INTRODUCTION
The obligatory intracellular apicomplexan parasite Toxoplasma gondii is one of the most successful parasites on this planet. It infects members of Felidae as definitive hosts, and has a wide range of intermediate hosts, including warm-blooded animals and humans (Tenter et al. Reference Tenter, Heckeroth and Weiss2000; Dabritz and Conrads, Reference Costa, Pautas, Ernault, Foulet, Cordonnier and Bretagne2010). Approximately one third of the human population is infected with T. gondii worldwide, but despite the high prevalence, in the vast majority of cases disease is benign and asymptomatic (Barratt et al. Reference Barratt, Harkness, Marriott, Ellis and Stark2010). However, clinical problems can arise in 2 situations. First, in individuals with an immature immune system or in immune-compromised patients infection can lead to toxoplasmic encephalitis (TE) or even death. TE occurs as a result of the reactivation of brain cysts that encapsulate slowly proliferating bradyzoites, which then undergo bradyzoite-to-tachyzoite stage conversion, and is common in chronically infected patients, which receive immunosuppressive therapy or suffer from human immunodeficiency virus (HIV) infection (Tenter et al. Reference Tenter, Heckeroth and Weiss2000; Barratt et al. Reference Barratt, Harkness, Marriott, Ellis and Stark2010; Henriquez et al. Reference Henriquez, Woods, Cong, McLeod and Roberts2010). Secondly, congenital toxoplasmosis poses a major health risk upon primary maternal infection during pregnancy, leading to placental or fetal infection (or both), which often results in abortion or damage of the fetal tissues (Feldman et al. Reference Feldman, Timms and Borgida2010). In addition, T. gondii is also a highly significant economic and veterinary medical concern in the livestock industry, and as such represents an important zoonosis (Dubey, Reference Dubey2009).
Toxoplasmosis begins generally mildly or is asymptomatic, with an acute phase in which tachyzoites invade the cells, divide rapidly, and disseminate throughout the host. With the onset of the host immune response, the proliferative tachyzoite stage is replaced by the quiescent bradyzoites, which form tissue cysts predominantly located in the central nervous system (CNS) and muscle tissue, where they do not cause apparent immunopathology and can persist for many years, up to a lifetime ( Carruthers and Suzuki, Reference Carruthers and Suzuki2007; Laliberté and Carruthers, Reference Laliberté and Carruthers2008). The chemotherapeutical options for the treatment of toxoplasmosis are limited. A synergistic combination therapy comprised of sulfadiazine and pyrimethamine, targeting the synthesis and the reduction of folic acid in tachyzoites, has been shown to be the most efficient option (Montoya and Liesenfeld, Reference Montoya and Liesenfeld2004). However, adverse reactions can occur, which include haematological toxicity and hypersensitivity. Another option employs clindamycin-pyrimethamine combination therapy, but this treatment is also prone to side effects (Pereira-Chioccola et al. Reference Pereira-Chioccola, Vidal and Su2009). The major problem in the treatment of T. gondii infection is the non-effectiveness of compounds against bradyzoites that are enclosed in tissue cysts (Barratt et al. Reference Barratt, Harkness, Marriott, Ellis and Stark2010).
Pentamidine and its analogues represent a class of broad-spectrum antimicrobial compounds, with activities against a wide range of intracellular and extracellular protozoan parasites (Soeiro et al. Reference Soeiro, De Souza, Stephens and Boykin2005; Wilson et al. Reference Wilson, Tanious, Mathis, Tevis, Hall and Boykin2008; Buckner and Navabi, Reference Buckner and Navabi2010). Since its discovery, pentamidine has been successfully applied to treat African trypanosomiasis, leishmaniasis, and malaria in humans, and the pentamidine-derivative diminazene aceturate is commonly used for trypanosome chemotherapy in livestock (Werbovetz, Reference Werbovetz2006). More recently, novel analogues, known as arylimidamides, with a more favourable pharmacokinetic profile, improved bioavailability and lower host toxicity were shown to be effective against Leishmania donovani and Trypanosoma cruzi in vitro and in vivo (Wang et al. Reference Wang, Zhu, Srivastava, Liu, Sweat, Pandharkar, Stephens, Riccio, Parman, Munde, Mandal, Madhubala, Tidwell, Wilson, Boykin, Hall, Kyle and Werbovetz2010; Batista et al. Reference Batista, Batista, de Oliveira, do Amaral, Lannes-Vieira, Britto, Junqueira, Lima, Romanha, Sales Junior, Stephens, Boykin and Soeiro2010a).
The in vitro efficacy of pentamidine and some pentamidine analogues against T. gondii has been demonstrated previously (Lindsay et al. Reference Lindsay, Blagburn, Hall and Tidwell1991), and proliferation inhibitory properties were reported at concentrations of around 10 μg/ml. More recently, Leepin et al. (Reference Leepin, Stüdli, Brun, Stephens, Boykin and Hemphill2008) demonstrated the in vitro efficacy of a set of arylimidamides (DB750, DB766, DB786, DB811, formerly named reversed diamidines), which inhibited the proliferation of T. gondii and the closely related Neospora caninum with IC50s at submicromolar concentrations ranging between 0·16 and 0·66 μ m (=0·14–0·5 μg/ml), indicating the potential of these di-cationic compounds, especially DB750 and DB786, for chemotherapeutical purposes. DB811 was recently reported to inhibit the proliferation of the related apicomplexan Besnoitia besnoiti in Vero cells at an IC50 of 0·08 μ m (Cortes et al. Reference Cortes, Muller, Boykin, Stephens and Hemphill2011).
Here, we further explore the in vitro characteristics of DB750 in relation to T. gondii. We demonstrate that, despite low IC50 in 3-day growth assays, Toxoplasma tachyzoites could readily adapt to this compound during culture within a few days, while this was not possible for the closely related N. caninum. Screening of additonal di-cationic compounds for anti-Toxoplasma activity lead to the identification of DB745, which inhibited the proliferation of T. gondii tachyzoites at an IC50 of 0·03 μ m. Also, the effects of DB745 and DB750 were evaluated by transmission electron microscopy analysis of DB750-adapted and non-adapted T. gondii parasites.
MATERIALS AND METHODS
Culture media, buffers and reagents
Unless otherwise stated, all tissue-culture media were purchased from Gibco-BRL (Zurich, Switzerland) and biochemical reagents were from Sigma (St Louis, MO, USA). The di-cationic compounds used in this study were synthesized at the Department of Chemistry and Center for Biotechnology and Drug Design, Georgia State University, USA. They were kept as a dry powder or as stock solutions of 1 mg/ml in dimethyl sulfoxide and were stored at −20°C.
