carbons (octanoic acid). Also, like the alcohols, the positive chemotactic responses of DJs to caprylic and caproic acids, are substantially decreased with 1[ratio ]50 and 1[ratio ]100 dilutions. However, the substitution of the –OH group in the alcohols by the –COOH group of the acids reduce the attractiveness of these organic acids to H. bacteriophora DJs.

Fig. 2. The chemotactic responses of Heterorhabditis bacteriophora DJs to straight-chain alcohols. Dilutions were made in water. Each data point represents the mean±S.E. of 6 assays. For each data point means followed by the same letter are not significantly different (ANOVA P[les ]0·05).

Fig. 3. The chemotactic response of Heterorhabditis bacteriophora DJs to isomers of 1-heptanol (A) and 1-nonanol (B). Controls (black bars) were set-up using M9 buffer instead of alcohol. Each bar represents the mean of 6 replicates±S.E. For each data point means followed by the same letter are not significantly different (ANOVA P[les ]0·05).
Thiazoles and pyrazines are strongly odorant aromatic compounds which are commonly used in the beverage, food and fragrance industries. Six of the compounds tested were attractive to H. bacteriophora DJs, with the compound 4,5-dimethyl thiazole having a high chemotaxis index, comparable to that of 1-heptanol (Fig. 4). H. bacteriophora DJs displayed a positive chemotactic response to CO2 when 3 different assays were used. The strongest response was observed when a point source of gaseous CO2 was provided to the assay plates (chemotaxis index, 0·43). Subliming dry ice was also attractive to the DJs (chemotaxis index, 0·28), while a weaker response was detected towards carbonated water (chemotaxis index, 0·12).

Fig. 4. Chemotactic responses of Heterorhabditis bacteriophora DJs to various thiazoles and pyrazines. Each data point represents the mean±S.E. of 6 assays. For each data point means followed by the same letter are not significantly different (ANOVA P[les ]0·05).
Larvae of the greater wax-moth G. mellonella provided a source of attraction for H. bacteriophora DJs. G. mellonella cadavers 4 days post-infection by H. bacteriophora DJs elicited statistically similar chemotactic responses to those of uninfected G. mellonella larvae (paired t-test P[les ]0·01). Uninfected cadavers have a chemotaxis index of 0·436, and infected cadavers show a chemotaxis index of 0·458. However, these chemotaxis indices obtained were not as high as those obtained for some individual volatile chemicals.
Saturation assays (Bargmann et al. 1993) were used to investigate whether chemotaxis by H. bacteriophora DJs to chemical cues resulted from a non-specific response to organic molecules or were a specific chemical recognition of particular odorants. When 5 μl of 1-heptanol were spotted onto the heptanol saturated plates we found that the DJs did not chemotax to a source point of heptanol. When 4,5-dimethylthiazole was spotted onto 1-heptanol saturated plates H. bacteriophora DJs could still chemotax to this source point (Fig. 5). This result was also found in reciprocal assays – when 1-heptanol was spotted onto 4,5-dimethylthiazole saturated assay plates (Fig. 5) the DJs still chemotaxed to the point source of 1-heptanol. The chemotactic response of H. bacteriophora DJs towards a source point of CO2 was normal when tested on either 1-heptanol or 4,5-dimethylthiazole saturated agars. Thus, these saturation assays provide evidence for the existence of at least 3 classes of odorant binding chemoreceptors in H. bacteriophora DJs.

Fig. 5. Chemotaxis indices representing the saturation of the odorant response of Heterorhabditis bacteriophora DJs with high concentratrions of 1-heptanol and 4,5-dimethylthiazole. The odorant which was spotted as a point source onto the 1-heptanol- or thiazole-saturated plate is denoted on the x-axis. Each bar represents the mean±S.E. of 6 replicates. Statistical analysis was performed within each group. For each within group data point means followed by the same letter are not significantly different (paired t-test P[les ]0·01).
All stages of H. bacteriophora except the DJs occur in a nutrient-rich environment inside the insect cadaver and do not have to forage for food. The chemotactic responses of a mixed population of parasitic juveniles of H. bacteriophora nematodes to representatives of the 3 main types of molecule to which DJs of H. bacteriophora are strongly attracted (alcohol, thiazole and organic acid) were compared to those of H. bacteriophora DJs (Fig. 6). This experiment showed that the chemotactic responses of H. bacteriophora parasitic stages are severely reduced relative to those of the DJ stage. Similar chemotactic responses were obtained from young hermaphrodite adult stages. The chemotactic responses of the parasitic and DJ stages to a mixture of symbiont bacteria and digested host tissue both displayed a weak response (Fig. 6). In this experiment we also tested parasitic-stage juveniles of H. bacteriophora for their chemotactic response to diacetyl, a bacterial metabolite which is a very strong attractant for C. elegans and is the ligand for the C. elegans ODR-10 receptor (Sengupta, Chou & Bargmann, 1996). Neither the H. bacteriophora parasitic stages nor DJs find diacetyl attractive, providing further evidence that H. bacteriophora responds to a different range of odorants than C. elegans.

