INTRODUCTION
Parasites may influence ecosystem function by shaping host populations, altering interspecific competition and influencing energy flow (Hudson et al. Reference Hudson, Dobson and Lafferty2006). Recent studies suggest that approximately 75% of the links in food webs involve parasites of free-living organisms (Dobson et al. Reference Dobson, Lafferty, Kuris, Hechinger and Jetz2008), making them the ‘ultimate missing link’ in food web studies (Lafferty et al. Reference Lafferty, Allesina, Artim, Briggs, De Leo, Dobson, Dunne, Johnson, Kuris, Marcogliese, Martinez, Memmott, Marquet, Mordecai, Pascual, Poulin and Thieltges2008). Among these known parasites, approximately 70 000 species live on the external surface of their host. These ectoparasites are distributed among 5 animal phyla and infest vertebrate and invertebrate hosts in terrestrial, freshwater and marine ecosystems (Poulin, Reference Poulin2007). Among arthropod ectoparasites about 14 000 species from 400 genera feed largely or exclusively on vertebrate blood (Graca-Souza et al. Reference Graca-Souza, Maya-Monteiro, Paiva-Silva, Braz, Paes, Sorgine, Oliveira and Oliveira2006). The best known of these haematophagous arthropods include mosquitoes, ticks and fleas that may ingest 10–100 times their initial weight in a single blood meal (Friend et al. Reference Friend, Choy and Cartwright1965; Romoser, Reference Romoser, Beaty and Marquardt1996). Many may also act as vectors of smaller parasites such as protozoa, bacteria, viruses, cestodes and nematodes. These combined effects can have major impacts on the growth, survivorship and reproductive output of their hosts and therefore have a great impact on populations and ecological communities (Hatcher and Dunn, Reference Hatcher and Dunn2011).
One of the most widely studied topics in host–parasite ecology is the degree to which hosts vary in their susceptibility to particular parasites (McCoy et al. Reference McCoy, Boulinier, Tirard and Mickalakis2001; Fast et al. Reference Fast, Ross, Mustafa, Simes, Johnson and Burka2002; Giorgi et al. Reference Giorgi, Arlettaz, Guillaume, Nussle, Ossola, Vogel and Christie2004; Bandilla et al. Reference Bandilla, Hakalahti, Hudson and Valtonen2005; Jones et al. Reference Jones, Nagel, Hughes and Grutter2007; Nagel and Grutter, Reference Nagel and Grutter2007; Walker et al. Reference Walker, Harris, van der Velde and Wendelaar Bonga2008; Seneviratne et al. Reference Seneviratne, Fernando and Udagama-Randeniya2009). Factors that may influence host susceptibility to parasites include host specificity of the parasite, spatial and temporal activities of the host and host defences (Jaenike, Reference Jaenike1990; Combes, Reference Combes1997). The degree of host specialization of a parasite will be influenced by variation in the fitness benefits (difference between benefits and costs) accrued by feeding on particular hosts (Tschirren et al. Reference Tschirren, Bischoff, Saladin and Richner2007), which are expected to be a function of the availability and predictability of hosts (McCoy et al. Reference McCoy, Boulinier, Tirard and Mickalakis2001; Poulin et al. Reference Poulin, Krasnov and Shenbrot2008), their ability to initiate and sustain an immune response against the parasite (Jones, Reference Jones2001; Kubanek et al. Reference Kubanek, Whalen, Engel, Kelly, Henkel, Fenical and Pawlik2002) and the quality of the meal they provide (Christe et al. Reference Christe, Giorgi, Vogel and Arlettaz2003; Nagel and Grutter, Reference Nagel and Grutter2007). Even for generalist parasites, potentially suitable hosts may vary in morphological, immunological or behavioural traits that influence their value. For example, some individual hosts in poorer condition may have less energy to devote to costly defence mechanisms (Martin et al. Reference Martin, Han, Lewittes, Kuhlman, Klasing and Wikelski2006), leaving parasites to balance between the nutritive resources and the immunity of the host.