Cell culture and parasite purification
Vero cells were maintained in RPMI 1640 medium supplemented with 5% fetal calf serum (FCS), 2 mm L-glutamine, 50 U of penicillin/ml, and 50 μg of streptomycin/ml at 37°C with 5% CO2 in tissue-culture flasks and were trypsinized 3 times a week. Human foreskin fibroblasts (HFF) were maintained in Dulbecco's modified Eagles's medium (DMEM) with 10% FCS, 50 U of penicillin/ml, and 50 μg of streptomycin/ml at 37°C with 5% CO2 in tissue-culture flasks. Cultures were trypsinized once a week. Toxoplasma gondii Me49, T. gondii Rh, T. gondii-β-gal, (a transgenic strain with Rh-background expressing β-galactosidase; McFadden et al. Reference McFadden, Seeber and Boothroyd1997) were cultured in Vero cells (Scheidegger et al. Reference Scheidegger, Vonlaufen, Naguleswaran, Gianinazzi, Müller, Leib and Hemphill2005). Intracellular parasites were harvested by trypsinization of infected Vero cells, followed by repeated passages through a 25-gauge needle at 4°C, and separation from cell debris on a Sephadex-G25 column as described previously (Hemphill, Reference Hemphill1996). Purified tachyzoites were used to infect HFF monolayers as described below.
In vitro drug treatment assays employing T. gondii Me49 and DB750-adapted T. gondii_DB750 by quantitative real time PCR
HFF cells were grown to confluency in 24-well tissue-culture plates, either adherent to glass coverslips or directly on the plastic surface. Each well was infected with 5×104 cell culture-derived and freshly purified T. gondii tachyzoites re-suspended in DMEM containing 5% FCS, 50 U of penicillin/ml, and 50 μg of streptomycin/ml (=full medium). Following incubation for 1 h at 37°C in a 5% CO2 athmosphere, unbound parasites were removed by washing in DMEM, and 1 ml of full medium was added, containing the compounds at concentrations as indicated in the individual experiments. In some experiments, DB750 (1 μ m) was added to parasites and/or host cells already prior to infection, as indicated below. For a list of some of these compounds refer to Table 1. Each experiment included controls such as (i) parasite-infected HFF in full medium containing respective concentrations of the DMSO-solvent and (ii) uninfected HFF monolayers in drug-containing full medium to assess selective toxicity. The cultures were maintained at 37°C in a 5% CO2 atmosphere during different periods of time as indicated below, and were inspected daily by light microscopy. Samples for quantitative real time PCR analysis were taken by removal of the medium and addition of a mixture of 200 μl of phosphate-buffered saline (PBS), 180 μl of lysis buffer and 20 μl of proteinase K (DNAeasy Kit, QIAGEN, Basel, Switzerland). Samples were stored at −20°C until further analysis was carried out. The quantification of parasites was done by qPCR according to previously described protocols (Costa et al. Reference Costa, Pautas, Ernault, Foulet, Cordonnier and Bretagne2000; Scheidegger et al. Reference Scheidegger, Vonlaufen, Naguleswaran, Gianinazzi, Müller, Leib and Hemphill2005). As external standards, samples containing the DNA from 0, 10, 100, and 1000 T. gondii tachyzoites were included. The parasite numbers in the experimental samples were deduced by interpolation from the standard curve.
Table 1. IC50 determination of selected compounds against different Toxoplasma gondii strains, and assessment of HFF toxicity

* Values are given for exposure of HFF to drugs for 2×3 days at 37°C, 5% CO2, and induction of 50% or greater cell death.
** Values are in a similar range to that previously determined for T. gondii Rh (0·16 μ m) by real time PCR (Leepin et al. Reference Leepin, Stüdli, Brun, Stephens, Boykin and Hemphill2008).
In vitro drug treatment assays employing T. gondii β-gal
These assays were done essentially as previously described (Müller et al. Reference Müller, Limban, Stadelmann, Missir, Chirita, Chifiriuc, Nitulescu and Hemphill2009). HFF monolayers were grown to confluency in 96-well flat-bottommed microtitre plates. For each assay, 8 wells were used. Uninfected control-HFF received 200 μl of full medium, all other monolayers were infected with 100 μl of full medium containing 2×103 freshly purified T. gondii β-gal tachyzoites as above. After 1 h, drug- and solvent-control samples were added, and plates were incubated for 72 h at 37°C/5% CO2. In some experiments, DB750 or DB745 (1 μ m) were added already prior to infection, either to the host cells or to the parasites, as indicated below. Subsequently, plates were centrifuged at 80 g for 5 min at 20°C, the medium was removed, and wells were washed once with 200 μl of PBS and centrifuged again. Each well received 90 μl of PBS containing 0·05% Triton-X-100 and 10 μl of the enzyme substrate (5 mm chlorophenylred-β-galactoside in PBS), and the absorption was read at various time-points. As determined by McFadden et al. (Reference McFadden, Seeber and Boothroyd1997), the initial velocity (ΔA570/min) was proportional to the number of tachyzoites down to 50 per well.
Determination of IC50 values
IC50 values of selected compounds (see Table 1) were determined in cultures treated for 72 h with drugs at different concentrations ranging from 0 to 2 μ m. IC50 values were calculated after the logit-log-transformation of the relative growth (RG; control=1) according to the formula ln[(RG/(1-RG)]=a×ln(drug concentration)+b and subsequent regression analysis by the corresponding software tool contained in the Excel software package (Microsoft, Seattle, WA, USA).
Assessment of host cell toxicity
HFF were seeded into 24-well tissue-culture plates and were grown to confluency. Medium was removed and 2 ml containing the compounds of interest (DB702, DB745, DB1127 and DB1282) were added at concentrations between 0·5, 1, 2, and 3 μ m. Controls received corresponding amounts of DMSO-solvent. After 3 days of culture, the medium was replaced with fresh medium containing the same amounts of the drugs, and culture was repeated for another 3 days. Monolayers were inspected microscopically on a daily basis and, at day 6, cell death was assessed by trypsinization, staining of cells with Trypan blue, and counting the live and the dead cells in a Neubauer chamber.
