Introduction
Fatigue is one of the most common and vexing symptoms in cancer patients, with prevalence rates ranging from 40% to 90% (Hofman et al., Reference Hofman, Ryan and Figueroa-Moseley2007). In cancer patients, fatigue has been described as a symptom which is subjective and apparent only to the affected individual. It is a phenomenon experienced by a person that is not directly observable by another but instead becomes known only through the report of the person being assessed. A symptom has many meanings and dimensions not limited to physical or psychological definitions.
The etiology of fatigue is believed to be multifactorial (Neefjes et al., Reference Neefjes, van der Vorst and Blauwhoff-Buskermolen2013). Fatigue is often described in terms of physical, mental, and emotional tiredness by those who experience it. It is sufficiently consistent which can be presented in unidimensional manner or it can be sufficiently distinct in their expression which can be characterized as different dimensions of fatigue (De Raaf et al., Reference De Raaf, de Klerk and Van der Rijt2013).
The possible consequences of fatigue are reflected in its detrimental effect on the activity level. In female cancer patients, fatigue and weakness were the symptoms that interfered most with self-care activities (Rhodes et al., Reference Rhodes, Watson and Hanson1988). To limit the expenditure of energy, activities and work were scheduled, nonessential activities were decreased and patients reported an increasing dependence on others for home management activities, including meal preparation, grocery shopping, and cleaning.
Fatigue is having high prevalence and increased acknowledgment of negative effect on the patient's well-being which has resulted in fatigue being important research variable. Fatigue is being investigated as a symptom, side effect, as a precursor of disease (Appels and Mulder, Reference Appels and Mulder1988), as a diagnostic criterion (Fernandes et al., Reference Fernandes, Stone and Andrews2006), and as an outcome variable (Fawzy et al., Reference Fawzy1990). Instruments available for the assessment of fatigue can be unidimensional or multidimensional. Whatever the reason for including fatigue in research, reliable, and valid instrument should be used.
The Cancer-Related Fatigue Guidelines developed by the National Comprehensive Cancer Network (Mock et al., Reference Mock, Atkinson and Barsevick2005) and the International Classification of Disease 10th Revision criteria for cancer-related fatigue (CRF) proposed by the Fatigue Coalition (Portenoy and Itri, Reference Portenoy and Itri1999; Cella et al., Reference Cella, Davis and Breitbart2001; Sadler et al., Reference Sadler, Jacobsen and Booth-Jones2002; Van Belle et al., Reference Van Belle2005) emphasized assessment as the key to identifying and managing the CRF symptom (Bender et al., 2002; Ressel, Reference Ressel2003).
The National Institutes of Health State of the Science panel further stressed that the assessment of fatigue is an important step in treating cancer patients (National Institute of Health Sate of the Science Panel, 2003, 2004). This recent development shows greater receptivity of health professionals to assessing CRF and increased recognition of the importance of incorporating the patients’ perspective in the CRF assessment.
CRF instruments in breast cancer patients lack a systematic report of their psychometric properties. Psychometric testing should have two types of reliability, at least one type of content validity and at least one type of criterion related or construct validity (Norbeck, Reference Norbeck1985; Table 1).
Table 1. Explanation of psychometric properties of an ideal scale (Taherdoost, Reference Taherdoost2016)

In this review, CRF instruments were identified which have been used in breast cancer patients with the detailed description about the instruments in terms of dimensions, domains, scoring, and interpretation. The psychometric properties of the identified CRF instruments were evaluated.
Methods
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines was followed for performing this review. A search was conducted from January 2000 to April 2020 from electronic databases such as Pubmed, Cochrane, Embase, and Google Scholar. The search strategies used for the review were Breast cancer patients, Breast cancer, Breast neoplasms, Neoplasms, Breast carcinoma, Cancer of the Breast, Mastectomy, Post Breast surgery, Radiation therapy, Chemotherapy, Cancer-related fatigue, Fatigue, CRF, Instruments, Measures, Scale, Assessment, Questionnaire, and Surveys. From this search, studies were identified in which instruments were used to measure fatigue. The fatigue instruments which were used in these studies were reviewed for their validation in breast cancer patients. The literature search procedure is given in Figure 1.

