Introduction
There is increasing evidence that the Southeast Asian liver fluke Opisthorchis viverrini may serve as a carrier of Helicobacter pylori and implicate this bacterium in carcinogenesis of opisthorchiasis-associated cholangiocarcinoma (CCA) (Boonyanugomol et al., Reference Boonyanugomol, Chomvarin, Sripa, Bhudhisawasdi, Khuntikeo, Hahnvajanawong and Chamsuwan2012; Deenonpoe et al., Reference Deenonpoe, Chomvarin, Pairojkul, Chamgramol, Loukas, Brindley and Sripa2015, Reference Deenonpoe, Mairiang, Mairiang, Pairojkul, Chamgramol, Rinaldi, Loukas, Brindley and Sripa2017; Sripa et al., Reference Sripa, Deenonpoe and Brindley2017). Helicobacter pylori and O. viverrini may have evolved which facilitates conveyance of the bacillus into human bile duct during the migration of the juvenile fluke, following ingestion of the metacercaria in raw or undercooked cyprinid fish and establishment of infection (Plieskatt et al., Reference Plieskatt, Deenonpoe, Mulvenna, Krause, Sripa, Bethony and Brindley2013; Deenonpoe et al., Reference Deenonpoe, Chomvarin, Pairojkul, Chamgramol, Loukas, Brindley and Sripa2015; Suyapoh et al., Reference Suyapoh, Tirnitz-Parker, Tangkawattana, Suttiprapa and Sripa2021). Since H. pylori bacteria have been shown to localize on the gut epithelium and the tegument of the fluke (Deenonpoe et al., Reference Deenonpoe, Chomvarin, Pairojkul, Chamgramol, Loukas, Brindley and Sripa2015; Suyapoh et al., Reference Suyapoh, Tirnitz-Parker, Tangkawattana, Suttiprapa and Sripa2021), the H. pylori bacillus might be passively transported by the migrating parasite via these organs (Suyapoh et al., Reference Suyapoh, Tirnitz-Parker, Tangkawattana, Suttiprapa and Sripa2021). Once the fluke reaches the biliary tract, the H. pylori may further colonize to the cholangiocytes and induce disease in similar ways as described in the gastric epithelium (Boonyanugomol et al., Reference Boonyanugomol, Chomvarin, Hahnvajanawong, Sripa, Kaparakis-Liaskos and Ferrero2013; Hatakeyama, Reference Hatakeyama2014; Backert et al., Reference Backert, Neddermann, Maubach and Naumann2016). However, mechanism(s) by which and how H. pylori might colonize the gut epithelium and the tegument of O. viverrini have not been described.
Establishment of infection by H. pylori requires adhesion and colonization on the gut epithelium as those described in the mammalian stomach (Chmiela and Kupcinskas, Reference Chmiela and Kupcinskas2019; Matos et al., Reference Matos, Amorim, Magalhaes, Haesebrouck, Gartner and Reis2021). The colonization involves interaction between the bacterial adhesins and host receptors (Fagoonee and Pellicano, Reference Fagoonee and Pellicano2019). There are several adhesins in H. pylori including blood-antigen binding protein A (BabA) and sialic acid-binding adhesin (SabA), neutrophil-activating protein (NAP), heat-shock protein 60 (Hsp60), adherence-associated proteins (AlpA and AlpB), H. pylori outer membrane protein (HopZ) and lacdiNAc-binding adhesin (LabA) (Kao et al., Reference Kao, Sheu and Wu2016; Matos et al., Reference Matos, Amorim, Magalhaes, Haesebrouck, Gartner and Reis2021) that interact with host tissues and cells. BabA mediates binding of the bacteria to Lewis B antigens (Ilver et al., Reference Ilver, Arnqvist, Ogren, Frick, Kersulyte, Incecik, Berg, Covacci, Engstrand and Boren1998) and related terminal fucose residues found on blood group O (H antigen), A and B antigens (Aspholm-Hurtig et al., Reference Aspholm-Hurtig, Dailide, Lahmann, Kalia, Ilver, Roche, Vikstrom, Sjostrom, Linden, Backstrom, Lundberg, Arnqvist, Mahdavi, Nilsson, Velapatino, Gilman, Gerhard, Alarcon, Lopez-Brea, Nakazawa, Fox, Correa, Dominguez-Bello, Perez-Perez, Blaser, Normark, Carlstedt, Oscarson, Teneberg, Berg and Boren2004). Similar to BabA, the SabA has affinity for sialyl-Lewis X (Mahdavi et al., Reference Mahdavi, Sonden, Hurtig, Olfat, Forsberg, Roche, Angstrom, Larsson, Teneberg, Karlsson, Altraja, Wadstrom, Kersulyte, Berg, Dubois, Petersson, Magnusson, Norberg, Lindh, Lundskog, Arnqvist, Hammarstrom and Boren2002). Toll-like receptor 4 (TLR4) is a known receptor for bacterial lipopolysaccharide (Pachathundikandi et al., Reference Pachathundikandi, Tegtmeyer and Backert2013).
