Published online by Cambridge University Press: 14 September 2004
The mechanism by which Giardia lamblia exerts its pathogenicity is likely to be multifactorial. A 58 kDa enterotoxin was purified and characterized from the excretory–secretory product (ESP) of the parasite (Kaur et al. 2001). In the present study an attempt has been made to elucidate the mechanism of action of the ESP, a potentially important enterotoxin. A 41 kDa glycoprotein was identified in the mouse enterocyte membrane fraction with which the ESP interacted in a GM1-specific manner. The GTPase activity was reduced in enterocytes stimulated with the ESP, resulting in an increase in the level of adenylate cyclase-dependent cyclic adenosine monophosphate (cAMP). The activity of protein kinase A (PKA) in the enterocytes was also upregulated after ESP treatment. Ultimately, a significant increase in intracellular Ca2+ concentration and decrease in cytosolic Cl− level were noticed in ESP-stimulated mouse enterocytes. Thus it is possible that the enterotoxic ESP could bind to the 41 kDa glycoprotein (receptor?) on the enterocytes and activate the G-protein-mediated signal transduction pathway resulting in alteration of electrolyte transport.
Giardia lamblia, is a waterborne parasite with a worldwide distribution (280 million estimated clinical cases per year) and is the most common intestinal parasite of humans in developed countries. Although usually self-limiting, infections can be life threatening in nutritionally deprived or immunocompromized individuals, or in the very young or elderly (Lloyd, Ralphs & Harris, 2002).
The pathophysiological processes responsible for small intestinal malabsorption and diarrhoea in giardiasis remain unclear (Faubert, 2000; Buret et al. 2002). A large number of studies have been performed to elucidate the mechanism(s) involved in the regulation of mammalian small intestinal electrolyte secretion (Hardcastle, Hardcastle & Noble, 1984; Binder, 1989; Field, Rad & Chang, 1989). Previous studies established that giardiasis causes a shortening of epithelial microvilli and that the resulting loss of overall absorptive surface area reduces the activities of disaccharidases, sodium-coupled glucose absorption, active glucose uptake and water absorption along the small intestine (Buret et al. 1992, 2002). Samra et al. (1988) reported that a crude preparation of ESPs of G. lamblia could stimulate net secretion of Na+ and Cl− in normal intestinal segments of mice. Impairment of the electrolyte transport leading to fluid secretion has also been reported in this infection (Ganguly et al. 1987; Gorowara et al. 1991). Intracellular messengers proposed to regulate directly the small intestinal electrolyte transport include cAMP, cGMP and Ca2+ (Hardcastle et al. 1984; Donowitz et al. 1985, 1989; Binder, 1989), the levels of which have been found to be altered by the extracellular messengers like hormones, microbial toxins through a receptor-mediated activation. The intracellular messengers are thought to activate protein kinases, which phosphorylate the membrane proteins, thereby affecting the transport carriers or the conductance channels (de Jonge, 1983; Donowitz et al. 1989; Asaoka et al. 1992). The intracellular messengers like cAMP; Ca2+ and PKC have also been implicated in electrolyte imbalance in G. lamblia-induced infection (Gorowara et al. 1998).
In diarrhoeagenic conditions toxins are considered to play an important role in the induction of fluid secretion (Fromm et al. 1974; Buret et al. 2002). When the toxins are released into the media of the host, they can interact with or even enter the host's cells and interfere with normal physiological functions by disrupting the delicate balance of various intracellular processes. In giardiasis the identity of a possible ‘toxin’ released by the parasite, though proposed, remained elusive. Recently, a novel 58 kDa excretory–secretory product (ESP) of G. lamblia having enterotoxic activity has been identified and characterized from our laboratory (Kaur et al. 2001). Antibody against this toxin has been found to cross-react with the binding subunit of the cholera toxin (CT). Moreover, the predilection of the ESP for GM1, the well-known receptor for the CT gave us an indication of its probable receptor (Shant et al. 2003). All the findings have led to speculations about the mechanism of action of this toxin on animal cells. Thus, the aim of this study was to trace the G-protein mediated signal transduction pathway in the ESP stimulated mice enterocytes.
Inbred Balb/c mice (10–15 g) obtained from Central Animal House of Postgraduate Institute of Medical Education and Research (Chandigarh, India) were used for the study. The animals were checked for any parasitic infection by formal ether concentration of 3 consecutive stool specimens (Kaur et al. 1999). Animals free of any infection were used for the study.
The trophozoites of G. lamblia, Portland-1 (P-1) strain, were maintained axenically in modified TYI-S-33 medium at 37 °C (Diamond, Harlow & Cunnick, 1978). The mass cultivation of the parasite was done according to the method as described by Farthing et al. (1982) with slight modifications (Kaur et al. 2001).
