INTRODUCTION
Parasite dispersal between host populations is probably one of the most important factors affecting the dynamics of host-parasite interactions and the spread of diseases (Price, Reference Price1980; Poulin, Reference Poulin2007). For ectoparasites with low intrinsic mobility, such as ticks, dispersal is highly dependent on host movements (McCoy et al. Reference McCoy, Boulinier, Tirard and Michalakis2003). Although some parasites may manipulate or influence their host's behaviour and movements (e.g. Thomas et al. Reference Thomas, Poulin and Brodeur2010), it is likely that in ectoparasites such as ticks, dispersal is mainly a passive process. They can nevertheless exert some control by attaching at a time when hosts are most likely to cover greater distances, and adjusting the duration of attachment and the timing of separation from the host to ensure detachment in a habitat suitable for the continuation of the parasite's life cycle (Matuschka et al. Reference Matuschka, Richter and Spielman1991).
Ixodid ticks are a common group of haematophagous ectoparasites that exhibit a variety of attachment durations and detachment behaviour according to their ecology and that of their hosts (Sonenshine, Reference Sonenshine1993; Stachurski and Adakal, Reference Stachurski and Adakal2010). In open-field generalist ticks, such as Ixodes ricinus, there is relatively little variation in the timing of tick detachment (Matuschka et al. Reference Matuschka, Richter, Fischer and Spielman1990b; Sonenshine, Reference Sonenshine1993; Heylen and Matthysen, Reference Heylen and Matthysen2010), as the chances of detaching in a suitable habitat are quite high. By contrast, optimal detachment behaviour is crucial for nidicolous and/or specialist ticks, as habitats where they may encounter their next host or reproduce are often more restricted (Heylen and Matthysen, Reference Heylen and Matthysen2010).
Ixodes arboricola is a specialist, nidicolous tick with very low intrinsic mobility and with an entire life cycle restricted to natural tree holes. This tick species mainly parasitizes birds, such as great tits Parus major, that breed and roost in such cavities (Hillyard, Reference Hillyard1996). As in all ixodid ticks, it feeds once per life stage during a non-stop period of several days (Sonenshine, Reference Sonenshine1993). After feeding, the engorged tick detaches and starts to develop to the next life stage. In this study, we experimentally infested great tits with I. arboricola larvae or nymphs and subsequently manipulated the winter roosting behaviour of the birds. In the experimental treatment, hosts were prevented from roosting in a nest box for varying lengths of time, whereas in the control treatment, hosts had free access to the nest boxes. This experiment had 3 main aims: (1) to investigate whether I. arboricola adjusts the duration of attachment on the host according to the roosting behaviour of their hosts; (2) if ticks do extend their attachment on hosts, to determine whether this has any fitness consequences for the ticks and (3) to compare the detachment strategies and associated costs in I. arboricola larvae and nymphs, the stages most frequently found on hosts during the winter months (Heylen, Reference Heylen2011).
Because detaching outside of the nest box is likely to lead to a dramatic fitness reduction for a nidicolous tick such as I. arboricola, we expected both larvae and nymphs from the experimental group to delay detachment as long as the host had no access to nest boxes. Furthermore, we expected prolonged attachment to have adverse effects on the ticks, due to the increased likelihood of suffering from host defence mechanisms, such as grooming or acquired resistance.
MATERIALS AND METHODS
Study system
Ixodes arboricola, generically known as the tree-hole tick, is widely distributed in the Palearctic region (Liebisch, Reference Liebisch, Mitchell, Horn, Needham and Welbourn1996) and is frequently found parasitizing great tits (Hudde and Walter, Reference Hudde and Walter1988). Larvae and nymphs take 3–4 days, on average, to feed and fully engorge (Heylen and Matthysen, Reference Heylen and Matthysen2011). After detaching from the host, engorged individuals typically climb to the top of the cavity (negative geotropism, Heylen and Matthysen, Reference Heylen and Matthysen2010). The larvae of this tick species most actively parasitize their preferred hosts during autumn and winter (from October to March), the nymphs are most prevalent during the host pre-laying and breeding season (January to May) whereas the adults mostly parasitize host nestlings (May to July) (Heylen, Reference Heylen2011).
