Introduction
Australia's mosquito fauna consists of over 300 species, many of which transmit pathogens of human and veterinary concern (Webb et al., Reference Webb, Russell and Doggett2016). Australian mosquito-borne pathogens such as Ross River virus (RRV) and Barmah Forest virus (BFV) can cause chronic debilitation in affected patients, while Murray Valley encephalitis virus (MVEV) can be potentially fatal, and West Nile virus (Kunjin strain) is of both human and veterinary importance (Burrow et al., Reference Burrow, Whelan, Kilburn, Fisher, Currie and Smith1998; Harley et al., Reference Harley, Sleigh and Ritchie2001; Jacups et al., Reference Jacups, Whelan and Currie2008). Australia is also at risk to exotic pathogens such as Japanese encephalitis virus, which has been identified as a threat in northern Australia where suitable mosquito species and reservoir hosts are present (Mackenzie et al., Reference Mackenzie, Johansen, Ritchie, Van Den Hurk, Hall, Mackenzie, Barrett and Deubel2002; Ritchie et al., Reference Ritchie, Van Den Hurk, Zborowski, Kerlin, Banks, Walker, Lee, Montgomery, Smith and Pyke2007). Exotic mosquito species have already become established in Australia, including Aedes aegypti [the key vector of dengue viruses, chikungunya virus and Zika virus (Jansen and Beebe, Reference Jansen and Beebe2010; Hall-Mendelin et al., Reference Hall-Mendelin, Pyke, Moore, Mackay, McMahon, Ritchie, Taylor, Moore and van den Hurk2016)], Culex pipiens molestus (Kassim et al., Reference Kassim, Webb and Russell2012) and Cx. gelidus (Williams et al., Reference Williams, Ritchie and Whelan2005). Exotic mosquito incursions are increasingly common at Australia's ports, where international cargo ships and aircraft can provide a source of foreign species – both immature and adult stages – with Ae. aegypti and Ae. albopictus of particular concern (van den Hurk et al., Reference van den Hurk, Nicholson, Beebe, Davis, Muzari, Russell, Devine and Ritchie2016). The development of strategic response plans for these species requires appropriate surveillance methods (Webb and Doggett, Reference Webb and Doggett2016) which rely on accurate and rapid mosquito specimen identification.
Mosquito and arbovirus surveillance in Australia is primarily performed using traditional morphological taxonomy, which can be time consuming, requires specialist skills and is challenging when specimens are immature or damaged. Mosquito and arbovirus surveillance continues to evolve in Australia with new technologies for mosquito collection (Flies et al., Reference Flies, Toi, Weinstein, Doggett and Williams2015) and arbovirus detection (van den Hurk et al., Reference van den Hurk, Hall-Mendelin, Townsend, Kurucz, Edwards, Ehlers, Rodwell, Moore, McMahon and Northill2014) as well as the development and application of molecular methods for specimen identification (Batovska et al., Reference Batovska, Lynch, Cogan, Brown, Darbro, Kho and Blacket2017). New technologies for mosquito and arbovirus surveillance aim to provide rapid and accurate data collection as well as easily translatable results to guide public health interventions.
Matrix-Assisted Laser Desorption/Ionization–Time-of-Flight Mass Spectrometry (MALDI-TOF MS) is an important rapid identification tool in microbiology and is traditionally used for the identification and analysis of large biomolecules including proteins, peptides and nucleic acids (Lin and Cai, Reference Lin and Cai2018). MALDI-TOF MS is based on the ionization of the biomolecules (such as proteins) of an organism of interest, co-crystallized with a chemical acidic matrix. The molecules are accelerated in a flight tube according to their mass-to-charge ratio and their time-of-flight is measured by an MS detector. Each detected molecule is associated with a peak, on a global spectrum which will be the specific fingerprint signature of the sample (Yssouf et al., Reference Yssouf, Almeras, Raoult and Parola2016). MALDI-TOF MS is now validated and utilized for identification of mammals (Tran et al., Reference Tran, Aboudharam, Gardeisen, Davoust, Bocquet-Appel, Flaudrops, Belghazi, Raoult and Drancourt2011), Archaea (Dridi et al., Reference Dridi, Raoult and Drancourt2012), giant viruses (La Scola et al., Reference La Scola, Campocasso, N'Dong, Fournous, Barrassi, Flaudrops and Raoult2010) and a range of arthropods relevant to human and animal health including Culicoides biting midges (Kaufmann et al., Reference Kaufmann, Schaffner, Ziegler, Pflueger and Mathis2012), phlebotomine sand flies (Dvorak et al., Reference Dvorak, Halada, Hlavackova, Dokianakis, Antoniou and Volf2014), tsetse flies (Hoppenheit et al., Reference Hoppenheit, Murugaiyan, Bauer, Steuber, Clausen and Roesler2013), ticks (Karger et al., Reference Karger, Kampen, Bettin, Dautel, Ziller, Hoffmann, Süss and Klaus2012; Yssouf et al., Reference Yssouf, Flaudrops, Drali, Kernif, Socolovschi, Berenger, Raoult and Parola2013a), fleas (Yssouf et al., Reference Yssouf, Socolovschi, Leulmi, Kernif, Bitam, Audoly, Almeras, Raoult and Parola2014b), triatomine bugs (Laroche et al., Reference Laroche, Bérenger, Gazelle, Blanchet, Raoult and Parola2017b) and mosquitoes (Müller et al., Reference Müller, Pflüger, Wittwer, Ziegler, Chandre, Simard and Lengeler2013; Yssouf et al., Reference Yssouf, Socolovschi, Flaudrops, Ndiath, Sougoufara, Dehecq, Lacour, Berenger, Sokhna, Raoult and Parola2013b; Schaffner et al., Reference Schaffner, Kaufmann, Pflüger and Mathis2014; Raharimalala et al., Reference Raharimalala, Andrianinarivomanana, Rakotondrasoa, Collard and Boyer2017; Mewara et al., Reference Mewara, Sharma, Kaura, Zaman, Yadav and Sehgal2018).