The ESP of G. lamblia was purified by the method of Shant et al. (2002). Briefly, the culture supernatant in minimum essential medium was fractionated with different percentage saturations of ammonium sulphate according to Dixon's nomogram (Dixon, 1953). The biologically active fractions were further purified on a Superdex 200 HR 10/30 gel filtration coloumn in the FPLC system (Pharmacia, Sweden).
Enterocytes were isolated from the small intestine as described by Pinkus (1981) with modifications (Toyoda, Lee & Labenthal, 1985). Briefly, the animals were sacrificed and the small intestine of each animal was quickly excised. The intestine was opened longitudinally, cut into small pieces and enterocytes were isolated by chelation-elution. The viability of cells was checked by trypan blue exclusion (Khurana et al. 1991).
Mice enterocytes extracted from the small intestine were suspended in saline. The cells were sonicated and the mixture was centrifuged (3000 g, 10 min) to remove the debris. The cell lysate containing a cocktail of protease inhibitors (Boehringer Mannheim) was further centrifuged (105000 g, 1 h) at 4 °C and the pellet containing the total membrane fraction of the enterocytes was suspended in 10 mM Tris/HCl (pH 7·2) containing 150 mM NaCl (TBS).
The total membrane preparation was subjected to sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS–PAGE) under reducing conditions (Laemmli, 1970) and the protein bands on the gel were electrophoretically transferred onto NCP by the method of Towbin, Staehelin & Gordon (1979). The NCP strips were blocked in TBS containing 2% BSA (BSA-TBS), overnight at 4 °C and washed thrice with TBS. These were then incubated separately with the purified ESP (2·5 μg/ml) both in the absence and in the presence of GM1 (1·0 μg/ml) for 2 h at 25 °C. After extensive washing with TBS containing 0·05% Tween-20 (TBST) and then TBS, the NCP strips were treated with immunoglobulin G (IgG) isolated from the purified ESP (IgGES, diluted to 1[ratio ]250 in BSA-TBS) for 2 h at 37 °C. This was followed by washing and incubation with horse-radish peroxidase-conjugated goat anti-rabbit IgG (diluted to 1[ratio ]1500 in BSA-TBS) at 37 °C for 1 h. Finally, the strips were developed with 3,3′-diaminobenzidine tetrahydrochloride (DAB) in TBS containing 30% H2O2 (1 μg/ml) for 30 min. The reaction was stopped by washing the NCP strips with water.
The enterocytes were isolated separately from various parts (duodenum, jejunum and ileum) of the small intestine of the mice and the membranes were prepared as described above. The membrane pellet obtained from each of the 3 parts of intestine was suspended in TBS. A 96-well flat-bottomed enzyme immunoassay plate was coated overnight at 4 °C with enterocyte membrane suspension (100 μl, 50 μg protein/ml) in 50 mM carbonate/bicarbonate buffer (pH 9·6). The unbound proteins were washed out with TBS. The residual binding sites of the wells were blocked by the addition of 200 μl of BSA-TBS followed by incubation at 37 °C for 2 h.The plates were washed thrice with TBS. Subsequently, the ESP (2·5 μg/ml) was added to each well and incubated for 1 h at 25 °C. For inhibition studies, the ESP (2·5 μg) pre-incubated with GM1 (1 μg) for 30 min was added to the enterocyte membrane-coated wells. The control wells were coated with the ESP only. The wells were washed with TBST and incubated with IgGES (diluted to 1[ratio ]1500 in BSA-TBS) for 2 h at 37 °C. Following washing, 100 μl of HRP-conjugated swine anti-rabbit IgG (diluted to 1[ratio ]1000 in BSA-TBS) was added to it. After incubation for 1 h at 37 °C, the plates were washed successively with TBST and TBS followed by incubation with 100 μl of ortho-phenylenediamine [OPD, 0·05% in 0·1 M citrate–phosphate buffer (pH 5·0) containing H2O2 (1 μl/ml)]. The reaction was stopped by addition of 6 M H2SO4 and the optical density (OD) of the colour reaction was recorded in an ELISA reader at 492 nm.
The viable enterocytes (106 cells/ml) were triggered with the required amount of purified ESP for different time-periods. Enterocytes without the ESP served as negative control in all the assays. The enterocytes triggered with commercial CT were taken as positive control. All the assays were done in the presence and absence of GM1 (1 μg) or IgGES (diluted to 1[ratio ]2500 in TBS) to assess the effect of the ESP on different parameters of signal transduction. Further, specific inhibitors of different intracellular mediators were used in respective assays to confirm the authenticity of the results. Finally, the cytosolic free Na+ and Cl− concentrations were estimated in order to correlate the electrolyte imbalance in enterocytes due to the effect of the ESP. All the assays were performed in triplicate.
