INTRODUCTION
Alveolar echinococcosis (AE) is a severe, life-threatening parasitic disease caused by primarily intrahepatic growth of the metacestode (larval stage) of Echinococcus multilocularis (Gottstein and Hemphill, 1997), a small tapeworm using mainly foxes as definitive hosts. The natural life-cycle of the parasite involves not only rodents but also humans as an intermediate host, both becoming infected through ingestion of parasite eggs. This can subsequently result in asexual proliferation of the metacestode in the liver and – at a later stage of infection – in the formation of metastases in adjacent or even remote organs. Natural inclusion of both humans and rodents in biology of the parasite provides an ideal situation to study experimentally the host-parasite interplay using laboratory mice.
In both murine and human AE, massive, tumour-like growth of the larval parasite tissues results in clinical symptoms similar to those of hepatic carcinoma. Thus the disease progression depends on kinetics of the metacestode growth and proliferation, which again depend on the presence or absence of restrictive periparasitic host effector events, predominantly immune reactions (Dai et al. 2004). This, again, may be modulated by the parasite itself (Rakha et al. 1991). It was shown that the E. multilocularis metacestode grows more rapidly in athymic nude mice or in severely compromised immunodeficient (SCID) mice when compared with wild type control animals (Playford and Kamiya, 1992; Playford et al. 1992). These observations were largely confirmed by Dai et al. (2004), who also showed that a major carbohydrate antigen of E. multilocularis induces T cell-independent IgG responses, thus modulating the periparasitic environment in favour of the parasite. As larval E. multilocularis tissue exhibits various growth kinetics upon different environmental conditions, differences are also anticipated to be found at gene expression levels of genes that are directly or indirectly involved in the regulation of the proliferative performance.
The present study aimed to discover and characterize parasite molecules, whose expression levels correlate with viability status and also with growth activity of E. multilocularis metacestode tissue. We focused on 2 proteins synthesized by the parasite, 14-3-3 and II/3-10. 14-3-3 proteins are a family of highly conserved proteins among all eukaryotic organisms studied so far (Aitken, 1996). They are intracellular proteins and play key roles in basic cellular events related to cellular proliferation, including signal transduction, cell-cycle control, cell differentiation and cell survival (Fu, Subramanian and Masters, 2000; van Hemert, Steensma and van Heusden, 2001). With regard to Echinococcus spp., 5 different 14-3-3 isoforms have been isolated and sequenced so far, although only some of them have been studied in more detail (reviewed by Siles-Lucas and Gottstein, 2003). Among them, an E. multilocularis 14-3-3 isoform, close to the zeta isoform in higher eukaryotes, was shown to exhibit higher expression level in the metacestode stage of E. multilocularis compared to its expression level in adult worms (Siles-Lucas et al. 1998) or in metacestodes of E. granulosus (Siles-Lucas, Nunes and Zaha, 2001). Therefore, this specific 14-3-3 isoform represents a putative growth regulator of the larval parasite, as generally described for other eukaryotic organisms. II/3-10 (Müller et al. 1989) is a fragment of the II/3-molecule, which was shown to be specifically expressed in E. multilocularis metacestodes (Vogel et al. 1988). II/3-10 and other identical molecules share homologies with mammalian ezrin, radixin, and moesin (ERM) protein family (Frosch et al. 1991; Hemmings and McManus, 1991; Ito, Wang and Liu, 1993; Brehm et al. 1999; Sako et al. 2002; reviewed by Ito and Craig, 2003). This protein family is involved in several key processes related to ‘cellullar architecture’, including cell-cell adhesion, membrane trafficking, microvillus formation, trans-membrane signalling, and cell division (Bretscher et al. 2000; Louvet-Vallée, 2000; Bretscher, Edwards and Fehon, 2002; Gautreau, Louvard and Arpin, 2002). Therefore, E. multilocularis II/3-10 is also assumed to play a key role in similar processes although its functional role has not been elucidated so far. A putative correlation between parasite viability status and humoral immune response to II/3-10-antigen was recently demonstrated in a retrospective clinical study including 57 cases of AE (Ammann et al. 2004).