MALDI-TOF MS has been shown to be highly effective for the rapid identification of endemic (Yssouf et al., Reference Yssouf, Parola, Lindström, Lilja, L'Ambert, Bondesson, Berenger, Raoult and Almeras2014a) and invasive (Schaffner et al., Reference Schaffner, Kaufmann, Pflüger and Mathis2014) mosquito species in Europe, North India (Mewara et al., Reference Mewara, Sharma, Kaura, Zaman, Yadav and Sehgal2018) and Madagascar (Raharimalala et al., Reference Raharimalala, Andrianinarivomanana, Rakotondrasoa, Collard and Boyer2017). However, there has been no research into the creation and use of MALDI-TOF MS protein spectra for the identification of Australian mosquitoes. Currently, MALDI-TOF MS is primarily used for microbiology diagnostics in Australia and expansion of MALDI-TOF MS utilization to arthropods would be beneficial for vector surveillance. This study aimed to investigate the viability of using MALDI-TOF MS for rapid mosquito identification for vector surveillance and research purposes in Australia. To address this, we used a three-part approach to taxonomic classification that allowed a performance comparison between morphological identification, DNA barcoding and MALDI-TOF MS. We not only benchmark the use of MALDI-TOF MS for identification of Australian mosquitoes but also show the utility of using a range of taxonomic tools for mosquito classification.
Materials and methods
Sample collection
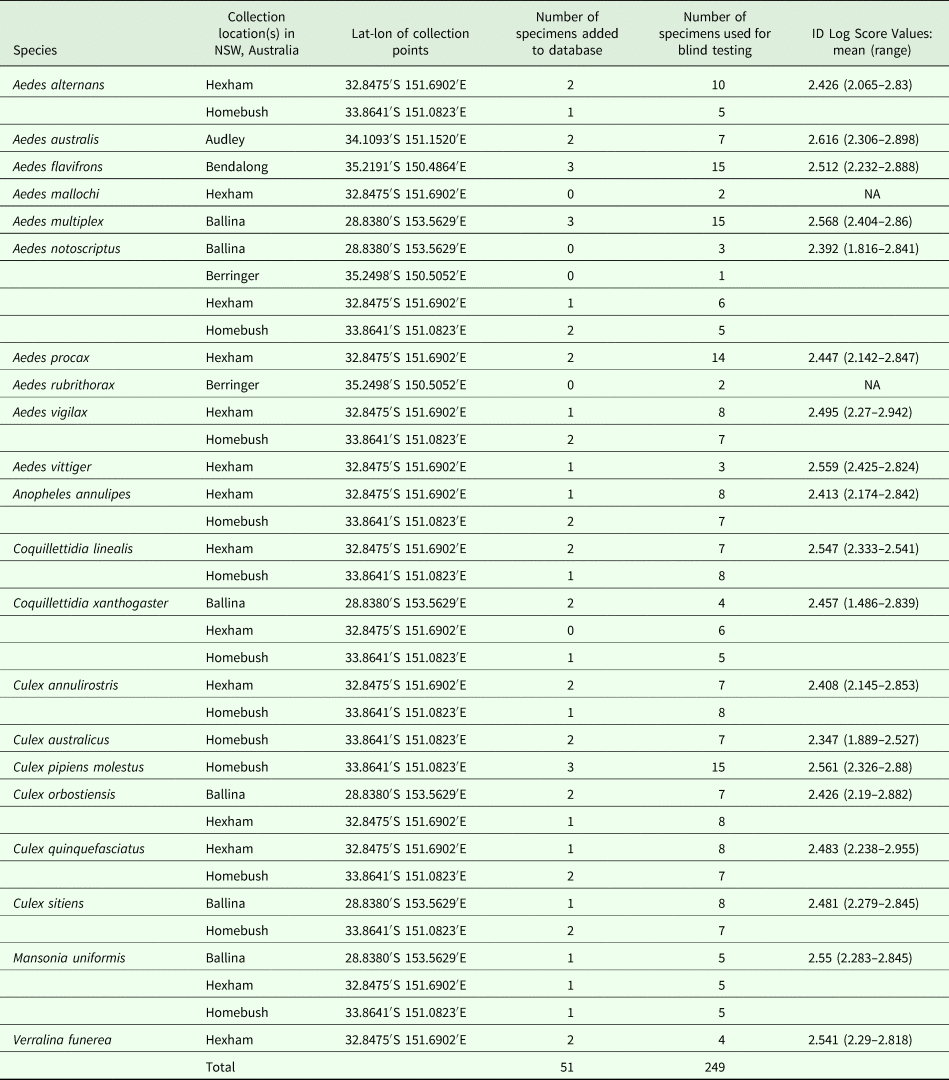
Adult mosquitoes were collected using battery-powered, CO2-baited light traps [Encephalitis Virus Surveillance (EVS) traps (Australian Entomological Supplies, NSW] from six locations in south-eastern Australia from January to October 2016 (Table 1). Mosquito larvae and pupae were collected from two of the six locations (Berringer and Audley, NSW) and reared to adulthood in the laboratory. A single mosquito sample (92-B103189; Cx. australicus from Homebush, NSW) collected in December 2014 was used in this study. After collection or emergence, mosquitoes were immediately stored at −20 °C in petri dishes until morphological identification was performed on a cold bench. Mosquitoes were identified to species level with the aid of various locally relevant keys and descriptions (Dobrotworsky, Reference Dobrotworsky1965; Lee et al., Reference Lee, Hicks, Debenham, Griffiths, Marks, Bryan and Russell1989; Russell, Reference Russell1993, Reference Russell1996). It is important to note that in many parts of the world, there is great difficulty in differentiating Cx. p. molestus from other mosquitoes in the C. pipiens group based on morphological characteristics. However, in Australia where Cx. p. pipiens is not present, Cx. p. molestus can readily be differentiated from other mosquitoes in the C. pipiens group (e.g. Cx. quinquefasciatus and Cx. australicus) based on morphological characteristics. Mosquito specimens were placed in individual tubes for storage at −80 °C. Only female mosquitoes were included in this study.