The larvae used in the experiment originated from the eggs laid by 4 adult female ticks that fed on P. major nestlings in spring 2010 and were kept in the laboratory. The nymphs used were all collected unfed from nest boxes used by blue tits or great tits in 2010. All ticks were kept at 25°C and 83% relative humidity in a dark environment until infestation.
In the winter months of 2010–2011, P. major individuals were caught at night while roosting in nest boxes in 3 sites near Antwerp, Belgium. All birds were ringed, weighed, measured and fitted with a passive integrated transponder (PIT) tag. All birds were devoid of ticks at the time of capture. Each bird was then placed in an individual 8 m3 cage in an outdoor aviary. All cages were provided with shelter, small trees and 2 clean nest boxes. Bird roosting behaviour was monitored every night using a portable PIT tag reader allowing us to determine which nest box, if any, was used for roosting, without having to open the box or enter the cage. Birds were provided with peanuts, sunflower seeds, live mealworms, lard balls containing mixed millet seeds and fresh water ad libitum. Birds were kept captive for a maximum of 35 days (range 16–35 days) under license from the Agency for Nature and Forests (Flemish Government, Belgium), and released at the site where they were caught. The infestation procedure (see below) was approved by the Ethics Committee for Animal Experiments of the University of Antwerp (Belgium).
Experimental design
After an average habituation period of 10 days (range: 7–20 days) and once all birds roosted in the nest boxes on a daily basis, we infested the birds with either larvae or nymphs by placing them underneath the crown feathers. Birds infested with larvae received 20–30 individuals, while birds infested with nymphs received 10–12 individuals, numbers which correspond to the natural infestation levels of these instars in I. arboricola (Heylen, Reference Heylen2011). After infestation, birds were immediately placed in a cotton-bag for 1·5 h and then released in their respective cages. All ticks that did not attach and remained in the bag were counted. Each bird was then randomly assigned to one of two treatments: an experimental treatment where the entrances to nest boxes were blocked, and a control treatment where the birds had free access to the nest boxes. A total of 18 birds were infested with larvae, including 9 experimental birds and 9 control birds. Due to limited aviary space, larva infestations were carried out in 2 batches, 8 birds (4 control and 4 experimental) in mid-November 2010 and 10 birds (5 control and 5 experimental) in mid-December 2010. Within the experimental group, nest boxes were re-opened after varying periods: 7 days (n=2 birds), 11 days (n=2), 12 days (n=4) and 14 days (n=1; see Fig. 2a). A further 9 birds were infested with nymphs in February, including 3 control birds and 6 experimental birds (2 birds with no access to the nest box for 7 days, 1 bird for 9 days, 1 bird for 10 days and 2 birds for 14 days, Fig. 2b).
The number of ticks attached on the birds’ heads was first monitored 5 days after infestation (no checks were performed before then to avoid stress on birds and ticks during the natural engorgement period) and then every 2–3 days thereafter. In experimental birds, the number of attached ticks was also checked on the morning following the re-opening of the nest box. Additionally, engorged ticks that detached in the nest box and that were found on the lid and upper walls were collected on a daily basis, weighed to the nearest 0·01 mg (Mettler-Toledo XA105DU Analytical Balance, with a 0·01 mg fine range precision) immediately after collection and kept in the laboratory. Engorged ticks collected on the same day from the same nest box were all weighed together (producing an average engorgement weight per box.day−1) in order to reduce measurement error. Tick engorgement weight is known to be proportional to the amount of absolute blood consumed (Balashov, Reference Balashov1972), is a good measure of feeding success and is known to decrease with increased host resistance (Rechav, Reference Rechav1992). We further considered the proportion of engorged ticks found on the lid and upper walls of the nest box in relation to the total number that detached (henceforth termed ‘recovery rate’). Nest box lids and upper walls were checked for ticks daily for at least 2 weeks after the end of the experiment. After this period, all nest boxes were thoroughly checked and no ticks were found (bodies had supposedly decayed within the fecal matter or had been eaten by the birds) indicating that all individuals not collected on the lid and upper walls of the nest boxes had effectively died. Recovery rate thus seems to comprise a reliable index of post-detachment survival in this tick species. A period with low temperatures (max. −2°C, min. −12°C) occurred between 13 and 21 December 2010, during which only 7% of all detached larvae (n=10 birds) were found on the nest box lids (versus 50% in less harsh conditions, see Fig. 3), supposedly due to abnormally high mortality rates. We therefore decided to exclude larvae collected during that period from the tick post-detachment recovery rate analyses (see below).