Fig. 1. Flowchart of methodology in accordance with PRISMA (preferred reporting items for systematic reviews and meta-analyses) statement criteria.
Inclusion and exclusion criteria
The studies were included if the instrument was used to measure fatigue in breast cancer patients and its description and psychometric properties reported in breast cancer patients. The search was limited to studies in the English language and use of English version of instruments. Randomized clinical studies focusing on breast cancer treatments and review studies evaluating CRF in breast cancer patients were excluded. Conference abstracts, dissertations, commentaries, editorials, or summary reports were not included. The studies were included if the study population consisted of breast cancer patients only or mixed cancer patients in which breast cancer patients were included. Psychometric properties available on other cancer patients or general patients were not included in the study.
Data extraction
Data extraction was recorded on performa in which study title, population, the number of participants, and instrument used for fatigue was mentioned. After collecting all studies in which fatigue instruments used in breast cancer patients, description of scale, and psychometric properties were evaluated for instruments. The instruments were evaluated only if they have mentioned criteria for inclusion.
Result
Among 34 CRF instruments, 9 instruments were included according to inclusion and exclusion criteria. From nine instruments, six were multidimensional, two were unidimensional, and one instrument was quality-of-life (QOL) subscale. Table 2 gives details about instrument's domains, items, scaling, and administration. Table 3 provides details about validation study of instrument and the number of studies performed by using a particular instrument. Table 4 described the psychometric details about the instruments.
Table 2. Description of cancer-related fatigue instruments in breast cancer patients
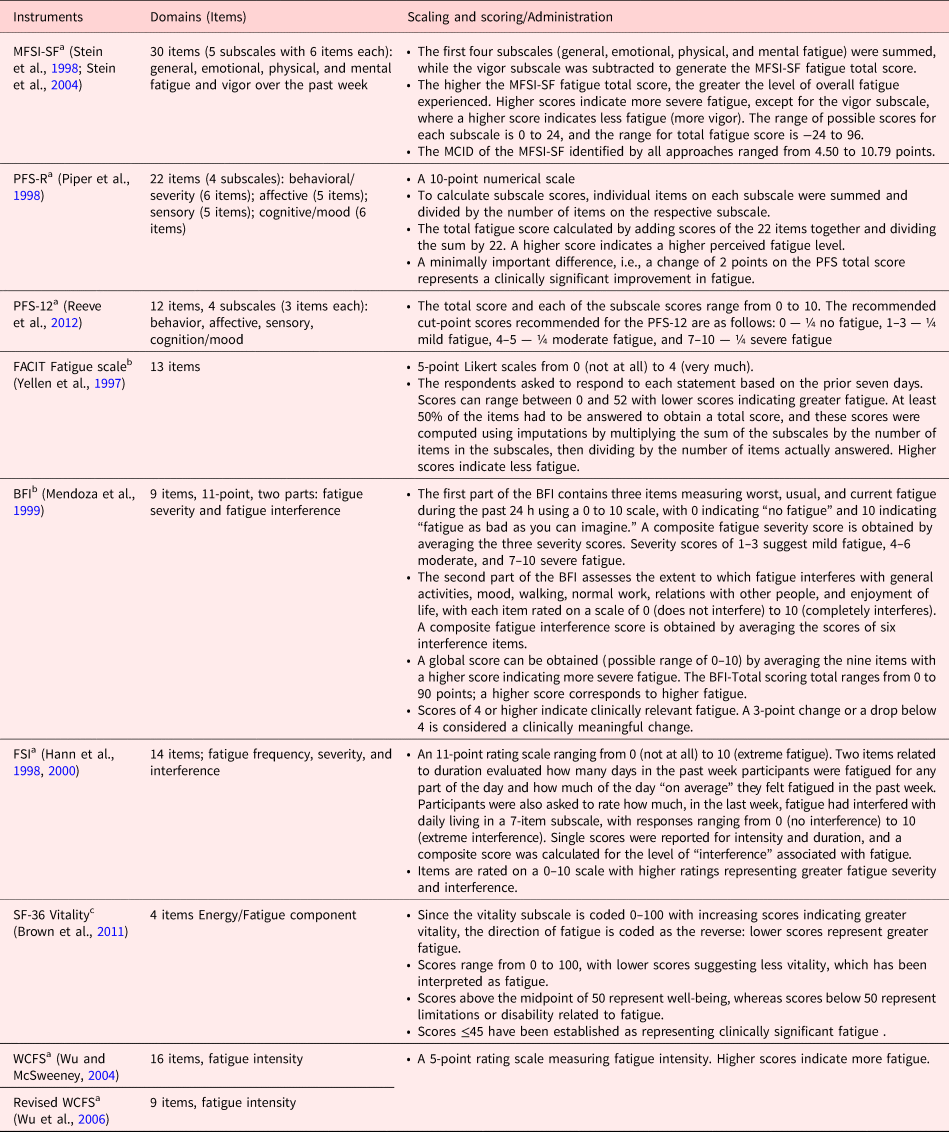
Abbreviations: MFSI-SF, Multidimensional Fatigue Symptom Inventory — Short Form; MCID, minimum clinically important difference; PFS-R, Piper Fatigue Scale — Revised; PFS-12, Piper Fatigue Scale-12; FACIT Fatigue Scale, The Functional Assessment of Chronic Illness Therapy — Fatigue Scale; BFI, Brief Fatigue Inventory; FSI, Fatigue Symptom Inventory; WCFS, Wu Cancer Fatigue Scale.
a Multidimensional.
b Unidimensional.
c Subscale of quality-of-life scale.
Table 3. Cancer-related fatigue instruments in breast cancer patients
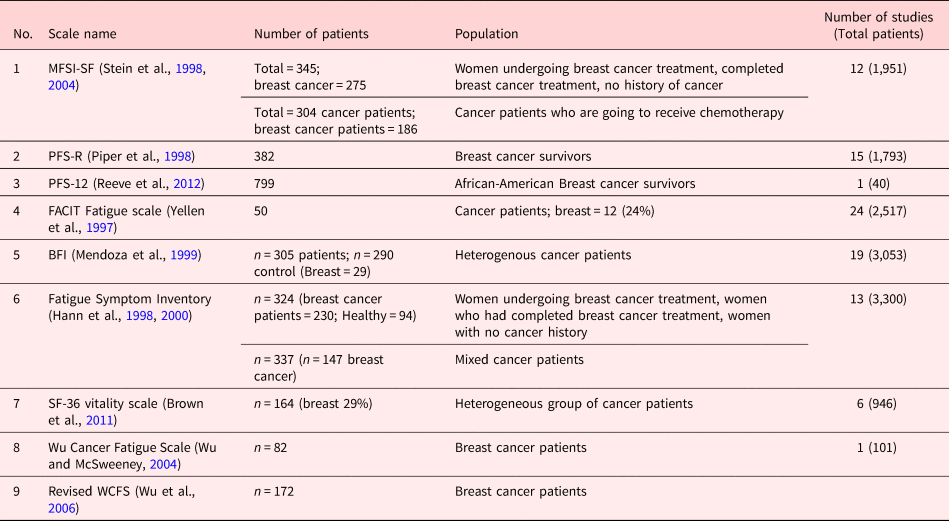
Abbreviations: MFSI-SF, Multidimensional Fatigue Symptom Inventory — Short Form; PFS-R, Piper Fatigue Scale — Revised; PFS-12, Piper Fatigue Scale-12; FACIT Fatigue Scale, The Functional Assessment of Chronic Illness Therapy — Fatigue Scale; BFI, Brief Fatigue Inventory; FSI, Fatigue Symptom Inventory; WCFS, Wu Cancer Fatigue Scale.