Table 1. Australian mosquitoes used for MALDI-TOF analysis including reference spectra creation and blind test specimens

NB, Log Score Values >1.9 were considered adequate for MS identification; NA, not submitted to blind test analysis.
MALDI-TOF MS sample preparation and mass spectrometer parameters
A total of two to 18 specimens were subjected to MALDI-TOF MS for each species depending on sample availability (Table 1). For species that were collected in more than one location, multiple specimens from each of the collection locations were tested to evaluate any populational variations within the species due to geographical dissociation. Prior to MALDI-TOF MS testing, mosquito samples were thawed and the legs were removed using forceps and placed into individual 1.5 mL Eppendorf tubes. The remaining body of the mosquito was placed in 70% ethanol for DNA isolation and molecular analysis. A solution containing 15 µL of 50% acetonitrile, 15 μL of 70% formic acid and a small amount (roughly half the liquid volume) of glass beads (⩽106 µm; Sigma-Aldrich, St Louis, Missouri, USA) was added to each tube containing mosquito legs. Mosquito legs were crushed as previously described (Yssouf et al., Reference Yssouf, Socolovschi, Flaudrops, Ndiath, Sougoufara, Dehecq, Lacour, Berenger, Sokhna, Raoult and Parola2013b) using the TissueLyser II (Qiagen, Hilden, Germany) with three cycles of 1 min at a frequency of 30 Hz. The homogenates were centrifuged for 30 s at 20 784 × g after which 1 µL of each sample solution was spotted onto a steel target plate (Bruker Daltonics, Germany) in quadruplicate. Where possible, each plate contained two to four phylogenetically similar species (when known) to minimize between run bias and ensure that any variation exhibited was natural and not a result of variation between runs. Additionally, variation