MATERIALS AND METHODS
Experimental design
The present study included 2 separate experimental approaches: one was based on in vivo experiments using experimentally infected athymic nu/nu mice or genetically matching wild type controls (BALB/c). Gene expression levels of the parasite-specific molecules 14-3-3 and II/3-10 were quantitatively evaluated in relation to a housekeeping molecule beta-actin, and they were directly compared with growth activity of the parasite as determined by parasite weight at the end-point of the experiments. Parasite metacestode tissue was collected from 4 different host groups: athymic nude mice at 1 month (n=7) and 2 months p.i. (n=7), wild type controls at 1 month (n=5) and 2 months p.i. (n=6). A parasite sample obtained from each mouse was individually subjected to measurement of total parasite weight and gene expression analyses by real-time reverse transcription (RT)-PCR as described below. In addition, samples were morphologically investigated by light microscopy. The second approach employed in vitro cultures of larval E. multilocularis (Hemphill and Gottstein, 1995), aiming to elucidate a potential correlation between parasite viability and respective gene expression levels of the parasite-derived molecules, 14-3-3 and II/3-10. Changes in gene expression levels within the parasite tissue were quantitatively manipulated during the course of in vitro treatment by the use of an anti-echinococcal drug, nitazoxanide, anticipating that the drug treatment would dramatically reduce the parasite viability as evidenced by morphological alterations in previous studies (Stettler et al. 2003, 2004). Transcription levels of the two selected molecules were compared with that of a housekeeping molecule, beta-actin, which is supposed to be expressed constitutively in normal living cells (Naora and Naora, 1995; for experimental details, see below).
Maintenance and isolation of E. multilocularis metacestodes
E. multilocularis metacestodes (KF5 isolate; Gottstein, Deplazes and Aubert, 1992) were maintained by serial transplantation passages in gerbils (Meriones unguiculatus). Parasite microcyst suspensions were prepared according to Hemphill and Gottstein (1995). The microcyst suspension was adjusted to a density of approximately 100 intact vesicles per 100 μl Hank's Balanced Salt Solution just before use for experimental infection of mice. The identical microcyst preparation was also used for setting up in vitro cultures.
Experimental infection of mice
Female 8-week-old athymic nude mice and genetically matching wild type BALB/c mice were purchased from Harlan Ltd (The Netherlands). All animals were housed and fed according to the Swiss federal animal protection guidelines. Animals were intraperitoneally injected with 100 freshly prepared metacestode microcysts, which were processed as described above. At the time of necropsy, the infected animals were euthanized by CO2. The parasite tissues were removed from the peritoneal cavity and subsequently assessed for its weight. These parasite tissues were also used for gene expression analyses by real-time RT-PCR, morphological characterization and immunoblotting (see below). A part of the parasite tissues recovered from the infected mice was fixed in 10% formalin, embedded in paraffin and stained with haematoxylin and eosin for light microscopic observation.
In vitro drug treatment
E. multilocularis microcysts were prepared as described above. The freshly prepared parasite microcysts were divided into separate culture flasks, each one containing approximately 2600 intact microcysts in 10 ml of complete culture medium (Hemphill and Gottstein, 1995). One flask containing the parasite microcyst-suspension was prepared for each time-point of culture (days 0, 2, 4, 6, 8, and 10) with or without nitazoxanide-treatment, respectively. In vitro treatment of the larval parasite with nitazoxanide was carried out as previously described (Stettler et al. 2003). Nitazoxanide was obtained from Romark Laboratories (Tampa, USA) and prepared as a stock solution of 10 mg/ml in dimethyl sulfoxide (DMSO). The stock solution was added to the cultures, yielding a final concentration of 10 μg/ml. For each experiment, the appropriate control included a culture containing an equal amount of DMSO alone. The parasite microcysts were incubated at 37 °C in the presence of 5% CO2. After defined time-points, as indicated above, the parasite microcysts in the culture medium were collected and centrifuged at 1000 g for 15 min at 4 °C. The microcyst pellet obtained was treated with TRIzol reagent (Invitrogen, Basel, Switzerland) according to the manufacture's instructions before being subjected to gene expression analyses by real-time RT-PCR as described below.
Gene expression analysis by real-time RT-PCR
Pieces of parasite tissue were treated with TRIzol reagent at a proportion of 50 mg parasite tissue per 1 ml of TRIzol. Subsequently, total cellular RNA was extracted according to the instructions of the manufacturer. For RNA purification, 1 ml of sample suspension containing 50 mg parasite-tissue was used. The total extracted RNA was then precipitated with isopropanol in the presence of a high salt solution (0·8 M sodium citrate and 1·2 M NaCl) as recommended for materials containing high levels of polysaccharide components (Hülsmeier et al. 2002). Subsequently, residual genomic DNA was removed from sample RNA by performing a 180-min incubation at 37 °C in the presence of RQ1 RNase-free DNase I (Promega, Madison, USA). After heat inactivation (10 min at 80 °C) of the DNase, RNA preparations were subjected to cDNA synthesis by applying a random oligonucleotide-hexamer primer (Promega) and AMV reverse transcriptase (Promega). Finally, complementary DNA (cDNA) was purified by using a spin column from High Pure PCR product purification kit (Roche Diagnostics, Rotkreuz, Switzerland) and then eluted with 100 μl of water before use in RT-PCR analysis.