Determination of the effects of DB745 treatment on extracellular T. gondii β-gal
T. gondii β-gal tachyzoites (2×105 parasites in 1 ml) were re-suspended in pre-warmed (37°C) or pre-cooled (4°C) full medium, and were incubated at the respective temperature for 30 min. Subsequently, tubes were complemented with 1 μ m DB745, or the respective concentration of solvent (DMSO), and were further incubated for either 2 h or 4 h at 4°C and 37°C, respectively. Subsequently, the tachyzoites were centrifuged at 300 g for 10 min at 4°C, and were re-suspended in 1 ml of full medium. Then 100 μl of the suspension was used per well of a 96-well plate to infect a HFF monolayer grown to confluency, and 8 wells were used for 1 experimental assay. The 96-well plates were placed at 37°C in 5% CO2 for 72 h. Assessment of tachyzoite numbers was done by detection of β-galactosidase activity as described above (McFadden et al. Reference McFadden, Seeber and Boothroyd1997; Müller et al. Reference Müller, Limban, Stadelmann, Missir, Chirita, Chifiriuc, Nitulescu and Hemphill2009).
In vitro adaptation of T. gondii Me49 to increased concentrations of DB750 and DB745
Confluent HFF monolayers were infected with T. gondii Me49 in small tissue-culture flasks and were exposed to a stepwise increase in drug concentration over time, starting with 0·05 μ m for DB750 and 0·02 μ m for DB745. The drug concentration was elevated stepwise every 3–4 days by 0·05 and 0·02 μ m, respectively, with daily evaluation by light microscopy. The drugs reached maximum concentrations of 1·2 μ m for DB750 and 0·46 μ m for DB745. Every 6–9 days, parasites were passaged onto fresh HFF by trypsinization of infected cultures, washing them in PBS, and adding them to fresh monolayers previously grown overnight. For both compounds a further increase in drug concentration had to be terminated due to host cell toxicity as judged by microscopical assessment.
Proliferation of non-adapted T. gondii tachyzoites and DB750-adapted T. gondii_DB750 in the presence and absence of DB750
Confluent HFF monolayers, grown in 24-well tissue-culture plates, were infected with 103 freshly purified non-adapted T. gondii or DB750-adapted T. gondii_DB750 in full medium for 1 h at 37°C/5% CO2. Subsequently wells received DB750 to a final concentration of 1·2 μ m, or the appropriate amount of solvent (DMSO) alone, and were further incubated at 37°C/5% CO2 . Sample collection was done at 12 h, 36 h, 60 h, 84 h, 108 h, 134 h and 158 h following initiation of drug treatment. For this, the supernatants were removed, wells were trypsinized, and cells were collected by centrifugation at 340 g for 10 min at 4°C. DNA was purified and samples were processed for quantitative real-time PCR, both as described above.
Differential immunofluorescence staining of extracellular and intracellular T. gondii Me49 tachyzoites
The protocol previously applied for N. caninum (Hemphill et al. Reference Hemphill, Gottstein and Kaufmann1996; Naguleswaran et al. Reference Naguleswaran, Müller and Hemphill2003) was used. In short, non-adapted T. gondii Me49 and T. gondii_DB750 were resuspended in full medium to 5×107 parasites/ml. Parasites were treated or not with 1 μ m DB745 for 1 h at 37°C. Subsequently, 100 μl of parasite suspensions were allowed to settle onto HFF cell monolayers grown on poly-L-lysine (100 μg/ml) coated glass coverslips in 24-well tissue-culture plates at 37°C and 5% CO2 for 40 min. Subsequently, coverslips were rinsed in PBS and were placed into fixation buffer containing PBS/3% paraformaldehyde/0·05% glutaraldehyde for 10 min at 22°C. The coverslips were washed extensively in PBS and were incubated in PBS containing 1% bovine serum albumin (BSA) and 50 mm glycine (blocking buffer) for 30 min. The first antibody layer (polyclonal rabbit anti-T. gondii antiserum; Scheidegger et al. Reference Scheidegger, Vonlaufen, Naguleswaran, Gianinazzi, Müller, Leib and Hemphill2005) was applied at a dilution of 1:500 in blocking buffer for 25 min, followed by 3 washes in PBS, and the secondary antibody (goat anti-rabbit-Texas red (Becton Dickinson Immunocytometry Systems)) at a 1:200 dilution for 25 min. After washing in PBS (3 times×5 min), cells were permeabilized by placing the coverslips into pre-cooled methanol and acetone (×20°C) for 5 min each. After rehydration in PBS, coverslips were placed into blocking buffer, and were labelled with the same polyclonal rabbit anti-Toxoplasma gondii antiserum (1:500), and with a goat anti-rabbit-FITC (Becton Dickinson Immunocytometry System) at a 1:200 dilution. Specimens were then washed extensively (1 min in 2×concentrated PBS for 1 min, followed by additional rinses 5×5 min in PBS). Finally, coverslips were mounted onto glass slides using Vectashield mounting medium containing DAPI (Vector Laboratories, Burlingame, USA). Results were obtained by inspection of 20 randomized chosen fields (with at least 15 host cells) at 40 times magnification on a Nikon ECLIPSE 80i fluorescence microscope using all 3 channels. By counting the FITC-labelled parasites the overall number of T. gondii tachyzoites was determined. The number of parasites located on the surface of the HFF cells was determined by counting the Texas red immunolabelled tachyzoites. Finally, the number of intracellular parasites was calculated.
Transmission electron microscopy (TEM)
HFF monolayers grown in 25 cm2 tissue-culture flasks were infected with T. gondii Me49 or with T.gondii_DB750. At 2 days post-infection, treatment with DB750 or DB745 were initiated, both at 1 μ m. Samples were collected 24 h later, by removing the medium, washing the monolayers twice with 100 mm sodium cacodylate buffer (pH 7·2), and fixation in 100 mm sodium cacodylate buffer, pH 7·3, containing 2·5% glutaraldehyde for 2 h. Then specimens were washed twice with 100 mm cacodylate buffer, scraped off with a rubber policeman, and centrifuged at 100 g for 10 min at 4°C. Post-fixation was done in cacodylate buffer containing 2% OsO4 at 22°C. Subsequently, specimens were washed in water pre-stained in 1% uranyl acetate in water for 30 min, followed by an extensive wash with water. The samples were dehydrated in a graded series of ethanol (30, 50, 70, 90, and 100%), and were embedded in Epon 820 epoxy resin as described by Leepin et al. (Reference Leepin, Stüdli, Brun, Stephens, Boykin and Hemphill2008). The resin was polymerized at 65°C for 24 h. Ultrathin sections (∼80 nm) were cut on a Reichert and Jung ultramicrotome and were loaded onto 300-mesh copper grids (Plano GmbH, Marburg, Germany), and stained with uranyl acetate and lead citrate (Hemphill et al. Reference Hemphill, Vonlaufen, Naguleswaran, Keller, Riesen, Guetg, Srinivasan and Alaeddine2004). Grids were viewed on a Philips 400 transmission electron microscope (TEM) operating at 80 kV.