Understanding variation in the susceptibility of hosts to ectoparasites requires disentangling the effects of exposure of hosts due to differences in encounter rates and other factors that influence the probability of infestation. The majority of published studies on components of host susceptibility by terrestrial (Clark et al. Reference Clark, Oliver, Grego, James, Durden and Banks2001; McCoy et al. Reference McCoy, Boulinier, Tirard and Mickalakis2001; Giorgi et al. Reference Giorgi, Arlettaz, Guillaume, Nussle, Ossola, Vogel and Christie2004; Seneviratne et al. Reference Seneviratne, Fernando and Udagama-Randeniya2009; Stapp et al. Reference Stapp, Salkeld, Franlin, Kraft, Tripp, Antolin and Gage2009) and aquatic ectoparasites (e.g. Fast et al. Reference Fast, Ross, Mustafa, Simes, Johnson and Burka2002; Bandilla et al. Reference Bandilla, Hakalahti, Hudson and Valtonen2005; Jones et al. Reference Jones, Nagel, Hughes and Grutter2007; Nagel and Grutter, Reference Nagel and Grutter2007; Walker et al. Reference Walker, Harris, van der Velde and Wendelaar Bonga2008; Hadfield et al. Reference Hadfield, Smit and Avenant-Oledwage2009) are based solely on either cultured, laboratory-reared parasites or field observations of parasites found on wild-caught hosts. While these studies are instructive, they do not present the full suite of potential hosts, or control for the effects of host habitat utilization, and often fail to control for temporal variation in the activity of ectoparasites.
Research on host–ectoparasite interactions in the ocean lags well behind research on terrestrial and even freshwater systems. Like their terrestrial counterparts, marine fishes are known to harbour multiple ectoparasites that may have negative effects on their growth, survival and reproduction (Adlard and Lester, Reference Adlard and Lester1994; Bunkley-Williams and William, Reference Bunkley-Williams and Williams1998) including about 450 of the approximately 4000 species of known parasitic isopods (Ravichandran et al. Reference Ravichandran, Rameshkumar and Balasubramanian2010). Among these, isopods in the family Gnathiidae are perhaps the best studied. Gnathiid isopods feed on the blood, lymph or mucus of host fishes (Smit and Davies, Reference Smit and Davies2004) and have been referred to as micropredators; natural enemies attacking more than one victim in their life, but not necessarily killing it (Lafferty and Kuris, Reference Lafferty and Kuris2002; Jones et al. Reference Jones, Nagel, Hughes and Grutter2007; Grutter et al. Reference Grutter, Pickering, McCallum and McCormick2008). Gnathiid isopods are unique in their life history compared with most parasitic marine crustaceans in that they are only parasitic during their three larval stages (protelean parasitism), and the adults do not feed (Monod, Reference Monod1926; Smit and Davies, Reference Smit and Davies2004; Tanaka, Reference Tanaka2007). Larval gnathiids emerge from the substratum and use piercing mouthparts to penetrate host fish skin and gills, enabling them to feed on blood and tissue, then return to the substratum to digest the meal and moult into the next stage (Monod, Reference Monod1926; Tanaka and Aoki, Reference Tanaka, Aoki, Watanabe and Fusetani1998; Smit and Davies, Reference Smit and Davies2004). These parasitic larvae can lower the hosts’ blood volume (Jones and Grutter, Reference Jones and Grutter2005), cause serious tissue damage and can even kill the host fish (Paperna and Por, Reference Paperna and Por1977; Bunkley-Williams and William, Reference Bunkley-Williams and Williams1998; Penfold et al. Reference Penfold, Grutter, Kuris, McCormick and Jones2008).
Although gnathiids can be found in the benthos from the Antarctic to the Arctic, they have been reported mostly in warm temperate and tropical waters as one of the most common ectoparasites of coral reef fishes (Grutter, Reference Grutter1994; Grutter and Poulin, Reference Grutter and Poulin1998) and they play a major role in cleaning symbiosis as the main food source of cleaners (Côté, Reference Côté2000; Grutter, Reference Grutter2002). They also appear to transmit haemogregarine blood parasites to host fishes (Davies et al. Reference Davies, Smit, Seddon and Wertheim2004; Smit et al. Reference Smit, Grutter, Adlard and Davies2006). Thus, data on the susceptibility of potential host species to gnathiid isopod infestation are essential for future studies on the role of these parasites in coral reef trophic dynamics and transmission of bloodborne parasites.