The [Ca2+]i was estimated in enterocytes by the method of Pace & Galan (1994). Briefly, the enterocytes were suspended in 20 mM HEPES buffer (pH 7·4) containing 145 mM NaCl, 5 mM KCl, 1 mM Na2HPO4, 1 mM CaCl2, 0·5 mM MgSO4 and 5 mM glucose. The cells were triggered with different doses of the purified ESP for 1 min, washed (1000 g, 10 min at 4 °C) and loaded with 2 μM Fura-2/AM (Sigma, Chemicals, dissolved in DMSO) at 37 °C for 45 min. Unabsorbed dye was removed by washing the cells in HEPES buffer (thrice). Finally, the enterocytes were suspended in the same buffer and fluorescence measurements of the cell suspension were performed at an excitation wavelength of 340 nm. The emission spectrum was recorded at 510 nm. The intracellular free Ca2+ concentration was calculated by taking the Kd value of Fura-2/AM as 224. The optimum dose of the ESP was then used to trigger the enterocytes for different time-periods and the [Ca2+]i was estimated. Further, the [Ca2+]i in the enterocytes was also determined in the presence of GM1 (1 μg)/IgGES (diluted to 1[ratio ]2500 in TBS)/dantrolene (20 μM, a drug known to trap Ca2+ in intracellular stores).
The GTPase activity in the membrane fraction of the enterocytes was measured by the method of Sweeney (1995). The assay is based on the measurement of the hydrolysis of guanosine triphosphate (GTP) to inorganic phosphate (Pi) and guanosine diphosphate (GDP), a reaction catalysed by GTPase using [γ-32P] GTP. Thus, any GTPase induced hydrolysis results in the 32P label appearing as 32Pi. 32Pi can be separated from unhydrolysed [γ-32P] GTP and easily measured using liquid scintillation counting. The amount of 32Pi is directly proportional to the amount of [γ-32P] GTP hydrolysed and, therefore, proportional to the activity of GTPases in the preparation.
Briefly, the membrane suspension was taken into ADP-ribosylating buffer (25 mM Tris/HCl, pH 7·4, 1 mM EDTA, 1 mM DTT, 1 mM MgCl2, 1 mM ATP, 10 mM thymidine, 10 mM NAD+, 10 mM nicotinamide and 100 μM GTP), incubated with the ESP (2 μg) for different time-periods and finally resuspended in homogenizing buffer (10 mM Tris/HCl, pH 7·4, 1 mM EDTA, 5 mM DTT, 2 mM benzamidine, 50 μM chlorpromazine, 50 μM leupeptin, 0·25 TIU/ml aprotinin and 10 μM PMSF) for GTPase assay. The assay mixture contained 20 μl of this membrane suspension, 30 μl of water and 50 μl of GTPase reaction buffer [20 mM Tris/HCl (pH 7·4) containing 100 mM NaCl, 2 mM EDTA, 5 mM MgCl2, 2 mM DTT, 1 mM ATP, 1 mM creatine phosphate, 1 mM ouabain, 0·5 μM GTP and γ-32P GTP (final concentration 2·27×104 mCi/ml)]. Further, each tube was centrifuged (1500 g, 10 min, 4 °C) after addition of ice-cold activated charcoal suspension (2%, 900 μl). The supernatant (200 μl) from each reaction mixture was removed, counted for 32P content in a liquid scintillation counter and the rate of GTP hydrolysis was expressed in pmol/mg protein/min. The activity of GTPase was measured in the membrane fraction of enterocytes in the presence of GM1/IgGES/ouabain (100 μM), a non-specific inhibitor of GTPase.
The adenylate cyclase-dependent cAMP level was measured in the enterocytes by using the Biotrak™ cAMP [125I] assay system (Amersham Pharmacia Biotech, Code RPA 509). This system utilizes [125I] 2′-O-succinyl-cAMP tyrosine methyl ester as tracer with a high specific activity, together with a highly specific and sensitive antiserum. The method allows high sensitivity without the interference from ATP to which other adenylate cyclase assays are prone. The assay is based on the competition between unlabelled cAMP and a fixed quantity of 125I-labelled cAMP for a limited number of binding sites on a cAMP-specific antibody.
In this assay, the enterocytes were triggered with the ESP (2 μg). The cells were washed and the cAMP from the cells was extracted with cold 0·1 M HCl (0·1 ml) (15 min, 37 °C). The cell debris was removed by centrifugation (500 g, 10 min). The supernatant was titrated to pH 7·2 with 0·1 M NaOH and used for the estimations. Results were expressed as fmol of cAMP/mg protein. A separate set of experiments were conducted to measure the activity of cAMP in presence of GM1/IgGES/2′, 5′-dideoxy adenosine (DDA, a specific inhibitor of adenylate cyclase).
