Introduction
Acanthamoeba species are free-living amoebae that commonly inhabit the environment, especially in soil and water. However, some species can be isolated from plants, animals and humans (Khan, Reference Khan2006; Bunsuwansakul et al., Reference Bunsuwansakul, Mahboob, Hounkong, Laohaprapanon, Chitapornpan, Jawjit, Yasiri, Barusrux, Bunluepuech and Sawangjaroen2019). Acanthamoeba spp. are transmitted to humans by different routes, such as ocular, nasal and injured skin that still cause a mild to severe diseases (Neelam and Niederkorn, Reference Neelam and Niederkorn2017; de Lacerda and Lira, Reference de Lacerda and Lira2021). In immunocompromised individuals, the clinical presentations may be more severe and may present cutaneous lesions (Morrison et al., Reference Morrison, Morris, Shannon, Lauer, Guarner and Kraft2016), chronic sinusitis (Kim et al., Reference Kim, Syms, Holtel and Nauschuetz2000) and granulomatous amoebic encephalitis (Matson et al., Reference Matson, Rouah, Lee, Armstrong, Parke and Baker1988) depending on the site of infection. Acanthamoeba keratitis is the most frequent ocular disease caused by this protozoan parasite in healthy individuals and has recently been increased among contact lens wearers (Lorenzo-Morales et al., Reference Lorenzo-Morales, Khan and Walochnik2015; Neelam and Niederkorn, Reference Neelam and Niederkorn2017; Khan et al., Reference Khan, Anwar and Siddiqui2019). According to genotypic diversity of Acanthamoeba species, the T4 genotype has a major impact on human health as it causes infection in several organs (Juarez et al., Reference Juarez, Tártara, Cid, Real, Bermúdez, Rajal and Palma2018). Acanthamoeba triangularis is one of the T4 members widely distributed in the environment and has been shown to cause eye infection in humans (Xuan et al., Reference Xuan, Chung, Hong, Kong, Hahn and Chung2008; Hasni et al., Reference Hasni, Andréani, Colson and La Scola2020). According to the life cycle of an amoeba, trophozoite is a metabolically active/infectious form that can be transmitted to humans. Upon infection, the trophozoite may expand further or become a cyst under stressful conditions. This dormant form is a major obstacle for Acanthamoeba treatment, as it is less sensitive to most of the available drugs. The preventing transmission of Acanthamoeba is as simple as learning and practicing healthy habits, especially for those who wear contact lenses. However, the available drug for Acanthamoeba treatment is quite limited. Chlorhexidine and polyhexamethylene biguanide (PHMB) are the two most common drugs used to treat Acanthamoeba spp. However, a recent report on the use of PHMB showed that A. castellanii from clinical isolates were resistant to this drug (Huang et al., Reference Huang, Shih, Chang, Huang, Shin and Lin2017).
Autophagy is an important intracellular mechanism for cell homoeostasis. Damage or defective organelles, including large protein aggregates, are degraded by the autophagy mechanism (Yorimitsu and Klionsky, Reference Yorimitsu and Klionsky2005; Feng et al., Reference Feng, He, Yao and Klionsky2014). The target proteins are enclosed in a double-membrane structure called autophagosome, which is further fused with lysosome for degradation. The biogenesis of the autophagosome is involved with several autophagy-related (Atg) proteins. In yeast and mammals, more than 30 Atg proteins have been reported (Feng et al., Reference Feng, He, Yao and Klionsky2014) whereas in the amoeba, a partial list of these Atg proteins, core machinery, is conserved (Picazarri et al., Reference Picazarri, Nakada-Tsukui and Nozaki2008). Based on evidence so far, a role for Acanthamoeba autophagy has not been clearly demonstrated. Some Atg proteins have been partially characterized and reported to be associated with the Acanthamoeba encystation (Moon et al., Reference Moon, Chung, Hong and Kong2011, Reference Moon, Hong, Chung and Kong2013; Song et al., Reference Song, Han, Moon, Lee, Yu, Jha, Danne, Kong, Chung and Hong2012; Kim et al., Reference Kim, Moon, Hong, Chung and Kong2015). Thus, further characterization of the autophagy pathway, including other cellular pathways related to encystation under stress conditions, are of interest. Altogether, evidence of drug resistance and knowledge of Acanthamoeba autophagy encourage researchers to pay more attention to drug development and the search for a natural compound or plant extract containing anti-Acanthamoeba activity (Elsheikha et al., Reference Elsheikha, Siddiqui and Khan2020) as to its ability to induce the amoeba encystation.
In this study, seven plant extracts from West Asia were screened for amoebicidal activity. The extract of Cassia angustifolia demonstrated a significant amoebicidal activity against A. triangularis trophozoite. Scanning electron microscopy (SEM) images also represented a morphological change in the cell membrane that can lead to cell death. The drug combination was also performed to explore if chlorhexidine could provide a synergistic effect with the plant extract. In addition to the amoebicidal activity, the encystation of surviving Acanthamoeba under a microscope and the autophagic response of Acanthamoeba at the transcriptional level were investigated. These data opened up another view of the plant extract apart from its cidal activity. Understanding the molecular mechanism in response to stress induced by the plant extract can provide an insight into the future drug development and treatment.
Materials and methods
Plant collection and preparation of extracts
Dried medicinal plants were purchased from a perfume market in the eastern province of Azerbaijan, and the species was identified and authenticated in the botany section of the Agricultural Research Center in eastern Azerbaijan: Artemisia absinthium L., Cuscuta epithymum, Achillea millefolium, Ferula assa-foetida, Teucrium polium, Melissa officinalis and C. angustifolia. The whole dried plant was mechanically powdered using an electrical blender. The ethanolic extracts of plants were prepared by macerating 370 g of dry powdered plants in 70% aqueous ethanol for 3 days at room temperature (RT), and then filtered through filter paper (Whatman Ltd., Buckinghamshire, UK). The alcohol in the sample was removed by using a rotary vacuum evaporator. The extract was concentrated in an incubator at 37°C to obtain the dry powder extract.
Acanthamoeba cultivation
Acanthamoeba triangularis strain WU19001 (Mitsuwan et al., Reference Mitsuwan, Bunsuwansakul, Leonard, Laohaprapanon, Hounkong, Bunluepuech, Chalermpol, Mahboob, Sumudi Raju and Dhobi2020) was cultured in 25 cm2 cell culture flasks containing PYG medium [2% (w/v) proteose peptone, 0.1% (w/v) yeast extract, 400 μ m CaCl2, 4 mm MgSO4, 2.5 mm Na2HPO4, 2.5 mm KH2PO4, 50 μ m (NH4)2Fe(SO4)2, 100 mm glucose] at 25°C in the dark without shaking (Taravaud et al., Reference Taravaud, Loiseau and Pomel2017). The amoeba trophozoite was kept in PYG medium and replaced with fresh medium every 2 days until the harvest of Acanthamoeba. Regarding a cyst induction medium by Aqeel et al. (Reference Aqeel, Siddiqui, Iftikhar and Khan2013), a ready-to-use Page's saline (PAS) powder (HiMedia, Mumbai, India) that contained NaCl, MgSO4⋅7H2O, CaCl2⋅2H2O, Na2HPO4, KH2PO4 and supplemented with 10% glucose for cyst induction in our laboratory and cultured for 5–7 days was performed. Then, the cysts were harvested by centrifugation.