Orthologues of the human cell receptors and antigens have yet to be described from the epithelium of the gastrodermis of the gut and/or the tegument of O. viverrini or related flukes. Here we examined the expression of host receptors including Lewis B, sialyl-Lewis X, TLR4 and L-fucose in the adult stage of O. viverrini and investigated role of certain glycans in host–pathogen interaction.
Materials and methods
Liver fluke samples
Opisthorchis viverrini adult worms and infected hamster livers were sourced from an ongoing project (Animal ethics #AEKKU 55/2554). Syrian golden hamsters were infected with 50 O. viverrini metacercariae via intragastric intubation as described (Suyapoh et al., Reference Suyapoh, Tirnitz-Parker, Tangkawattana, Suttiprapa and Sripa2021). Adult worms were recovered from the bile ducts of infected hamster livers 12 weeks post-infection and processed as below. Formalin-fixed tissues of O. viverrini were used for the histochemistry and immunolocalization studies.
DNA extraction and PCR amplification for H. pylori
DNA was extracted from 50 individual O. viverrini adult worms using Gentra Puregene kit (Qiagen, Germany) according to the manufacturer's instructions. Briefly, each worm was homogenized in 300 μL of a lysis solution, the lysates were incubated at 65°C for 60 min after which RNase was added and protein was precipitated. After clarification by centrifugation, the supernatant was transferred to 300 μL isopropanol to precipitate the genomic DNA. The DNA pellet was washed by 70% ethyl alcohol and dissolved in DNA hydration solution, and stored at −20°C until used.
Detection of H. pylori in the O. viverrini worms was undertaken using nested PCR amplification of the H. pylori-specific ureA gene using ureA-specific primers (Deenonpoe et al., Reference Deenonpoe, Mairiang, Mairiang, Pairojkul, Chamgramol, Rinaldi, Loukas, Brindley and Sripa2017) (Table 1). The reaction mixture contained 5 mm dNTP, 37.5 mm MgCl2, 2.5 U of Taq DNA polymerase and 0.2 mm primers. For the outer primer, the analysis settings included 30 s of initial denaturation at 94°C, 40 cycles of 30 s at 62°C and 30 s at 72°C for annealing and elongation steps. For the inner primer, amplification conditions were 30 s of initial denaturation at 94°C, 40 cycles of 30 s at 59°C and 30 s at 72°C for annealing and elongation steps. The PCR was performed in an automated thermocycler GeneAmp® PCR system 9700 (Applied Biosystems CO., LTD, USA). The amplified products were sized by electrophoresis through 1% agarose gel, stained with ethidium bromide and visualized and documented under UV illumination.
Table 1. Primer sequences for Helicobacter pylori-specific ureA (Hp-ureA).