between runs was assessed by appraising the reproducibility of spectra for the same species run on different plates using the gel view tool of the ClinProTools software. One microliter of matrix overlay containing saturated α-cyano-4-hydroxycinnamic acid (Sigma-Aldrich), 100% acetonitrile, 100% trifluoroacetic acid and high-performance liquid chromatography-grade water was then spotted directly onto the dried sample plate. The matrix solution was allowed to dry completely at room temperature before launching the plate with the MALDI-TOF Mass Spectrometer (Microflex LT, Bruker Daltonics, Germany) to obtain protein mass profiles. Step-by-step instructions are available at Protocols.io (doi: dx.doi.org/10.17504/protocols.io.md5c286). Parameters were set according to a previous study (Laroche et al., Reference Laroche, Bérenger, Gazelle, Blanchet, Raoult and Parola2017b). Spectrum profiles were obtained then visualized using Flex analysis v.3.3 software (Fig. 1). The profiles were exported to ClinProTools software v.2.2 and MALDI-Biotyper v.3.0. (Bruker Daltonics) for data processing (smoothing, baseline subtraction and peak picking) as previously described (Yssouf et al., Reference Yssouf, Socolovschi, Flaudrops, Ndiath, Sougoufara, Dehecq, Lacour, Berenger, Sokhna, Raoult and Parola2013b).
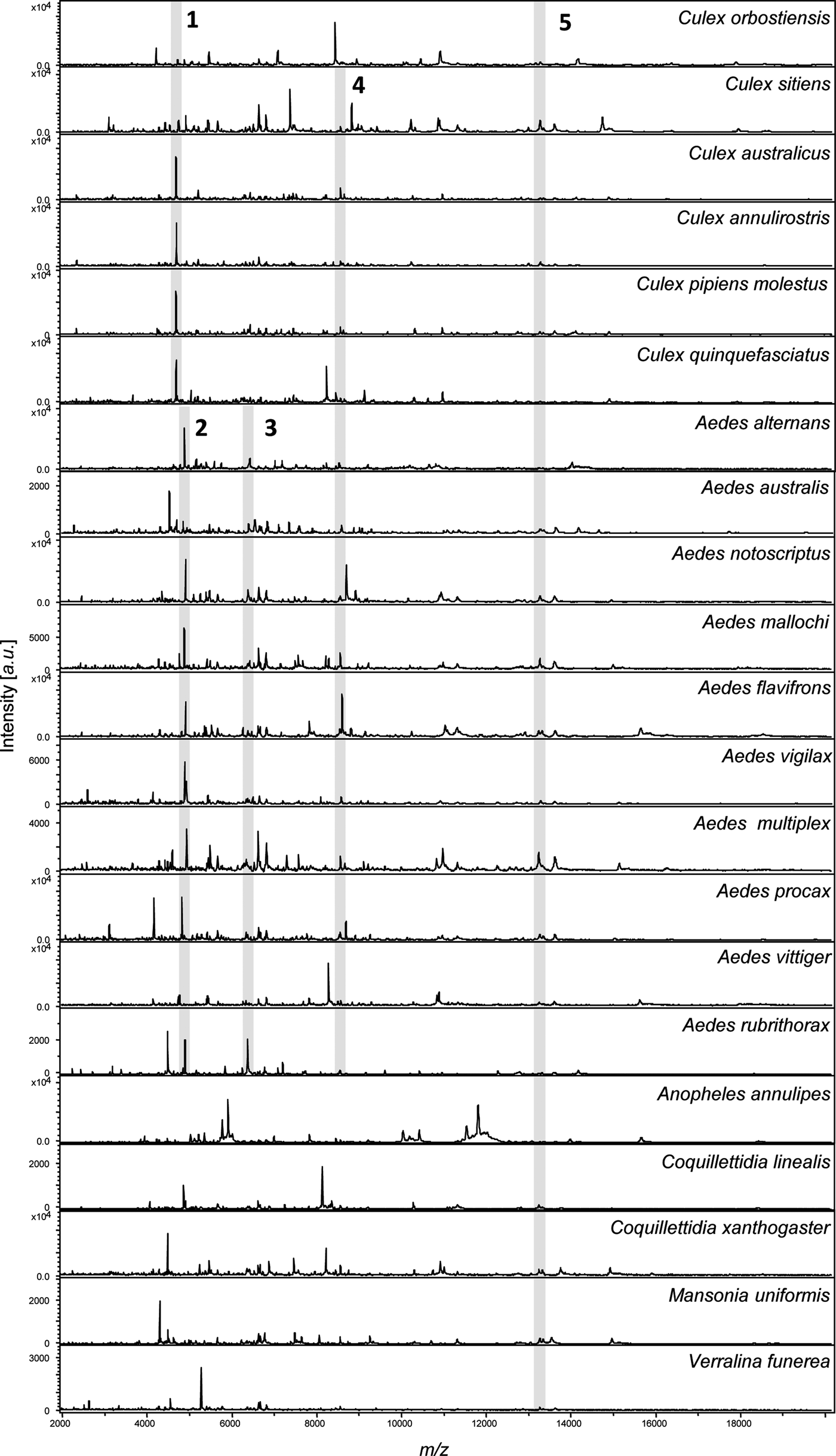
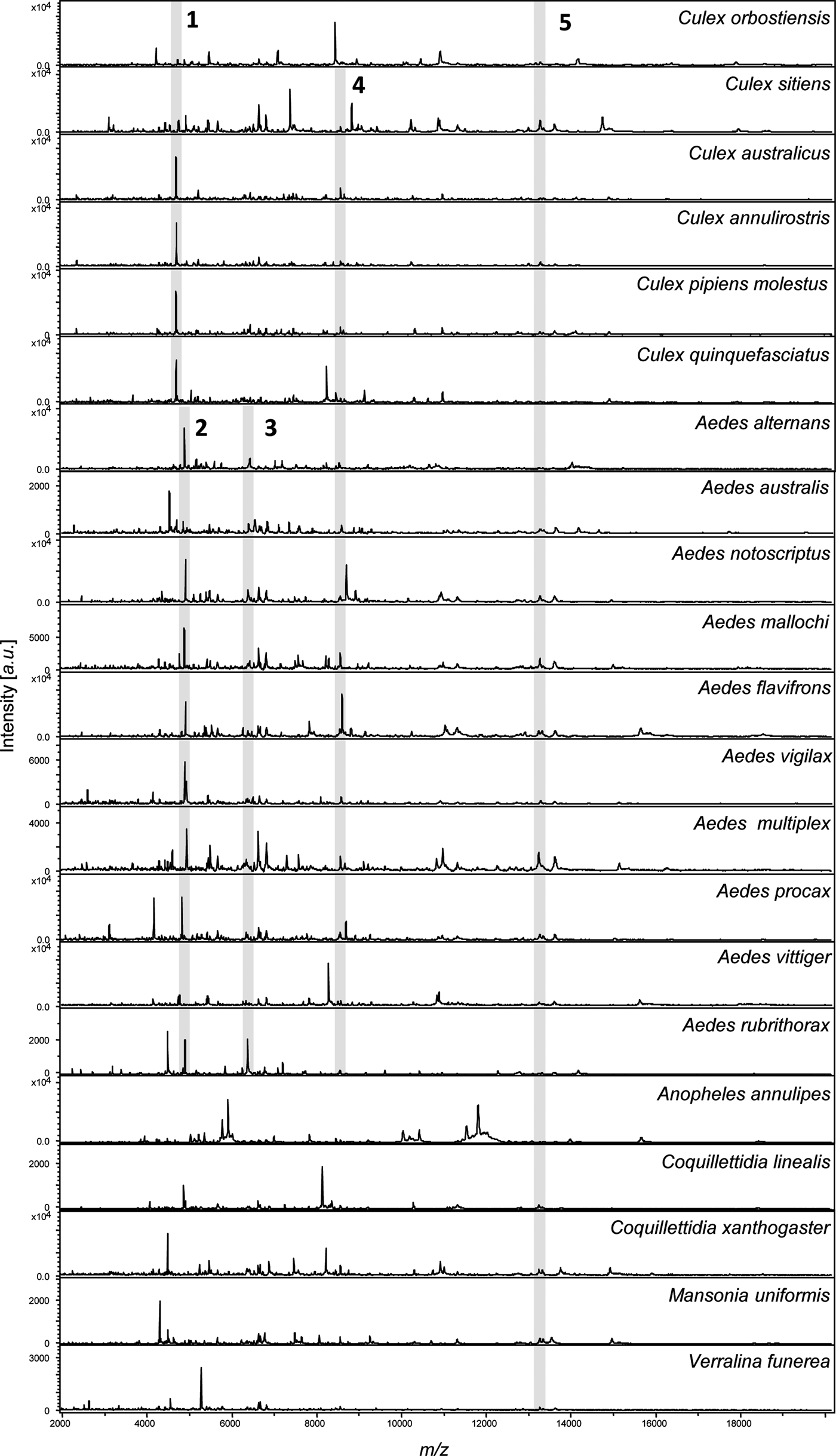
Fig. 1. MALDI-TOF MS spectra for 21 mosquito species. Spectra were obtained from homogenization of the legs of each species. Species name is indicated for each spectrum. Intensity is measured in a.u. (arbitrary units) and m/z denotes the mass-to-charge ratio. Shaded regions indicate common peaks shared across all Culex spp. (1), Aedes spp. (2 and 3), both Culex spp. and Aedes spp. together (4), as well as all species in the analysis (5).