RESULTS
In vitro activity of DB750 against T. gondii Rh and T. gondii Me49
As determined by real time PCR quantification, DB750 was previously shown to be active against T. gondii Rh (Rh-strain) tachyzoites, with an IC50 of 0·16 μ m (Leepin et al. Reference Leepin, Stüdli, Brun, Stephens, Boykin and Hemphill2008). In order to investigate whether T. gondii Me49 (Me49-strain) tachyzoites exhibit a similar susceptibility, infected HFF monolayers were subjected to increasing concentrations of DB750 (0–2 μ m) for a period of 72 h. Real time PCR showed that DB750 inhibited growth of T. gondii Me49 with an IC50 of 0·13 μ m, which was in the same range, as previously reported for T. gondii Rh tachyzoites (Leepin et al. Reference Leepin, Stüdli, Brun, Stephens, Boykin and Hemphill2008).
To further characterize the effects of DB750 in T. gondii tachyzoites, and to facilitate screening for other potentially interesting compounds in a more cost-effective manner, a T. gondii transgenic strain (T. gondii β-gal; Rh-background) was employed that constitutively expressed β-galactosidase (McFadden et al. Reference McFadden, Seeber and Boothroyd1997). DB750 inhibited proliferation of T. gondii β-gal with an IC50 of 0·11 μ m (see Table 1). Thus, T. gondii Rh, T. gondii β-gal, and T. gondii Me49 exhibited similar susceptibility to the action of DB750.
Further investigations were carried out employing T. gondii β-gal (Fig. 1). As expected, culture of T. gondii β-gal tachyzoites in HFF for 72 h in the presence of 1 μ m DB750 did not result in tachyzoite proliferation, regardless of whether the drug was added 1 h after infection or during the invasion phase (see Fig. 1A, B). As previously observed for N. caninum (Leepin et al. Reference Leepin, Stüdli, Brun, Stephens, Boykin and Hemphill2008), incubation of uninfected HFF monolayers with 1 μ m DB750 for 12 h prior to infection, and subsequent infection with T. gondii tachyzoites and culture for 72 h, also did not result in any parasite growth (see Fig. 1C). In order to investigate whether the activity of DB750 was maintained in the supernatant of HFF monolayers after 12 h of treatment, freshly purified T. gondii tachyzoites were suspended in the medium supernatant originating from HFF cultures treated for 12 h, and were used for infection and culture on fresh HFF monolayers for 72 h. T. gondii tachyzoites readily proliferated in that pre-used medium (Fig. 1D).

Fig. 1. Effects of DB750 against Toxoplasma gondii β-gal employing 72 h in vitro-growth assays. (A) T. gondii tachyzoites were allowed to infect HFF monolayers and 1 μ m DB750 was added 1 h later. (B) DB750 was added already during the invasion phase. (C) HFF monolayers were cultured for 12 h in medium containing 1 μ m DB750, washed extensively with medium, and freshly isolated T. gondii tachyzoites were added subsequently. (D) Freshly purified T. gondii tachyzoites were re-suspended in the medium supernatant obtained from (C) and added to HFF monolayers. Note the inhibition of parasite growth in (A), (B) and (C), and extensive parasite proliferation in (D). Shown is 1 of 3 experiments with essentially identical outcome.
T. gondii tachyzoites can readily adapt to the effects of DB750 treatment in vitro
In order to establish whether T. gondii tachyzoites would be able to adapt, or possibly acquire resistance, to DB750, we cultured T. gondii Me49 tachyzoites in HFF monolayers, by increasing the drug concentration stepwise every 2–3 days, starting at 0·05 μ m and ending at 1·2 μ m. Upon exposure to concentrations of more than 1·2 μ m DB750 for more than 10 days, HFF monolayers exhibited aberrant morphological alterations, which indicated toxic effects upon longer-term exposure (data not shown). Nevertheless, a DB750-adapted T. gondii strain with Me49 background (T. gondii_DB750) was generated which still readily proliferated at 1·2 μ m drug concentration. The ability of T. gondii_DB750 to resist elevated drug concentrations was retained after freezing of infected cells in liquid nitrogen using a medium composed of FCS/10% DMSO, short-term storage in liquid nitrogen, and subsequent thawing and culture in DB750-containing medium. The proliferation of T. gondii_DB750 in the presence of different concentrations (0–2 μ m) of DB750 was assessed in 72 h-growth assays in HFF, and yielded an IC50 of 0·48 μ m, which was almost 4 times higher than that for the non-adapted strain (0·13 μ m; see Table 1).
Subsequently, the proliferation-kinetics of non-adapted T. gondii Me49 and T. gondii-DB750 within a time frame of 156 h were comparatively assessed by real time PCR in the presence and absence of 1·2 μ m DB750, respectively (Fig. 2). Within the first 84 h of culture in the absence and presence of DB750 both T. gondii strains exhibited similar growth kinetics. Subsequently, in the absence of DB750, the non-adapted T. gondii Me49 tachyzoites proliferated at a significantly higher rate (P<0·05) compared to T. gondii_DB750, until the time-point 156 h post-infection, at which the numbers of T. gondii_DB750 increased dramatically (Fig. 2A). In the presence of 1·2 μ m DB750 (Fig. 2B), the DB750-adapted strain was proliferating slightly more rapidly from 84 h post-infection onwards until the end of the assay. However, surprisingly, a substantial proliferation of non-adapted T. gondii Me49 tachyzoites could be observed from 84 h post-infection onwards, and the parasite numbers of the two strains were not significantly different at any time-point. Thus, DB750 did not exhibit parasiticidal activity against non-adapted T. gondii Me49 tachyzoites when applied at high (1·2 μ m) concentration. Conversely, T. gondii Me49 tachyzoites rapidly adapted to the action of the compound within the short time frame of 5–6 days of treatment. The same potential to readily adapt to DB750 treatment was observed for T. gondii β-gal tachyzoites (data not shown).

Fig. 2. Proliferation kinetics of Toxoplasma gondii Me49 and DB750-adapted T. gondii_DB750 in HFF monolayers. (A) Growth curves in the absence of DB750 and (B) in the presence of 1·2 μ m DB750. Parasite quantification was performed by quantitative real time PCR at different time-points as indicated in the x-axis over a period of 156 h. Shown is 1 of 2 experiments with essentially identical outcome. P values <0·05 indicate significant differences in parasite numbers of the two strains when cultured in the absence of DB750.