Quantitative RT-PCR was carried out on a LightCycler instrument (Roche Diagnostics) by using SYBR Green I (Qiagen, Basel, Switzerland). Amplifications of parasite gene sequences were performed by using the primer pairs listed in Table 1. Each PCR was done with 4 μl of sample cDNA (see above), which had been confirmed in advance to contain an appropriate amount of the target sequence in each PCR, by using Quantitect SYBR Green PCR kit (Qiagen) in a 10 μl standard reaction containing 0·5 μM forward and reverse primers. All PCRs were performed in triplicate for samples from in vivo experiments or in duplicate for samples from in vitro experiments. Furthermore, control PCRs using samples without RT reaction were performed to confirm that residual genomic DNA was not amplified. PCR was started by initiating the Hot-Start Taq DNA polymerase reaction at 95 °C (5 min). Subsequent DNA amplification was done in 50 cycles (denaturation [95 °C, 15 s], annealing [60 °C for actin; 55 °C for 14-3-3; 49 °C for II/3-10, 20 s], and extension [72 °C, 20 s]; temperature transition rates were 20 °C/s in all cycle steps). Amplification products were quantitatively assessed by applying the standard software of the LightCycler™ instrument (version 3.5.3). As external standards, serial 10-fold dilutions (4 μl aliquots) of amplification products previously generated from different target sequences were included in the quantitative PCR analyses. Standard curves from the different assays (14-3-3, II/3-10, and beta-actin PCRs) were independently run in duplicate and contained 5 log units within a linear range that essentially covered the maximal and minimal concentrations of the target cDNA sequences within the different samples. Linearity among the standard reactions was reflected by the correlation coefficient, which was calculated by a computer program to be high (1·00 in all PCRs) for all of the PCR assays applied. Overall specificity of the reactions and lack of unwanted primer-dimer formation were confirmed by a DNA melting profile assay using LightCycler™ standard software (version 3.5.3) and subsequent agarose gel electrophoresis in 3% gels (data not shown). For each primer set, absence of cross-reactivity with the host tissue-derived sequences was confirmed by PCR using a cDNA sample prepared from mouse liver. Furthermore, efficiencies of the quantitative PCRs were revealed to be nearly identical and exhibited high amplification rates (1·739, 1·586, and 1·782 for 14-3-3, II/3-10, and beta-actin PCR, respectively). With parasite materials obtained ex vivo from experimentally infected animals, 14-3-3- and II/3-10-mRNA levels were relatively quantified upon the mRNA level of a parasite housekeeping molecule, the beta-actin homologue of the parasite. This approach was necessary since the parasite tissues obtained from different animals were likely to be differently contaminated with host materials. Due to the specificity of this relative quantification procedure (use of parasite-specific PCR primer sets), a compensation for the variability in input RNA amounts and for the efficiencies of RT became possible. Respective mean values from triplicate determinations were taken for the calculation of relative 14-3-3 and II/3-10 mRNA levels (14-3-3 or II/3-10 mRNA level relative to beta-actin mRNA level). In experiments in vitro, mRNA levels of 14-3-3, II/3-10, and beta-actin were determined based on the standard curves separately determined using serial 10-fold dilutions of amplification products (see above).
Table 1. Primer pairs used for amplification of parasite gene sequences (These primer sets were designed based on nucleotide sequence information available in GenBank™ (Accession nos. L07773 for beta-actin, U63643 for 14-3-3 and U05573 for II/3-10, respectively).)

Affinity purification of monospecific antibodies against Echinococcus 14-3-3
Mono-specific antibodies against 14-3-3 were affinity purified from either rabbit or mouse serum obtained by immunization with recombinant Echinococcus 14-3-3 protein (Siles-Lucas et al. 1998). The band corresponding to the recombinant Echinococcus 14-3-3 protein was cut out from the nitrocellulose membrane following a parallel identification by SDS-PAGE and Western blotting of recombinant 14-3-3. After blocking of non-specific binding sites with PBS/3% BSA/0·3% Tween 20, the antiserum was applied at a dilution of 1[ratio ]10 in PBS/0·3% BSA/0·3% Tween 20 overnight at 4 °C. After washing the strip in PBS/0·3% Tween 20 three times for 10 min each, the specifically bound antibodies were eluted in 900 μl of low pH buffer (50 mM Tris, 50 mM glycine, pH 2·6) for 5 min on ice with occasional vortexing. Then the strip was removed, and 100 μl of 1 M Tris base was immediately added. The eluted antibody fraction was centrifuged (10000 g, 20 min) before BSA was added to the supernatant fraction to a final concentration of 0·1%. Specificity of the purified antibody was checked by Western blotting using crude soluble antigen prepared from metacestode material.
