INTRODUCTION
Cancer pain (CP) is a common and distressing symptom. Approximately 70% of patients with advanced cancer experience pain at some point during their disease trajectory (Portenoy & Lesage, Reference Portenoy and Lesage1999). Studies have shown that 42% of patients seen in outpatient oncology centers have inadequate analgesic prescriptions (Cleeland et al., Reference Cleeland, Gonin and Hatfield1994). Another study showed that as many as 50% of hospitalized cancer patients have uncontrolled pain (Holtan et al., Reference Holtan, Aass and Nordoy2007). Patients, especially those with more complex pain syndromes, fail to attain acceptable pain control. These patients often require more intense therapeutic interventions, resources, and time to achieve adequate pain control (Nekolaichuk et al., Reference Nekolaichuk, Fainsinger and Lawlor2005). To date, there is no standardized and universally accepted pain classification system for assessment and management of cancer pain in both clinical practice and research studies (Knudsen et al., Reference Knudsen, Aass and Fainsinger2009). The use of a standardized system would improve interpretation and comparison of study results, and potentially enhance the success of therapeutic interventions (Bruera et al., Reference Bruera, Schoeller and Wenk1995; Caraceni et al., Reference Caraceni, Cherny and Fainsinger2002).
In response to this gap in clinical assessment, the Edmonton Staging System (ESS) was developed by Bruera and colleagues (Reference Bruera, MacMillan and Hanson1989; Reference Bruera, Schoeller and Wenk1995). The ESS was limited because of some difficulties identified with interpreting the definitions of various features. It was further refined by an expert panel consisting of physicians and researchers at the Edmonton Regional Palliative Care Program and renamed the revised Edmonton Staging System (rESS). Subsequently, Fainsinger and colleagues conducted a number of validation studies and appraisals of the rESS, and the assessment tool was again reworked and renamed the Edmonton Classification System for Cancer Pain (ECS–CP) (Fainsinger et al., Reference Fainsinger, Nekolaichuk and Lawlor2012). The ECS–CP has demonstrated value in predicting pain management complexity based on five features: pain mechanism, incident pain, psychological distress, addictive behavior, and cognitive function (Fainsinger et al., Reference Fainsinger, Nekolaichuk and Lawlor2005). The tool containing the various features is shown in Table 1, and the detailed definitions and guidelines for use were similar to those reported in other studies (Fainsinger et al., Reference Fainsinger, Nekolaichuk and Lawlor2010; Fainsinger & Nekolaichuk, Reference Fainsinger and Nekolaichuk2008).
Table 1. The Edmonton Classification System for Cancer Pain

$ Sufficient impairment to affect patient's ability to provide accurate present and/or past pain history.
& Patient unresponsive, delirious, or demented to the stage of being unable to provide any present and past pain history.
This classification system still needs more validation studies in order to become a universally accepted prognostic indicator. Also, to date, there are no studies that have utilized a composite score to evaluate whether patients with more negative prognostic features have higher pain intensity compared to those with fewer negative features. Furthermore, there is limited information about the utilization of the ECS–CP in an outpatient population and the feasibility of its use as part of a thorough palliative care consultation. At our institution in the outpatient supportive care center, the ECS–CP is employed as part of a comprehensive pain assessment.
The objective of our study was to assess the utility of the ECS–CP as a tool for predicting pain-related outcomes in patients seen in the outpatient supportive care center at a comprehensive cancer center. We determined the relationships between the ECS–CP features and ESAS pain intensity, opioid use, need for opioid rotation, and number of adjuvant medication use. We also determined the association between the composite sum of negative prognostic features in a patient and pain intensity at the initial clinic visit.
We hypothesized that patients with negative prognostic features would have higher pain intensity scores, higher opioid use, a need for opioid rotation, or use more adjuvant medications, compared to patients without the negative features. Also, patients with more negative ECS–CP features would have higher pain intensity than those with fewer features.