In vitro screening of di-cationic pentamidine derivatives against T. gondii tachyzoites leads to the identification of DB745
T. gondii β-gal tachyzoites were used for the screening of a series of selected di-cationic pentamidine derivatives, including 10 di-arylimidamides (DB666, DB667, DB702, DB710, DB745, DB780, DB786, DB865, DB891, or DB930), 6 diamidines (DB1282, DB1341, DB1362, DB1407, DB1450 or DB1479), 15 newly generated mono-arylimidamides (DB1980, DB1996, DB1997, DB2001, DB2002, DB2006, DB2007, DB2018, DB2036, DB2045, DB2048, DB2074, DB2079, DB2081, or DB2083), and the di-guanidino analogue DB1127, all at an initial concentration of 1 μ m. Evaluation of β-galactosidase activity after 72 h of exposure to these drugs revealed that the mono-arylimidamides tested did not show any adverse effects on T. gondii proliferation nor on host cells, but proliferation-inhibitory effects were imposed by the di-arylimidamides DB702 and DB745, the di-guanidino-analogue DB1127, and the di-amidine DB1282 (see Table 1).
Assessment of host cell toxicity by light microscopical inspection of uninfected HFF monolayers exposed to increasing concentrations of the drugs (0, 0·5, 1, 2 or 3 μ m) twice/day for 3 days revealed that DB1282 induced over 50% cell death already at 1 μ m, and DB1127 lead to 80% HFF death at a concentration of 0·5 μ m. Fifty % of viable HFF compared to untreated samples were found in cultures treated with 2 μ m DB702 (Table 1). For DB745, treatments with 0·5–2 μ m did not result in adverse reactions in HFF monolayers, while at 3 μ m a 50% reduction in viable HFF was observed. Thus, DB745, with an IC50 for T. gondii β-gal of 0·03 μ m, and favourable selective toxicity was selected for further study (see Table 1).
Characterization of the effects of DB745 on proliferation and host cell invasion of non-adapted (T. gondii Me49) and DB750-adapted (T. gondii_DB750) tachyzoites
DB750 and DB745 exhibit a similar molecular structure (Fig. 3A). They differ in molecular weight (590·46 and 655·60, respectively), and in contrast to DB750, DB745 possesses an ethyl-moiety on each of the 1′-positions of the aryl rings. The proliferation of non-adapted T. gondii Me49 and T. gondii_DB750 in the presence of 1 μ m DB745 was assessed in 72 h growth-assays, and compared with T. gondii_DB750 tachyzoites treated with 1·2 μ m DB750 (Fig. 3B). As expected, DB745 completely blocked the proliferation of non-adapted T. gondii Me49 tachyzoites (IC50=0·03 μ m; see Table 1). The DB750-adapted T. gondii_DB750 tachyzoites readily underwent proliferation in the presence of 1·2 μ m DB750 (IC50=0·48 μ m; see Table 1), but were significantly impaired in the presence of DB745 (IC50 of 0·07 μ m; see Table 1). However, the inhibition of proliferation was significantly less pronounced as for non-adapted T. gondii Me49.

Fig. 3. Properties of DB750 and DB745. (A) Structures and molecular weights of DB750 and DB745. (B) Proliferation inhibitory effects of DB750 and DB745 over a period of 72 h. Parasite quantification was done by quantitative real time PCR. In (B) the proliferation of Toxoplasma gondii Me49 over a period of 72 h was completely inhibited by the presence of 1 μ m DB745 (left). T. gondii_DB750 proliferation was not inhibited by the presence of 1·2 μ m DB750 (middle), but significantly impaired by the presence of 1 μ m DB745 (right). Shown is 1 of 2 experiments with essentially identical outcome.
In order to investigate whether DB745 also had an impact on extracellular T. gondii tachyzoites, T. gondii β-gal tachyzoites were separated from their host cells and exposed to DB745 (1 μ m) for periods of 2 h and 4 h, either at 4°C or at 37°C (Fig. 4A). Subsequently, the drugs were removed, and parasites were added to HFF monolayers, and proliferation of tachyzoites was assessed after 72 h. Upon incubation of isolated tachyzoites at 4°C, DB745 already had a profound impact on the infectivity of the parasites, yielding a reduction of tachyzoite numbers of approximately 60% after both 2 and 4 h exposure-time to the drug. At 37°C, the impact of DB745 on parasite infectivity was even more pronounced, yielding a reduction in parasite numbers of 80% after 2 h, and over 90% upon 4 h of extracellular exposure to DB745 (Fig. 4A). This suggested that DB745 impaired the viability of extracellular parasites in a time- and temperature-dependent manner.

Fig. 4. Effects of DB745 on the infectivity of extracellular Toxoplasma gondii tachyzoites. (A) T. gondii β-gal tachyzoites were incubated in the presence or absence of 1 μ m DB745 for 2 h or 4 h, either at 4°C or at 37°C. They were then used for infection and proliferation in HFF monolayers for a period of 72 h prior to quantification of β-galactosidase activity. Results in terms of parasite proliferation are presented as a percentage in relation to treatments without drug (given as 100% β-galactosidase activity). The assays were done in quadruplicate. (B) Assessment of DB745-mediated inhibition of invasion of T. gondii Me49 tachyzoites by immunofluorescent distinction of intracellular and extracellular parasites. Results were obtained by differential staining and subsequently counting the parasites in 20 randomly chosen fields at 40 times magnification. Extracellularly located parasites fluoresce with Texas-red and the overall number of parasites was determined by FITC-staining. Note the reduction of invasive parasites after incubation with 1 μ m DB745 for 1 h. Shown are the results of 1 of 2 experiments with essentially identical outcome.
The effect of DB745 on host cell invasion was assessed using T. gondii Me49 and employing immunofluorescence microscopy and applying a differential staining procedure that allowed distinction of intracellular and extracellular parasites (Hemphill et al. Reference Hemphill, Gottstein and Kaufmann1996; Naguleswaran et al. Reference Naguleswaran, Müller and Hemphill2003). Following exposure of non-adapted T. gondii Me49 tachyzoites to 1 μ m DB745 for 1 h, parasites were washed and allowed to interact with HFF monolayers for 1 h (Fig. 4B). As a control, non-treated parasites were subjected to the same procedure without compound. Of 857 non-treated tachyzoites, 173 (20·2%) were located within host cells. In contrast, in DB745-treated samples the overall number of counted parasites was reduced to 664 tachyzoites and only 66 (11·2%) were found to be intracellular (Fig. 4B). No such effect was seen when T. gondii-DB750 tachyzoites were exposed to DB745 treatment, and when non-adapted T. gondii Me49 tachyzoites were exposed to DB750 (data not shown).