Immunohistochemistry detection of H. pylori receptors
Formalin-fixed and paraffin-embedded tissue of 100 O. viverrini parasites were used (Suyapoh et al., Reference Suyapoh, Tirnitz-Parker, Tangkawattana, Suttiprapa and Sripa2021). Histological sections (4 μm) were deparaffinized and rehydrated. For the antigen retrieval, the slides were immersed in a pressure cooker for 5 min in citrate buffer (pH 6) and then allowed to cool for 20 min. After washing with phosphate-buffered saline, pH 7.4 (PBS), the endogenous peroxidase activity was inactivated in solution containing 3% hydrogen peroxide in methanol. Next, the slides were blocked with 5% normal horse serum in PBS. Primary antibody specific for TLR4 (dilution 1:100) (Santa Cruz Biotechnology, Inc., USA), Lewis B (dilution 1:50) (Abcam, UK) and sialyl-Lewis X (dilution 1:50) (Thermo Fisher Scientific, USA) was added and incubated overnight at 4°C. After washing with PBS, the sections were incubated with appropriate secondary antibody conjugated with horse radish peroxidase (HRP) (dilution 1:100) (Sigma, USA) for 30 min at room temperature. The slides were rinsed thoroughly with PBS and then developed with 3-3′-diamino benzidine tetrahydrochloride (DAB) (Sigma, USA), counterstained with Mayer's haematoxylin, dehydrated, cleared in xylene and mounted with Permount®. L-fucose was histochemically stained with biotinylated Ulex europaeus agglutinin I (Vector Laboratories, USA) overnight at 4°C, followed by streptavidin-HRP (Vector Laboratories, USA), and developed with DAB, counterstained, cleared and mounted as above. Control experiments with omission of the primary antibody or lectin conjugate were parallelly performed.
Helicobacter pylori strains and growth media
Helicobacter pylori Thai strains (BT112) were cultured in brain heart infusion medium with 5% human blood or sheep blood, at 37°C in a microaerophilic condition for 3–5 days (Suyapoh et al., Reference Suyapoh, Tirnitz-Parker, Tangkawattana, Suttiprapa and Sripa2021). Bacterial pellets were washed and labelled with fluorescein isothiocyanate (FITC) as described (Falk et al., Reference Falk, Roth, Boren, Westblom, Gordon and Normark1993).
In vitro assay of H. pylori localization to liver fluke sections
Tissue sections of O. viverrini were deparaffinized in xylene, rehydrated in a series of ethanol alcohol, rinsed in tap water followed by PBS for 5 min. The sections were then blocked for non-specific staining with 0.2% BSA/TWEEN for 15–30 min. The FITC-labelled bacterial suspension diluted in blocking buffer (OD600 = 0.05) was placed onto the sections and incubated for 60 min at room temperature. After thorough washing in PBS, the slides were viewed and photographed using confocal microscopy (ZEISS, model LSM800). Control experiments with unlabelled H. pylori were parallelly done.
Specificity verification of fucose receptor
In order to test the specificity of the putative receptor, we employed the glycosidase, fucosidase, which catalyses the hydrolysis of L-fucose. Individually, 27 adult O. viverrini liver flukes were separated into 3 treatment groups of 9 worms each and exposed to increasing concentrations of fucosidase (New England BioLabs, Ltd., UK), specifically 0 (control), 40 and 100 U mL−1. Three worms of each treatment group were incubated at 37°C with the fucosidase for 2, 16 or 24 h, respectively, after which the worms were transferred to DNA extraction buffer (above). Subsequently, qPCR for ureA of H. pylori was undertaken by calculation of copy number with the ureA-plasmid standard curve. The qPCR reaction was performed in a 96-well microtitre plate using 12.5 μL of Master mix 2× [Thermo Scientific Maxima SYBR Green/ROX qPCR Master Mix (2×)] containing Maxima® Hot Start Taq DNA polymerase and dNTPs (dATP, dCTP, dGTP and dTTP) in an optimized PCR buffer, 0.5 μ m forward/reverse primer mix and 10 ng of DNA template, in free nuclease water to a final volume of 25 μL. The PCR was performed in duplicate in the Applied Biosystems® QuantStudio™ 6 Flex Real-Time PCR System (Life Technologies, Singapore) with the following condition; 95°C, 30 s; 55°C, 30 s; 72°C, 30 s × 35 cycles and the melting curve condition with 95°C, 30 s; 55°C, 60 s; 95°C, 15 s. Details of primer sets and product sizes are shown in Table 1. The qPCR output was expressed as number of DNA copies per reaction.