METHODS
Electronic charts of 100 patients were retrospectively screened between February of 2008 and March of 2010 at the outpatient supportive care clinic in a comprehensive cancer center. To be eligible for inclusion, patients had to be 18 years or older with a diagnosis of cancer and have a documented ECS–CP assessment at the initial consultation. Patients with nonmalignant pain syndrome were excluded from the study. The following information was collected: baseline characteristics (such as patient age, gender, race, and cancer diagnosis), initial ECS–CP assessment, the morphine equivalent daily dose (MEDD), opioid rotation, Edmonton Symptom Assessment Score (ESAS), Memorial Delirium Assessment Scale (MDAS) score, the Cut-Down, Annoyed, Guilty, and Eye-Opener (CAGE) questionnaire, performance status, use of adjuvant analgesics, and additional interventions such as palliative radiotherapy. The ECS–CP documentation was done using the tool as shown in Table 1. The institutional review board approved the study and provided a waiver of informed consent. Due diligence was taken to protect patient confidentiality.
Process and Instruments
The supportive care team at our clinic is comprised of board-certified palliative care (PC) physicians, PC-trained registered nurses, pharmacists, nutritionists, chaplains, social workers, psychiatric nurse counselors, and wound care nurses. A standardized management plan is followed in care delivery. The patient and his or her family are initially assessed by the nurse using a template comprised of various tools employed as part of the routine clinical practice at the center. These include the ESAS, the ECS–CP, the CAGE questionnaire, and the MDAS (Breitbart et al., Reference Breitbart, Rosenfeld and Roth1997; Fadul et al., Reference Fadul, Kaur and Zhang2007). The findings are then discussed with the palliative care physician, who then conducts an interview with the patient and family, does a physical examination, and subsequently formulates the assessment and treatment plan. The palliative care physician will ensure completion of any missing vital information. Other members of the team are then involved in the care of the patient as and when necessary.
The ECS–CP is a standardized assessment tool utilized to characterize pain complexity based on five prognostic features or indicators—namely, pain mechanism (whether neuropathic or nociceptive), incident pain, psychological distress, addictive behavior, and cognitive dysfunction (Fainsinger et al., Reference Fainsinger, Nekolaichuk and Lawlor2012; Reference Fainsinger, Nekolaichuk and Lawlor2005). The presence of any of these features is predictive of high pain complexity and difficulty in achieving adequate analgesia in such patients. This approach is still undergoing research appraisals in order to become a universally accepted assessment tool.
The ESAS is a validated tool used to screen for the presence and severity of symptoms in advanced cancer patients. It is comprised of a numerical rating scale, with a range from 0 to 10 (0 being the absence of symptoms and 10 being the worst symptoms imaginable). It assesses symptoms of pain, fatigue, nausea, depression, anxiety, drowsiness, and appetite, and sensations of well-being, financial distress, and spiritual pain (Bruera et al., Reference Bruera, Kuehn and Miller1991; Chang et al., Reference Chang, Hwang and Feuerman2000; Philip et al., Reference Philip, Smith and Craft1998).
The CAGE questionnaire is a screening tool for alcoholism and may reveal the possibility of maladaptive behavior when there is an exaggerated and erroneous need for opioid medication. It consists of a four-item questionnaire. A score of 2 out of 4 or more by a patient is considered positive and raises concerns about potential opioid misuse and chemical coping.
The MDAS is a validated tool employed to screen patients for delirium (Breitbart et al., Reference Breitbart, Rosenfeld and Roth1997; Fadul et al., Reference Fadul, Kaur and Zhang2007). It is a 4-point severity rating scale of 10 items: awareness, disorientation, short-term memory, digit span, attention, disorganized thinking, perception, delusions, psychomotor activity, and sleep–wake cycle disturbance. It yields a global score of from 0 to 30. If the score is more than 7 out of 30 in cancer patients, it is considered positive for presence of delirium.