Immunofluorescence and immunogold labelling
For immunolocalization of 14-3-3 within the larval E. multilocularis tissues in situ, host tissue-free parasite materials were obtained from in vitro cultures (Hemphill and Gottstein, 1995) and fixed in 3% paraformaldehyde/0·05% glutaraldehyde. The parasite samples were then dehydrated and embedded in LR-White resin (Sigma, USA) as described previously (Hemphill and Croft, 1997), and sections 1–2 μm in thickness were prepared for immunofluorescence. Sections were loaded onto poly-L-lysine-coated coverslips and air-dried before incubated for 2 h in PBS/1·5% BSA. Following a wash in PBS, the sections were incubated with the affinity-purified monospecific mouse antibody against the recombinant Echinococcus 14-3-3 (see above) diluted 1[ratio ]2 in PBS/0·15% BSA for 1 h, washed in PBS for 15 min, and then incubated with goat anti-rabbit FITC conjugate diluted 1[ratio ]200 in PBS/0·15% BSA for 1 h at room temperature. Following another 15-min wash in PBS, the sections were incubated in Hoechst 32558 (1 μg/ml in PBS) for 2 min, rinsed in water, embedded in Fluoroprep (bioMérieux, Geneva, Switzerland), and then viewed on a Nikon Eclipse E 800 digital confocal fluorescence microscope. Processing of images was performed with Openlab software (version 2.0.7; Improvision, Heidelberg, Germany). For immunogold labelling of 14-3-3, ultra-thin sections of approximately 80 nm were labelled with the affinity-purified monospecific mouse antibody against the recombinant Echinococcus 14-3-3 (see above) without dilution, and bound antibodies were detected by using goat anti-mouse antibody conjugated to 10-nm gold particles (Amersham, Zürich, Switzerland). Grids were stained with lead citrate and uranyl acetate (Hemphill and Croft, 1997) before being observed by transmission electron microscopy as described above. Immunolocalization of II/3-10 and beta-actin was not carried out due to the lack of appropriately validated tools.
SDS-PAGE and immunoblotting
For the demonstration of 14-3-3 expression in E. multilocularis metacestodes at protein level, immunoblotting was performed using the parasite materials obtained from in vivo experiments, basically following the procedure described by Dai et al. (2004). Briefly, soluble fractions of total parasite extracts (Matsumoto et al. 1998) were subjected to SDS-PAGE in 15% gels, in which 4 parasite samples from wild type mice and 5 from nude mice at 2 months p.i. were loaded at final concentrations of 60 μg protein per lane for each individual sample. Subsequently, separated proteins were blotted onto a nitrocellulose membrane, which was then incubated with the monospecific anti-E. multilocularis 14-3-3 antibody (see above). The immunoblot reaction was completed by incubation with an alkaline phosphatase-conjugated rabbit IgG, and developed as described previously (Siles-Lucas et al. 1998). The intensity of the immunoreactive-specific band, in each parasite extract, was measured by densitometry using NIH Image software. Detection of II/3-10 and beta-actin at the protein level was not carried out due to the lack of appropriately validated tools.
Statistics
We used a factorial analysis of variance to search for significant differences in expression levels between different time-points and mouse strains for each gene. Parasite masses and the expression values for each gene were log-transformed (natural logarithm) to normalize their distribution. A one-way analysis of variance was then carried out to evaluate significant expression differences among all the sample groups. Fisher's protected least significant differences was performed to evaluate for differences between two groups. All statistics were performed with StatView (version 5.0) package.
RESULTS
Parasite growth in experimentally infected animals
To define the growth activities and proliferation kinetics of E. multilocularis metacestodes in hosts with different degrees of susceptibility to infection, T cell-deficient nude mice and corresponding wild type BALB/c controls were intraperitoneally injected with approximately 100 parasite microcysts. One month after the parasite injection, total masses of recovered parasites were relatively low and comparable for both nude mice (0·57±0·22 g) and wild type BALB/c controls (0·94±0·28 g). Two months after infection, clear differences were observed between the two groups: in nude mice, the recovered metacestode mass (6·21±2·87 g) was significantly higher than that found in nude mice at 1 month p.i. (P<0·0001) and higher than that found in wild type controls at 2 months p.i. (P=0·0005). On the other hand, the parasite mass did not significantly increase in wild type controls from 1 month to 2 months p.i. (1·85±1·34 g). These experiments were carried out twice, and repeatability of experiments was demonstrated by identical findings, respectively.
Morphological observations by light microscopy also revealed clear differences between nude and wild type controls at 2 months p.i. In nude mice, the parasite exhibited an unrestricted, active proliferation of germinal layers, together with an internal formation of young brood capsules, resulting in the establishment of a mesh-like appearance of the metacestode (Fig. 1E–F). In addition, thick, multi-layered germinal cell masses were occasionally found, indicative of massive cellular proliferation within germinal layers (Fig. 1H). Conversely, the parasite growth appeared to be restricted in wild type controls, with an only a thin layer of germinal cells adjacent to laminated layers (Fig. 1B and D). The metacestode tissue was surrounded by a peri-parasitic host inflammatory response, which was still relatively light at 1 month p.i., but much heavier at 2 months p.i. Most parts of the metacestode tissue were characterized by the presence of a marked granulomatous infiltration, including multinucleated giant cells and a marked fibrosis around them, which was totally absent in nude mice.