Statistical analysis
Descriptive statistical analysis was carried out using percentages. Specific verification of fucose receptors was analysed by ANOVA followed by Dunnett's test for multiple comparison. Analysis was accomplished using GraphPad Prism version 7 software (GraphPad, San Diego, CA). P values <0.05 were considered to be statistically significant.
Results
Detection of H. pylori ureA in O. viverrini adult worms
To determine the presence of H. pylori in O. viverrini adult worms, nested PCR for ureA was employed. The results revealed that the ureA with approximately 359 bp was identified in 41 out of 49 (79%) genomic DNAs, each from individual O. viverrini. Representative nested PCR amplification of ureA gene products in 1% agarose gel is shown in Fig. 1.

Fig. 1. Representative PCR products amplified by using Helicobacter pylori ureA gene (product size = 359 bp). M, marker; C, positive control (H. pylori) and lane 1–24 are individual O. viverrini worms. Data representative of PCR products amplified from DNA extracted from 49 O. viverrini adult worms.
Expression of H . pylori -specific receptors in O . viverrini gut epithelium
Immunohistochemical analysis of 94 adult worm sections revealed certain specific receptors for H. pylori presented along the gut epithelium of O. viverrini. Lewis B, sialyl-Lewis X and TLR4 were detected in 3.2% (3/93), 3.1% (6/97) and 18.2% (16/88) of the samples, respectively. Notably, L-fucose residue was detected along the gut epithelium of O. viverrini in 70.2% (66/94) of the sections. Figure 2A–D provides representative micrographs of the expression of these receptors in the epithelium of gut and in the tegument of the liver fluke.
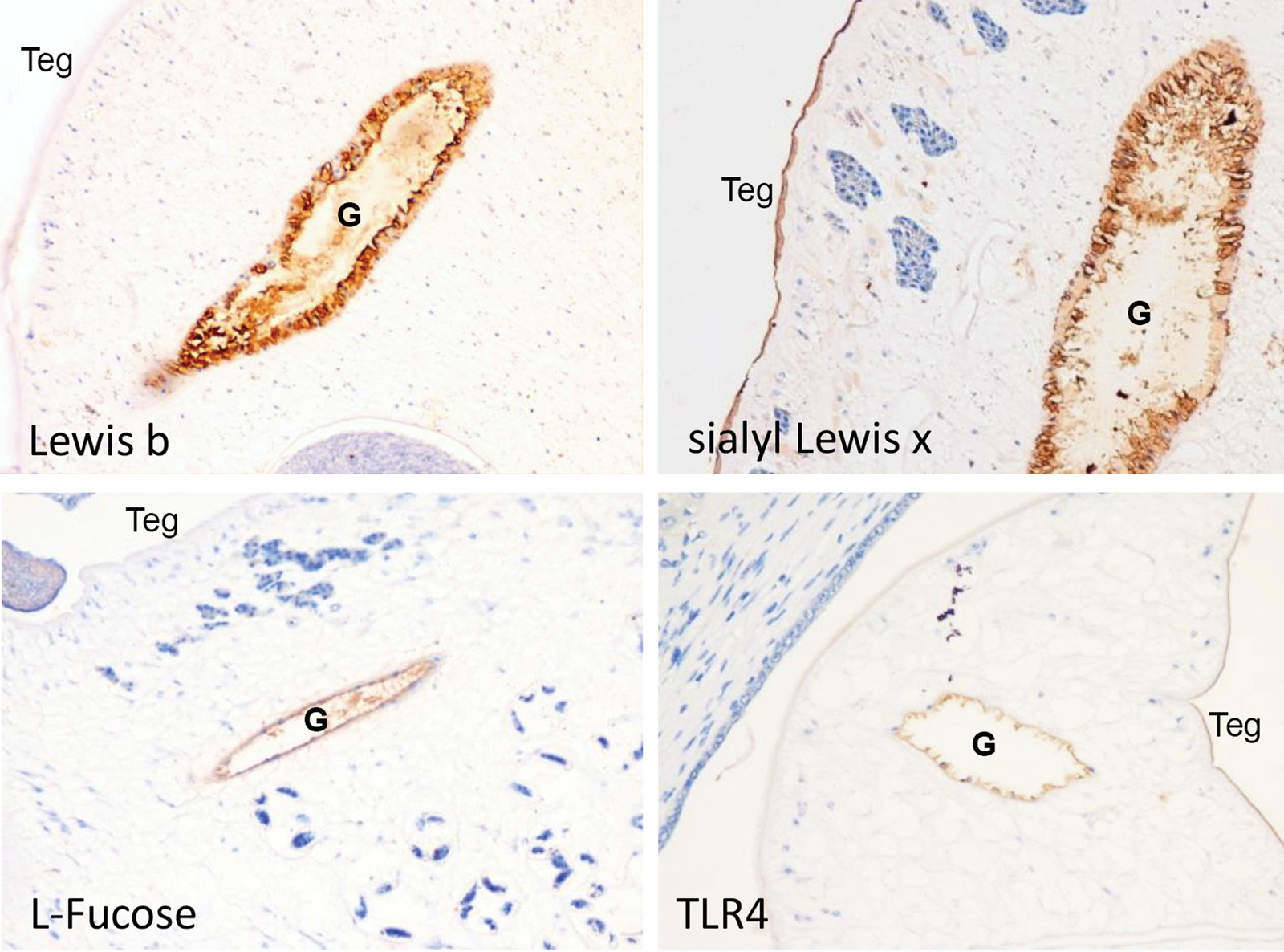