Plant extract preparation and determination of anti-Acanthamoeba activity
All plant extracts were dissolved in 100% dimethyl sulphoxide (DMSO) and prepared at a stock concentration of 100 mg mL−1. The screening of amoebicidal activity was performed on a 96-well black plate (SPL Life Sciences, Seoul, Korea). The plant extract was added at a final concentration of 1000 μg mL−1. Acanthamoeba trophozoites and cysts were harvested, washed twice with PAS buffer and counted using a haemocytometer. The trophozoite suspension was prepared in PYG medium whereas the cyst suspension was prepared in PAS containing 10% glucose. Amoebae trophozoites were added to the final cell number of 2 × 104 cells per well. Cells treated with 5 μg mL−1 chlorhexidine, 1% DMSO and medium alone were included as controls. Edge wells were filled with PAS buffer to reduce the evaporation rate. After 24 h of treatment, the cells were stained with PrestoBlue® dye (Invitrogen, Waltham, USA), a new reagent based on resazurin to assess cell viability and cytotoxicity, in the final dilution of 1:20 for 30 min in an incubator at 37°C, followed by a fluorescence signal quantified by using a microplate reader (BioTek Synergy™ MX microplate reader, VT, USA) at excitation/emission wavelength of 535/615 nm. The percentage viability of the parasite was then calculated using Prism 5 software (GraphPad Software, CA, USA). DMSO-treated condition was set to 100% cell viability. Then, the percentage of anti-Acanthamoeba activity was calculated using the formula: % amoebicidal activity = 100 − % cell viability. All experiments were conducted in triplicate with three independent experiments.
Determination of minimal inhibitory concentration
The C. angustifolia extract which demonstrated the greatest amoebicidal activity was selected for further studies. The minimum inhibitory concentration (MIC) was identified using the microtiter broth dilution method (Mitsuwan et al., Reference Mitsuwan, Bunsuwansakul, Leonard, Laohaprapanon, Hounkong, Bunluepuech, Chalermpol, Mahboob, Sumudi Raju and Dhobi2020). The plant extract was prepared in a transparent 96-well plate (SPL Life Sciences, Seoul, Korea) with a 2-fold serial dilution. The extract concentrations were 2048, 1024, 512, 256 and 128 μg mL−1, respectively. Then, 100 μL of trophozoites and cysts of 2 × 105 cells were added into each well. The plates were incubated at RT in the dark for 24, 48 and 72 h. Then, the cells were stained with Trypan Blue dye, and cell viability was quantified using a light inverted microscope, Eclipse TE2000-S (Nikon, Tokyo, Japan). The MIC value of the extract was defined as the lowest concentration with Acanthamoeba growth inhibition >90%.
Scanning electron microscopy
To further investigate the effect of the C. angustifolia extract on the amoeba, the trophozoite and cyst forms were treated separately with 0.25 MIC concentration of 250 μg mL−1 of C. angustifolia extract in a 24-well plate. Two pieces of sterile glass with high cell-binding affinity, 3 × 3 mm2 in size, were added to the well, the cells were resuspended and allowed to stand for 24 h. The amoeba-coated glass was fixed with 2.5% glutaraldehyde overnight and dehydrated with a series of graded ethanol (20, 40, 60, 80, 90 and 100% ethanol). The samples were mounted on aluminium stubs, dried in a critical point dryer and coated with gold particles (Cressington 108 sputter coater, MA, USA). Then, the amoeba surface structure was observed under SEM (Gemini, Oberkochen, Germany).
Analysis of cyst formation and vacuolization
The Acanthamoeba trophozoites were treated with a C. angustifolia extract of 250 μg mL−1 and the surviving trophozoites were evaluated for cyst formation and vacuolization under stress induced by the plant extract 24 h after treatment. Cells were stained with Trypan blue and viable cells were analysed. At least 200 cells per condition were analysed for cyst formation and at least 100 surviving trophozoites per condition were examined for vacuole formation under the light microscope. The vacuole with a diameter ⩾5 μm was considered as an enlarged vacuole and the trophozoite containing at least one enlarged vacuole was counted as 1. Three independent experiments were performed.
Drug combination
For the drug combination study, the experiment was set-up in the same manner as the MIC determination. The 2-fold serial dilution of the drug and plant extract was prepared and combined in a 96-well plate. The highest concentration was the MIC of drug/plant extract. Then, 2 × 105 trophozoites were added and incubated at RT for 24 h in the dark. Then, the cells were stained with Trypan blue and analysed under an inverted microscope.
Preparation of total RNA and cDNA synthesis
Acanthamoeba trophozoites of 2 × 105 cells per 1 mL PYG medium per well were cultured in a 24-well plate in the presence or absence of the C. angustifolia extract at a concentration of 250 μg mL−1. The plate was incubated at RT for 24 h. The cells were harvested at 6, 12, 18 and 24 h after treatment. At each time point, untreated and C. angustifolia extract-treated cells, a total of two wells were collected into 1.5 mL Eppendorf tubes and kept on ice. The tubes were centrifuged and the culture medium was discarded. The cell pellet was vortexed and a 500 μL TRI reagent (Molecular Research Center, Cincinnati, USA) was immediately added to the cells to preserve parasite RNA. The total RNA was extracted using a RNA extraction kit (Vivantis Technologies, Selangor, Malaysia). The total 100 ng mRNA was then converted to cDNA by the Viva cDNA synthesis kit (Vivantis Technologies, Selangor, Malaysia) following the manufacturer's protocol. The cDNA was kept at −20°C until use.
Validation of polymerase chain reaction (PCR) primers
Specific primers targeting genes of interest were obtained from published research on Acanthamoeba spp. as shown in Table S1. Genes of interest were ATG3, ATG8b, ATG16, including the housekeeping gene, 18S rRNA. These primers were tested with DNA from the A. triangularis strain WU19001. The amplicon was purified, commercially sequenced (Apical Scientific Sdn. Bhd., Selangor, Malaysia) and blasted against A. castellanii NCBI databases before performing a real-time PCR.
Analysis of gene expression by quantitative PCR
Real-time PCR was performed in duplicate with three independent experiments using an iTaq Universal SYBR Green Supermix kit (Bio-Rad, Hercules, USA). The qPCR reaction was conducted as follows. Ten microlitres of iTaq supermix, 2× concentration, were added to the PCR tube followed by 100 ng cDNA, 1 μL of 200 mm F + R primers and the total volume adjusted with DEPC water up to 20 μL. The software setting for the thermal cycler, StepOnePlus Real-time PCR systems (Applied Biosystems, Waltham, USA), was as follows: holding stage 95°C for 30 s, cycling stage for 40 cycles at 95°C for 15 s, 60°C for 60 s, and then, melting curve stage at 95°C for 15 s, 60°C for 60 s, 95°C for 15 s with a temperature increase of 0.3°C. The average ∆Ct, ∆∆Ct and the relative expression of the mRNA were calculated. The 18S rDNA was used as an internal control.