All birds were weighed on the day of infestation and at regular intervals between day 5 after infestation and the day of release, which allowed us to measure change in weight (relative condition) according to treatments. Any stress due to handling was therefore equal between experimental and control birds.
Statistical analysis
Statistical analyses were performed with a generalised linear model (MIXED procedure, SAS Institute Inc., 9.2), unless stated otherwise. When analysing tick engorgement weights, we included host identity as a random effect to account for the non-independence of the data. The sample size for each average engorgement weight per box.day−1 was accounted for, using the ‘weight’ option in the mixed model. In the analyses on detachment and recovery rates, proportions were normalised by arcsine transformation. As the proportions of detached larvae were still not normally distributed after transformation, we performed a non-parametric analysis of variance (Kruskal-Wallis). In all other analyses, homoscedasticity and normality of the residuals complied with standard model assumptions. As the larvae originated from 4 different adult females (systematically distributed among treatments and birds) and because the experiment was carried out in 2 batches, we included batch and female identity as covariates in all relevant models and found no significant batch detachment rate (χ 21,19=0·85, P=0·36; engorgement weight F1,8=3·27, P=0·11) or female detachment rate (χ 23,19=0·90, P=0·83; engorgement rate F3,8=5·5, P=0·07; recovery rate F1,8=0·44, P=0·57) effects. We thus do not mention batch or female effects in the Results section. Sample values are expressed as mean±s.e. throughout.
RESULTS
After infestation, all the control birds continued to sleep every night in their nest boxes. All experimental birds returned to roost in the nest box on the first night after nest boxes were re-opened, regardless of the duration of the treatment (i.e. the period during which the bird had no access to the nest box). We found no effect of the nest box treatment (GLM, F1,17=0·07, P=0·79) or duration of treatment (F1,17=0·04, P=0·84) on bird body condition.
One of the birds infested with larvae lost a large proportion of its crown feathers during the experiment, presumably due to the bird repeatedly flying against the wire of the cage roof. As this may have caused the loss of a number of attached ticks, this bird was excluded from further analyses.
Tick detachment behaviour according to treatment
When we checked birds infested with larvae after 5 days, we found that almost all ticks had detached from the control birds (mean proportion of infested larvae still attached: 0·03±0·02) while a large proportion was still attached to the experimental birds (0·71±0·09). This difference was highly significant (Kruskal-Wallis, χ 21,19=13·94, P=0·0002, Fig. 1). The same pattern was found in nymphs (GLM, F1,7=14·16, P=0·007, Fig. 1) although a higher proportion was still attached to control hosts after 5 days (0·26±0·04). The remaining nymphs of the control group all detached within 7 days, indicating longer natural feeding durations in nymphs as observed in most ixodid ticks (see Balashov, Reference Balashov1972).

Fig. 1. Mean proportion of Ixodes arboricola larvae and nymphs still attached on the host 5 days after experimental infestation according to host treatment: hosts with access to nest boxes (grey bars) and hosts with no access to nest boxes (black bars). Error bars represent s.e.