In coral reef habitats where biodiversity of fishes is high, gnathiids indeed have the opportunity to specialize on hosts. Because they have been found on a wide range of host fishes (Jones et al. Reference Jones, Nagel, Hughes and Grutter2007; Nagel and Grutter, Reference Nagel and Grutter2007; Tanaka, Reference Tanaka2007; Soares et al. Reference Soares, Bshary and Côté2008) and have rather brief interactions with their hosts, they have been regarded as generalist micropredators that may exhibit host preferences (Jones et al. Reference Jones, Nagel, Hughes and Grutter2007; Nagel and Grutter, Reference Nagel and Grutter2007). However, because the parasitic larvae are difficult to identify, it is unclear whether gnathiids have a wide host range within species or consist of a large number of specialist species.
The diversity of host and parasite species in the Caribbean is low compared with the tropical Indo-Pacific fauna from which it is derived (Rhode and Stauffer, Reference Rhode and Stauffer2005). Thus far, only three species of gnathiids have been described from the north-eastern Caribbean, and in recent studies of gnathiid–host interactions in the eastern Caribbean and Bahamas, only one gnathiid species (Gnathia marleyi) has been found in shallow reef habitats (Sikkel et al. Reference Sikkel, Ziemba, Sears and Wheeler2009, Reference Sikkel, Sears, Weldon and Tuttle2011; Farquharson et al. Reference Farquharson, Smit and Sikkel2012). This presents an opportunity to conduct experimental field studies, investigating host susceptibility to a single ectoparasite species. This study investigates variation in the susceptibility of some common Caribbean reef fishes to infestation by gnathiid isopods in these shallow reef habitats. To our knowledge this is the only manipulative experimental field study to examine variation in susceptibility of multiple host species to an ectoparasite in any terrestrial or aquatic system.
MATERIALS AND METHODS
Study sites
This study was conducted at 3 shallow (<6 m depth) reef sites within Great Lameshur Bay, St. John, United States Virgin Islands (18°19′N, 65°44′W) during the late spring and summer (May–August) of 2010 and 2011. Bottom topography at the 3 sites included encrusting hard granite, patches of scleractinian coral and large amounts of crustose coralline algae and algal turf (Edmunds, Reference Edmunds2000, Reference Edmunds2002). The 3 sites were separated by at least 300 m that included the west and east margins of the bay (‘West’ and ‘East’ Lameshur, respectively) and a small inlet south of East Lameshur (‘Donkey Bight’). We chose Great Lameshur Bay because it has high densities of gnathiids (Sikkel et al. Reference Sikkel, Schaumburg and Mathenea2006, Reference Sikkel, Ziemba, Sears and Wheeler2009, Reference Sikkel, Sears, Weldon and Tuttle2011) and thus far only one species of gnathiid (G. marleyi; Farquharson et al. Reference Farquharson, Smit and Sikkel2012) has been found there. We chose the 3 sites within Great Lameshur Bay because they are easy to access, differ in relative abundance of substratum types and fish species, and collectively provide a good representation of shallow water reef habitat in the Virgin Islands. Supplemental data were collected in 2012 at another site within Great Lameshur Bay located southwest of Donkey Bight (‘Tektite’: see below).
Sampling protocol
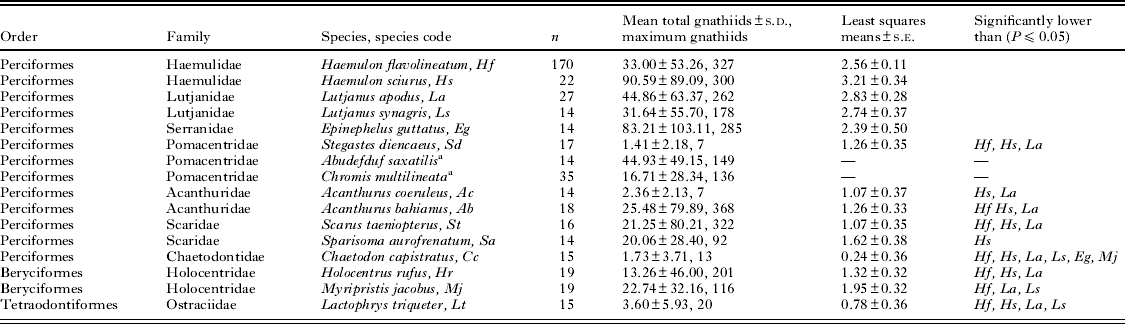
To quantify host susceptibility to parasitism by gnathiids (G. marleyi), 16 fish species from 9 families and 3 orders were used in caging experiments (Table 1). These species were chosen because they are common and representative of the diversity of potential host fishes available at our study sites. Because it was not possible to collect and deploy all comparison species simultaneously, each species was compared sequentially with French grunts (Haemulon flavolineatum) that served as a standard. French grunts were used as a standard because they are abundant, easy to catch, and have been shown to be susceptible to infestation by G. marleyi (Sikkel et al. Reference Sikkel, Sears, Weldon and Tuttle2011). The French grunt standards used in each trial allowed us to account for spatiotemporal variation in availability and activity of gnathiids during each trial (see Statistical analyses below). At each of the 3 sites, ⩾5 individuals from one comparison species were compared to an equal number of the French grunt standard for a total of n⩾15 for each host species. This was the minimal sample size deemed necessary for sufficient statistical power. For 9 species the total sample size exceeded 15 because the additional individuals were used for another study, and for 5 species the sample size was 14 because of a missing sample (Table 1).