Statistical data analysis
All data from the extract testing assay were recorded, entered and edited using Microsoft Excel 2016 (Microsoft Corporation, Washington, USA) and data were obtained from three independent experiments with three technical replicates. The results were pooled to determine the mean ± s.d. or mean ± s.e.m. depending on the experiment and analysed using a two-tailed unpaired Student's t-test by Prism 5 (GraphPad Software, San Diego, USA). P values <0.05 were considered statistically significant.
Results
Anti-Acanthamoeba activity of plant extracts
Seven plant extracts (Table 1) at the final concentration of 1000 μg mL−1 were selected for an anti-Acanthamoeba activity against A. triangularis trophozoites. All plant extracts tested demonstrated amoebicidal activity to varying degrees. The percentage of cidal activity was in the range of 25–65% compared to the DMSO control. Among these plants, the extract of C. angustifolia demonstrated the highest cidal activity followed by A. millefolium, F. assa-foetida, A. absinthium, T. polium, respectively, whereas for the extracts of C. epithymum and M. officinalis, the percentage was below 35%. However, statistical analysis data revealed that the C. angustifolia extract was the only extract that significantly killed the A. triangularis trophozoite (Fig. 1).

Fig. 1. Screening of amoebicidal activity of the plant extract against Acanthamoeba triangularis trophozoites. Acanthamoeba trophozoites were treated with these extracts for 24 h at a final concentration of 1000 μg mL−1. Chlorhexidine of 5 μg mL−1 and 1% DMSO were included as a positive and a negative control, respectively. The cells were stained with PrestoBlue dye and the fluorescence intensity was analysed. Cell viability in the DMSO control was set to 100% and the percentage of the extract-treated conditions was adjusted accordingly. Then, the percentage of amoebicidal activity was calculated using 100% cell viability. The experiment was performed in triplicate with three independent experiments. Bar graphs show mean ± s.e.m. **P value < 0.01.
Table 1. List of plants used in this study

Cassia angustifolia was further identified with its MIC against the A. triangularis trophozoite and cyst forms. In the 24 h treatment, the C. angustifolia extract had an inhibitory activity against the trophozoite with an MIC value of 1024 μg mL−1, whereas in the 48 and 72 h treatments, the MIC was 2048 μg mL−1. However, the inhibitory effect against cysts, their MICs at different time points were >2048 μg mL−1 (Table S2).
Ultrastructural effects of C. angustifolia
A morphological change of the amoeba upon exposure to a concentration of 0.25 MIC, 250 μg mL−1, of C. angustifolia extract was observed. The SEM was performed. The amoebae of both trophozoite and cyst forms were treated separately with the extract for 24 h, at RT in the dark. The amoeba was harvested and processed for SEM. Under the conditions treated with C. angustifolia, almost the entire surface of the trophozoites was covered by the plant extract. The cell membrane was ruptured and the formation of pores in the membrane was clearly seen (Fig. 2). This damage to the membrane can lead to the leakage of cytoplasmic content and finally result in the death of A. triangularis cells. In addition, acanthopodia of most of cells disappeared after 24 h of treatment, cells were much smaller compared to the untreated control, but did not shrink as expected. However, in part of cell membrane of some cells, the structure was still stabilized, which probably indicates the slow action of the extract.
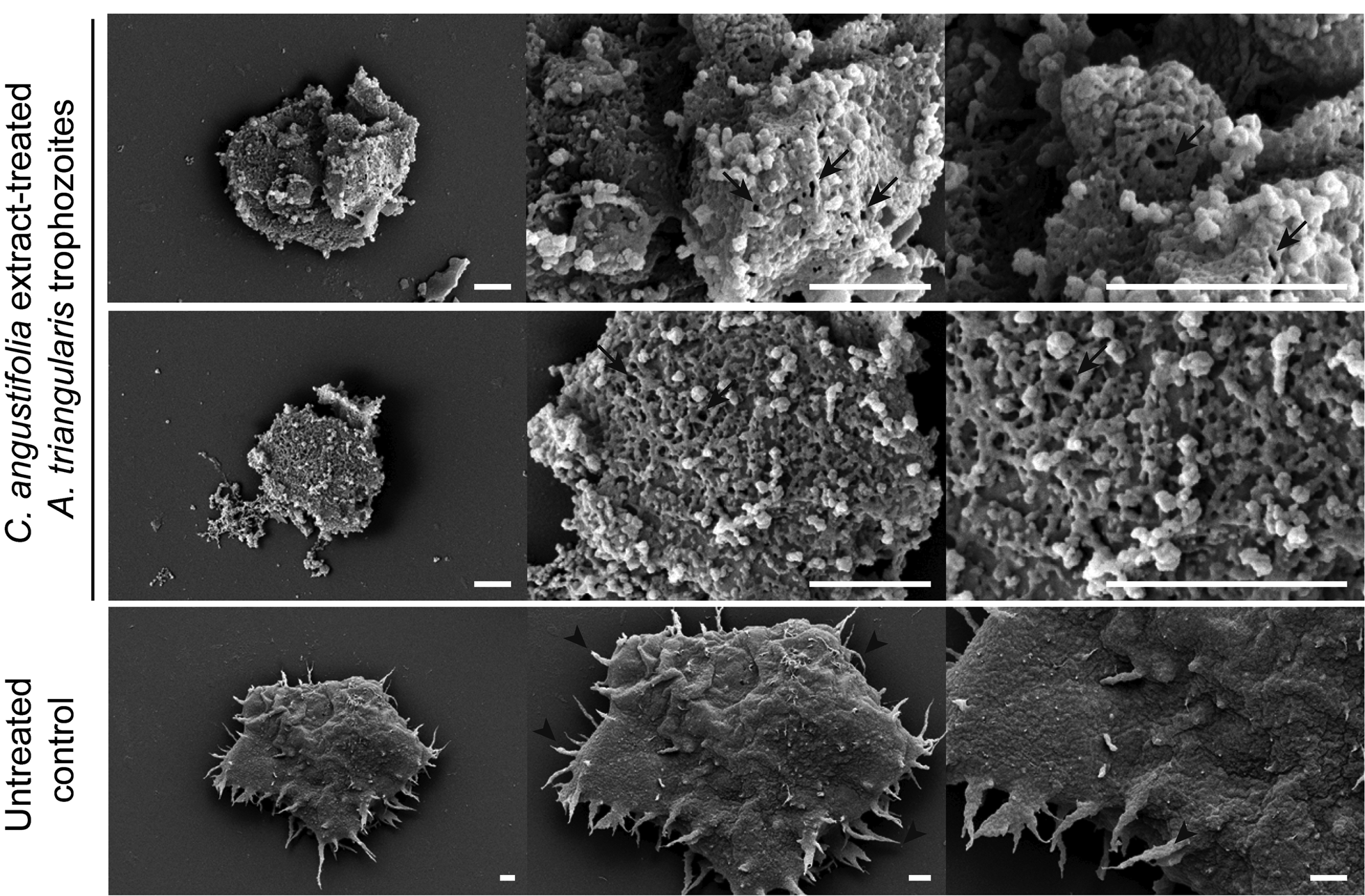
Fig. 2. SEM imaging of A. triangularis trophozoites treated with Cassia angustifolia extract. Cells were treated with the compound for 24 h in a 24-well plate. Cells were then fixed and processed for SEM. Arrows indicate porous membrane, whereas arrow heads indicate acanthopodia. Scale bars = 1 μm.