We then monitored the number of ticks attached to the experimental hosts from 5 days onwards. For both larvae and nymphs, detachment happened at a very slow rate as long as the bird was prevented access from the nest box (average daily detachment: 1·75% in larvae, 1·05% in nymphs). However, when the bird was given access to the box the number of attached ticks dropped to 0 in all cases (Fig. 2), indicating that all ticks detached from the birds on the first night they roosted in the box.

Fig. 2. Proportion of Ixodes arboricola larvae (a; n=9 birds) and nymphs (b; n=6 birds) attached on the experimental birds according to time since infestation. Dashed circles indicate the day on which each bird was given access to its nest box.
Tick recovery rates and engorgement weight according to treatment
In larvae, there was no significant difference in recovery rates between control ticks and those for which we experimentally extended attachment duration (GLM, F1,4=0·64, P=0·47, Fig. 3). In nymphs, however, ticks detached from control birds had significantly higher recovery rates than those for which detachment was delayed (F1,7=10·22, P=0·015, Fig. 3). Furthermore within the experimental group, the proportion of nymphs collected was negatively related to the detachment delay (i.e. duration of experimental treatment), although this was only marginally significant (F1,7=4·15, P=0·054).

Fig. 3. Mean proportion of detached engorged Ixodes arboricola larvae and nymphs found and collected on the nest box lid according to the treatment: the control group in which ticks detached within 5–6 days (grey bars) and the experimental group in which tick detachment was delayed (black bars). Error bars represent s.e.
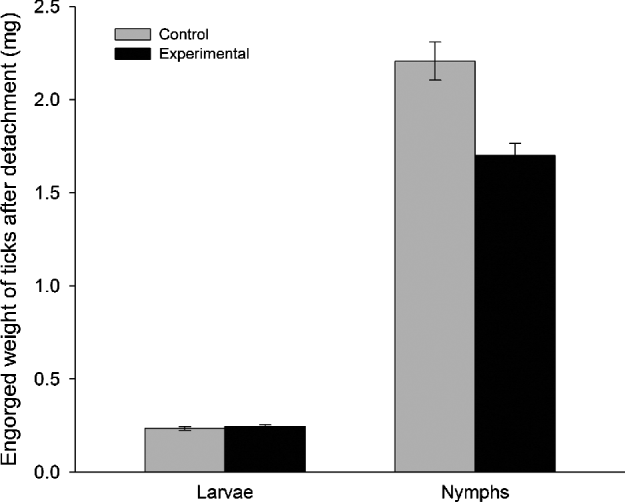
Similarly we found that nymphs for which detachment was delayed had significantly lower engorgement weights than control nymphs (GLMM, F1,15=11·53, P=0·004, Fig. 4), whereas no such difference was detected in larvae (F1,4=0·21, P=0·67, Fig. 4).

Fig. 4. Mean engorged weight after detachment (mg) of Ixodes arboricola larvae and nymphs according to the treatment: the control group in which ticks detached within 5–6 days (grey bars) and the experimental group in which tick detachment was delayed (black bars). Error bars represent s.e.
DISCUSSION
Our findings provide experimental evidence that the immature instars of the tree-hole tick, Ixodes arboricola, adjust the duration of their attachment to hosts in order to detach in optimal habitat. Ticks on birds that had regular access to nest boxes detached rapidly (less than 5 days after infestation), whereas those on birds with no access delayed detachment (up to 14 days after infestation) until the bird roosted in a nest box.
Our results join the existing body of literature showing different detachment strategies adapted to the specific ecology of the ticks and that of their hosts. While a majority of studies have focused on detachment behaviour in relation to photoperiod and circadian rhythms (e.g. Amin, Reference Amin1970; Rechav, Reference Rechav1978; Belozerov, Reference Belozerov, Obenchain and Galun1982; Dutoit et al. Reference Dutoit, Fourie and Horak1994; George et al. Reference George, Pound and Davey1998; Heylen and Matthysen, Reference Heylen and Matthysen2010) or the timing of host activity patterns (Mather and Spielman, Reference Mather and Spielman1986; Matuschka et al. Reference Matuschka, Richter, Fischer and Spielman1990a, Reference Matuschka, Richter, Fischer and Spielmanb), experimental evidence that detachment behaviour may be associated with the host's immediate environment is largely lacking (but see Matuschka et al. Reference Matuschka, Richter and Spielman1991; Bianchi and Barre, Reference Bianchi and Barre2003). Here our results clearly demonstrate that in I. arboricola, detachment behaviour is associated with roosting cavities.