Statistical Analysis
Descriptive statistics were used to summarize demographic data and symptoms. A paired t test was employed to analyze symptom scores. Each of the five ECS–CP components was tested against baseline measurements such as the ESAS, using the Wilcoxon rank sum tests or the Kruskal–Wallis test. The Spearman correlations test was utilized to determine the association between baseline ECS–CP measures and baseline ESAS measures. We developed a simple model whereby a numerical value of 1 was assigned to each negative ECS–CP feature. The sum of all negative features for each patient represented a composite score. The score ranged from 1 to 5. The mean pain intensity for all patients with the same composite score was computed. We then summarized the association between composite scores and pain intensity levels using the Wilcoxon rank sum test, as illustrated in Figure 1.

Fig. 1. Association between composite score and mean pain intensity.
RESULTS
Some 91 out of 100 charts contained full ECS–CP information and were therefore eligible for analysis. The patient demographic and clinical characteristics are shown in Table 2. The mean age was 58 years; 55% were female. The most common primary cancer was gastrointestinal (22.1%). Patients were predominantly Caucasian (70.3%). Most patients had metastatic or refractory disease (79.1%). The median performance status was 2, and the median MDAS score was 1.
Table 2. Patient characteristics
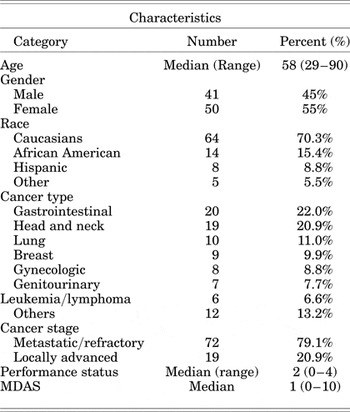
MDAS = Memorial Delirium Assessment Scale; performance status = Zubrod performance status.
Table 3 summarizes the baseline information on pain and other cancer-related symptoms as measured by the ESAS. Some 67% were on at least one adjuvant medication at the initial clinic visit; opioid rotation was done in 62.6% of patients. The median MEDD was 45. The median baseline pain intensity was 6. Incident pain was the predominant ECS–CP feature (60.4%), and cognitive dysfunction was the least frequent feature (2.2%). The worst cancer-related symptom was fatigue, with a median score of 6, while nausea was the least unpleasant symptom (1).
Table 3. Baseline information on pain and other cancer-related symptoms
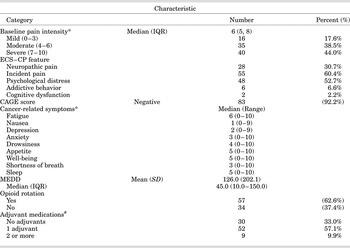
Abbreviations. CAGE = Cut down, Annoyed, Guilty, Eye-opener; ECS–CP = Edmonton Classification System for Cancer Pain; SD=standard deviation; IQR = interquartile range; MEDD = morphine equivalent daily dose.
# Nonsteroidal anti-inflammatory drugs, corticosteroids, anticonvulsants, antidepressants, atypical muscle relaxants, N-methyl-D-aspartate antagonists, topical analgesics.
* As measured by the Edmonton Symptom Assessment Scale.
Table 4 shows the relationship between ECS–CP features and baseline pain intensity, as well as the MEDD. Neuropathic pain was significantly associated with median pain intensity (presence of neuropathic pain compared with absence of neuropathic pain was 7 vs. 5, p = 0.01) and median MEDD requirement (83 vs. 30, p = 0.01). Psychological distress was also significantly associated with median pain intensity (7 vs. 5, p = 0.04). There was a trend toward association between incident pain and median pain intensity (6 vs. 5, p = 0.06).
Table 4. Association between ECS–CP features, pain, and MEDD

* Kruskal–Wallis test.
Backward stepwise regression was performed on a multivariable linear model containing variables that had been found to be significantly or nearly significantly associated with pain intensity. These included MEDD, neuropathic pain, psychological distress, and incidental pain. The model found that pain intensity was associated with MEDD (p < 0.0001) and neuropathic pain (p = 0.02). The presence of neuropathic pain was associated with a 2.44-point increase in pain intensity, and each 100-mg increase in MEDD was associated with a 3.14-point increase in pain intensity.