MALDI-TOF MS spectra analysis and reference database creation
Initially, all spectra were tested against the in-house database containing reference spectra for 31 species of European and African mosquitoes to observe whether any cross-matching could occur between the mosquitoes in the database and the test spectra [the most recent list of specimens comprising this database can be found in Boucheikhchoukh et al. (Reference Boucheikhchoukh, Laroche, Aouadi, Dib, Benakhla, Raoult and Parola2018)]. Spectra from two cosmopolitan species (Mansonia uniformis and Cx. quinquefasciatus) from Europe and Africa were already present in the database. Additionally, spectra for Cx. pipiens pipiens – a closely related taxon to Cx. p. molestus – was also present in the database. Spectra reproducibility was tested through visual inspection of gels, dendrograms and comparison of mean spectra for each sample using ClinProTools 2.2 and Flex analysis v.3.3 software (Bruker Daltonics). Virtual gel views are a representation of an alignment of spectra generated by ClinProTools 2.2 that allow a visual assessment of reproducibility for a group of spectra. Dendrograms are based on the results of Composite Correlation Index matrix (CCI), a parameter that defines the distance between spectra. CCIs are calculated by dividing spectra into intervals and comparing the correlation of these intervals across a dataset. A CCI match value of 1 represents complete correlation, whereas a CCI match value of 0 represents an absence of correlation (Laroche et al., Reference Laroche, Bérenger, Gazelle, Blanchet, Raoult and Parola2017b).
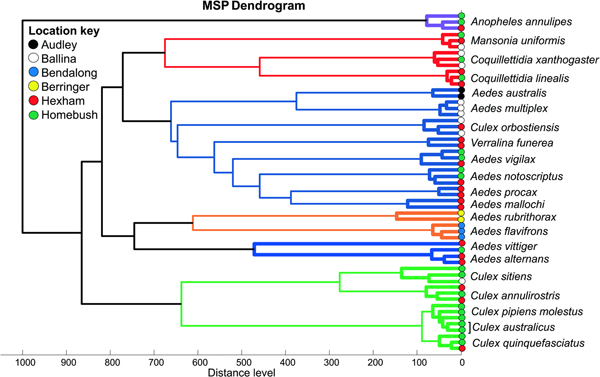
MALDI-Biotyper software v3.0 (Bruker Daltonics) was used to create reference spectra (MSP, Main Spectrum Profile) for each sample to capture biological variation by taking into account the peak intensity, positioning and frequency in an automated algorithm. Spectral dendrograms were created to aid in selecting representative spectra from the dendrogram clusters. Two to three spectra were added to the reference database where possible, depending upon sample availability and spectra reproducibility (Supplementary Dataset S1). For samples with the highest reproducibility, only two reference spectra were selected. Only one reference spectrum was added for the species Ae. vittiger due to low sample number (n = 4). Following the addition of the reference spectra to the reference database for each species, the remaining samples were screened against the database containing the new reference spectra to test identification power. A further six specimens for each species (excluding four species with low sample number – Table 1) were subjected to MALDI-TOF MS and screened against the upgraded reference database. The spectrum for each sample quadruplicate was graded to produce a Log Score Value (LSV). For each spectrum, LSVs correspond to the degree of similarity in mass spectra peaks and signal intensities between the query spectra and the reference spectra. LSVs range from 0.0 to 3.0, and allow the evaluation of reproducibility between a queried spectrum and a reference spectrum, with higher scores representing greater similarity between two spectra (Laroche et al., Reference Laroche, Bérenger, Gazelle, Blanchet, Raoult and Parola2017b). Although strict thresholds are established for bacterial identification, no threshold is definitely validated for arthropod identification. Nevertheless, in this study LSVs >1.9 were considered adequate for relevant identification. Correct identification in the blind test was determined by the highest scoring spectrum within the quadruplicates of each sample. A final dendrogram displaying hierarchical clustering of all reference spectra was produced to visualize the distances and grouping between each species (Fig. 2).

Fig. 2. Dendrogram of 21 mosquito species based on cluster analysis of MALDI-TOF MS spectra. All specimens selected for reference spectra (MSP) were included with two to three spectra per species. The spectra of two species (Ae. rubrithorax and Ae. mallochi) where reference spectra were unable to be created due to low sample numbers were included in the cluster analysis. Branch colours highlight the clustering pattern among species.
DNA isolation and sequencing of cox1
All DNA barcoding analysis was performed using the bodies (without legs) of 58 mosquitoes, including all specimens used to create the MALDI-TOF MS reference spectra. Total DNA was extracted by adding 150 µL of QuickExtract DNA Extraction Solution (Epicentre, USA) and following the manufacturer's instructions. The extracted DNA was quantified using a NandoDrop 1000 spectrophotometer (Thermo Scientific, USA) and diluted to 40 ng µL−1 for use in conventional PCR.
A 710 bp region of the cox1 gene was targeted and amplified using the universal cox1 barcoding primers LCO1490 5′ GGT CAA CAA ATC ATA AAG ATA TTG G 3′ and HCO2198 5′ TAA ACT TCA GGG TGA CCA AAA AAT CA 3′ (Folmer et al., Reference Folmer, Black, Hoeh, Lutz and Vrijenhoek1994). The total PCR volume was 25 µL and included 15.8 µL of 0.1 mg mL−1 bovine serum albumin; 2.5 µL 10× ThermoPol Reaction Buffer (New England Biolabs, USA); 2 µL 2.5 µ m dNTPs; 1.25 µL of each 10 µ m L−1 primer; 0.2 µL 1.0 U Taq DNA polymerase; and 2 µL template DNA. The cycling conditions were as previously described (Batovska et al., Reference Batovska, Blacket, Brown and Lynch2016) and all PCRs were performed using a Veriti Thermal Cycler (Applied Biosystems, USA). Products were resolved on 2% agarose gel and PCR products showing unambiguous single bands were sequenced bi-directionally on an ABI3730XL (Thermo Fisher Scientific, USA) by Macrogen Inc. (Korea).