Table 1. Infestation of gnathiids on caged Caribbean reef fishes retrieved at dawn

a Species not compared to the H. flavolineatum standard.
Fishes used in our experiment were handled and maintained in accordance with the National Research Council Guide for the Care and Use of Laboratory Animals and all fishes were treated in the same manner. Fishes were collected either during the day by free divers using modified casting nets, or at night using SCUBA, underwater lights and aquarium nets. Fishes were held for 24–48 h in 1500 L tanks containing running seawater before placing them in individual mesh cages and deploying them on the reef. This time in the holding tanks allowed us to ensure that they were in good condition before being used in the experiment and to allow time for any gnathiids that might be on them to dislodge. The tanks were cleaned and flushed every 3 days to avoid any build-up of gnathiids. Periodic examination of filtrate from the input source over a 5-year period has revealed no gnathiids, and no gnathiids have been found on fish removed from the holding tanks during this period. The French grunt standards were collected at the same time and held in the holding tanks for the same duration as the test species with which they were matched. The mesh cages used to deploy fishes on the reefs were similar to those used in previous studies at these sites (Sikkel et al. Reference Sikkel, Schaumburg and Mathenea2006, Reference Sikkel, Ziemba, Sears and Wheeler2009). Each cage was constructed using black plastic mesh (1·5 cm mesh width) ranging in length from 50–100 cm depending upon fish size. To prevent sharks from biting through the mesh, additional protective cages were constructed out of plastic lattice and were custom-fit to encase each black mesh cage. Cages were secured to the substratum with a 1·4–2·3 kg dive lead attached to the outside of each cage using plastic cable ties.
Prior to placing in cages, each fish was carefully netted from a holding tank and visually inspected to verify that no gnathiids were attached. Each fish was then individually placed in its own mesh cage. Fish cages were then placed in separate buckets containing fresh seawater (from the input spouts of the holding tanks) for transport to the deployment site. The fish cages were deployed on the reef at dusk (approximately 1900 h) 3–4 m away from any other cages on the reef. Initially all fishes were deployed on the reef for 2 different trials, the first trial was retrieved during the dawn peak in gnathiid activity (05.30–06.00 h) and the second trial retrieved during the night peak (22.30 and 23.30 h) (Sikkel et al. Reference Sikkel, Schaumburg and Mathenea2006). However, because of some mortality from holding the fishes between dawn and night trials and because the number of gnathiids retrieved at dawn was overall higher than at night (Repeated measures ANOVA: F 1,113=6·156, P=0·015) we discontinued the night retrievals.
During retrieval, surface divers lifted the cages slowly from the water and the mesh cages were then extracted from the shark protection cages and placed inside a large bucket filled with seawater to be transported to the laboratory. Fish remained in their individual buckets for approximately 3 h to allow the gnathiids to finish feeding and then dislodge from the fish. Air stones were placed in the buckets during this time to provide sufficient oxygen. Mesh cages containing fish were removed from the buckets, the fish thoroughly rinsed to remove any remaining gnathiids, and then transferred back to the holding tank to be used again the next day for the second time block or to be released at the site where they were collected (all fish were released after a maximum of 2 trials).