In vitro adaptation of T. gondii Me49 tachyzoites to DB745
In order to explore the potential of T. gondii Me49 tachyzoites to adapt to DB745 treatment, the concentration of the drug during the culture of infected HFF monolayers, starting at 0·02 μ m, was increased stepwise by 0·02 μ m every 3–4 days (see Table 2). As for DB750, T. gondii Me49 tachyzoites exhibited an astonishing capacity to also adapt to DB745, albeit at a much slower speed, but finally still undergoing proliferation up to a concentration of 0·46 μ m. Rapid adaptation to increasing concentrations of DB745 as previously observed for DB750 was not possible. At higher concentrations, exposure to DB745 during periods of 10 days or longer lead to deterioration of the HFF monolayers. Thus, besides exhibiting a higher anti-parasitic activity compared to DB750, DB745 also showed an increased host cell toxicity. The IC50 of DB745 for this DB745-adapted strain was not determined.
Table 2. Schedule for the establishment of a DB745-adapted Toxoplasma gondii Me49 line

Effects of DB745 on the ultrastructure of T. gondii tachyzoites
Differences in efficacy of DB745 against non-adapted and DB750- adapted T. gondii_DB750 tachyzoites were also detectable at the ultrastructural level. HFF monolayers infected with T. gondii_DB750 or non-adapted T. gondii were treated with 1 μ m DB750 and DB745, respectively, for 24 h, and were processed for TEM (Figs 5 and 6). In non-adapted T. gondii Me49 both, DB750 (Fig. 5B) and DB745 (Fig. 5C, D) induced severe morphological alterations in parasite ultrastructure, as can be seen by, for example, the formation of increased cytoplasmic vacuoles with membranous and often granular content, the presence of membrane stacks, and electron-dense inclusions. In DB750-adapted T. gondii_DB750 tachyzoites (Fig. 6) there were no obvious differences observed in the ultrastructure of untreated (Fig. 6A) and DB750-treated T. gondii_DB750 tachyzoites (Fig. 6B), confirming the complete adaptation of these parasites to the drug. In contrast, populations with different degrees of structural alterations were detected in cultures infected with T. gondii_DB750 tachyzoites and treated with DB745. In some instances seemingly viable parasites with normal or only slightly aberrant ultrastructural organization (Fig. 6C and D) could be seen, often in the direct vicinity of tachyzoites that exhibited extensive cytoplasmic distortions such as increased vacuolization and membranous inclusions (Fig. 6C, E). These aberrant structural changes point towards severe metabolic impairment of affected tachyzoites. In addition, numerous non-viable tachyzoite-remnants were detected in DB745-treated cultures (Fig. 6F).
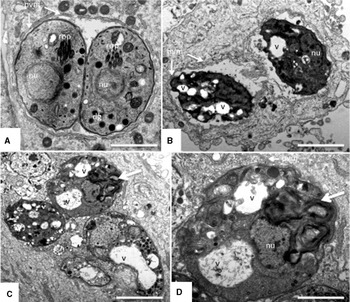
Fig. 5. TEM of the effects of DB750 and DB745 (1 μ m, 24 h) against Toxoplasma gondii Me49 tachyzoites cultured in HFF. (A) Control without drug treatment, with T. gondii Me49 tachyzoites surrounded by a parasitophorous vacuole membrane (pvm) and exhibiting cytoplasmic organelles such as nucleus (nu), rhoptries (rop) and dense granules (dg). Scale bar=0·7 μm. (B) T. gondii Me49 tachyzoites incubated in the presence of DB750, exhibiting extensive vacuolization, and substantial degradation of the cytoplasmic architecture (v). Scale bar=0·9 μm. (C) Shows a group of T. gondii Me49 tachyzoites incubated in the presence of DB745 (Scale bar=0·9 μm), and (D) displays a higher magnification view of the parasite indicated by the large arrow (Scale bar=0·4 μm). In (D), note the presence of cytoplasmic vacuoles, either seemingly empty or filled with a meshwork of filamentous and granular material of unknown origin (v) and the large stacks of membrane adjacent to the nucleus (nu). In all drug-treated samples, the pvm appeared still largely intact and clearly discernible.

Fig. 6. TEM of the effects of DB750 and DB745 (1 μ m, 24 h) against DB750-adapted Toxoplasma gondii-DB750 tachyzoites cultured in HFF. (A) Control, displaying T. gondii_DB750 tachyzoites without drug treatment. Typical components such as the parasitophorous vacuole membrane (pvm), rhoptries (rop), dense granules (dg) and micronemes (mic) at the apical pole of the tachyzoites are clearly discernible. The arrow points towards the conoid. Scale bar=1 μm. (B) T. gondii_DB750 tachyzoites incubated in the presence of DB750. Note that these parasites seem largely unaffected by the drug treatment. (C–F) T. gondii_DB750 cultured in the presence of DB745; in (C) tachyzoites exhibiting only moderate alterations and still exhibiting a largely intact cytoplasmic organization (vertical arrow), and another tachyzoite with increased vacuole formation (horizontal arrow) are shown. (D) Higher magnification view of (C). Scale bars in (C)=0·9 μm, in (D)=0·4 μm. (E) and (F) Show tachyzoites that exhibit more severe alterations due to drug treatment such as intracytoplasmic vacuoles (v), lipid droplet formation (ld) and a general desintegration of the cellular architecture. Scale bars in (E) and (F)=0·5 μm. In all drug-treated samples, the pvm appeared still intact and clearly discernible.
DISCUSSION
Although pentamidine was synthesized already in the 1930s, it has remained the only aromatic diamidine that is widely used in the clinic, especially against human stage 1 African trypanosomiasis caused by Trypanosoma brucei gambiense, but also against Pneumocystis pneumonia caused by Pneumocystis jiroveci, and against leishmaniasis, and Candida albicans infections (Werbovetz, Reference Werbovetz2006). A number of arylimidamides, including DB702, DB750, DB766, DB786, DB811 and DB889 exhibited good in vitro activity against Trypanosoma cruzi, N. caninum, B. besnoiti or L. donovani (Stephens et al. Reference Stephens, Brun, Salem, Werbovetz, Tanious, Wilson and Boykin2003; Silva et al. Reference Silva, Batista, Mota, de Souza, Stephens, Som, Boykin and Soeiro2007a, b; Leepin et al. Reference Leepin, Stüdli, Brun, Stephens, Boykin and Hemphill2008; Cortes et al. Reference Cortes, Muller, Boykin, Stephens and Hemphill2011), and DB766 effectively reduced the parasite burden in the blood and heart of T. cruzi-infected mice at a dose of 100 mg/kg/day given orally and intraperitoneally (Batista et al. Reference Batista, Batista, de Oliveira, do Amaral, Lannes-Vieira, Britto, Junqueira, Lima, Romanha, Sales Junior, Stephens, Boykin and Soeiro2010a). Thus, the goal of this investigation was to study the interactions of T. gondii tachyzoites with DB750, which had shown interesting properties in a previous study (Leepin et al. Reference Leepin, Stüdli, Brun, Stephens, Boykin and Hemphill2008), and possibly identify other compounds with good in vitro activity. This included effects of these compounds on intracellular proliferation, host cell toxicity, parasiticidal activity, the involvement of the host cells in drug activity, and the potential of these parasites to adapt to the adverse conditions during in vitro drug treatment.