Fig. 6. Comparison of the chemotactic responses of parasitic juvenile stages, infective-stage DJs and young adult hermaphrodites of Heterorhabditis bacteriophora to 4 odorant molecules and an infected cadaver exudate. Each data point represents the mean±S.E. of 6 independent assays. Statistical analysis was performed within each group. For each data point means followed by the same letter means are not significantly different (paired t-test P[les ]0·01).
A large majority of the volatile chemicals investigated in this study are also water soluble. We tested the effect on the chemotaxis index of impeding the solubilization of these odorants on the agar surface by putting the volatile chemical being tested into a heat-sealed 10 μl pipette tip which was inserted into the layer of agar, thereby blocking direct contact between the agar and chemical. We tested 4 attractants using the volatile chemotaxis assay, and in each case a large reduction in chemotaxis index was observed (Fig. 7). In the case of 4,5-dimethylthiazole, we observed a reduction in the chemotaxis index from 0·82 to 0·145. With heptanol we detected a drop in chemotaxis index from 0·796 to 0·272. Caprylic acid was also tested and displayed a decrease in chemotaxis index from 0·49 to 0·03. For each of the 4 compounds tested, the mean difference in chemotaxis index between the chemotaxis and volatile assays was statistically significant (paired t-tests P[les ]0·01).