Gnathiids were recovered from each bucket by filtering the seawater through 55-μm plankton mesh and transferring the contents to a Petri dish with seawater. The number of gnathiids was counted using a dissecting (stereo) scope and recorded for each fish. The fork length (distance from the tip of the snout to the fork of the tail) of each fish was measured and tracings were made of the entire body of the fish (including fins) before they were released at the reefs from which they were originally collected. These tracings were then used to calculate the surface area of the fish using Image J® (Schneider et al. Reference Schneider, Rasband and Eliceiri2012). The number of gnathiids per fish was divided by the calculated surface area to determine the density of gnathiids (gnathiids per cm2) on each French grunt at every trial. The average density of gnathiids among the 5 French grunt standards was then calculated for every trial to be used in the analyses. The supplemental data collected on Chromis multilineata and Abudefduf saxatilis in 2012 was collected in the same method as above. However, individuals of these species were deployed without French grunt standards and therefore could not be compared with the other 14 species in the analyses (Table 1).
Statistical analyses
Statistical analyses were performed using SYSTAT 13.0. Variation in the number of gnathiids among host fish species was examined using an ANCOVA. To meet the assumptions of the analysis and to reduce the impact of extreme values, the dependent variable (number of gnathiids) was natural log (+1) transformed. Independent categorical variables included species (14 levels: see Table 1 for a list of the 14 comparison species) and deployment site (3 levels: 3 different reef sites). To control for possible effects of host body size on gnathiid loads (Grutter, Reference Grutter1999; Muñoz et al. Reference Muñoz, Grutter and Cribb2006), surface area was used as a covariate. The average density of gnathiids on the 5 French grunts deployed during each trial was also included as a covariate to account for spatial and temporal differences in the availability of gnathiids. There was a significant interaction between species and deployment site (F 26,350=1·81, P=0·010); however, this was attributable to differences in the magnitude and not the direction of the differences between species at the 3 sites. Thus, deployment site was removed from the model and data were pooled among deployment sites. Tukey's Honestly Significant Difference was used as a post-hoc test to compare differences in the number of gnathiids among host fish species.
RESULTS
All 16 species deployed were susceptible to infestation by gnathiids, with gnathiid loads on a single host ranging from as few as zero (at least 1 for all host fish species), to ⩾300 for Haemulon flavolineatum, Haemulon sciurus, Acanthurus bahianus and Scarus taeniopterus (Table 1). Gnathiids from all 16 host fish species were reared in the laboratory and the larval gnathiids that metamorphosed into males were identified as G. marleyi.
There was a significant effect of the average density of gnathiids on French grunt standards (F 1,378=58·190, P<0·001), host fish surface area (F 1,378=13·133, P<0·001) and host species (F 13,378= 9·315, P<0·001) on the number of gnathiids (ln x+1 transformed) on fish deployed in cages. Collectively, species from the families Haemulidae and Lutjanidae had the highest levels of gnathiid infestation among species (least-squared means 2·556±0·113 s.e. to 3·211±0·339 s.e.). Although none of these species were significantly different from each other, all other species except Epinephelus guttatus had significantly lower gnathiid loads than at least one of these species, and nearly all of the significant pairwise differences were a result of species from these families having higher gnathiid infestation than other host species (Table 1). The only exception was that Chaetodon capistratus (least-squared mean 0·243±0·361) also had significantly lower gnathiid loads than Myripristis jacobus and E. guttatus (Table 1).
DISCUSSION
At our Caribbean study sites, most diurnal reef fishes rest at night near reef structures or the reef–sand interface, while nocturnal species are actively searching for food at night. Thus, many potential hosts are available during times of peak gnathiid activity. However, among coral reef fishes generally, availability of any given species varies considerably in space and time, even in similar habitats (Holbrook et al. Reference Holbrook, Forrester and Schmitt2000; Shima et al. Reference Shima, Osenberg and St. Mary2008). In our study, trials were conducted at the same 3 reef sites within a large bay. However, each site differed in absolute and relative abundance of alternative (uncaged) host species, and some species that were common at one site were completely absent at another.
All of the 16 species examined in this study were infested by G. marleyi, which has been previously confirmed to infest 10 of the host species examined in this study, as well as 3 other host species (Farquharson et al. Reference Farquharson, Smit and Sikkel2012). This study extends the total known number of species infested by G. marleyi to 19, from 3 orders and 9 families. Among previous studies where the gnathiids infesting bony fishes were identified, the highest number of hosts recorded for a single gnathiid species is 18 (8 families and 2 orders) for Gnathia auriomaculosa from the Great Barrier Reef (Ferreira et al. Reference Ferreira, Smit, Grutter and Davies2009). However, Ota et al. (Reference Ota, Hoshino, Hirose, Tanaka and Hirose2012) reported 25 different elasmobranch hosts for Gnathia trimaculata off Japan.