These findings naturally raise questions on the proximate mechanisms that explain such behaviour. The pattern we observe excludes the possibility of detachment being solely influenced by circadian rhythms endogenous to the ticks, or exogenous cues linked to light/dark cycles, as ticks of both treatments were submitted to the same photoperiod conditions. Birds roosting inside or outside nest boxes may have exhibited slight differences in sleeping behaviour thus providing a possible cue for detachment. However we are not aware of any studies showing differences in sleeping patterns according to nest box use. The idea that, in I. arboricola, detachment behaviour is neither related to photoperiod or host sleeping patterns is corroborated by the anecdotal observation that all ticks detached from a host that was released in a nest box during the day and remained there for less than 30 minutes (J. White, personal observation). One could argue that preventing birds from roosting in nest boxes may have had physiological consequences (e.g. differences in hypothermia or stress) thus affecting tick detachment behaviour. Differences in host nocturnal hypothermia are, however, an unlikely explanation, as it has been shown that captive birds do not use hypothermia, unless they are submitted to food deprivation (Nord et al. Reference Nord, Nilsson, Sandell and Nilsson2009), As all birds were fed ad libitum, we can reasonably assume that no individuals used hypothermia during our experiment. Further, the lack of difference in host weight according to treatment suggests that the experimental birds did not experience severe stress (e.g. Thomson et al. Reference Thomson, Tomas, Forsman, Broggi and Monkkonen2010). However, we cannot exclude that a slight increase in stress levels may have contributed to inhibiting detachment in experimental ticks. In sum, it appears likely that detachment from hosts by I. arboricola larvae is triggered by one or a combination of cues related to hosts and/or intrinsically linked to tree-hole cavities, such as those pertaining to confined spaces (lack of air movement or slight increase in CO2 levels), lower light levels, specific humidity levels or chemical cues (bird feces, wooden cavities, specific fungi, etc.). Future experiments are needed to determine the exact cues that trigger detachment in I. arboricola.
One of the remarkable findings of this study is the capacity of I. arboricola to survive prolonged periods of attachment on the host. Larvae and nymphs of this species have been found to detach within an average 4 days when parasitizing nestlings (Heylen and Matthysen, Reference Heylen and Matthysen2011). Further, the duration of attachment rarely exceeds 4–5 days in larvae and 6–7 days in nymphs when parasitizing adult hosts in natural conditions (Driessen, K. and Heylen, D., personal observations), as is the case for most ixodid ticks (Sonenshine, Reference Sonenshine1993). However, in the absence of a suitable habitat, I. arboricola larvae and nymphs were able to extend the normal duration of attachment to the host up to 14 days (a 2 to 3-fold increase, see also Heylen and Matthysen, Reference Heylen and Matthysen2010). Although there have been anecdotal observations of ticks extending the duration of attachment in unsuitable habitat (e.g. Balashov, Reference Balashov1972), our study is the first, to our knowledge, to provide experimental evidence of such behaviour and especially for such long durations. Such plasticity in the detachment strategy of I. arboricola is likely to be an adaptation of this strictly nidicolous tick species allowing it to optimize host encounter rates and to avoid unfavourable abiotic conditions and/or predation.
Nymphs seemed to have a similar, if not higher, capacity to remain attached as larvae. This may be explained by a higher capacity of nymphs to resist removal by the host despite their larger size (e.g. larger, sturdier hypostome than larvae, Haarlov, Reference Haarlov1962). However, this ability to remain attached to the host for extended periods does not exclude associated costs. Our findings suggest that extending the duration of attachment had more deleterious effects on nymphs than on larvae. The costs incurred seemed not to be due to host grooming behaviour, which would have resulted in tick removal or injury (although we did observe one partially engorged nymph that died of injury after 9 days of attachment), but rather translated into lower post-detachment survival and lower engorgement weights.