Fig. 7. Chemotaxis indices of Heterorhabditis bacteriophora to 4 attractants in volatile and chemotaxis assays. Each bar represents the mean±S.E. of 6 replicates. Statistical analysis was performed within each group. The mean difference in chemotaxis index between the chemotaxis and volatile assays was statistically significant for each of the compound tested (paired t-tests P[les ]0·01).
H. bacteriophora DJs live at an air–water interface in the soil environment where they are exposed to water-soluble and volatile chemicals. These infective stages must be able to detect and interpret the olfactory cues provided by these molecules to locate suitable insect hosts. H. bacteriophora DJs are highly motile and actively seek out suitable hosts in the soil using host-specific cues. This kind of host-seeking strategy has been described as cruise foraging (Lewis, Gaugler & Harrison, 1992; Lewis, Glazer & Gaugler, 1996). Some species of Heterorhabditis and Steinernema use an ambush foraging strategy in which they tend to remain stationary but, by raising most of their bodies off the substrate in a behavioural activity known as nictation, they endeavour to attach themselves to passing insect hosts. Lewis (2002) has reviewed the literature on foraging and host recognition in Heterorhabditis and Steinernema DJs. He proposes that ambusher nematodes respond to host cues in a hierarchical order, with host volatile cues only becoming important after the DJ had made contact with the insect cuticle, whereas remote volatile cues are more important for cruise forager nematodes.
The paired amphids located on either side of a nematode's mouth are its primary chemosensory and thermosensory organs. The functions of each of the 12 amphidial neurons in C. elegans have been identified by laser ablation studies (Bargmann & Horvitz, 1991; Bargmann et al. 1993; Bargmann & Mori, 1997): 8 neurons which have cilia exposed to the environment through the amphid pore detect water-soluble chemicals; 3 neurons which are indirectly exposed to the external environment detect volatile odorants and a single enclosed neuron detects thermal cues. Ashton, Li & Schad (1999) have found that the positions of the amphidial neuronal cell bodies in several vertebrate nematode parasites are very similar to the positions of these neuronal cell bodies in C. elegans and they have also found that functional homologies have been conserved between certain amphidial neurons in Strongyloides stercoralis and C. elegans. In C. elegans, insects and vertebrates odour molecules are sensed by large families of G protein coupled receptors (Buck & Axel, 1991; Sengupta et al. 1996; Clyne et al. 1999; Vosshall et al. 1999). In contrast to vertebrates, each chemosensory neuron in C. elegans recognizes a variety of chemicals (Bargmann & Horvitz, 1991) and expresses multiple receptor genes (Troemel et al. 1995).
C. elegans has been shown to respond to a very wide spectrum of chemicals varying from alcohols to aromatic compounds, among them many metabolites of bacterial metabolism. We observe a similar but more restricted pattern in Heterorhabditis, which also responds chemotactically to alcohols and some aromatic compounds as well as host metabolites such as uric acid and CO2. The most notable difference in the chemotactic responses of these two nematodes is that H. bacteriophora DJs are unresponsive to a large number of compounds which C. elegans finds highly attractive. The latter compounds are typical by-products of bacterial metabolism and include aldehydes, esters, ketones and short-chain alcohols. Chemotaxis experiments and saturation studies have identified 7 different classes of volatile molecules to which C. elegans shows a positive chemotactic response (Bargmann et al. 1993). When H. bacteriophora DJs were tested with a similar panel of odorants, our data indicate that just 3 classes of molecules were recognized – 1-heptanol, 4,5-dimethylthiazole and CO2. Most of the chemicals which we assayed have both volatile and water-soluble properties. In the closed Petri dish system used for these chemotaxis assays, the head space above the agar would become saturated with volatile molecules within seconds. When the solublilization of selected odorants on the agar surface was impeded, a large reduction in chemotaxis index was observed. These data indicate that the DJs are chemotaxing along a water-soluble gradient of the tested odorants. Dilution experiments indicate that the response to individual compounds is concentration specific. Each compound has a characteristic vapour pressure and solubility and this will influence the characteristics and concentration of the odorant in the gradient along which the nematodes are chemotaxing.
The chemotactic responses of C. elegans juveniles and adults, including the DJ stages, are similar (Ward, 1973) but investigations on C. elegans chemotaxis are normally carried out using adult nematodes. Where the transition from larval to adult life is associated with morphological transformation and/or with a change in food utilization, then the olfactory preferences of the adult and larval stages may differ (Dubin et al. 1995; Shaver et al. 1998; Consoulas et al. 2000). H. bacteriophora DJs rely on olfactory cues for host finding whereas the parasitic stages of H. bacteriophora are confined within the host cadaver, in a nutrient-rich broth of symbiotic bacteria and digested host tissues and do not have to forage for food. Our data indicate that the parasitic stages of H. bacteriophora show a very weak chemotactic response to 3 classes of molecules that DJs find highly attractive (1-heptanol, 4,5-dimethylthiazole and caprylic acid). Neither adults nor DJs of H. bacteriophora were attracted to diacetyl, an odor-intense by-product of bacterial metabolism which C. elegans finds highly attractive. Surprisingly, the chemotactic response of the parasitic and DJ stages of H. bacteriophora to a mixture of symbiotic bacteria and digested host tissue is very weak. Since an acute chemotactic response to their food source is not required by the parasitic stages of H. bacteriophora which do not need to forage for food, it appears that this sensory modality has been down-regulated in these stages.
Our data show that H. bacteriophora DJs are attracted to host related stimuli (CO2 and uric acid) and to long-chain alcohols and various thiazoles. They are not attracted to the volatile by-products of bacterial metabolism and are only very weakly attracted to volatile or water-soluble products released from their own bacterial symbiont or from digested insect host tissues. Previous workers have shown that various Steinernema species are attracted to host-released stimuli such as CO2 (Gaugler et al. 1980), insect faecal products (Schmidt & All, 1978), insect gut contents (Grewal, Gaugler & Lewis, 1993) and insect plasma (Khlibsuwan, Ishibashi & Kondo, 1992). Carbon dioxide gradients are very important in host finding in other nematode parasites (Robinson, 1995; Sciacca et al. 2002). Our data also indicate that H. bacteriophora DJs are attracted to CO2. The chemotaxis index of H. bacteriophora DJs towards CO2 (0·43) was similar to that towards G. mellonella (0·45); however, these chemotaxis indices were substantially lower than those obtained for certain volatile chemicals such as 1-heptanol (0·80), 1-nonanol (0·72) or dimethylthiazole (0·82).
Van Tol et al. (2001) have shown that plant roots damaged by vine weevil larvae release volatile chemicals that are attractive to Heterorhabditis megidis. The chemical nature of these volatiles has not been determined. However, it is known that plants damaged by herbivore feeding release a variety of volatile compounds including alcohols (principally hexanol, but also octanol and nonanol), carboxylic acids and terpenoids, among others (Geervliet et al. 1997; Bate et al. 1998; Dicke, 1999). Several carnivorous arthropods and insect parasitoids are known to exploit these plant-provided cues to locate their insect hosts (Han & Chen, 2002; Meiners, Wackers & Lewis, 2002). The alcohols 1-heptanol and 1-hexanol and the primary aldehydes nonanal and hexanal were found, using electroantennograms, to induce strong olfactory responses in Podisus sp., predatory bugs of insects (Sant'Ana & Dickens, 1998). 1-Heptanol, 1-hexanol and 1-nonanol were also among the chemicals which elicited an olfactory response in the whip spider, Phrynus parvulus an insect predator (Hebets & Chapman, 2000). While C. elegans finds long-chain alcohols (e.g. 1-heptanol and 1-octanol) repellent and short-chain alcohols highly attractive, H. bacteriophora DJs are strongly attracted to 1-hexanol, 1-heptanol, 1-octanol and 1-nonanol and find the short-chain alcohols ethanol 1-propanol and 1-butanol to be only slightly attractive. It may be possible that, like carnivorous arthropods and insect parasitoids, H. bacteriophora DJs use the longer chain alcohols as chemical cues to guide them to insect-damaged plants. This hypothesis could be further tested by investigating the chemoattraction to H. bacteriophora DJs of other molecules which are released by insect damaged plant tissues.
Data from phylogenetic analyses suggest that Heterorhabditis may have evolved from bacterial feeding nematodes which acquired a necromenic life-style and subsequently developed a symbiotic association with an entomopathogenic bacterium (Sudhaus, 1993; Blaxter et al. 1998). Our data indicate that associated with the adoption of a parasitic mode of life by Heterorhabditis there was an adaptive change in chemotactic behaviour. This change has resulted in a decreased sensitivity to volatile by-products of bacterial metabolism and an increased sensitivity towards long-chain alcohols and other insect-specific molecules, and possibly also to herbivore-induced plant volatiles.
This work was funded by the Irish Higher Education Authority Programme for Research in Third Level.
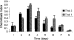
Fig. 1. The effects of day of emergence from the host cadaver on the chemotactic behaviour of Heterorhabditis bacteriophora DJs to 1-heptanol. Each data point represents the mean±S.E. of 6 separate assays. The experiment was repeated with a second batch of nematodes to analyse batch variation (Mann–Whitney U test at 95% C.I., no significant difference between batches).