The protein kinase A activity in the enterocytes was measured by using a PepTag® non-radioactive cAMP-dependent protein kinase assay kit (Promega, USA, Cat. No. V5340) according to the manufacturers' instructions. The PepTag® assay utilizes brightly coloured, fluorescent peptide substrate i.e. PepTag® A1 Peptide, LRRASLG (Kemptide), that is highly specific for the kinase. Phosphorylation by PKA of this specific substrate alters the peptide's net charge from +1 to −1. This change in the net charge of the substrate allows the phosphorylated and the non-phosphorylated forms of the substrate to be rapidly separated and detected by agarose gel electrophoresis. The phosphorylated substrate is extracted from gel, heated at 95 °C, solubilized, acidified with glacial acetic acid and finally evaluated by measuring the optical density at 550 nm.
For this assay, the enterocytes were incubated with the ESP (2 μg) for different time-periods. The cells were washed, resuspended in TBS and sonicated. The debris was removed by centrifugation (500 g, 10 min) and the supernatant fraction of each tube was used for the estimation of PKA. The activity of PKA was expressed as units/106 cells. The activity of PKA was also measured in the presence of GM1/IgGES/ the peptide inhibitor (TTYADFIASGRTGRRNAIHD, 10 μM) specific for PKA.
The fluorescent indicator N-(6-methoxyquinolyl) acetoethyl ester (MQAE, Molecular Probe Co.) having the highest Cl− sensitivity among the fluorophores prepared to date, was used for the estimation of the level of Cl− within the enterocytes according to the method of Verkman et al. (1989).
In this assay, the enterocytes were incubated with the ESP (2 μg) for different time-periods and washed. The MQAE (5 mM) was loaded onto the cells by 3 min incubation in hypotonic solution (0·1% sodium citrate containing 0·1% Triton X-100) at 37 °C. The cells were centrifuged and resuspended in DMEM. The cells were loaded into a FACScan (Becton, Dickinson, USA) using the CELL QUEST programme and the analysis of the cell suspension was performed at an excitation wavelength of 360 nm. The emission spectrum was recorded at 410 nm. The results were expressed as the mean fluorescence intensity of labelled enterocytes, which could be directly correlated to the level of cytosolic free Cl−. The level of cytosolic free Cl− was also estimated in the presence of GM1/IgGES/chlorotoxin (1 μg), an inhibitor of chloride channels.
The fluorescent Na+ indicator, sodium binding benzofuran isophthalate (SBFI) from Molecular Probes Co (Eugene, OR) having high sensitivity to Na+ was used for the estimation of the level of Na+ within the enterocytes by the method of Borin & Siffert (1990).
In this assay, in one set of experiments the enterocytes were incubated with the ESP (2 μg) for different time-periods in sodium-free K+-HEPES buffer [10 mM HEPES/5 mM KH2PO4 (pH 7·4), containing 150 mM KCl, 1 mM MgSO4 and 5 mM glucose]. In another set of experiments, the enterocytes were incubated in the presence of the ESP in Na+-HEPES buffer [10 mM HEPES/5 mM KH2PO4 (pH 7·4) containing 145 mM NaCl, 5 mM KCl, 1 mM MgSO4 and 5 mM glucose]. The cells were washed in the same buffer and suspended in hypotonic solution (0·1% potassium citrate containing 0·1% Triton X-100) containing a final concentration of 10 μM of SBFI-AM. The cells were incubated for 3 min at 37 °C, centrifuged and resuspended in K+-HEPES/Na+-HEPES buffer. The cells were put through the FACScan (Becton Dickinson, USA) using the CELL-QUEST programme and the analysis of the cell suspension was performed at an excitation wavelength of 385 nm. The emission spectrum was recorded at 490 nm. The results were expressed as the mean fluorescence intensity of labelled enterocytes, which could be directly correlated to the level of cytosolic free Na+. The level of Na+ in the enterocytes was also estimated in the presence of GM1/IgGES/amiloride (1 μM), an inhibitor of Na+/K+ ATPase and known to be a blocker of transmembrane Na+ entry.
The data were analysed by standard statistical methods [mean, S.D., analysis of variance (ANOVA) and unpaired Student's t-tests wherever applicable]. In comparing groups P<0·05 was taken as significant.
ESP purified by the method of Kaur et al. (2001) with slight modification (Shant et al. 2003) was used in the present study. The interaction of the purified ESP with the membrane proteins was visualized as a darkly stained band of Mr 41 kDa on the nitrocellulose paper in Western blotting. However, the intensity of the band was found to be greatly reduced in the presence of GM1 (Fig. 1). The purified ESP could bind with the membrane fractions of the enterocytes obtained from 3 different regions (duodenum, jejunum and ileum) of the mice small intestine to almost the same extent. However, maximum inhibition of binding was observed in the membrane fraction of the enterocytes obtained from the jejunum region in the presence of ESP pre-incubated with GM1 (Table 1).