Table 4. Psychometric properties of cancer-related fatigue instruments in breast cancer patients

Abbreviations: MFSI-SF, Multidimensional Fatigue Symptom Inventory — Short Form; MCID, minimum clinically important difference; PFS-R, Piper Fatigue Scale — Revised; PFS-12, Piper Fatigue Scale-12; FACIT Fatigue Scale, The Functional Assessment of Chronic Illness Therapy — Fatigue Scale; BFI, Brief Fatigue Inventory; STAI, The State-Trait Anxiety Inventory; CES-D, The Center for Epidemiologic Studies Depression Scale; MC 20, a short form of the Marlow–Crowne Social Desirability Scale; POMS-F, Profile of Mood States Fatigue Scale; FSI, Fatigue Symptom Inventory; SF-36, The Medical Outcomes Study 36-Item Short Form; ECOG, The Eastern Collaborative Oncology Group; FSI, Fatigue Symptom Inventory; WCFS, Wu Cancer Fatigue Scale; PHQ fatigue, fatigue from the Patient Health Questionnaire; SCFS, Schwartz Cancer Fatigue Scale; SCL-20, The 20-item depression scale of the Hopkins Symptom Checklist; GDS, Geriatric Depression Scale; SLDS-C, the Satisfaction with Life Domains scale-Cancer.
Discussion
Fatigue is the most common and problematic side effects of breast cancer treatment (Winningham et al., Reference Winningham, Nail and Burke1994). It is endemic in persons with cancer. Those who are dealing with breast cancer patients are familiar with fatigue developed by the patients and disablement caused by it (Mendoza et al., Reference Mendoza1999). The reliable and valid fatigue instruments for breast cancer patients will provide way for epidemiological studies on fatigue. It will improve communication about fatigue between patients and those who care for them and will facilitate clinical trials for the development of new treatment for fatigue.
The research has shown that CRF may manifest in a wide range of symptom domains, including behavioral, cognitive, somatic, and affective (Knobf, Reference Knobf1986; Rhodes et al., Reference Rhodes, Watson and Hanson1988; Cimprich, Reference Cimprich1993). It should be ascertained first that what aspects of fatigue are to be measured. If the measure is for screening purpose, then a long multidimensional scale would be necessary. Multidimensional fatigue instruments detect multiple characteristics and manifestations of fatigue and its impact on function. However, it will be too demanding for those patients who are having the increased level of fatigue due to the more comprehensive assessment of fatigue that they provide. They are also lengthy in terms of the number of items they include and sometimes may have complicated response formats. Unidimensional fatigue instruments provide information only about the severity or intensity of the symptoms. These instruments do not provide the full spectrum of the fatigue symptom profile. The final choice of measure should take into consideration about the detail required and the practical issues of completion. The scale must be sensitive enough to detect change over time for using it as an outcome measure in an intervention study or to detect disease progression.
The MFSI-SF can be administered at frequent intervals as it is keyed to a one-week time frame; thus, it allows the clinicians for the assessment of fatigue during the course of the cancer treatment. The advantage of the MFSI-SF is that it does not assume the presence of fatigue. Because of that, the MFSI-SF can be used to obtain baseline data from the patients who are going to receive treatments which may develop fatigue. There are favorable estimates of internal consistency and of test–retest reliability for MFSI-SF. It has also shown to demonstrate concurrent, convergent, and divergent validity. The internal consistency of the MFSI-SF scale is within acceptable to good range in both the validation study. The clinically significant worsening of CRF can be identified by a ≥10% increase or a deterioration of 4.50–10.79 point in the MFSI-SF fatigue scale (Rowe et al., Reference Rowe, Fontaine and Lauver2016; Chan et al., Reference Chan, Yo and Wang2018).