Fig. 1. Light microscopic observations of Echinococcus multilocularis metacestodes obtained from athymic nude mice (nude; E–H) and wild type BALB/c controls (WT; A–D) after i.p. injection with 100 parasite microcysts. In nude mice, the parasite exhibited thick, multi-layered germinal cell masses with young brood capsule formation, which was observed at 2 months p.i. (H and G). Conversely, the parasite growth appeared to be restricted, with only a thin monolayer of germinal cells inside laminated layers in wild type controls (B and D), where the parasite was surrounded by host responses around the parasite tissues (A–D). Haematoxylin and eosin stain.
Gene expression levels of parasite-specific molecules (ex vivo)
mRNA expression levels of the selected parasite-specific molecules in E. multilocularis metacestodes were quantitatively assessed by real-time RT-PCR for each individual mouse. 14-3-3 and II/3-10 gene expressions were quantified in relation to a housekeeping gene, beta-actin, in order to compensate for variations in input RNA amounts and efficiencies of RT among each sample, thus allowing direct comparison between individual values (Fig. 2). With regard to 14-3-3 expression levels at 1 month p.i., there was no significant difference between the metacestodes recovered from nude (84·0±39·5) versus wild type BALB/c controls (140·8±130·4). At 2 months p.i., however, the parasites grown in nude mice showed significantly higher 14-3-3 expression levels (292·3±115·3) when compared to the parasites recovered from wild type mice (122·9±72·5; P=0·0060) at the same time-point. In addition, statistical analysis revealed that the increase in E. multilocularis 14-3-3 expression moderately correlated with the increase of the parasite mass (Pearson's r=0·44, P=0·025).

Fig. 2. Gene expression levels of the parasite-specific molecules, 14-3-3 and II/3-10, in Echinococcus multilocularis metacestodes as evaluated by quantitative reverse transcription-PCR. Each value is standardized in relation to the gene expression level of a housekeeping molecule, beta-actin homologue of the parasite. The parasite tissues were recovered from athymic nude (nude) and wild type control (BALB/c) mice (WT) at 1 and 2 months after secondary infection with the parasite. Open circles represent mRNA levels of 14-3-3 and II/3-10 in individual parasite samples obtained from infected mice. Respective mean values from triplicate determinations were taken for the calculation of the relative mRNA levels. Bars represent mean values±S.D. of each group. Single and double asterisks indicate statistically significant differences (P=0·0060 and P<0·0001, respectively) between the two groups.
Conversely, E. multilocularis II/3-10 gene expression in the parasites from nude mice at 1 month p.i. (29·9±14·1), from nude mice at 2 months p.i. (33·7±14·7) and from wild type controls at 1 month p.i. (32·1±29·9) did not show any differences. At 2 months p.i., however, II/3-10 gene expression was significantly lower in the parasites grown in wild type controls (6·38±4·46) when compared with nude mouse group (P<0·0001) at the same time-point.
Detection of 14-3-3 expression in E. multilocularis by immunoblotting (ex vivo)
In addition to the quantitative assessment of E. multilocularis 14-3-3 mRNA transcription, 14-3-3 expression was also demonstrated at the protein level by immunoblotting, using mono-specific antibodies directed against recombinant E. multilocularis 14-3-3 protein. As demonstrated in Fig. 3, all parasite samples obtained from both wild type and nude mice basically exhibited similar protein profiles in SDS-PAGE (upper image) and contained E. multilocularis 14-3-3 protein, as shown by the presence of a specific band with a molecular mass of approximately 25 kDa in immunoblotting (lower image). 14-3-3 protein was expressed at higher levels in the metacestodes recovered from nude mice when compared with those from wild type controls. Semi-quantification of the immunoblot reaction was achieved by densitometric measurement of the 14-3-3 band staining intensities. For the comparative purposes, an arbitrary value of 1·0 was assigned to intensity corresponding to the specific 14-3-3 band in a parasite isolated from a wild type mouse. The metacestode samples isolated from nude mice contained significantly higher amounts of 14-3-3 protein (10·59±1·52) than those isolated from wild type controls (1·29±0·70; Student's t-test, P<0·0001). Detection of II/3-10 and beta-actin at the protein level was not carried out due to the lack of appropriately validated tools.