The associations between ECS–CP features and such other variables as cancer type, ESAS symptoms, CAGE score, and MDAS score were also analyzed. Patients with breast cancer and genitourinary cancer were more likely to have neuropathic pain than a different type of pain. Neuropathic pain was also associated with worse median sleep scores (7 vs. 5, p = 0.01). Psychological distress was associated with median anxiety (4 vs. 2, p = 0.02) and appetite (6 vs. 3, p = 0.02), and also showed a trend for an association with depression (3 vs. 1, p = 0.08) and adjuvant use (68 vs. 65%, p = 0.08). Cognitive impairment was significantly associated with a higher fatigue score (10 vs. 6, p = 0.04) and median MDAS score (6 vs. 1, p = 0.01).
Figure 1 is a box plot depicting the relationship between composite score and pain intensity, which shows that the mean pain intensity for a composite score of 1 was 4.5 and that for a composite score of 5 was 9.5. Higher composite scores were associated with higher pain intensities (p = 0.01).
DISCUSSION
In this study, we report on the use of the ECS–CP to assess cancer pain in routine clinical practice. We illustrate the feasibility of the tool in routine clinical practice as demonstrated by the fact that 91 of the 100 patients were successfully evaluated by the clinician at the initial visit using this tool. Neuropathic pain and psychological distress were associated with higher pain intensity. Similar trends were seen in patients with incident pain. Patients with neuropathic pain were taking higher opioid doses than those without it. These findings are similar to those of a multicenter study by Fainsinger et al. (Reference Fainsinger, Nekolaichuk and Lawlor2010) in which patients with neuropathic pain, incident pain, psychological distress, or higher pain intensity required more adjuvants and a higher MEDD. Neuropathic pain and sleep disturbance were found to be significantly associated in our study. Chronic pain is associated with sleep disturbance in reciprocal fashion. Pain can decrease the quality and quantity of sleep. This in turn can increase the subjective experience of pain and lower the pain threshold, thereby creating a vicious cycle (Lavigne et al., Reference Lavigne, McMillan, Zucconi, Kryger, Roth and Dement2005; Roehrs et al., Reference Roehrs, Hyde and Blaisdell2006). Neuropathic pain has been specifically found to have an impact on sleep disturbance (Gustorff et al., Reference Gustorff, Dorner and Likar2008; Zelman et al., Reference Zelman, Brandenburg and Gore2006).
In the multivariate analysis, we found that higher pain intensity was associated with higher MEDD and higher neuropathic pain. Because this was a one-time assessment, we were unable to determine the predictive effect of the ECS–CP on pain response, as has been the case in other studies (e.g., Fainsinger et al., Reference Fainsinger, Nekolaichuk and Lawlor2010). One possible limitation of a multivariate analysis of baseline pain is that not all of these patients had been managed for their pain by a pain or palliative medicine specialist prior to their initial consultation. Therefore, pain intensity in some of these patients might have simply been the result of inappropriate pain assessment or undertreatment rather than failure to control the pain using appropriate methods.
Our study did not find an association between ECS–CP cognitive dysfunction or ECS–CP addictive behavior and pain intensity or MEDD. It is possible that some prognostic features may be less predictive in certain clinical settings as compared to others. Our study was conducted with patients in an outpatient setting where the prevalence of cognitive dysfunction was known to be only about 3.6% (Kuriya et al., Reference Kuriya, Yennurajalingam and de la Cruz2014), as compared with patients in an inpatient setting where delirium ranges from 13.3 to 42.3% at admission, 26 to 62% during admission, and increases 58.8–88% in the weeks and hours preceding death (Hosie et al., Reference Hosie, Davidson and Agar2013). The lack of an adequate sample size in this patient population in our study (2.2%) might have made it difficult to obtain the power to detect a significant association. Similarly, the number of patients exhibiting addictive behavior in our study was very low (6.6%). Previous studies have found that patients who score positive on the CAGE questionnaire require a longer time to achieve pain control (Fainsinger et al., Reference Fainsinger, Nekolaichuk and Lawlor2010; Reference Fainsinger, Nekolaichuk and Lawlor2005), receive a higher opioid dose upon referral to the supportive care center (Parsons et al., Reference Parsons, Delgado-Guay and El Osta2008), and require a considerably longer time to discontinue opioid analgesics after treatment of their disease (Kwon et al., Reference Kwon, Hui and Chisholm2013). Dev and colleagues found that only 13% of patients who were CAGE-positive had been identified as alcoholics before their palliative care consultation (Dev et al., Reference Dev, Parsons and Palla2011).