DNA sequence analysis
All sequences were trimmed and assembled using Geneious version 8.1 (Kearse et al., Reference Kearse, Moir, Wilson, Stones-Havas, Cheung, Sturrock, Buxton, Cooper, Markowitz, Duran, Thierer, Ashton, Meintjes and Drummond2012). All sequence chromatographs had unambiguous reads. Assembled sequences were first queried against the Barcode of Life Database (BOLD) using the ‘Species Level Barcode Records’ search (accessed 13 December 2017) to verify molecular identity. MEGA version 6 (40) was then used to align edited sequences (627 bp) with the ClustalW algorithm, calculate sequence divergence using pairwise distance and create a neighbour-joining tree with 1000 bootstrap replicates (Supplementary Dataset S2). All sequences were deposited in GenBank (National Centre for Biotechnology Information, NCBI) under the following accession numbers: MG712511–MG712568, and in BOLD: MONSW001-17–MONSW058-17.
Results
Mosquito samples
A total of 300 female mosquitoes representing 21 species were collected, morphologically identified and analysed in this study including Ae. flavifrons, Ae. multiplex, Ae. notoscriptus, Ae. vigilax, Anopheles annulipes, Coquillettidia linealis, Cq. xanthogaster, Cx. annulirostris, Cx. australicus, Cx. p. molestus, Cx. orbostiensis, Cx. quinquefasciatus, Cx. sitiens, M. uniformis (n = 18 specimens for each species); Ae. procax (n = 16); Ae. alternans, Ae. australis (n = 9 specimens for each species); Verralina funerea (n = 6); Ae. vittiger (n = 4); Ae. mallochi and Ae. rubrithorax (n = 2 specimens for each species). Sample collection locations and sample details are displayed in Table 1.
Evaluation of sample MS spectra
The MS profiles were highly reproducible within each of the 21 mosquito species analysed. All spectra displayed high peak masses ranging from 2000 to 16 000 kDa with a range of 76 to 139 peaks per species (Fig. 1). Visual inspection of the spectra showed little variation between specimens from the same species collected from different locations in Australia. This is evident in Fig. 2, a dendrogram created with the reference spectra, where all specimens within a species clustered together regardless of location.
Initial screening of spectra for the Australian mosquito species that were not already present in the database (n = 18) produced incorrect identifications as expected. Due to the genetic similarity between Cx. p. molestus and Cx. p. pipiens, it was assumed that Cx. p. molestus would match to Cx. p. pipiens in the initial blind test. This was true for all specimens, but with low LSV ID scores (below 1.7). Likewise, the identification scores for M. uniformis already present in the database were below 1.7.
Creation of reference spectra and MALDI-TOF MS blind test validation
Reference spectra were created for 19 mosquito species (Supplementary Dataset S1). To account for within-species spectra diversity, at least four to six specimens are required for creating reference spectra, however less are required if the spectra are highly reproducible. The reference spectra are then used for the identification of the remaining specimens during blind testing. There was an insufficient number of samples for the remaining two species (Ae. rubrithorax and Ae. mallochi; n = 2 for each) to include them in the blind testing, therefore reference spectra could not be created. In the blind test analysis, 100% of the specimens from each species were correctly identified with LSVs >2 for the spot with the highest score and a range of 1.486–2.955 for all spots across all species (Table 1). Despite the spectral similarity between Cx. australicus and Cx. p. molestus from Homebush, NSW, both species were identified to the correct species, validating the existence of variation in their protein profiles. Similarly, once reference spectra were added for Cx. p. molestus, no samples matched to the Cx. p. pipiens already in the database and all were 100% correctly identified as Cx. p. molestus. Our results demonstrate that MALDI-TOF MS can clearly differentiate these taxa. As shown in the Supplementary Data 3, the MS profiles of these two mosquito species are significantly different with several visible specific peaks, as confirmed by the principal component analysis. All Australian M. uniformis and Cx. quinquefasciatus were 100% correctly identified to Australian reference spectra only and not to existing European and African spectra for these species. Reference spectra that were representative of the intraspecific variation were selected and a dendrogram was created to demonstrate the clustering of all species based on similarities and differences in their reference spectra. All reference spectra from the same species clustered together, supporting the reproducibility of the spectra and the delineation of each species (Fig. 2).
Molecular identification by DNA barcoding
A cox1 sequence was generated for 58 mosquito specimens, representing all 21 species (Supplementary Dataset S2). A BOLD search of the cox1 barcodes was able to correctly identify 15 of the 21 species with >98% match to reference sequences (Supplementary Table 1). Of the six species that were not identified, four were species that have not been previously barcoded (Fig. 3 and Supplementary Table 1) and have now been added to the BOLD and NCBI reference databases. The closest similarity match of these new species with other named species on BOLD was between 90% and 96%. The remaining two species that could not be identified using cox1 DNA barcoding were C .p. molestus and Cx. quinquefasciatus. These two species are part of the Cx. pipiens complex, and are known to have low genetic diversity (Batovska et al., Reference Batovska, Blacket, Brown and Lynch2016). A further species, Ae. flavifrons, now has three publically available barcode sequences as a result of our study, as no public barcodes were available previously for this species.

Fig. 3. Neighbour-joining tree of genetic relationships obtained from cox1 DNA sequences from 21 mosquito species. A summarized neighbour-joining tree based on p-distance comparisons between 58 mosquito cox1 sequences (627 bp in length) with bootstrap support values shown (%). The * symbol indicates new DNA barcode reference species; the ** symbol indicates a species complex which could not be resolved using the cox1 DNA barcode.
These results are reflected in the neighbour-joining tree, where the majority of species formed distinct clusters, correlating with morphological identification (Fig. 3). Again, only Cx. p. molestus and Cx. quinquefasciatus were not distinguishable, with both species generating 100% identical cox1 sequences and forming a single cluster. The neighbour-joining tree also highlighted the differences in intraspecific diversity between species. For instance, Ae. rubrithorax, Cq. xanthogaster and Cx. orbostiensis all have no intraspecific diversity in this dataset. However, Ae. notoscriptus and An. annulipes have high intraspecific diversity. These two diverse species groups have been previously documented (Foley et al., Reference Foley, Wilkerson, Cooper, Volovsek and Bryan2007; Endersby et al., Reference Endersby, White, Chan, Hurst, Rašić, Miller and Hoffmann2013) and further investigation would be required to elucidate which specific clades these specimens belong to.