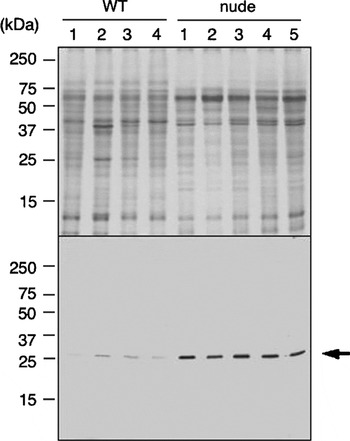
Fig. 3. Upper panel: Coomassie-stained SDS-PAGE image of 15% polyacrylamide gel run under reducing conditions showing soluble protein fraction of larval Echinococcus multilocularis obtained from wild type BALB/c mice (WT; n=4) and nude mice (nude; n=5), respectively. An equal amount (60 μg protein) of Triton X-100-soluble extract was loaded on each column. Lower panel: immunoblot of the same protein fractions as those used in SDS-PAGE (upper panel), labelled with the affinity-purified monospecific rabbit antibody against Echinococcus 14-3-3. The arrow indicates the specific reactivity against native E. multilocularis 14-3-3 protein with a respective molecular mass (~25 kDa).
In vitro experiments
In vitro experiments were set up in order to investigate putative changes in the gene expression levels of the selected parasite-derived molecules, 14-3-3 and II/3-10, together with a housekeeping molecule beta-actin, in the presence of an anti-echinococcal drug nitazoxanide. These experiments were repeated twice, yielding identical results. As shown in Fig. 4, the transcription level of a housekeeping gene, beta-actin, decreased gradually until it reached an extremely low level on day 8 of culture, indicating that in vitro treatment with nitazoxianide resulted in a marked reduction of parasite viability. Transcription levels of the selected molecules 14-3-3 and II/3-10 exhibited comparable kinetics to that of beta-actin during in vitro treatment. On the other hand, non-treated control samples exhibited only a slight and non-significant decrease in gene expression levels.

Fig. 4. Changes in gene expressions of the parasite-specific molecules, 14-3-3, II/3-10, and actin, in larval Echinococcus multilocularis during the course of in vitro treatment of an anti-echinococcal drug, nitazoxanide, at a concentration of 10 μg/ml. The parasite microcysts were cultured with (closed circles) or without (open squares) nitazoxanide-treatment, collected at different time-points of the treatment, and then subjected to quantitative evaluation of each gene expression level by real-time reverse transcription-PCR. mRNA levels of 14-3-3, II/3-10, and beta-actin genes represent logarithmic values from duplicate determinations.
Immunolocalization of Echinococcus 14-3-3 in situ
Metacestode tissue localization of 14-3-3 protein was performed by in situ immunofluorescence (microscopy) and immunogold labelling (TEM) analyses (Fig. 5A–C) on in vitro-generated metacestode materials. As a result, intense signals were observed on almost all cell types localized within proliferative germinal layers. Notably, reaction was absent within acellular laminated layers. Immunolocalization of II/3-10 and beta-actin was not carried out due to the lack of appropriately validated tools.

Fig. 5. Immunofluorescence (A) and immunogold labelling (B, C) of 14-3-3 proteins in in vitro-generated Echinococcus multilocularis metacestode. Thin sections were reacted with affinity-purified monospecific mouse antibodies against Echinococcus 14-3-3, and subsequently detected with goat-anti-mouse IgG conjugated with FITC (A) or with 10 nm gold particles (B, C). Note that labelling is found exclusively on proliferative germinal layers (GL) but not on acellular laminated layers (LL) of the parasite.
DISCUSSION
The aim of the present study was to determine whether expression levels of specific molecules synthesized by E. multilocularis metacestodes were affected by different (restrictive/non-restrictive) growth conditions. This would open new avenues for the development of molecular tools to assess viability and growth activity of the parasite. The parasite-derived molecules chosen for this study were E. multilocularis 14-3-3 and II/3-10.
In vivo restricting and non-restricting conditions were achieved by secondary infection of mice, either with or without immune deficiency. Our results confirm previous studies (Playford and Kamiya, 1992; Playford et al. 1992; Dai et al. 2004), which demonstrated that E. multilocularis metacestodes exhibited more active growth in athymic nude and SCID mice when compared to wild type control animals. These findings suggest that host immune responses, as orchestrated by T cell-populations, play a crucial role in controlling unlimited proliferation of E. multilocularis metacestodes. Similar findings were obtained in human AE cases. Surveys of human populations at risk of infection have indicated that the metacestode can exhibit various growth activities and that human patients can show different degrees of resistance against the parasite: as little as 10% of those persons exposed to infection may actually develop AE, and the majority will never develop the disease (Gottstein, 1992; Gottstein et al. 2001). Conversely, a rapid and fatal outcome of the infection has been observed in an E. multilocularis-infected patient suffering from acquired immune deficiency syndrome (AIDS) (Sailer et al. 1997). Furthermore, appropriate treatment of an AIDS patient resulted in recovery of the T cell population, allowing control of the dramatic growth of the parasite that was observed before treatment (Zingg et al. 2004). The question remains as to how parasite proliferation was initially controlled even in nude mice at 1 month p.i. One explanation is that innate immune mechanisms partially controlled the initial phase of infection as proposed previously (Dai and Gottstein, 1999; Dai et al. 2004). Once established (at 2 months p.i.), the parasite was then able to down-regulate these innate immune mechanisms. In contrast, wild type mice were in control of a much wider arsenal of T cell-dependent effectors, which allow – to some extent – restriction of the metacestode growth.