The di-cationic molecules used here have been designed to have improved pharmacokinetic properties by including aryl groups on one of the amidine nitrogen atoms (Wang et al. Reference Wang, Zhu, Srivastava, Liu, Sweat, Pandharkar, Stephens, Riccio, Parman, Munde, Mandal, Madhubala, Tidwell, Wilson, Boykin, Hall, Kyle and Werbovetz2010) and DB750 was an obvious drug candidate due to its low IC50 against T. gondii Rh (0·23 μ m), and low toxicity in HFF monolayers (Leepin et al. Reference Leepin, Stüdli, Brun, Stephens, Boykin and Hemphill2008). Previous studies had also shown that the HFF host cells actually participated in the activity against closely related N. caninum tachyzoites, most likely by taking up the drug and providing the host cell with a ‘memory effect’, thus exerting the anti-parasitic activity against intracellular tachyzoites even in the absence of added drug (Leepin et al. Reference Leepin, Stüdli, Brun, Stephens, Boykin and Hemphill2008). However, DB750 did not affect the viability and invasive capacities of extracellular N. caninum and T. gondii Rh tachyzoites (Leepin et al. Reference Leepin, Stüdli, Brun, Stephens, Boykin and Hemphill2008). Moreover, recent in vivo studies in mice experimentally infected with the N. caninum tachyzoites showed that DB750-treatment, applied intraperitoneally at 2 mg/kg/day for a period of 14 days, had a benefitial impact by reducing clinical signs of neosporosis and diminishing the cerebral parasite load (Debache et al. 2011). Thus, we attempted to characterize the effects mediated by DB750 in 2 different strains of T. gondii, Rh and Me49, which represent strains of high and low virulence in mice, respectively (Dardé, Reference Dardé2008).
By employing 72 h-growth assays, DB750 IC50 values for the two Toxoplasma strains, T. gondii β-gal (Rh background) and T. gondii Me49, were shown to be in the similar low range (0·11–0·16 μ m) of the previously reported IC50 for T. gondii Rh (Leepin et al. Reference Leepin, Stüdli, Brun, Stephens, Boykin and Hemphill2008). Thus, the assessments of parasite proliferation by a colourimetric assay based on the quantification of β-galactosidase activity (McFadden et al. Reference McFadden, Seeber and Boothroyd1997) and the quantification of parasite numbers by quantitative real time PCR produced similar results.
Similar to what was previously reported for N. caninum, DB750 retained its anti-parasitic activity against T. gondii also upon pre-treatment of host cells for prior to infection and subsequent culture for 72 h in the absence of the drug. In addition, no anti-parasitic activity could be seen in the corresponding HFF-medium supernatant. The lack of DB750-activity in medium supernatant after 12 h of incubation with HFF could be potentially explained (i) by metabolic degradation of DB750 or (ii) by highly efficient drug-uptake by the host cells. The fact that the anti-parasitic effect was retained in these pre-treated HFF cells but was no longer evident in the corresponding medium supernatant supports the notion of DB750 being actively taken up by HFF monolayers and subsequently retained in the host cell cytoplasm. How this is achieved is not known; passive and active transport could be involved. Ming et al. (2008) identified a human organic cation transporter for which pentamidine and furamidine act as substrates, and possibly such transporters could mediate DB750 uptake into HFF. In addition, intracellular accumulation of DB750 within the HFF monolayers could be responsible for the toxicity that was detected upon culture of infected fibroblasts for more than 10 days in the presence of DB750 at concentrations higher than 1·2 μ m.
Although the promising IC50 values achieved in 72 h assays indicated that T. gondii tachyzoites were highly susceptible to DB750 treatments, it became clear that these parasites exhibited a surprising ability to rapidly adapt to approximately 10 times higher drug concentrations within 5–6 days following initiation of treatment. The resulting DB750-adapted T. gondii strain, T. gondii_DB750, had an IC50 of 0·48 μ m and could still undergo proliferation in HFF at a drug concentration of 1·2 μ m. In comparison to T. gondii, the closely related N. caninum (Nc-1 and Nc-Liv isolates) did not exhibit the ability to rapidly adapt to such high concentrations of DB750. DB750 adaptation of Neospora populations could only be achieved after several weeks of careful and stepwise increase of drug concentrations up to 0·2–0·3 μ m. Thus, although phylogenetically closely related, the two species seem to exhibit a different adaptation profile. Most likely, T. gondii has a more sophisticated metabolic machinery to deal with adverse life conditions, and it would be interesting to investigate the molecular mechanisms involved. In any case, the lower potential of Neospora to react to environmental changes could be a determining factor that limits the range of potential intermediate hosts of this parasite in comparison to T. gondii (Hemphill et al. Reference Hemphill, Vonlaufen and Naguleswaran2006; Dubey et al. Reference Dubey, Schares and Ortega-Mora2007). Thus, a higher degree of flexibility on the transcriptional and translational level (reviewed by Bougdour et al. Reference Bougdour, Braun, Cannella and Hakimi2010) may have enabled T. gondii tachyzoites to adapt to the arylimidamide compound DB750 within this short time span.
By monitoring the growth of T. gondii Me49 and T.gondii_DB750 in the presence of DB750 over time, the capacity of T. gondii_DB750 to proliferate at a slightly higher rate compared to non-adapted parasites was demonstrated. However, the fact that T. gondii Me49 tachyzoites were able to adapt to 1·2 μ m DB750 and resume proliferation in such a short time frame was surprising. Adaptation could be accompanied by an increased expression of genes coding for components of the Toxoplasma detoxification machinery, such as e.g. ATP-binding cassette (ABC) transporters, which represent an important family of membrane proteins involved in drug resistance and other biological activities (Sauvage et al. Reference Sauvage, Aubert, Escotte-Binet and Villena2009). A prominent ABC transporter, P-glycoprotein (TgABC.B1 and TgABC.B2) potentially mediating transport of the drug out of the cell, are associated with the membrane in tachyzoites, and is constitutively expressed in all 3 virulent types. On the other hand, another adaptation mechanism could involve the reduced uptake of drug, due to the down-regulation of certain adenosine transporter activities, which are involved in diamidine uptake (Matovu et al. Reference Matovu, Stewart, Geiser, Brun, Mäser, Wallace, Burchmore, Enyaru, Barrett, Kaminsky, Seebeck and de Koning2003; Witola et al. Reference Witola, Inoue, Ohashi and Onuma2004). For instance, homologues to the P2 aminopurine transporter in T. brucei are found in the Toxoplasma genome (www.toxodb.org). This protein normally transports essential adenosine and adenine, but is also responsible for the efficient transfer of pentamidine and the diamidine DB75 into the trypanosome interior (Lanteri et al. Reference Lanteri, Stewart, Brock, Alibu, Meshnick, Tidwell and Barrett2006). Down regulation of the expression of such adenosine transporters would then, besides limiting diamidine uptake, also limit the uptake of other essential molecules such as aminopurines, which are important for proliferation. This could explain the initial reduced proliferation of T. gondii_DB750 compared to T. gondii Me49 in the absence of DB750.