While our findings suggest that G. marleyi has a broad range of suitable hosts, there was significant variation in levels of infestation among the species tested. Only 2 previous studies have attempted to quantify variation in the likelihood of infestation among specific gnathiid hosts. Laboratory host choice experiments by Nagel and Grutter (Reference Nagel and Grutter2007) found that although the gnathiid species G. auromaculosae was capable of feeding on all 6 host species they tested (from 3 families), it appeared to show a preference for wrasses (Labridae). Jones et al. (Reference Jones, Nagel, Hughes and Grutter2007) assessed apparent gnathiid host ‘specificity’ on the Great Barrier Reef using DNA analysis of gnathiids caught in light traps. They found that 4 host fish families were shared between 2 gnathiid species, suggesting gnathiids at their study sites are ‘micropredators with a preference’.
To our knowledge, the present study is the first manipulative field study investigating host susceptibility to a gnathiid isopod (or any other blood-feeding arthropod). By placing fish in cages during times of peak gnathiid activity and comparing all species to the same standard, we effectively controlled for the effects of habitat utilization by hosts and variation in gnathiid abundance on exposure to and the likelihood and intensity of host infestation by gnathiids. Because gnathiids and other ectoparasites attack the outside of hosts, larger hosts provide larger targets and may therefore be more susceptible (Grutter and Poulin, Reference Grutter and Poulin1998; Valera et al. Reference Valera, Hoi, Darolová and Kristofic2004). In our study, host surface area was a significant predictor of variation in gnathiid infestation and when its effects were statistically removed, significant differences still emerged among comparison species. In particular, grunts (Haemulidae) and snappers (Lutjanidae) experienced the highest infestation levels of gnathiids, reflecting an apparent higher level of susceptibility among members of these families compared with other species. These families are closely related phylogenetically and exhibit many similarities in anatomy, physiology and behaviour (Friedlander and Monaco, Reference Friedlander and Monaco2007; Rocha and Molina, Reference Rocha and Molina2008; Hitt et al. Reference Hitt, Pittman and Brown2011a, Reference Hitt, Pittman and Nemeth2011b).
As with other host–parasite interactions, host susceptibility to gnathiids may result from some combination of parasite preference and host defence, and a comprehensive understanding of the exploitation of different hosts by ‘generalist’ parasites such as gnathiids must go beyond host phylogeny to include examination of the functional traits of hosts (Poulin et al. Reference Poulin, Krasnov and Mouillot2011). Parasites may show a preference for hosts that provide the optimum nutrients for growth, survival and reproduction (Tschirren et al. Reference Tschirren, Bischoff, Saladin and Richner2007). For example, ectoparasitic mites have shown preference to feeding on bat hosts that provide the most nutritious blood meal (Christe et al. Reference Christe, Giorgi, Vogel and Arlettaz2003). Thus, haemulids and lutjanids may offer a more nutritional blood meal compared with other hosts. Data are needed on the reproductive success of the gnathiids recovered from these different host species to test this possibility.
As with other organisms, teleost fish can reduce their susceptibility to infestation by ectoparasites through a combination of external barriers and internal defences (Jones, Reference Jones2001). For example, mucosal epithelium may be secreted in excess to minimize the chances that an ectoparasite may penetrate the host and, in some cases, may be lethal to the ectoparasite (Harris et al. Reference Harris, Soleng and Bakke1998). However, during attachment some crustacean and monogenean ectoparasites may interfere with the host fish's ability to secrete mucus (Wells and Cone, Reference Wells and Cone1990; Nolan et al. Reference Nolan, Reilly and Wendelaar Bonga1999). Scale morphology can vary greatly in fishes and many exhibit different scale modifications. For example, some fish can easily shed scales and others may have scales modified into sharp scutes, all providing different levels of external protection (Helfman et al. Reference Helfman, Collette, Facey and Bowen2009). Fish may also initiate coagulation in response to infestation by isopod ectoparasites (Horton and Okamura, Reference Horton and Okamura2003). However, parasitic arthropods can inject anticoagulants into their victims to facilitate feeding (Stark and James, Reference Stark and James1996; Ribeiro and Francischetti, Reference Ribeiro and Francischetti2003). Manship et al. (Reference Manship, Walker, Jones and Davies2012) detected anticoagulants in the gnathiid isopod Pragnathia formica suggesting gnathiids could suppress immunological, inflammatory and haemostatic responses in host fishes during feeding. Skin toxins in the form of venom or crinotoxins are common in many teleost families (Halstead, Reference Halstead1978; Randall et al. Reference Randall, Oshima and Hoshimato1981) and may aid in deterring predators and micropredatory parasites (Kubanek et al. Reference Kubanek, Whalen, Engel, Kelly, Henkel, Fenical and Pawlik2002) and by limiting the site of parasitic attachment (Munday et al. Reference Munday, Schubert, Baggio, Jones, Caley and Grutter2003). Haemulids and lutjanids share similar scale morphology, and may lack particular innate immune responses to ectoparasites as well. Members of both families have scale-less snouts with haemulids also having scale-less lips (Kells and Carpenter, Reference Kells and Carpenter2011). Gnathiids likely take advantage of these easy to penetrate areas. We routinely witnessed blood marks on the bodies of haemulids and lutjanids, and in the cases where hosts died, there were many visible blood markings on the body from gnathiid bites.