In the present study, we provide evidence that quantification of gene expression levels of the selected parasite molecules enables a direct measurement of growth activity of the parasite. Firstly, 14-3-3 expression in E. multilocularis metacestode positively correlated with metacestode growth in infected animals. Our findings are in line with the hypothesis that 14-3-3 protein is involved in the regulation of metacestode growth activity (Siles-Lucas et al. 1998, 2001). Recently, Dai et al. (2004) had demonstrated that an increased level of 14-3-3 expression could be observed at the protein level in immunodeficient nude mice, which was associated with an increased parasite load observed at 2 months p.i. In the present study also, immunoblotting for the detection of 14-3-3 protein in the soluble fraction of larval E. multilocularis demonstrated that 14-3-3 was over-expressed in an actively growing parasite. During this former semi-quantitative determination of 14-3-3 protein expression, we could actually not fully exclude inaccuracies originating from differences due to host tissue contamination. These previous findings had included only the protein expression level. In the present study now, we additionally demonstrated a positive correlation between 14-3-3 over-expression and active proliferation of larval E. multilocularis at the transcriptional level by a comparative assessment using a parasite-specific housekeeping gene, beta-actin, as a reference reaction. This method enabled us to exactly address the expression levels of parasite target molecules.
Among 5 distinct 14-3-3 isoforms identified so far in the genus Echinococcus, the first described Echinococcus 14-3-3 protein, close to the zeta isoform in mammals, appeared over-expressed at the metacestode stage of E. multilocularis when compared to corresponding adult stages (Siles-Lucas et al. 1998) and to the closely related E. granulosus metacestode (Siles-Lucas et al. 2001). An association between high expression levels of the 14-3-3 zeta isoform and tumour cell growth has also been repeatedly found in human malignancies (Nakanishi et al. 1997). In addition, co-expression and/or in vitro interaction of 14-3-3 protein with Raf has been demonstrated in several organisms, including a parasitic helminth Schistosoma mansoni (Freed et al. 1994; Rittinger et al. 1999; McGonigle, Loschiavo and Pearce, 2002). Raf is a kinase that has a pivotal role in growth factor-mediated signal transduction. The interaction between 14-3-3 and Raf, upon receipt of appropriate signals, increases Raf activity. Recently, Spiliotis et al. (2005) first cloned and characterized Ras- and Raf-homologues in larval E. multilocularis. The fact presented in their paper, together with our findings described in this report, strongly suggests that the E. multilocularis metacestode actually employs a signalling system involving Ras, Raf, and 14-3-3, which would be involved in growth control and development of the parasite tissues as found in other eukaryotic animals.
In contrast to 14-3-3, II/3-10 was not over-expressed in any of the parasite groups investigated, but the gene expression levels were significantly reduced in parasites from wild type controls at 2 months p.i. compared to all other groups. Considering the fact that parasite growth is largely restricted in wild type mice, it will be interesting to address a question whether II/3-10-protein takes over an active part in this biological restriction, or whether this just reflects a general down-regulation of biological activities of the parasite. II/3-10 was earlier shown to be localized within germinal layers, and sequence analyses revealed that this molecule exhibited significant sequence homologies to the ERM protein family (Frosch et al. 1991; Brehm et al. 1999). The reduced expression of II/3-10 in metacestodes grown in wild type mice at 2 months p.i. could simply reflect the fact that in these mice, parasites exhibited largely reduced, thus rather thin, germinal layers, with a clearly lower cell count compared to parasites grown in nude mice.
So far, little knowledge has been acquired with regard to biological function(s) of II/3-10. II/3 antigen was originally identified by Vogel et al. (1988), and subsequently by other groups (Frosch et al. 1991; Hemmings and McManus, 1991; Sako et al. 2002; reviewed by Ito and Craig, 2003). The ERM-protein family consists of 3 closely related proteins, which were originally characterized as structural components of cell cortex in mammals. Specifically concentrated in actin-rich surface structures such as microvilli, filopodia, and membrane ruffles, these factors have been shown to be involved in several key processes such as cell-cell adhesion, membrane trafficking, microvilli formation, transmembrane signalling, and cell division (Bretscher et al. 2000; Louvet-Vallée, 2000). Frosch et al. (1991) proposed a possible role for the E. multilocularis ERM homologue in active tumour-like growth of E. multilocularis metacestodes. On the other hand, however, Felleisen and Gottstein (1993) detected II/3 antigen homologues also in E. granulosus and some Taenia species, all of which do not demonstrate chronic invasive growth characteristics. Further detailed comparative studies on II/3-10 and its homologues are required to reveal the expression pattern and biological function(s) of this ERM homologue in parasite development.