Evidently, these findings on DB750 did not allow high expectations in terms of a potential in vivo efficacy of DB750 against T. gondii infection. As a consequence, we aimed to search for additional active di-cationic compounds with increased anti-parasitic activity, leading to the identification of DB745.
DB750 and DB745 exhibit a highly similar molecular structure. However, the change of the hydroxyl groups on the benzene ring to ether-coupled ethyl groups could have provided DB745 with an increased metabolic resistance and membrane permeability, since the compound is slightly more lipophilic (log P at pH 7·4=3·82; Wang et al. Reference Wang, Zhu, Srivastava, Liu, Sweat, Pandharkar, Stephens, Riccio, Parman, Munde, Mandal, Madhubala, Tidwell, Wilson, Boykin, Hall, Kyle and Werbovetz2010), which could in turn increase the chances of the drug passing several layers of membrane, including the host cell plasma membrane, parasitophorous vacuole membrane, parasite membrane, and the membrane of a potential target organelle. Which target is actually affected by DB745 is not clarified to date. Current evidence suggests that aromatic diamidines bind to AT-rich sites in the DNA minor groove, and thus inhibit transcription or the interaction with DNA-binding enzymes such as topoisomerases or nucleases (Wilson et al. Reference Wilson, Tanious, Mathis, Tevis, Hall and Boykin2008). This indicates that these compounds could influence gene expression, and thus many diverse cellular functions could be affected. In kinetoplastids, fluorescence microscopy had demonstrated that these drugs associate with the kinetoplast DNA, which is in agreement with the observed mitochondrial swelling seen in T. cruzi upon incubation with diamidines (Batista et al. Reference Batista, Pacheco, Kumar, Branowska, Ismail, Hu, Boykin and Soeiro2010b). However, neither DB750 nor DB745 exhibit fluorescent properties, thus it was not possible to localize these drugs, neither within the host cells nor within the parasites.
DB745 did not only affect the proliferation of intracellular T. gondii tachyzoites, but also exhibited pronounced effects upon treatment of extracellular parasites. This showed that, in order to be active DB745 is not required to be metabolized by the host cell. In contrast, DB750 did not affect the viability of extracellular N. caninum and T. gondii Rh tachyzoites (Leepin et al. Reference Leepin, Stüdli, Brun, Stephens, Boykin and Hemphill2008) nor did it have an impact on the invasive properties of T. gondii Me49 tachyzoites. Thus, the decrease in the IC50 (from 0·13 μ m (DB750) to 0·03 μ m (DB745)) against T. gondii Me49 could be due to an added effect such as the impairment of extracellular parasites. As a consequence, T. gondii Me49 adaptation to the effects of DB745 treatment took place much less efficiently and only after extended periods of time. Rapid adaptation to DB745, as seen for DB750, was not possible.
The impact of pre-treatments on the viability of T. gondii Me49 tachyzoites with DB745 at 37°C was time dependent, and these effects were less pronounced upon pre-treatment at 4°C and, at lower temperature, time independent. By employing an adhesion/invasion assay that allowed distinction between adhering tachyzoites and intracellular tachyzoites, the negative impact of DB745-treatments of extracellular tachyzoites was confirmed microscopically. The fact that the IC50 of DB745 for T. gondii_DB750 was about 2 times higher compared to the IC50 for T. gondii Me49 (0·07 μ m versus 0·03 μ m) can be explained by the similarities in structure of DB750 and DB745, thus adaptation to DB750 must have had some cross-protective effect. HFF monolayers infected with T. gondii Me 49 and T. gondii_DB750 were exposed to either DB750 or DB745, both at 1 μ m for 24 h, and the effects were monitored by TEM. As expected, DB750 did not affect T. gondii_DB750, while DB745 clearly had a much higher impact. However, seemingly structurally unharmed parasites were occasionally visible. This indicates and confirms that a certain degree of cross-adaptation had taken place in some tachyzoites, but not in all. This also clearly shows that DB745, although highly effective in 3 day-growth assays, does not readily kill all parasites, even at high concentrations, allowing adaptation to take place in some tachyzoites.
In conclusion, we have demonstrated an astonishing ability of the apicomplexan parasite T. gondii to adapt to the effects of the arylimidamide DB750 within a time span of 5–6 days in culture. DB750 had previously provided encouraging data on selective anti-Toxoplasma efficacy in vitro. Thus our findings question the value of short-term (e.g. 72 h) in vitro assays for the evaluation of anti-Toxoplasma properties, and corresponding investigations that include longer-term in vitro drug exposure could provide more reliable results. T. gondii could also adapt to (less) elevated levels of DB745, although only after many weeks in cell culture. Drug adaptation is also likely to happen in vivo, since chemotherapeutical treatment failures when employing pyrimethamine, sulfadiazine and clindamycine have been reported frequently (Dedicoat and Livesley, Reference Dedicoat and Livesley2006; Ribera Pasquet et al. Reference Ribera Pascuet, López Aldeguer, Pérez Elías and Podzamczer Palter1998). Clearly, Toxoplasma represents a true survival artist, and this illustrates the inherent difficulties in obtaining reliable and efficacious drugs for the treatment of toxoplasmosis. Arylimidamides, however, represent a class of compounds that could serve this cause. DB745 should be followed up in an appropriate in vivo model, and the molecular mechanisms of the outstanding adaptive potential of T. gondii must be elucidated in future studies.
ACKNOWLEDGEMENTS
Many thanks are addressed to Norbert Müller for critical comments on the manuscript. We are grateful to Furio Spano (Istituto Superiore di Sanità, Rome, Italy) for providing us with T. gondii Me49, and to David Sibley (Washington University, St Louis, MO, USA) for his gift of T. gondii β-gal. This study was financed through the Swiss National Science Foundation (grant no. 31003A_127374/1).