In cysts treated with C. angustifolia, even the amoebicidal activity was not as potent as in trophozoites, SEM images revealed some morphological changes of the cyst (Fig. 3). Most of the A. triangularis cysts were slightly shrunk, pronounced circular or polygonal edges were obviously seen and a venation was no longer present in the membrane. Emphasizing on the membrane pore, its number was similar to that of the untreated control, but its size was slightly larger. However, due to the complexity of the double cyst wall, this limits the ability of most drugs/compounds to enter the cyst form and becomes the main barrier to Acanthamoeba treatment (Lorenzo-Morales et al., Reference Lorenzo-Morales, Martín-Navarro, López-Arencibia, Arnalich-Montiel, Piñero and Valladares2013).

Fig. 3. SEM imaging of A. triangularis cysts treated with C. angustifolia extract. Cells were treated with the compound for 24 h in a 24-well plate, and then fixed and processed for SEM. Arrows indicate a pronounced edge, whereas arrow heads indicate venation. Scale bars = 1 μm.
Drug combination
Regarding a common anti-Acanthamoeba drug, chlorhexidine, we also combined the drug with the C. angustifolia extract against the trophozoite of A. triangularis. The concentrations of the drug/extract are shown in Table 2. At the maximum concentrations of the extract (MIC: 1024 μg mL−1) and chlorhexidine (MIC: 16 μg mL−1), the percent viability of the trophozoites fell into a range of 4–7%. Reduction of the concentration of chlorhexidine to 8 μg mL−1 in combination with different concentrations of the extract, the percentage viability was markedly increased to 20–44% and their percentages were similar to those of chlorhexidine alone, 25%. At lower concentrations of chlorhexidine (4, 2, 1 μg mL−1), a similar pattern of result was observed in which its percentage viability was at a level comparable to that of the drug alone. On the contrary, in the combination of fixed concentration of the extract, 512 μg mL−1, with varying concentrations of chlorhexidine, the percentage of viability was consistent in the range of 20–28% and close to the extract alone, 30%. When the concentration of the extract was reduced to 256, 128 and 64 μg mL−1, the percentage of viability was gradually increased to a level comparable to that of its own drug treated alone. Altogether, no synergistic, additive or antagonistic effects were observed in any combination of drugs that indicate their own inhibitory effects for A. triangularis.
Table 2. Activity of C. angustifolia in combination with chlorhexidine against Acanthamoeba triangularis

Autophagic response to C. angustifolia extract
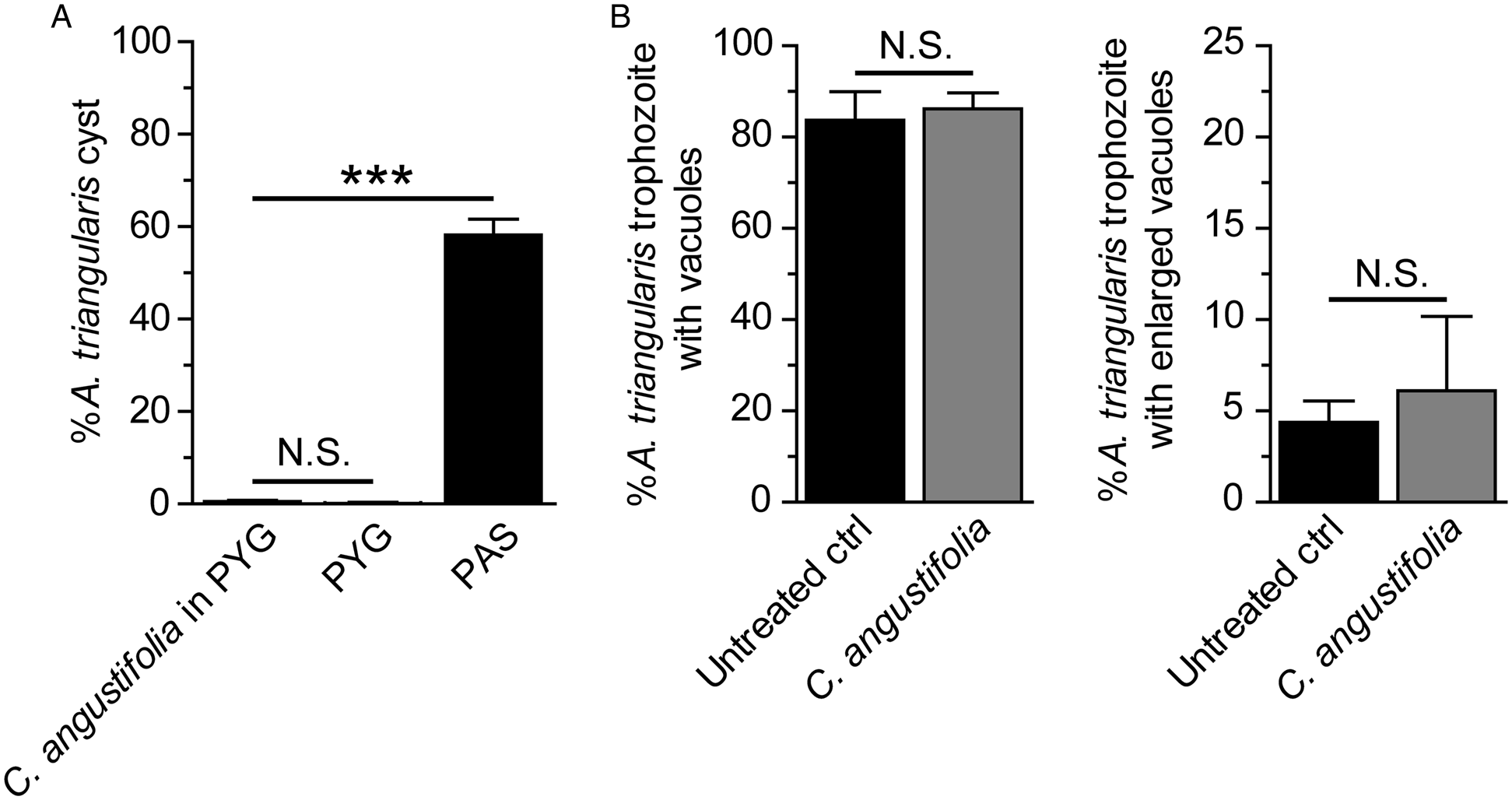
Acanthamoeba autophagy is not clearly identified. Acanthamoeba autophagy-related (Atg) proteins were partially characterized in A. castellanii and their functions have been reported to be associated with the amoeba encystation (Moon et al., Reference Moon, Chung, Hong and Kong2011, Reference Moon, Hong, Chung and Kong2013; Song et al., Reference Song, Han, Moon, Lee, Yu, Jha, Danne, Kong, Chung and Hong2012). According to our screening result of the plant extract for the anti-Acanthamoeba activity, a stress-induced encystation was not observed upon 24 h treatment of C. angustifolia. The surviving A. triangularis remained in the trophozoite stage (Fig. 4A). Further quantification of stress-induced vacuolization was conducted on surviving trophozoites. The results showed that the percentage of trophozoites containing vacuoles was at a level comparable to the untreated control, 80–90% (Fig. 4B, left). We also considered the size of the vacuole to explore if it was enlarged (a diameter of ⩾5 μm). However, the percentage of trophozoites with enlarged vacuole was not a significant difference between C. angustifolia-treated and untreated control (Fig. 4B, right). Regarding autophagy, a stress-sensing mechanism, we hypothesized that the extract may affect ATG gene expression which further impairs the encystation mechanism. Quantitative PCR was further performed. The validation of PCR primers targeting ATG3, ATG8b, ATG16, 18S rRNA genes in the A. triangularis genomic DNA was performed for the first time (Fig. S1). A single band from each PCR reaction was clearly seen on the agarose gel. These amplicons were subsequently sequenced to confirm a specific amplification, as shown in Table S3. Then, qPCR of A. triangularis treated with C. angustifolia was performed and the data are shown in Fig. 5.