In vitro experiments were carried out to study changes in 14-3-3 and II/3-10 gene expression levels within E. multilocularis metacestodes under restricting or non-restricting conditions by comparing nitazoxanide-treated versus non-treated cultures. Transcription levels of the selected molecules exhibited comparable kinetics to that of beta-actin as a house-keeping gene. This latter molecule is constitutively expressed in normal living cells, thus its transcription level is supposed to represent the normal viability status of the parasite materials examined. Stettler et al. (2003, 2004) had already demonstrated that nitazoxanide causes severe damage to the E. multilocularis metacestodes in vitro as well as in vivo. According to their report, in vitro treatment with nitazoxanide causes severe morphological damages to the parasite cysts during a relatively early phase of in vitro treatment: on day 7 of treatment, only cellular debris of the former germinal layer could be seen in the metacestode. In our present experiments, expression levels of the housekeeping beta-actin gene gradually decreased with time during drug treatment and reached extremely low levels by day 8. The kinetics of beta-actin expression correlated with the course of morphological alterations in treated parasite materials as demonstrated by Stettler et al. (2003), thus the beta-actin expression level appeared to quantitatively reflect the viability status of the metacestode. The two selected molecules 14-3-3 and II/3-10 exhibited similar kinetics to that of beta-actin. Consequently, a link to the viability status of the parasite became obvious also for the 14-3-3 and II/3-10 molecules. These findings may prove their importance when addressing the potential application of a gene expression analysis for monitoring treatment efficacy in human AE patients.
Assessing viability and growth activity of the larval parasite is of particular importance for determining biological status of the Echinococcus metacestode. Conventional approaches for the determination of parasite viability include various biological stains (Richards et al. 1988; Emery et al. 1995; Liance et al. 1992) and light and electron microscopic observations. The most sensitive procedure known so far is the inoculation of the parasite materials into highly susceptible rodents such as gerbils (Eckert and Jacquier, 1991; Wilson et al. 1992). Clinically, novel approaches have developed including adequate in vivo imaging procedures (Reuter et al. 1999) and molecular techniques (reviewed by Siles-Lucas and Gottstein, 2001) such as a RT-PCR-based diagnosis (Kern et al. 1995). However, these methods still have disadvantage(s): some of them are time-consuming and/or unsuitable for quantitative evaluation, and others are not parasite-specific, and thus not applicable for metacestode materials recovered from patients or infected animals, which unavoidably contain host tissue components.
Based on findings in the present study, here we propose that a combined analysis of 14-3-3 and II/3-10, together with beta actin, gene expression levels would provide a powerful tool for ex vivo assessment of viability and growth activity of E. multilocularis metacestode tissue. The mRNA expression levels of the parasite-specific molecules were quantitatively linked with viability status and growth activity of larval parasite. In particular, increased 14-3-3 expression levels in E. multilocularis metacestodes were associated with active growth of the parasite, implying the usefulness of this molecule as a marker for the evaluation of proliferative activity of the parasite in studying, for example, the controlling factors of metacestode development. Further functional studies on E. multilocularis 14-3-3 protein, including the search for potential interaction partner(s) within the metacestode, may provide further insight into the role of 14-3-3 as a putative growth regulator of larval E. multilocularis.
Many thanks are addressed to Mirjam Walker (University of Bern), Karen L. Haag (Universidade Federal do Rio Grande do Sul) and Nariaki Nonaka (Hokkaido University) for many pieces of helpful advice during this study. We also thank Maja Suter and Toni Wyler (Institute of Veterinary Pathology and Institute of Zoology, University of Bern, respectively), as well as Phillippe Tregenna-Piggott and Beatrice Frey (Department of Chemistry and Biochemistry, University of Bern), for access to their electron microscopy facilities. Peter Deplazes, Hansueli Ochs, and Manuela Schnyder (University of Zürich) are thanked for the in vivo maintenance of E. multilocularis strains. This work was supported by grants from the following organizations: the 21st Century COE Program, ‘Program of Excellence for Zoonosis Control,’ and a Grant-in-Aid for Scientific Research (grant no. 17790274) both from the Ministry of Education, Culture, Sports, Science, and Technology of Japan; the Japan Health Sciences Foundation; the Swiss National Science Foundation (grant no. 31-63615.00), the Hans Sigrist Foundation of the University of Bern and the EU EchinoRisk-project QLK2-CT-2001-01995 (BBW no. 00.0586-1).