Discussion
We present the first MALDI-TOF MS reference spectra for the identification of a selection of Australian mosquito species. These species are known to be widely distributed, of pest or public health importance, or most commonly collected in mosquito surveillance programmes undertaken by health authorities on the east coast of Australia (Webb et al., Reference Webb, Russell and Doggett2016). Highly accurate differentiation of these species is vital for arbovirus surveillance and vector-borne disease risk management. A number of the species collected in our study (Ae. notoscriptus, Ae. vigilax, An. annulipes, Cx. annulirostris, Cx. p. molestus, Cx. quinquefasciatus, Cq. linealis, Cq. xanthogaster and M. uniformis) are important vectors of mosquito-borne pathogens in Australia such as RRV, BFV, Kunjin virus and MVEV (Mackenzie et al., Reference Mackenzie, Lindsay, Coelen, Broom, Hall and Smith1994; Russell, Reference Russell1995; Ryan and Kay, Reference Ryan and Kay1999; Selvey et al., Reference Selvey, Dailey, Lindsay, Armstrong, Tobin, Koehler, Markey and Smith2014; Claflin and Webb, Reference Claflin and Webb2015). We achieved 100% correct identification of all 19 species that were exposed to the blind testing. Despite the insufficient sample number for the remaining two species (Ae. rubrithorax and Ae. mallochi), the dendrogram created showed highly reproducible spectra (Fig. 2) that were clearly delineated from the other species; thus, had more samples been available, creation of reference spectra for these species would have undoubtedly been successful.
Current mosquito and arbovirus surveillance programmes in Australia rely on morphological identification of mosquito specimens, despite limitations in identifying immature and damaged specimens. Alternative methods are required to complement morphological taxonomy and overcome its limitations in existing programmes. Rapid identification of immature mosquito stages has been developed through real-time reverse transcription-polymerase chain reaction assays (Montgomery et al., Reference Montgomery, Shivas, Hall-Mendelin, Edwards, Hamilton, Jansen, McMahon, Warrilow and van den Hurk2017) but this is currently only being incorporated into limited surveillance programmes. MALDI-TOF MS running costs are extremely economical and could represent a suitable alternative given that successful identification of immature specimens has been demonstrated (Dieme et al., Reference Dieme, Yssouf, Vega-Rúa, Berenger, Failloux, Raoult, Parola and Almeras2014; Schaffner et al., Reference Schaffner, Kaufmann, Pflüger and Mathis2014).
Emerging technologies are being incorporated into surveillance programmes to provide rapid identification of infective mosquitoes (van den Hurk et al., Reference van den Hurk, Hall-Mendelin, Johansen, Warrilow and Ritchie2012) but these approaches often do not provide similarly rapid and cost-effective identification of mosquito specimens. The role of DNA barcoding and metabarcoding in mosquito surveillance programmes is increasing (Batovska et al., Reference Batovska, Lynch, Cogan, Brown, Darbro, Kho and Blacket2017; Lilja et al., Reference Lilja, Nylander, Troell and Lindström2017) but is not currently employed on a large scale. Internationally, the species and forms within the Cx. pipiens group are demonstrably difficult to differentiate using morphological and molecular tools (Fonseca et al., Reference Fonseca, Keyghobadi, Malcolm, Mehmet, Schaffner, Mogi, Fleischer and Wilkerson2004; Batovska et al., Reference Batovska, Blacket, Brown and Lynch2016; Shaikevich et al., Reference Shaikevich, Vinogradova, Bouattour and Gouveia de Almeida2016). While cox1 differentiation of Cx. quinquefasciatus and Cx. p. molestus has been unsuccessful in the past and in the current study, alternative gene regions, namely the acetylcholinesterase-2 (ace-2) gene have been successfully utilized for species differentiation (Smith and Fonseca, Reference Smith and Fonseca2004). To address and resolve the ongoing taxonomic problems, an integrated approach should be employed to glean as much information on the species identity as possible. Our work demonstrates that MALDI-TOF MS is a valuable method to add to the taxonomic tool kit for mosquitoes since differentiation of Cx. quinquefasciatus and Cx. p. molestus was achieved. Likewise, differentiation of individuals within the An. gambiae complex has also been achieved using MALDI-TOF MS protein profiling (Müller et al., Reference Müller, Pflüger, Wittwer, Ziegler, Chandre, Simard and Lengeler2013).
For the MALDI-TOF MS approach to be successful for mosquito identification in Australia, a rich database of specimens must be gathered and the issues surrounding geographic variability of mosquito species within Australia such as Ae. notoscriptus (Endersby et al., Reference Endersby, White, Chan, Hurst, Rašić, Miller and Hoffmann2013) must be considered as well as geographic variability of cosmopolitan species found worldwide. This is of increasing importance where the identification of less commonly encountered exotic specimens is required or where pathway analysis is sought to identify the country of origin. The differentiation between specimens of the same species sourced from different countries in this study (M. uniformis and Cx. quinquefasciatus) could have important implications for detecting incursions of cosmopolitan species from foreign countries to Australia and vice versa. This is significant since these specimens may carry exotic pathogens into a naïve region that has existing competent hosts (Whiteman Noah et al., Reference Whiteman Noah, Goodman Simon, Sinclair Bradley, Walsh, Cunningham Andrew, Kramer Laura and Parker Patricia2005; Tompkins and Gleeson, Reference Tompkins and Gleeson2006; Nett et al., Reference Nett, Campbell and Reisen2008). However, it should be mentioned that the MS spectra produced by MALDI-TOF MS are highly dependent on the specimen preparation techniques used prior to testing and the variation between the existing and the new spectra may be an artefact of the differing preparation techniques (Nebbak et al., Reference Nebbak, Willcox, Bitam, Raoult, Parola and Almeras2016). Despite this, the presence of distinct spectra across two countries within the same species has been observed previously, supporting our results (Raharimalala et al., Reference Raharimalala, Andrianinarivomanana, Rakotondrasoa, Collard and Boyer2017). Additionally, the differentiation of closely related taxa Cx. p. pipiens (already existent in the database) and Cx. p. molestus (new spectra) was not influenced by any preparation bias since were collected, stored and prepared using the methods described in this study.