Fig. 1. Identification of the ESP binding proteins on the enterocyte membrane. The ESP interacting molecule(s) on mouse enterocyte membrane were identified by Western blotting with IgGES. Lane A, molecular weight marker; lane B, enterocyte membrane proteins [SDS–PAGE (10%)]; lane C, ESP interacting protein on the enterocyte membrane and lane D, ESP interacting protein on the enterocyte membrane in the presence of GM1.
Table 1. Inhibition of the ESP binding to the enterocyte membrane from different parts of the mouse small intestine in the presence of GM1 (ELISA titre at OD490 corresponding to ~2·0 for each set was taken as 100% binding of ESP to that membrane fraction. The % inhibition in the presence of GM1 for each set was then calculated. Values are expressed as mean±S.D.)

The optimum dose of the purified ESP to cause an effective increase in the [Ca2+] (nM) in mouse enterocytes (106 cells) was found to be 2–5 μg (Fig. 2A). Thus, 2 μg ESP/106 enterocytes was used in the study of all other parameters. The [Ca2+]i in mouse enterocytes triggered with the ESP (2 μg) at different time-intervals revealed a 6·6-fold increase in [Ca2+]i at 1 min as compared to that in control enterocytes (Fig. 2B). In the presence of dantrolene (20 μM), the ESP-induced [Ca2+]i in the enterocytes at 1 min was significantly reduced to 122·0±1·3 nM. Further, in enterocytes triggered with ESP pre-incubated with IgGES or GM1 (1 min), the effective concentrations of the intracellular calcium ions were found to be 124·6±10·1 nM and 214·8±25·6 nM respectively. The CT (positive control) could induce a 4·8-fold increase in [Ca2+]i (370·0±8·0 nM) in the enterocytes as compared to that of control (76·7±3·6 nM).

Fig. 2. Values are expressed as mean±S.D. The [Ca2+]i levels in enterocytes (106) isolated from mice intestine and triggered with different doses of the purified ESP for 1 min (A), 2 μg of purified ESP at different times (B). The cells were washed and labelled with Fura2-AM as described in the Materials and Methods section. The [Ca2+]i was calculated by using KD value of Fura2-AM as 224.The optimum dose of the ESP was then selected. The results were expressed in mM/106 cells. *P<0·05, **P<0·01, ***P<0·001 as compared to control.
The GTPase activity (pmol/mg protein/min) in the membrane fraction of the ESP-triggered enterocytes was decreased significantly within 1 min (Fig. 3A). Partial inhibition in the reduction of the GTPase activity (28·52+1·35 or 25·74+0·61) was observed when the enterocytes were stimulated with the ESP pre-incubated with IgGES (1[ratio ]2500) or GM1 (1 μg). However, the activity increased to 30·69±0·39 in the presence of ouabain (100 μM). The CT-triggered enterocytes (positive control) showed a statistically significant (P<0·001) decrease in the GTPase activity (15·75±0·29) at 1 min (Fig. 3B).

Fig. 3. Values are expressed as mean±S.D. The mouse enterocyte membrane suspension was taken in ADP-ribosylating buffer and incubated with ESP (2 μg) at different times (A); ESP (2 μg) alone, ESP pre-incubated with GM1 (1 μg)/IgGES (diluted to 1[ratio ]2500 in TBS) separately as well as in the presence of ouabain (100 μM), a non-specific inhibitor of GTPase at 1 min (B). CT was used as the positive control. The rate of GTP hydrolysis was expressed in pmol/mg protein/min. **P<0·01, ***P<0·001 as compared to control (A); *P<0·05, as compared to ESP (B).
A significant increase (P<0·001) in the level of adenylate cyclase dependent cAMP (fmol/mg protein) was observed at 5 min (60·0±7·07) in the ESP as well as CT stimulated enterocytes (76·3±1·8, P<0·001) as compared to that of control enterocytes (non-detectable). Further, complete reduction in the levels of cAMP was noticed in the presence of IgGES/GM1/DDA at the above time.
The protein kinase A activity (units/106 cells) in the enterocytes triggered with the ESP was found to be maximum at 10 min. The increase was statistically significant (0·91±0·0) as compared to that of the control enterocytes (0·05±0·0) (Fig. 4) However, in the enterocytes treated with the ESP pre-incubated in the presence of IgGES/GM1/peptide inhibitor the PKA activity at 5 min and 10 min was reduced to a non-detectable range. The CT-stimulated enterocytes (positive control) showed a significant increase in the PKA activity (2·5±0·3) as compared to that of control cells.

Fig. 4. Values are expressed as mean±S.D. The PKA activity in mouse enterocytes(106 cells) was measured by using a PepTag® non-radioactive cAMP-dependent protein kinase assay kit (Promega, USA). For this assay, the enterocytes were incubated with the ESP for different time-periods and the PKA activity was estimated in the cell lysate as per manufacturer's instructions. The activity of PKA was expressed as units/106 cells. *P<0·05, ***P<0·001 as compared to control.