The post-detachment recovery rates showed that the longer the nymphs remained on the birds, the lower probability of finding them on the lid of the nest box. This implies that the nymphs died soon after detachment or did not have sufficient energy to climb up the walls of the nest box. The reduction in engorgement weight with duration of attachment may be a result of host resistance (Varma et al. Reference Varma, Hellerhaupt, Trinder and Langi1990; Rechav, Reference Rechav1992) which may increase when attachment durations are extended. Although the mechanisms that explain these patterns remain unclear, the lower engorgement weights and post-detachment survival show evidence for strong selective pressures on nymphs to optimize timing of detachment when a suitable habitat is available.
In contrast to nymphs, prolonging the duration of attachment seemed to have no apparent deleterious effects on larvae, whether in terms of attachment to hosts, engorgement weight or post-detachment survival. Larvae of I. arboricola are known to have a lower deleterious impact on the host than nymphs (Heylen and Matthysen, Reference Heylen and Matthysen2011) both in terms of total amount of blood ingested (10–20 times less than nymphs, Balashov, Reference Balashov1972) and quantity of salivary secretions and pathogens transferred. In that context, further studies investigating host reaction (e.g. immune response) according to infestation of varying durations by different instars would help elucidate why larvae seem to incur lower costs despite the extended attachment period.
This apparent contrast in the fitness consequences of extending attachment durations among the different tick stages has interesting implications for the ecology and life cycle of I. arboricola, especially when one considers its phenology and that of their main hosts, great and blue tits. Larvae of I. arboricola are predominantly found on hosts during autumn and winter months (Heylen, Reference Heylen2011), a period during which tits facultatively roost in cavities depending on ambient temperatures (Hinde, Reference Hinde1952) and carry out partial migration during cold spells (Cramp, Reference Cramp1993). The larvae's capacity to extend the duration of attachment with no apparent costs may thus be an adaptation for the survival of periods during which the birds do not roost in cavities or are migrating. More importantly, parasitizing hosts during the winter months may provide an opportunity for short-distance dispersal (as tits frequently change roosting sites, Hinde, Reference Hinde1952) or long-distance dispersal (during winter migration). In contrast, nymphs, for which extended attachment seems more detrimental, are predominantly found on the host during the bird's pre-laying period (Hudde and Walter, Reference Hudde and Walter1988; Heylen, Reference Heylen2011) when birds become more territorial and regularly use the same cavity. Adult female ticks, which suffer a high risk of injury by grooming of adult birds, almost exclusively parasitize nestlings during the breeding season (Heylen, Reference Heylen2011). We therefore hypothesize that, in this tick species, the larval instar is the stage during which dispersal is most effective. By being able to survive prolonged periods of attachment on the host during the winter months, I. arboricola larvae may be dispersed over much longer distances than might be expected from a strictly nidicolous tick. This may explain why I. arboricola is widely distributed in the Palaearctic region, from western Europe to extreme eastern Russia (Liebisch, Reference Liebisch, Mitchell, Horn, Needham and Welbourn1996). As I. arboricola has been found to be a carrier of pathogens such as Borrelia burgdorferi sensu lato (Heylen D. and Sprong H., unpublished data) and Rickettsia spp. (Spitalska et al. Reference Spitalska, Literak, Kocianova and Taragel'ova2011), this capacity for dispersal may also have implications for the epidemiology of Lyme borreliosis and rickettsiosis and for the contribution of non-migratory, cavity-nesting birds to the spread of these diseases.
ACKNOWLEDGEMENTS
We thank Joris Elst, Indra Jacobs and Frank Adriaensen for building the aviary and for assistance with the data collection. Funding was provided by FWO-project G.0049.10 awarded to E.M., and J.W. was supported by a FWO visiting Post-doctoral Fellowship.