The development of an Australian MALDI-TOF MS spectra database could enable the detection of incursions of Australian mosquito species into new regions (Williams Craig et al., Reference Williams Craig, Bader Christie, Williams Samantha and Whelan Peter2012). Moreover, as more and more countries such as India and Madagascar create and utilize MALDI-TOF MS spectra for arthropod identification, data sharing among laboratories could enable the development of comprehensive databases for invasive species monitoring on a global scale (Raharimalala et al., Reference Raharimalala, Andrianinarivomanana, Rakotondrasoa, Collard and Boyer2017; Mewara et al., Reference Mewara, Sharma, Kaura, Zaman, Yadav and Sehgal2018). Recent studies have shown MALDI-TOF MS has been utilized to identify mosquito blood meals in vitro (Niare et al., Reference Niare, Berenger, Dieme, Doumbo, Raoult, Parola and Almeras2016), and to identify Plasmodium infection within mosquito vectors (Laroche et al., Reference Laroche, Almeras, Pecchi, Bechah, Raoult, Viola and Parola2017a), demonstrating the future scope of MALDI-TOF for advanced vector screening worldwide. Furthermore, MALDI-TOF MS is able to differentiate between naïve, truly infected and exposed but uninfected mosquitoes for malaria; a potentially useful tool for the detection of exotic malaria threats, in Australia and other countries where malaria is not endemic (Laroche et al., Reference Laroche, Almeras, Pecchi, Bechah, Raoult, Viola and Parola2017a).
There are numerous factors to consider before MALDI-TOF MS can be incorporated into existing mosquito and arbovirus surveillance programmes in Australia, including the initial cost of the machine. However, due to the wide scope of MALDI-TOF MS for diagnostic identification across many different organisms, one machine can be used to service a variety of laboratories within an institution and initial costs can be shared as a worthwhile investment. Since MALDI-TOF MS is already utilized in Australia for microbiology diagnostics, the initial cost of the machine may be negated by collaborations between the microbiology laboratories with existing machines and entomology or arbovirus laboratories. Currently, three different MALDI-TOF mass spectrometer machines are used in microbiology diagnostics including the MALDI BioTyper™ (Bruker Daltonics, Germany) used in this study, the MALDI micro MX™ (Waters Corporation, Massachusetts, USA) and SARAMIS™ (Shimadzu & Anagnostec, Kyoto, Japan) (Seng et al., Reference Seng, Rolain, Fournier, La Scola, Drancourt and Raoult2010). This may pose a challenge for the direct transfer of reference spectra data between laboratories (between France and Australia, e.g.), since the utilization of these data for organism identification is dependent on the machine and software used (Flaudrops et al., Reference Flaudrops, Faye, Mediannikov, Sokhna, Lo, Fall, Sambe-Ba, Wade, Raoult and Fenollar2017). Thus, it is critical to standardize sample preparation as well as data processing to facilitate data exchange between laboratories (Nebbak et al., Reference Nebbak, Willcox, Bitam, Raoult, Parola and Almeras2016). It was recently demonstrated that spectra obtained from different instruments can be analysed together using a centralized database, enhancing the potential for global databases in the future (Mathis et al., Reference Mathis, Depaquit, Dvořák, Tuten, Bañuls, Halada, Zapata, Lehrter, Hlavačková, Prudhomme, Volf, Sereno, Kaufmann, Pflüger and Schaffner2015).
Rapid and cost-effective species identification of mosquito specimens is essential for the surveillance of mosquito vectors and vector-borne pathogens and our results show that the MALDI-TOF MS tool has significant potential in aiding Australian mosquito identification. The reference spectra produced in our study could act as a base for the creation of a rich database for the identification of Australian and exotic mosquito species. We have demonstrated the methodological pipeline required to build a database of reference spectra that may represent the foundation for utilizing this approach for endemic and exotic mosquito surveillance in Australia and worldwide.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0031182018001658.
Acknowledgements
We thank John Clancy, NSW Health Pathology, who aided with confirmation of mosquito specimen identification. We thank Jennifer Lawrence for her assistance in mosquito collection.
Author contributions
Study design: ALL, CEW, JŠ, PP, ML; provision of equipment, resources and material: PP, SEL, CEW; collection of mosquitoes: CEW, ALL; morphological identification of mosquitoes: CEW, ALL; specimen preparation for MALDI-TOF MS analysis: ALL, ML; analysis of spectra: ML, ALL; DNA isolation, PCR, sequencing of cox1 and sequence analysis: JB, SEL, MJB; discussion and consultation: ALL, ML, JB, CEW, SEL, MJB, JŠ, PP; writing of the manuscript: ALL, JB; preparation of manuscript figures: ALL; critical review, editing of manuscript drafts and approval of final version: ALL, ML, JB, CEW, SEL, MJB, JŠ, PP.
Financial support
The project leading to this publication was supported by the French Government under the ‘Investissements d'avenir’ (Investments for the Future) programme managed by the Agence Nationale de la Recherche (ANR, fr: National Agency for Research). Reference: Méditerranée Infection 10-IAHU-03. The DNA barcoding was funded by the Biosciences Research Innovation Fund Program through the Victorian Department of Economic Development, Jobs, Transport and Resources. ALL was a recipient of the University of Sydney, Sydney Medical School Edith Mary Rose Travel Scholarship and the University of Sydney William and Catherine McIlrath Scholarship for travel to Aix-Marseille University, France.
Conflict of interest
None.
Ethical standards
Not applicable.
Data accessibility
All data supporting the conclusions of this article are presented within the article and its supplementary files.