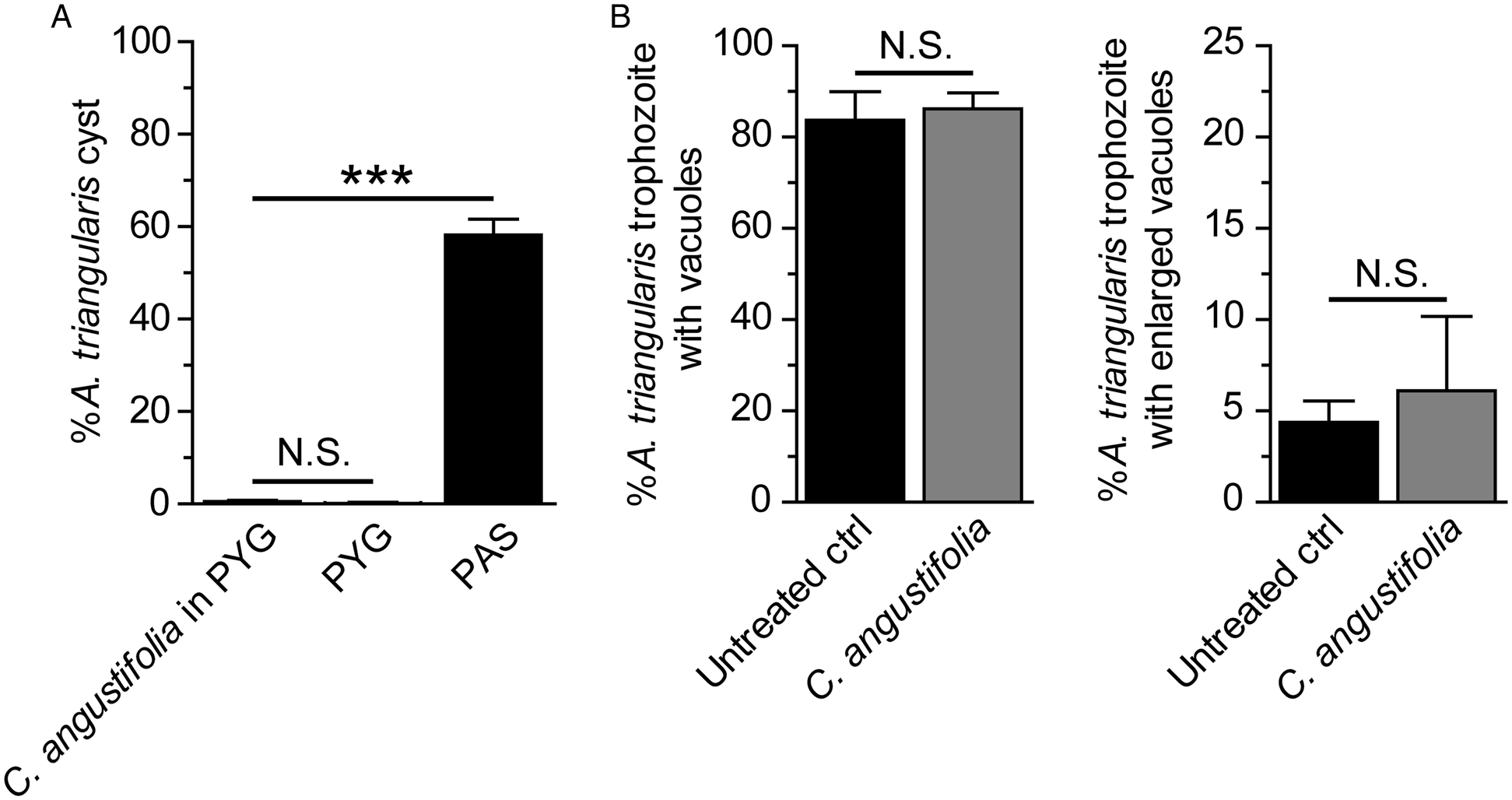
Fig. 4. Cyst formation and vacuolization in surviving trophozoites. Acanthamoeba triangularis trophozoites were cultured in PYG medium and treated with the 250 μg mL−1 C. angustifolia extract for 24 h. Trophozoites cultured in PYG and PAS media alone were included as negative and positive controls for encystation, respectively. (A) At least 200 cells per condition were analysed for cyst formation. (B) Surviving trophozoites of at least 100 cells per condition were examined for vacuole formation. Percentages of trophozoites containing vacuoles (left) and trophozoites with enlarged vacuole (diameter ⩾5 μm) (right) were analysed. The data were obtained from three independent experiments. Bar graphs show mean ± s.d. N.S., not significant. ***P value < 0.001.