Table 1. The volatile and water-soluble compounds with which Heterorhabditis bacteriophora DJs were screened for chemotaxis and the responses which were obtained

Fig. 2. The chemotactic responses of Heterorhabditis bacteriophora DJs to straight-chain alcohols. Dilutions were made in water. Each data point represents the mean±S.E. of 6 assays. For each data point means followed by the same letter are not significantly different (ANOVA P[les ]0·05).

Fig. 3. The chemotactic response of Heterorhabditis bacteriophora DJs to isomers of 1-heptanol (A) and 1-nonanol (B). Controls (black bars) were set-up using M9 buffer instead of alcohol. Each bar represents the mean of 6 replicates±S.E. For each data point means followed by the same letter are not significantly different (ANOVA P[les ]0·05).

Fig. 4. Chemotactic responses of Heterorhabditis bacteriophora DJs to various thiazoles and pyrazines. Each data point represents the mean±S.E. of 6 assays. For each data point means followed by the same letter are not significantly different (ANOVA P[les ]0·05).

Fig. 5. Chemotaxis indices representing the saturation of the odorant response of Heterorhabditis bacteriophora DJs with high concentratrions of 1-heptanol and 4,5-dimethylthiazole. The odorant which was spotted as a point source onto the 1-heptanol- or thiazole-saturated plate is denoted on the x-axis. Each bar represents the mean±S.E. of 6 replicates. Statistical analysis was performed within each group. For each within group data point means followed by the same letter are not significantly different (paired t-test P[les ]0·01).

Fig. 6. Comparison of the chemotactic responses of parasitic juvenile stages, infective-stage DJs and young adult hermaphrodites of Heterorhabditis bacteriophora to 4 odorant molecules and an infected cadaver exudate. Each data point represents the mean±S.E. of 6 independent assays. Statistical analysis was performed within each group. For each data point means followed by the same letter means are not significantly different (paired t-test P[les ]0·01).

Fig. 7. Chemotaxis indices of Heterorhabditis bacteriophora to 4 attractants in volatile and chemotaxis assays. Each bar represents the mean±S.E. of 6 replicates. Statistical analysis was performed within each group. The mean difference in chemotaxis index between the chemotaxis and volatile assays was statistically significant for each of the compound tested (paired t-tests P[les ]0·01).