Our results with respect to patient characteristics (age, gender, and cancer type) were comparable to previously published studies, with some minor variations (Bakitas et al., Reference Bakitas, Lyons and Hegel2009; Bruera et al., Reference Bruera, Michaud and Vigano2001; Follwell et al., Reference Follwell, Burman and Le2009; Strasser et al., Reference Strasser, Sweeney and Willey2004). Fatigue was found to be the worst cancer-related symptom, and nausea was the least bothersome. Follwell and colleagues (Reference Follwell, Burman and Le2009) in a similar study found that the mean fatigue score was 6.5 and the mean nausea score was 2.8. Similar findings were also shown by Strasser et al. (Reference Strasser, Sweeney and Willey2004). A study done by our team showed that pain was found to be one of the two most significant predictors of fatigue among patients seen at the initial clinic visit (Yennu et al., Reference Yennu, Urbauer and Bruera2012).
This study is the first one to look at the cumulative effect of negative ECS–CP features on pain intensity (Figure 1). This novel analysis postulates that the more negative features a patient has, the higher his pain intensity will be. In order to conclusively make this statement, one will have to show that each of the five individual ECS–CP features is independently associated with pain intensity, which has been well demonstrated in previous studies (Fainsinger et al., Reference Fainsinger, Nekolaichuk and Lawlor2010). It will be interesting to further determine whether such an effect is just additive or synergistic. This finding may eventually lead to further definitions and categorizations of pain based on the cumulative effect of the negative features. Also, future studies will be needed to investigate the cumulative effect of negative ECS–CP features on other cancer-related symptoms like fatigue, nausea, sleep, and dyspnea. Our new observations further add to the work done by previous researchers on pain classification and will be the subject of further research as ongoing efforts are being made to appraise and evaluate various aspects of the ECS–CP.
In a study assessing the ECS–CP, Fainsinger et al. (Reference Fainsinger, Nekolaichuk and Lawlor2010) had the participation of multiple international centers and involved a diverse patient population. That study proved that the ECS–CP can be utilized and standardized for use among a wide range of patients across the globe in different settings and under different circumstances. The international collaboration in that study points to the feasibility of the ECS–CP as a potential universally accepted tool for use both in clinical practice as well as for pain and palliative care research activities. A decision-making body in the field of palliative care has unanimously acknowledged the ECS–CP as a potential starting tool while researchers continue to work on a universally accepted international classification system for cancer pain (Hagen et al., Reference Hagen, Klepstad and Hjermstad2008).
The first limitation of our study was the retrospective design and a relatively smaller sample size when compared to similar studies. Efforts have been made to modify the symbols and simplify the documentation process at our center. These measures were taken in order to encourage all clinicians to document ECS–CP features on a more consistent basis. A study is currently being planned to assess the response of clinicians to the changes and interventions made at our center. Second, our study did not take into account such factors as age and genetic composition as possible contributors to patients' pain response. Further studies will need to look into such factors. Finally, our study only looked at patients in an ambulatory setting with relatively better functional status and cognitive function. Further studies will need to involve patients in the inpatient setting since they are likely to have different symptom profiles and severity and therefore different outcomes.
CONCLUSION
We conclude that the ECS–CP is a simple and feasible cancer pain assessment tool that can be employed in routine clinical practice. We have found that neuropathic pain and psychological distress are associated with higher pain intensity. In addition, we have demonstrated that neuropathic pain is associated with higher MEDD scores and that a higher sum of negative ECS–CP features is associated with higher pain intensity. Further studies will be needed to verify and explore these observations.