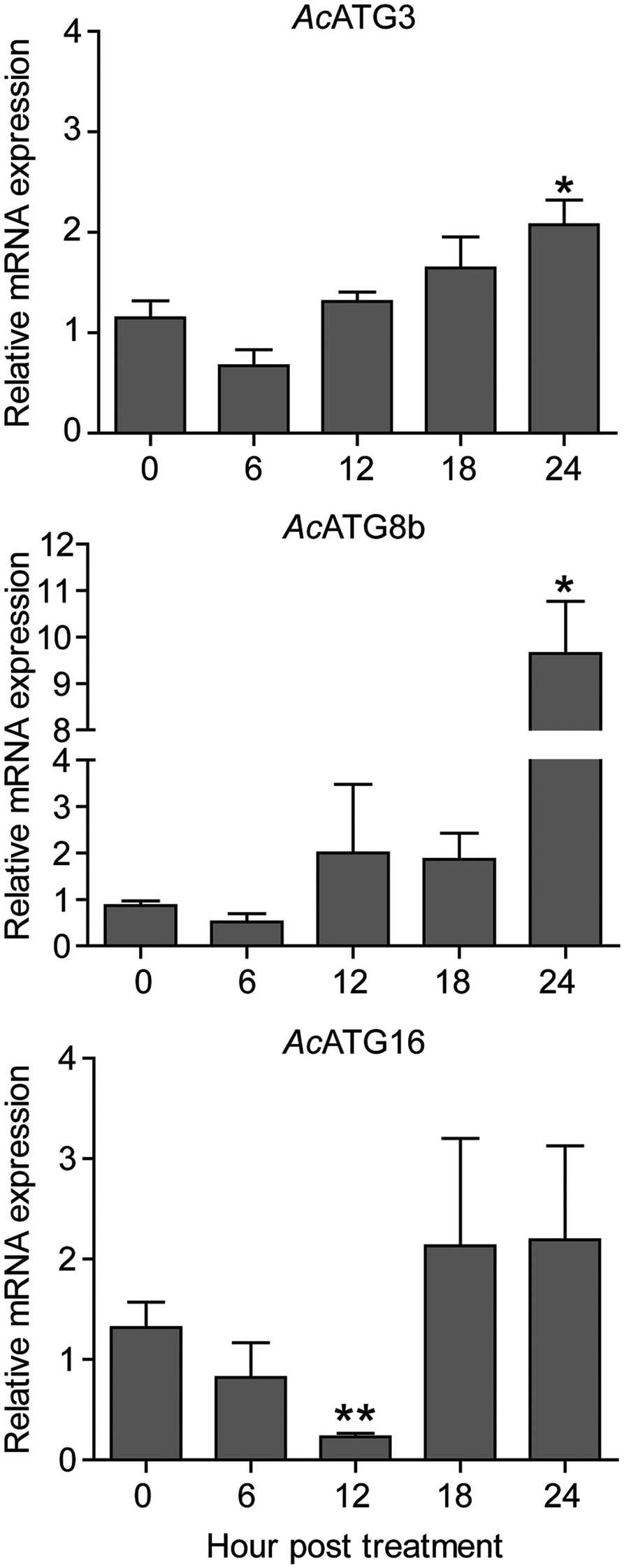
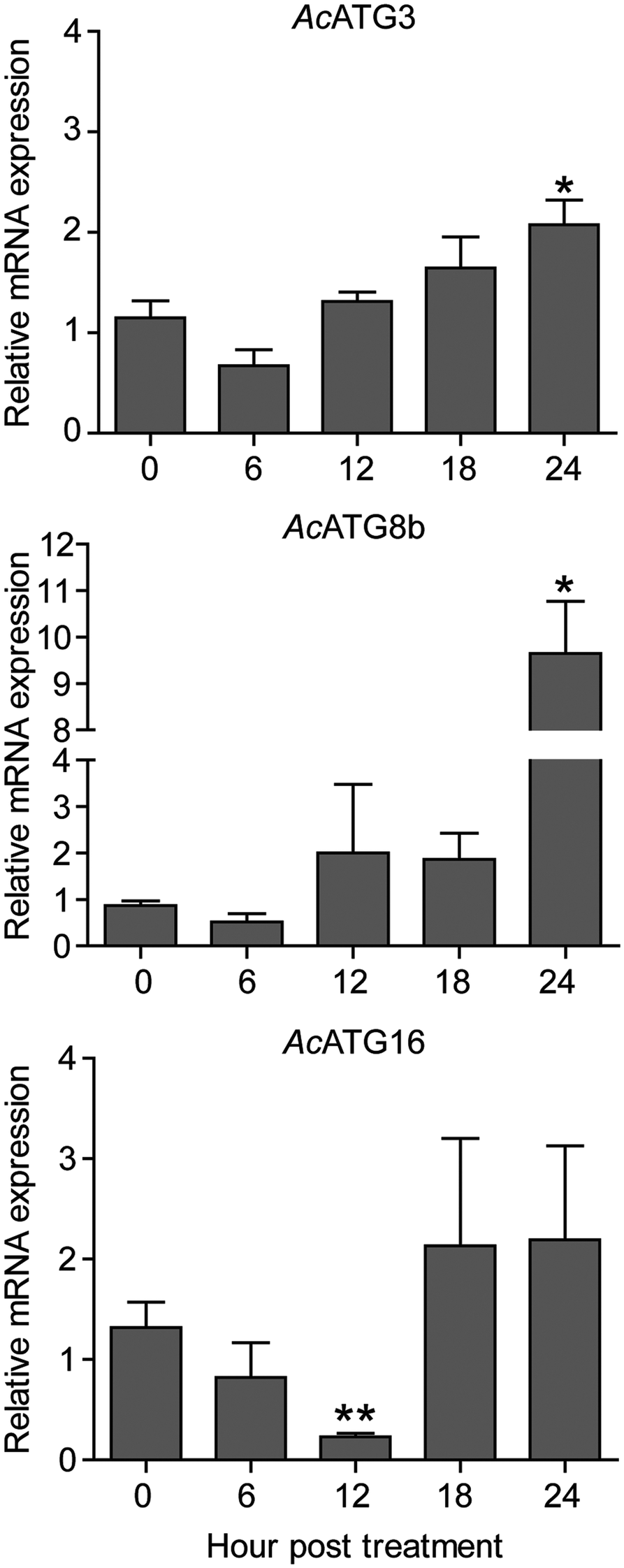
Fig. 5. Transcriptional expression of autophagy genes. Acanthamoeba triangularis trophozoites were cultured in PYG medium in the presence or absence of the extract and incubated for 24 h. The cells were harvested every 6 h and the relative changes in the mRNA levels of the ATG3, ATG8b and ATG16 genes were measured using quantitative PCR. The level of indicated transcripts from each time point was expressed as a relative mRNA expression. 18S rRNA was used as an internal normalization gene. The expression level was compared to that of time zero. The data were obtained from three independent experiments. Bar graphs show mean ± s.e.m. *P < 0.05; **P < 0.01.
Regarding Atg8b and Atg3 in Acanthamoeba spp., both were reported to be involved in the Acanthamoeba encystation (Moon et al., Reference Moon, Chung, Hong and Kong2011, Reference Moon, Hong, Chung and Kong2013). Our qPCR results showed that after exposure to C. angustifolia extract, mRNA expression of ATG3 and ATG8b was consistent for the first 18 h compared to 0 h and increased significantly within 24 h post-treatment. However, Atg16, which is part of Atg12-Atg5 complex and required for an efficient Atg8 conjugation in autophagosome biogenesis (Yorimitsu and Klionsky, Reference Yorimitsu and Klionsky2005; Fujita et al., Reference Fujita, Itoh, Omori, Fukuda, Noda and Yoshimori2008), our qPCR data demonstrated that AcATG16 mRNA expression decreased over a period of 12 h after treatment and a significant reduction was observed at 12 h. However, its expression was recovered 18 h post-treatment. This indicates the expression profile of selected ATG genes upon treatment with the C. angustifolia extract.