It is important to note that even among those species that averaged low levels of susceptibility, certain individuals experienced high levels of infestation. Although we controlled for the average habitat experienced by different species, certain individuals may have been placed on a ‘hot spot’ with a particularly high number of gnathiids. Alternatively, or in addition, these individuals may have been in a poorer physical condition and therefore unable to deter gnathiid infestation. Because of the confounding effects of phylogeny and host ecology, it is difficult to assess the contribution of ontogenetic changes and selective pressures in shaping any differences in susceptibility among species. For example, nutritionally superior hosts might also be better able to invest energy in antiparasitic defence mechanisms, which in turn could reduce the parasites’ reproductive success and thus the degree of infestation of the host (Bize et al. Reference Bize, Jeanneret, Klophenstein and Roulin2008). Thus, differences in infestation could reflect differences in the nutritional state of individuals of a given species at a given site, rather than inherent differences in species per se.
All of the diurnal fish species deployed had significantly lower gnathiid infestation compared with one or more of the species from the families Haemulidae and/or Lutjanidae (which were not different from each other). In contrast to the diurnal species, haemulids and lutjanids are typically ‘off’ the reef, feeding over seagrass beds and sand patches (Friedlander and Monaco, Reference Friedlander and Monaco2007; Appledoorn et al. Reference Appledoorn, Aguilar-Perera, Bouwmeester, Dennis, Hill, Merten, Recksiek and Williams2009; Grol et al. Reference Grol, Nagelkerken, Rypel and Layman2011; Hitt et al. Reference Hitt, Pittman and Brown2011a, Reference Hitt, Pittman and Nemethb) and exposure to gnathiids during the nocturnal and crepuscular periods may be lower in these habitats (Sikkel, unpublished results). If nocturnal habitat influences exposure to gnathiids, then species that are near the reef at night may experience higher exposure to gnathiids early in their ontogeny than other species. Early exposure to infection or infestation may lead to immune defences in hosts making them better able to survive secondary infestation (Sadd and Schmid-Hempel, Reference Sadd and Schmid-Hempel2006).
In addition, or alternatively, species that are near the reef during peak gnathiid activity may be under stronger selective pressure to develop other physiological, anatomical or behavioural defences. For example, parrotfishes secrete a mucous envelope around their bodies at night that has been shown to deter gnathiid isopods (Grutter et al. Reference Grutter, Rumney, Sinclair-Taylor, Waldie and Franklin2011). Because of this, we expected to find lower infestation levels on parrotfishes in this study. However, when we inspected fish at night (with a red light) we did not observe caged parrotfishes resting in a mucous envelope. Instead, there were pieces of the mucous envelope lying in the cages indicating an unsuccessful effort to encase themselves in the envelope. Of course, even if they had succeeded in producing a cocoon, the cocoons could have been shed at dawn (just prior to retrieval) when they would ordinarily have access to cleaners. Similarly, trunkfishes (Ostraciidae) have a protective carapace-like armour of thickened scale with only the mouth, eyes, gill slits, fins and caudal peduncle exposed and they can produce a poison in the mucous secretions of their skin when stressed (Thomson, Reference Thomson1964). Other species, such as chaetodontid butterflyfishes, may rely on heavy scales.