Fig. 2. Immunohistochemistry of adult O. viverrini tissue sections using antibodies raised against Helicobacter pylori-specific receptors. Lewis B (upper left), sialyl-Lewis X (upper right), L-fucose (lower left) and TLR4 (lower right) along the gut epithelium (G) and tegument (Teg) of Opisthorchis viverrini. G, gut; Teg, tegument; original magnification, 20×.
Colocalization between H. pylori and fucose residues along the gut epithelium of O. viverrini
Given that L-fucose expression showed the highest frequency mainly in the gut epithelium of the worms (70.2%), which was similar in frequency to the positive rates of H. pylori detection in O. viverrini DNA samples (79%), we proceed to investigate the co-localization of the bacillus and host cell fucose using an in vitro adhesion assay, which involved probing thin sections of worms in the hepatobiliary tract of hamsters with FITC-labelled H. pylori. The investigation revealed that H. pylori adhered to the epithelium of the O. viverrini gut where the L-fucose was presented (Fig. 3A and D). Adherence was not observed by H. pylori on the gut epithelium in L-fucose-negative O. viverrini worms (Fig. 3B and E). Intense adhesion of H. pylori was also observed in regions of the tegument where L-fucose-staining also was present (Fig. 3C and F).

Fig. 3. Co-localization between FITC-labelled Helicobacter pylori and L-fucose. Adherence of H. pylori was apparent along the gut epithelium of the liver fluke in regions positive for L-fucose (A, D). Adhesion by H. pylori was not observed in L-fucose-negative worms (B, E). Certain sites on the tegument showed intense H. pylori adhesion where L-fucose is positive (C, F). Upper panel (A, B, C) = adhesion assay using FITC-labelled H. pylori. Lower panel (D, E, F) = L-fucose histochemistry. G, gut epithelium; Teg, tegument; original magnification, 20×.