Discussion
The incidence of Acanthamoeba infection has increased annually, especially in contact lens wearers (Khan, Reference Khan2006; Ibrahim et al., Reference Ibrahim, Boase and Cree2009; Nielsen et al., Reference Nielsen, Ivarsen and Hjortdal2020; de Lacerda and Lira, Reference de Lacerda and Lira2021). Along with the reports on drug resistance (Iovieno et al., Reference Iovieno, Oechsler, Ledee, Miller and Alfonso2010; Huang et al., Reference Huang, Shih, Chang, Huang, Shin and Lin2017) and the limited anti-Acanthamoeba drugs available (Lorenzo-Morales et al., Reference Lorenzo-Morales, Khan and Walochnik2015; Szentmáry et al., Reference Szentmáry, Daas, Shi, Laurik, Lepper, Milioti and Seitz2019), these attract the researcher's attention to focus on the identification of new medicinal plants for therapeutic purposes of Acanthamoeba infection and a compound with greater inhibitory activity and less toxicity is urgently needed (Niyyati et al., Reference Niyyati, Dodangeh and Lorenzo-Morales2016; Mahboob et al., Reference Mahboob, Nawaz, Tian-Chye, Samudi, Wiart and Nissapatorn2018; Sanguan et al., Reference Sanguan, Wannasan, Junkum, Jitpakdi, Riyong, Champakaew and Pitasawat2018; Kolören et al., Reference Kolören, Kolören, Şekeroğlu, Colayvaz and Karanis2019; Elsheikha et al., Reference Elsheikha, Siddiqui and Khan2020). Besides, a treatment strategy of multidrug combination therapy may be needed for drug resistance cases (Tyers and Wright, Reference Tyers and Wright2019). Several phytochemicals extracted from many plants have been evaluated as a source of amoebicidal agent, for example Allium sativum, Origanum spp., Arachis hypogaea, Curcuma longa L., Pancratium maritimum L., Pterocaulon polystachyum, Croton isabelli, Artemisia argyi, Piper spp., Citrus spp., Murraya paniculata, Amomum uliginosum, Kaempferia pandurata, etc. (Sanguan et al., Reference Sanguan, Wannasan, Junkum, Jitpakdi, Riyong, Champakaew and Pitasawat2018; Kolören et al., Reference Kolören, Kolören, Şekeroğlu, Colayvaz and Karanis2019; Anwar et al., Reference Anwar, Ting, Anwar, ul Ain, Faizi, Shah, Khan and Siddiqui2020).
Cassia angustifolia is well known in West Asia, in particular in Yemen, a region in Southern Arabia, and is called Arabia Senna. The medicinal properties of Senna leaf are commonly used to treat constipation, loss of appetite, hepatomegaly, splenomegaly, indigestion, malaria, jaundice and anaemia (Tripathi, Reference Tripathi1999), antidiabetic, antioxidant and hepatopreventive activity of leaves of C. angustifolia has recently been reported (Bellassoued et al., Reference Bellassoued, Hamed, Ghrab, Kallel, Van Pelt, Makni Ayadi and Elfeki2019; Jani and Goswami, Reference Jani and Goswami2020). The anthelmintic activity of C. angustifolia leaves has also been reported against Heterakis gallinarum, Raillietina tetragona and Catatropis sp. which resulted in helminth paralysis and death after exposure to the plant extract (Kundu et al., Reference Kundu, Roy and Lyndem2014). A study on rat tapeworm, Hymenolepis diminuta reported that the anthelminthic activity of the C. angustifolia plant targeted the mitochondrial membrane and led to the loss of its architecture (Ukil et al., Reference Ukil, Roy, Nandi and Lyndem2018). In our study, the amoebicidal activity of C. angustifolia leaves extract was demonstrated against trophozoites and cysts of A. triangularis. The effect of C. angustifolia extract on trophozoites and cysts was observed by SEM. SEM images exhibited some morphological changes. Acanthopodia, a crucial component of the trophozoites for cell adhesion, movement and capture of food particles, has disappeared. A loss of membrane integrity was observed in the trophozoites membrane, including an obvious porous formation. These changes can lead to a leakage in the cytoplasmic content and death of the amoeba. This fact is in agreement with Heredero-Bermejo et al.'s (Reference Heredero-Bermejo, Martín-Pérez, Copa-Patiño, Gómez, de la Mata, Soliveri and Pérez-Serrano2020) finding that the membrane alteration led to the Acanthamoeba cell death. In addition, the presence of pores in the membrane after C. angustifolia treatment may indicate the amoebic cell death by necrosis (Zhang et al., Reference Zhang, Chen, Gueydan and Han2018). However, further investigation to rule out apoptotic cell death is needed. Different degrees of susceptibility to the plant extract, including anti-Acanthamoeba drugs available for trophozoites and cysts, are due to a different membrane architecture of the cells, resulting in different degrees of cell permeability. Although the MIC against the cyst form was higher, which means less potency compared to the trophozoites stage, but morphological changes were still presented, that is, cell shrinkage, much smaller cell compared to the untreated control and more pronounced edges of the cyst wall.