In the present study, the status of Na+ and Cl− was measured in mouse enterocytes using specific fluorescent probes. The maximum fluorescence intensity (M2 region, taken as 100%) was observed in control enterocytes labelled with MQAE. A decrease in the fluorescence intensity in this region was observed when enterocytes were triggered with the ESP for different time-periods (5 sec to 10 min) (Fig. 5). The maximum reduction was found at 5 min. However, when the enterocytes were pre-incubated with chlorotoxin (1 μg) and triggered with the ESP (5 min), a 9-fold increase in the fluorescence intensity (36·24±5·45%) was observed. Further, the ESP pre-treated with IgGES or GM1 could also enhance the fluorescence intensity (30·15±7·68% or 24·03±3·16%) of the enterocytes at 5 min. The cytosolic free sodium levels in the enterocytes were measured using SBFI. Maximum fluorescence intensity (M1 region, taken as 100%) was observed in control enterocytes. A minor decrease in the fluorescence intensity (97·9±0·05% or 93·9±0·05%) was observed when enterocytes were treated with ESP for 30 sec either in Na+-HEPES buffer or K+-HEPES buffer. However, the enterocytes pre-incubated with amiloride [(1 μM), a Na+/K+-ATPase inhibitor] followed by triggering with the ESP could not reveal any significant change in the fluorescence intensity of labelled cells. Further, the ESP pre-incubated with IgGES or GM1 could also not induce any significant change.

Fig. 5. Values are expressed as mean±S.D. The level of cytosolic Cl− in enterocytes triggered with ESP (2 μg) at different times was measured in view of % of MQAE (Cl− specific fluorophore)-labelled cells. The results were expressed as the mean fluorescent intensity of labelled cells, which was directly correlated to the level of cytosolic free Cl−. *P<0·05, **P<0·01, ***P<0·001 as compared to control.
G. lamblia, a unicellular, flagellated intestinal parasite is the most common cause of waterborne outbreaks of diarrhoea. It has been well established that Giardia could cause intestinal pathophysiology without invading the mucosal tissue, which indicated the probable release of a toxin by the parasite (Buret et al. 2002). Jimenez et al. (2004) have suggested that the ES antigens might be involved in the intestinal alterations associated with giardiasis. The 58 kDa ESP of G. lamblia (Portland-1) is the first immunogenic, potent enterotoxin (Kaur et al. 2001), which may be a major component, responsible for G. lamblia-induced diarrhoea. In the present study, an attempt has been made to understand the interaction of this toxin with the host cells (enterocytes) and its effect on intracellular signal transduction.
Glycosphingolipid (GSL)-enriched micro-domains are known as cellular binding sites for various pathogens. These attachment platforms are specifically associated with transducer molecules, so that binding of pathogens or their toxins to the host cell surface may result in the activation of signal transduction pathways. In the intestinal epithelium, such pathogen-induced dysregulations of signal transduction can elicit a severe disruption of enterocyte functions (Fantini et al. 2000).
In the present study, we identified a protein of Mr 41 kDa in the membrane fraction of mouse enterocytes, with which the ESP could interact in a GM1-specific manner. This observation has confirmed the glycoprotein nature of the 41 kDa molecule. Since it could not be highlighted in Western immunoblots in the presence of GM1, it can be assumed that the carbohydrate unit of this glycoprotein is somehow involved in the binding of the ESP to the mouse enterocytes. However, it is yet to be determined whether this molecule is the receptor of the ESP or not. The GM1-specific binding of the ESP was maximum in the membrane fraction of the enterocytes obtained from the jejunum. It may be possible that in the ileum as well as the duodenum membrane fraction, along with this specific glycoprotein (41 kDa), some other interacting moieties are also present with which the ESP could interact in a non-carbohydrate (non-GM1)-specific manner.
The binding of the ESP to the mouse enterocyte membrane could initiate a sequence of molecular events within the cells leading to electrolyte imbalance. In the present study, the effect of the specific inhibitor(s) of the respective intracellular mediator(s) of the diarrhoeagenic condition has also been assessed while studying the mechanism of action of the ESP in mouse enterocytes.
Calcium has been established as an intracellular regulator of small intestinal as well as colonic transmembrane electrolyte transport (Donowitz & Asarkof, 1982; Zimmerman, Dobbins & Binder, 1983). Khurana et al. (1991) have observed an enhancement in the level of [Ca2+]i in S. typhimurium enterotoxin-treated enterocytes. Further, the [Ca2+]i was found to be increased in enterocytes isolated from V. cholerae 0139-treated rabbit ileum (Gorowara et al. 1998). In the present study, the intracellular calcium stores seem to have major involvement since significant reduction in the concentration of cytosolic calcium was observed in enterocytes triggered with the ESP in the presence of dantrolene, known to act specifically on calcium channels by reducing the steady and phasic leakage of Ca2+ into the cytosol without changing the calcium flux at the plasma membrane (Gorowara et al. 1998). A similar effect with dantrolene was also observed in the case of V. cholerae non-01 heat-stable enterotoxin-treated rat enterocytes (Hoque et al. 2001).