The BFI is simple to apply to the patients. It has single word designation for the severity of fatigue and functionality domains which makes it very easy to understand. It has shown reliable result by correlation with measures of performance status and with physiological markers of anemia and nutritional status. A 3-point change or a drop below 4 is considered a clinically meaningful change in the BFI scale (Vickers et al., Reference Vickers, Straus and Fearon2004).
The 12-item PFS-12 has been developed by shortening of the 22-item PFS-R based on multiple criteria including reliability, validity, literacy demand, and response bias. It can be used without extensive response burden. PFS-12 will provide great value to capture the multidimensional aspects of fatigue experience. A minimally important difference, i.e., a change of 2 points on PFS total score shows a clinically significant improvement. The further validity and MCID for PFS-12 is planned for further research (Piper et al., Reference Piper, Dibble and Dodd1998).
The FACIT Fatigue scale is a psychometrically sound and places minimal burden on patients to answer and clinic staff to score and interpret. There is an availability of computer-administered assessment and scoring programs which make its routine use in clinical practice highly feasible. It has showed excellent internal consistency and test–retest reliability and group differences in hemoglobin level and performance status. The FACIT Fatigue scale measure a construct conceptually similar to that measured by the Piper Fatigue Scale but differ from it in important ways. They are briefer than the other measures, making them easier to administer and score. In this scale, patients do not necessarily have to experience fatigue to be able to answer all the questions, as in the Piper Fatigue Scale. This instrument addresses the implications or consequences of fatigue in addition to symptom expression. The FACIT Fatigue scale has its greatest utility in delineating both the physical and functional consequences of fatigue, which, in turn, have important implications for overall quality of life. Because the 13-item scale is psychometrically sound, can prove useful as an independent, brief assessment of fatigue. It has been found sensitive to change and its minimally important clinical difference is 3 points (Webster et al., Reference Webster, Cella and Yost2003).
The Fatigue symptom inventory (FSI) provides information about fatigue intensity, duration, and the interference of fatigue with various aspects of quality of life. The FSI had exhibited very good acceptability. For reliability, the interference subscale of the FSI showed excellent internal consistency. The estimates of the test–retest reliability were not as favorable and did not demonstrate that the FSI can reliably measure fatigue across a short period of time (two to four weeks) and a longer period of time (four to six weeks). Significant correlations with the POMS-F and the SF-36 vitality scale support the convergent validity of the scale. The comparisons also supported the divergent validity of the FSI. The construct validity was supported by significant correlation between the intensity items, duration items, and interference scale within each group. The construct validity of the FSI was demonstrated by moderate to high correlations with measures of anxiety and depression. A mean score of 3 or more has been found a clinically meaningful level of fatigue (Donovan et al., Reference Donovan, Jacobsen and Small2008).
The revised WCFS can be self-administered or read to the respondent. It is a reliable and valid instrument for measurement of CRF and will be useful in both clinical and research setting. It gives quantitative information on the fatigue symptom status to monitor the patient's condition and treatment progress. Convergent validity was supported by a strong correlation with the well-developed SCFS. A moderate correlation with the depression scores on the GDS Short Form was evidence to support the concurrent validity. A high Cronbach's alpha indicated that this instrument is consistently measuring the same construct, which supported its internal consistency reliability.
SF-36 Vitality Subscale score is determined by responses to 4 items: Did you feel full of life? Did you have a lot of energy? Did you feel worn out? Did you feel tired? The SF-36 Vitality (energy/fatigue) subscale is short (4 items) and has strong psychometric data supporting its reliability and validity.
This review will help healthcare providers who are dealing with breast cancer patients to acknowledge and better understand what their patients are experiencing. It will also allow them to use the most appropriate tool for holistic assessment of CRF. The appropriate instrument will help healthcare provider to monitor their patient's condition or treatment progress so it can be incorporated into treatment decisions for better management of fatigue.
Conflict of interest
There are no conflict of interest.