Specificity of fucose as a receptor for H. pylori in O. viverrini
The specificity of L-fucose was tested by using fucosidase enzyme that cleaves fucose residues and thus prevents H. pylori adhesion in the gut and tegument of the worm. After incubating O. viverrini parasites with increased concentrations of the fucosidase and for increasing intervals, 40 and 100 U mL−1 at 2, 16 and 24 h, the worms were harvested and copy numbers of ureA determined by qPCR. We observed a significantly decreased adhesion of H. pylori in both enzyme concentration- and time-dependent fashion (Fig. 4).

Fig. 4. Hydrolysis by fucosidase diminishes H. pylori adherence and colonization. Copy numbers of H. pylori were significantly decreased in a dose- and time-dependent fashion after exposure of O. viverrini worms to the fucosidase. Each time point represents copy numbers ± s.e. from 3 biological replicates (*P < 0.05; **P < 0.005).
Discussion
Helicobacter pylori and O. viverrini co-infections orchestrate pathogenesis of opisthorchiasis, specifically periductal fibrosis both in experimental rodent models (Dangtakot et al., Reference Dangtakot, Pinlaor, Itthitaetrakool, Chaidee, Chomvarin, Sangka, Wilailuckana and Pinlaor2017; Suyapoh et al., Reference Suyapoh, Tirnitz-Parker, Tangkawattana, Suttiprapa and Sripa2021) and in naturally infected people (Boonyanugomol et al., Reference Boonyanugomol, Chomvarin, Sripa, Bhudhisawasdi, Khuntikeo, Hahnvajanawong and Chamsuwan2012; Deenonpoe et al., Reference Deenonpoe, Mairiang, Mairiang, Pairojkul, Chamgramol, Rinaldi, Loukas, Brindley and Sripa2017), and are implicated in malignant transformation (Sripa et al., Reference Sripa, Deenonpoe and Brindley2017; Dangtakot et al., Reference Dangtakot, Intuyod, Chamgramol, Pairojkul, Pinlaor, Jantawong, Pongking, Haonon, Ma and Pinlaor2021). Given that the liver fluke is known to harbour H. pylori in its gut and tegument (Deenonpoe et al., Reference Deenonpoe, Mairiang, Mairiang, Pairojkul, Chamgramol, Rinaldi, Loukas, Brindley and Sripa2017; Suyapoh et al., Reference Suyapoh, Tirnitz-Parker, Tangkawattana, Suttiprapa and Sripa2021), H. pylori has, sine qua non, to bind to these helminth tissues via appropriate receptors. Although major H. pylori adhesins essential for colonization and establishment of infection have been described in human gastric epithelia (Chmiela and Kupcinskas, Reference Chmiela and Kupcinskas2019; Fagoonee and Pellicano, Reference Fagoonee and Pellicano2019; Matos et al., Reference Matos, Amorim, Magalhaes, Haesebrouck, Gartner and Reis2021), orthologues of the human cell receptors have yet to be reported in the gastrodermis of O. viverrini. Here, we report the adhesion of H. pylori to O. viverrini gut epithelium and the tegument via L-fucose binding adhesin.
In this study, we investigated the expression of 4 well-investigated receptors of H. pylori including Lewis B, sialyl-Lewis X, TLR4 and fucose residues in O. viverrini adult worms. Among these 4, L-fucose residue was detected in the gut in over 70% of the worms. The rate of fucose detection was close to the number of O. viverrini that carried H. pylori (79%). We have reported the expression of L-fucose in the gut of O. viverrini in an earlier study (Talabnin et al., Reference Talabnin, Aoki, Saichua, Wongkham, Kaewkes, Boons, Sripa and Tiemeyer2013). Thus, we hypothesized that this fucose residue might play a part in the H. pylori adhesion in O. viverrini adult worm, and accordingly we further investigated the relationship between H. pylori and fucose expression in O. viverrini tissues. An in vitro assay for adhesion revealed that H. pylori localized to the gut epithelium and tegument of O. viverrini where the fucose expression had also been detected. In parallel, the copy numbers of H. pylori-specific ureA gene were decreased in a dose- and time-dependent manner upon removal of fucose by fucosidase enzyme (P < 0.01). Altogether, these data provide increased support for our conjecture that the L-fucose is a candidate receptor for the adherence of H. pylori within the liver fluke, O. viverrini.