Trimeric guanine nucleotide-binding proteins (G-proteins) function as the key regulatory elements in a number of transmembrane signalling cascades where they convey information from agonist-activated receptors to effector molecules (Svoboda & Novotny, 2002). It has been reported that commercially available CT could act by the transfer of the ADP-ribose moiety of NAD+ to a specific arginine residue in the α-subunit of Gs protein leading to the inhibition of its intrinsic GTPase activity. This ultimately resulted in the activation of adenylate cyclase (Sears & Kaper, 1996). In the present study, it has been found that the ESP could reduce the GTPase activity in the mouse enterocytes, thereby suggesting a major involvement of the G protein in the ESP-induced transmembrane signalling. Further, an increase in the GTPase activity in enterocytes in the presence of ouabain, could reveal the authenticity of the previous observations.
It has been reported that in G. lamblia infection there was an increase in cAMP levels, a major intracellular second messenger. The cAMP could produce its effect by mobilizing calcium from intracellular stores, which in turn could regulate the electrolyte transport (Ganguly et al. 1984; Gorowara et al. 1992). The cAMP level has also been found to be elevated in Salmonella enterotoxin-treated enterocytes (Peterson et al. 1983). In the present study the increase in the adenylate cyclase-dependent cAMP level in the ESP-treated cells can be correlated indirectly to the increased level of adenylate cyclase in the system. There was a complete reduction in the level of cAMP in the presence of DDA, a specific inhibitor of adenylate cyclase. This confirmed the involvement of this enzyme in the toxin-induced disruption of the normal physiological functions of the cells.
One crucial step resulting from increased cAMP level is activation of protein kinase A. Kinase activity of the native PKA has been detected in crude extracts of G. lamblia using kemptide as a substrate (Abel et al. 2001). Chang & Rao (1991) have shown that the CT could increase the activity of PKA in isolated intestinal epithelial cells, which then phosphorylated numerous substrates within the cells. In the present study, although maximum increase in PKA activity was found at 10 min but from 1 min onwards PKA activity was observed in the cells. The earlier electrolyte imbalance in response to ESP may be because of other signal transduction pathways through which the ion disturbances could be triggered as also observed in the case of cholera toxin (CT) from V. cholerae. Further, cAMP which was upregulated at 1 min, could also be responsible for the increase in [Ca2+]i at 1 min. The ESP-induced activation of the PKA in mouse enterocytes was found to be inhibited in the presence of a PKA-specific peptide inhibitor. Hence, our data suggest that the molecular events initiated by the ESP in the enterocytes could increase adenylate cyclase activity in a G-protein dependent manner, leading to the activation of cAMP/PKA-mediated signal transduction pathway. This might further lead to phosphorylation of substrates involved in disturbance in ion transport.
In the pathophysiology of diarrhoeal disease, alterations in the fluid and electrolyte concentration within the cell and hence its transport are of prime importance. This is well documented in experimental salmonellosis, giardiasis, as well as different enterotoxin (cholera enterotoxin, E. coli heat-labile and heat stable enterotoxin)-induced diarrhoea (Khurana et al. 1991; Kanwar et al. 1994; Gerok, 2000). It has been reported that in the cholera toxin-induced diarrhoea an increased cAMP level could lead to increased Cl− secretion by the intestinal crypt cells and decreased NaCl – coupled absorption by villus cells (Kaper, Fasano & Trucksis, 1994). Impairment of transport of electrolytes and nutrients in brush border membrane vesicles of G. lamblia-infected albino mice has been reported earlier (Ganguly et al. 1984). It has been shown previously that GP 49, an invariant GP I-anchored antigen of G. lamblia altered the electrolyte fluxes thereby regulating the fluid secretion in the cultured human intestinal epithelial cell line, T84 (Das et al. 1994). Further, Gorowara et al. (1992) have observed that G. lamblia infection could result in decreased D-glucose absorption and increased Na+ and Cl− secretion in the intestines of mice. The present investigation assessed the effect of the ESP on intracellular electrolyte concentrations. A significant reduction in the fluorescence intensity of the enterocytes stimulated with the ESP (as assessed with MQAE) indicated an enhanced secretion of Cl− ions from the cells to the external milieu. Similarly, a decrease in the fluorescence intensity of the ESP-triggered enterocytes in the presence of SBFI depicted the level of Na+ secretion from cells, since this dye is known to trap cytosolic free Na+. The authenticity of these experiments was proved in the presence of chlorotoxin, the Cl− channel blocker and amiloride, the Na+/K+-ATPase inhibitor. These experiments directly suggested the involvement of Na+/K+-ATPase (the sodium pump) and the Cl− channels in the alteration of Na+ and Cl− level within the cells.