The other nocturnal species used in this study, M. jacobus and Holocentrus rufus (Holocentridae), were also significantly less susceptible to gnathiids than haemulids and lutjanids. In contrast to the latter, they remain on or just above the reef at night and therefore may be exposed to more gnathiids. The crepuscular E. guttatus (Serranidae) was not significantly different from haemulids and lutjanids. However, they were only significantly higher than the least susceptible species, C. capistratus. Epinephelus guttatus remains on the reef at night suggesting they may have more developed defences against gnathiid infestation compared with their more highly susceptible nocturnal counterparts. In controlling for differences in habitat utilization among fishes, individually caged fish (diurnal, crepuscular and nocturnal species) deployed on the reef at night may have been prevented from using their usual antiparasite behaviours. For instance, diurnal fishes were unable to hide in the reef and nocturnal species were unable to leave the reef to avoid gnathiids. Therefore, our results reveal susceptibility to gnathiid infestation after equalizing habitat utilization.
Two other Caribbean studies have quantified the number of gnathiids (Arnal et al. Reference Arnal, Côté and Morand2001) or total ectoparasites, including gnathiids (Côté and Molloy, Reference Côté and Molloy2003) on hosts. While the objective of these studies was not to investigate host susceptibility and they did not examine all of the same species, they did find variation in gnathiid infestation among host species, with a haemulid species exhibiting significantly higher levels of infestation in the morning compared with other species (Côté and Molloy, Reference Côté and Molloy2003).
Parasites are now believed to play a major role in trophic interactions in ecological communities (Hudson et al. Reference Hudson, Dobson and Lafferty2006; Lafferty et al. Reference Lafferty, Allesina, Artim, Briggs, De Leo, Dobson, Dunne, Johnson, Kuris, Marcogliese, Martinez, Memmott, Marquet, Mordecai, Pascual, Poulin and Thieltges2008). Fish moving between habitats may transport gnathiids between them, and some of the energy derived from nocturnal feeding may be transferred to reef environments when host fish visit cleaning stations where gnathiids are eaten (Johnson et al. Reference Johnson, Dobson, Lafferty, Marcogliese, Memmott, Orlofske, Poulin and Thieltges2010). Six of the species used in our study have been observed at goby cleaning stations (Arnal et al. Reference Arnal, Côté and Morand2001) and 8 of the families used in our study have been observed at shrimp cleaning stations (Heubner and Chadwick, Reference Huebner and Chadwick2012) in the tropical western Atlantic. Host fishes with high infestations of ectoparasites have been seen visiting cleaning stations more often (Arnal et al. Reference Arnal, Côté and Morand2001) and being inspected longer (Soares et al. Reference Soares, Bshary and Côté2008) than those with lower infestation. Chromis multilineata has been reported as being the most common visitor to cleaning stations at other localities in the tropical western Atlantic (Côté and Molloy, Reference Côté and Molloy2003). Because members of this genus feed on pelagic zooplankton during the day and take refuge in the reef at night (Allen, Reference Allen1991) they indeed play an important role in pelagic-benthic trophic linkages. The fact that they appear to be highly susceptible to reef-based gnathiids and are frequent visitors to cleaning stations suggests that gnathiids may contribute heavily to these linkages. Similarly, haemulids and lutjanids play a major role in trophic connectivity among coral reef habitats (Clark et al. Reference Clark, Pittman, Caldow, Christensen, Roque, Appeldoorn and Monaco2009) by feeding in seagrass beds or sand at night and returning to the reef at dawn. Our finding, that members of these families are highly susceptible to infestation by gnathiid isopods and also frequently visit cleaning stations suggests that gnathids may also be an important component of this trophic linkage. Current studies are examining these possibilities.
ACKNOWLEDGEMENTS
We would like to thank the staff of the Virgin Islands Environmental Resource Station (VIERS) for logistical support and use of their facilities. We would also like to thank John Artim, Elizabeth Brill, Marnaya Ellis, Kiesha Gray, Nissa Hughes, Josh Lukac, Will Jenkins, Hunter Owen, Kayla Parker, Rachel Welicky, Leah Wientrub and Sarah Wilson for assisting with the collection of data and Nico Smit for assistance with identification of gnathiid species. This is publication number 88 from the University of the Virgin Islands Center for Marine and Environmental Studies and is dedicated to Reggae Ambassador Amlak Tafari.
FINANCIAL SUPPORT
This work was funded in part by Arkansas State University, The Falconwood Corporation, and the US National Science Foundation (OCE-121615, P.C. Sikkel, PI).