Apart from the cidal activity of the C. angustifolia, encystation of the surviving trophozoites left by the treatment is a secondary concern. Our data showed that there was no induction of cyst formation. The percentage of surviving trophozoites containing vacuole and the cell with enlarged vacuole were not significantly different from the untreated control. This may indicate a specific stress-induced encystation in Acanthamoeba spp. Cyst form is metabolically inactive and more resistant to environmental stress. Under the condition of depleted nutrients, the amoeba immediately undergoes encystation (Chagla and Griffiths, Reference Chagla and Griffiths1974). The Acanthamoeba cyst consists of a double wall called ectocyst and endocyst (Anwar et al., Reference Anwar, Khan and Siddiqui2018). Several pathways are involved with the amoeba encystation (Picazarri et al., Reference Picazarri, Nakada-Tsukui and Nozaki2008; Bouyer et al., Reference Bouyer, Rodier, Guillot and Héchard2009; Moon et al., Reference Moon, Hong, Chung and Kong2012). Autophagy is one of the mechanisms that support this transformation and some autophagy proteins have been reported (Picazarri et al., Reference Picazarri, Nakada-Tsukui and Nozaki2008; Moon et al., Reference Moon, Chung, Hong and Kong2009; Song et al., Reference Song, Han, Moon, Lee, Yu, Jha, Danne, Kong, Chung and Hong2012; Moon et al., Reference Moon, Kim, Hong, Chung, Goo and Kong2015). Atg8 was first identified in A. castellanii and this protein was further characterized at the molecular level. Their data demonstrated that A. castellanii Atg8 (AcAtg8) was highly expressed during encystment and the fluorescence analysis data showed that AcAtg8 diffused into a cytosol in the trophozoites stage, whereas in the cyst, it formed as puncta structures. The Atg8 positive membrane was further identified as an autophagosome, as it showed a co-localization with LysoTracker (Moon et al., Reference Moon, Chung, Hong and Kong2009). Another isoform was identified later, AcAtg8b. This protein was slightly longer than the AcAtg8 protein. However, the AcAtg8b was highly expressed during encystation and its RNA interference data revealed that decreased expression of AcAtg8b mRNA significantly reduced Acanthamoeba encystation (Moon et al., Reference Moon, Hong, Chung and Kong2013). AcAtg3, a member of the Atg8 conjugation system, was further characterized in A. castellanii. Its molecular data showed that its mRNA expression level was not changed during the encystation. However, microscopic data revealed that the formation of mature cyst was significantly reduced in Atg3-depleted cells (Moon et al., Reference Moon, Chung, Hong and Kong2011). It was later reported that AcAtg16 was highly expressed during the A. castellanii encystation. The immunofluorescence analysis showed that the AcAtg16 protein was partially located in the autophagosome of the Acanthamoeba cyst. The reduction of this protein inhibited the formation of autophagosomes and further affected the efficiency of cyst formation (Song et al., Reference Song, Han, Moon, Lee, Yu, Jha, Danne, Kong, Chung and Hong2012). Our quantitative PCR data revealed that Acanthamoeba autophagy responded quickly to the C. angustifolia extract, which is similar to the autophagy mechanism in other organisms that respond immediately to cellular stress (Yorimitsu and Klionsky, Reference Yorimitsu and Klionsky2005; Gatica et al., Reference Gatica, Lahiri and Klionsky2018). The transcriptional expression of the autophagy genes was also affected. AcAtg16 was significantly downregulated 12 h after treatment, whereas transcripts of AcATG3 and AcATG8b were consistent during the first 18 h after treatment. However, at 24 h, Atg16 was recovered to a basal level, whereas transcripts of AcATG3 and AcATG8b were slightly increased. Regarding our data on cyst formation, there was no cyst induction after treatment with C. angustifolia, the reduction in ATG16, as well as a constant level of ATG8b can attenuate the induction signal for Acanthamoeba encystation. In agreement with data previously published by Moon, et al. (Reference Moon, Hong, Chung and Kong2013), that the increase in Atg8b expression was associated with the amoeba encystation (Moon et al., Reference Moon, Hong, Chung and Kong2013), our data also confirmed a previous study by Moon et al. (Reference Moon, Chung, Hong and Kong2011) that the level of ATG3 mRNA was consistent and expressed independently of encystment induction. However, the increase in AcATG3 and AcATG8b in 24 h was of interest. It is possible that one of the active compounds within the extract may be active at this time point, which initiates an encystation signal or the autophagy may be delayed in coping with the stress induced by the C. angustifolia extract. The latter, the autophagy mechanism, may work independently form the amoeba encystment. For those Atg, Atg3, Atg8 and Atg16 proteins, they normally work together on basal autophagy and autophagosome biogenesis upon autophagic induction. The latter requires an increased expression of all Atg proteins. According to our data, AcAtg16 was the only one affected. It is possible that Atg16 protein has an additional role in response to the stress and is probably to be specific or non-specific to this plant in which stress may enhance protein translation, function, recycling and/or degradation. Another possibility is that its transcription may be disrupted by any of biomolecules in the extract. Thus, the isolation and purification of bioactive molecules are needed to characterize a specific response related to a certain ATG transcript. Certainly, a study at the protein level provides more information on how each Atg protein works in Acanthamoeba spp. including its correlation with ATG mRNA. Further investigations of the ATG expression profile including ATG16 in response to other plant extracts are of interest. This is the first time that autophagy in A. triangularis, another important member of T4 genotype, was evaluated. The data provide insight into the autophagic mechanism of the Acanthamoeba in response to stress and an understanding of this mechanism may be useful for the development of drugs for Acanthamoeba infection.
As mentioned earlier on the limitation of commercially available drugs for the treatment of Acanthamoeba, monotherapy with chlorhexidine or PHMB has been used. As evidence of other infectious diseases in long-term treatment, combination therapy is usually superior to monotherapy for infections, especially in drug-resistant cases (Martínez-Sagasti et al., Reference Martínez-Sagasti, Gonzalez-Gallego and Moneo-Gonzalez2016; Tepekule et al., Reference Tepekule, Uecker, Derungs, Frenoy and Bonhoeffer2017) and synergistic effect of drug combinations is a promising approach to fight chronic diseases. This leads to our drug combination study, C. angustifolia extract and chlorhexidine. However, our results did not show any synergistic effect in any combination below its MIC, which indicates its own cidal activity against Acanthamoeba trophozoites. The investigation of the drug combination with an active compound purified from the plant extract is also needed.
Altogether, our study presents an extra property, amoebicidal activity, of the leave extract of C. angustifolia. The autophagy of Acanthamoeba was explored at the transcriptional level, indicating the expression profile of genes related to autophagy in response to the stress induced by this plant (Fig. S2). However, the autophagic response to other plants, as well as the response of other pathways related to the Acanthamoeba encystation are of interest and need further study. Moreover, investigations on active compounds, cytotoxicity against human cells, drug combination studies are essential for drug development and therapeutic purposes of protozoan infection, including free-living amoebae.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0031182021000718
Author contributions
R.B. and V.N. conceptualized the research; R.B. performed Acanthamoeba culture; R.B. performed compound treatment assay and quantitative PCR; S.S. performed drug combination study; W.M. and S.S. prepared Acanthamoeba sample for SEM; A.S. prepared plant extracts and provided information related to the plants; S.S., W.M., N.C., S.W., F.M., M.P., P.W., M.R., C.W., H.A.T. and K.G.D. provided critical suggestions; R.B. performed overall data analysis; R.B. wrote the manuscript and collated comments from all authors; V.N., P.W. and R.N. provided critical suggestions, comments on the manuscript, and financial support.
Financial support
We highly appreciate the support of The Royal Patronage of Her Royal Highness Princess Maha Chakri Sirindhorn – Botanical Garden of Walailak University, Nakhon Si Thammarat under the project entitled: Medicinal under-exploited Thai native plant against Acanthamoeba spp., Leishmania donovani and Plasmodium falciparum – Toward South East Asia collaboration initiative (WUBG020-2564), Thailand, Walailak University grant No. WU-IRG-63-073, Wasinee Poonsawat, the Research Institute of Health Science (RIHS) staff for the laboratory facilities and Benjaporn Somjit at the Center for Scientific and Technological Equipment for SEM imaging, Walailak University. We would also like to acknowledge the project CICECO-Aveiro Institute of Materials, UIDB/50011/2020 and UIDP/50011/2020, national funds by FCT/MCTES.
Conflict of interest
None.