The alteration in the levels of all the intracellular mediators could be inhibited in the presence of GM1 (1 μg) as well as IgGES (diluted to 1[ratio ]2500 in TBS). In most of the cases, the maximum inhibition was observed in the presence of IgGES. This observation has clearly indicated that the antibody binding epitope of the ESP might have a more significant contribution in cell signalling as compared to GM1 binding epitope. As per literature, the GM1 binding epitope might be involved only in the recognition of the cell surface receptor (Minke et al. 1999). Further, complete reversal of the level of intracellular mediators was not observed in enterocytes stimulated with the ESP pre-incubated in the presence of either IgGES or GM1 thereby indicating that somehow both the IgGES and GM1-specific epitopes of ESP are essential for transducing signals within the cells.
Thus it is possible that the enterotoxic ESP could bind to the 41 kDa glycoprotein (receptor?) on the enterocytes and activate signal transduction cascades resulting in alteration of the electrolyte transport. The present investigation has indicated that in the ESP-stimulated mouse enterocytes the reduced GTPase activity might be responsible for an increase in the level of adenylate cyclase-dependent cAMP and thereby PKA activity which might be involved in the ion disturbance within the cell (Fig. 6). Hence, it can be suggested that the knowledge of the pathophysiological mechanism of the disease as well as the receptor of the ESP may have importance in developing strategies to prevent the infection caused by this organism.

Fig. 6. Proposed mode of action of the ESP from Giardia lamblia in mice enterocytes.
The authors acknowledge the financial assistance provided by CSIR, New Delhi to J.S.

Fig. 1. Identification of the ESP binding proteins on the enterocyte membrane. The ESP interacting molecule(s) on mouse enterocyte membrane were identified by Western blotting with IgGES. Lane A, molecular weight marker; lane B, enterocyte membrane proteins [SDS–PAGE (10%)]; lane C, ESP interacting protein on the enterocyte membrane and lane D, ESP interacting protein on the enterocyte membrane in the presence of GM1.

Table 1. Inhibition of the ESP binding to the enterocyte membrane from different parts of the mouse small intestine in the presence of GM1
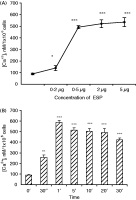
Fig. 2. Values are expressed as mean±S.D. The [Ca2+]i levels in enterocytes (106) isolated from mice intestine and triggered with different doses of the purified ESP for 1 min (A), 2 μg of purified ESP at different times (B). The cells were washed and labelled with Fura2-AM as described in the Materials and Methods section. The [Ca2+]i was calculated by using KD value of Fura2-AM as 224.The optimum dose of the ESP was then selected. The results were expressed in mM/106 cells. *P<0·05, **P<0·01, ***P<0·001 as compared to control.
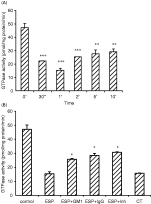
Fig. 3. Values are expressed as mean±S.D. The mouse enterocyte membrane suspension was taken in ADP-ribosylating buffer and incubated with ESP (2 μg) at different times (A); ESP (2 μg) alone, ESP pre-incubated with GM1 (1 μg)/IgGES (diluted to 1[ratio ]2500 in TBS) separately as well as in the presence of ouabain (100 μM), a non-specific inhibitor of GTPase at 1 min (B). CT was used as the positive control. The rate of GTP hydrolysis was expressed in pmol/mg protein/min. **P<0·01, ***P<0·001 as compared to control (A); *P<0·05, as compared to ESP (B).

Fig. 4. Values are expressed as mean±S.D. The PKA activity in mouse enterocytes(106 cells) was measured by using a PepTag® non-radioactive cAMP-dependent protein kinase assay kit (Promega, USA). For this assay, the enterocytes were incubated with the ESP for different time-periods and the PKA activity was estimated in the cell lysate as per manufacturer's instructions. The activity of PKA was expressed as units/106 cells. *P<0·05, ***P<0·001 as compared to control.

Fig. 5. Values are expressed as mean±S.D. The level of cytosolic Cl− in enterocytes triggered with ESP (2 μg) at different times was measured in view of % of MQAE (Cl− specific fluorophore)-labelled cells. The results were expressed as the mean fluorescent intensity of labelled cells, which was directly correlated to the level of cytosolic free Cl−. *P<0·05, **P<0·01, ***P<0·001 as compared to control.
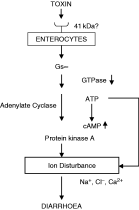
Fig. 6. Proposed mode of action of the ESP from Giardia lamblia in mice enterocytes.