Despite the result from this study suggesting that the L-fucose was essential for H. pylori adhesion, the source of the fucosylated oligosaccharides found in parasite tissues is still unclear. There is a possibility that these fucosylated oligosaccharides are the remains of host biomolecules ingested by worms, which could be acquired by the gut epithelium of the fluke. Our previous study showed that only the cagA-positive strain was detected in the gut of the worm and co-infection between cagA-positive H. pylori and O. viverrini resulted in a more severe biliary pathology and decreased E-cadherin expression in vivo and in vitro than those of the cagA-negative strain (Suyapoh et al., Reference Suyapoh, Tirnitz-Parker, Tangkawattana, Suttiprapa and Sripa2021). The present study demonstrated that the binding of Helicobacter to the gut of O. viverrini is fucose-dependent. Taking these results together, we hypothesize that fucose is a selective molecule for cagA-positive H. pylori binding in the worm's gut, which can promote H. pylori colonization and enhance the biliary pathogenesis and carcinogenesis.
To conclude, this report presents novel evidence that L-fucose, which is detected in the gut epithelium and tegument of the O. viverrini liver fluke, plays a role in adhesion of H. pylori to this liver fluke. These findings provide experimental support for our previously proffered hypothesis that co-migration of the 2 carcinogenic pathogens to the bile ducts orchestrates and exacerbates hepatobiliary disease including CCA (Deenonpoe et al., Reference Deenonpoe, Mairiang, Mairiang, Pairojkul, Chamgramol, Rinaldi, Loukas, Brindley and Sripa2017). Indeed, barriers to malignant transformation of the biliary tract epithelium may be compromised by this relationship involving helminth parasite and a bacterial pathogen (Dheilly et al., Reference Dheilly, Bolnick, Bordenstein, Brindley, Figueres, Holmes, Martinez Martinez, Phillips, Poulin and Rosario2017). One of the limitations of this study is that we cannot directly quantify the copy numbers of H. pylori and correlate with L-fucose expression in each individual worm.
Acknowledgements
We are grateful to Suwit Balthaisong for assistance with histological works, Wachiraporn Donsa for adhesion assay and Chalida Chuenchom for bacterial culture and maintenance.
Author contributions
P. T., S. S., C. P., Y. C., B. S. and P. J. B. conceived and designed the study. P. T. and B. S. performed the experiments. P. T., R. D. and B. S. analysed and interpreted the findings. P. T., R. D., S. S. and B. S. wrote the draft manuscript. B. S. and P. J. B. revised final draft manuscript. B. S. obtained funding. All authors read and approved the final version of the paper.
Financial support
This work was supported by the Higher Education Research Promotion and National Research University Project of Thailand, Office of the Higher Education Commission, through the Health Cluster (SHeP-GMS), Khon Kaen University, Thailand (award number I56110) (B. S.), Khon Kaen University (grant# RP64018) and partly by award R01CA164719 from National Cancer Institute, National Institutes of Health, USA (P. J. B.). P. T. acknowledges the support from the Royal Golden Jubilee PhD Program (grant number PHD/0013/2555), the Thailand Research Fund. B. S. is supported by KKU Senior Research Scholar Fund.
Conflict of interest
None.
Ethical standards
Animal procedures complied with guidelines of the Guide for the Care and Use of Laboratory Animals by National Research Council, USA, 8th edition and the Thai Institutional Animal Care and Use Committee.