Introduction
Cancer-related dyspnea is a common and distressing symptom for patients with cancer (Jones and Simone, Reference Jones and Simone2014). According to a meta-analysis by Solano et al. (Reference Solano, Gomes and Higginson2006), between 10% and 70% of patients may experience dyspnea. In addition to being a distressing symptom (Tishelman et al., Reference Tishelman, Petersson and Degner2007), dyspnea is also a predictor of shortened prognosis. Clinical treatment and advance care planning may change based on both dyspnea symptomatology and prognostic information (Pinna et al., Reference Pinna, Vargas and Moralo2009; Simone and Jones, Reference Simone and Jones2013).
A recent clinical practice guideline by the American Society of Clinical Oncology discusses nonpharmacologic interventions to relieve cancer-related dyspnea, including airflow interventions, supplemental oxygen, and other psychoeducational and self-management approaches (Hui et al., Reference Hui, Bohlke and Bao2021). For patients where nonpharmacologic interventions are insufficient, pharmacologic interventions including systemic opioids, short-acting benzodiazepines, systemic corticosteroids, and bronchodilators have been recommended. However, the strength of recommendations are weak to moderate given the paucity of randomized controlled trials (RCTs) conducted to date.
In an attempt to increase statistical power, a network meta-analysis can be used to generate indirect comparisons of pharmacologic interventions to one another. Through this, further clarity might be provided regarding which pharmacologic interventions may have the most promise for future clinical trials. The aim of this study was to conduct a systematic review and network meta-analysis of RCTs to determine the optimal pharmacologic interventions for the prophylaxis and treatment of cancer-related dyspnea.
Methods
Search strategy
A search was conducted in the databases of PubMed, Embase, and Cochrane CENTRAL (Appendix 1). The databases were searched through to May 4, 2021. No language restriction was placed.
Inclusion criteria
All articles from the search strategy and identified through backward reference screening underwent level 1 title and abstract screening. Articles were eligible for level 2 full-text screening if they reported on a clinical trial of pharmacologic treatments of dyspnea. Level 2 screening subsequently identified articles that were RCTs and reported exclusively on cancer patients. Eligible articles then underwent assessment for quantitative synthesis, and they were included in this review if they reported either (1) standardized mean differences (SMDs) of dyspnea symptoms from baseline to follow-up or (2) baseline and follow-up dyspnea scores. For articles where insufficient data were provided in the manuscript, corresponding authors were contacted twice, two weeks apart. In cases where no response was provided or no data were available, the study was excluded from this review.
Risk of bias assessment
Studies were assessed for risk of bias using the Cochrane Risk of Bias version 2 tool (Sterne et al., Reference Sterne, Savović and Page2019). Risk of bias was presented in this study using the Risk-of-bias VISualization package (McGuinness and Higgins, Reference McGuinness and Higgins2020).
Meta-analysis
Patient demographics and treatment characteristics were recorded for each included study. SMDs, as reported by studies or calculated from baseline and follow-up dyspnea scores, were amalgamated into a summary SMD and 95% confidence interval (CI) using a restricted maximum likelihood multivariate network meta-analysis. Meta-analyses were conducted separately for studies reporting on prophylaxis for exertional dyspnea and for studies reporting on treatment of everyday dyspnea. The selected study arms were grouped into three categories of treatments. We categorized treatments of buccal, nasal and sublingual fentanyl into rapid onset fentanyl, treatments of subcutaneous and intravenous fentanyl as parenteral fentanyl, and all treatments of morphine sulfate together. Although pharmacokinetics differed between treatments of different treatment routes, we pooled them together to increase statistical power. The underlying consistency assumption was assessed using an inconsistency model (White et al., Reference White, Barrett and Jackson2012). P-values less than 0.05 were defined as statistically significant. All analyses were conducted using Stata version 17.0 (StataCorp, College Station, TX, USA).
Results
A total of 1,444 records were identified through our database searches; 1 additional record was identified through backward reference screening. After 17 duplicates were removed, 60 of 1,426 records underwent level 2 full-text screening. Twenty articles were identified for potential quantitative synthesis, and 12 articles (Bruera et al., Reference Bruera, MacEachern and Ripamonti1993; Mazzocato et al., Reference Mazzocato, Buclin and Rapin1999; Charles et al., Reference Charles, Reymond and Israel2008; Gamborg et al., Reference Gamborg, Riis and Christrup2013; Hui et al., Reference Hui, Xu and Frisbee-Hume2014, Reference Hui, Kilgore and Frisbee-Hume2016a, Reference Hui, Kilgore and Park2016b, Reference Hui, Kilgore and Frisbee-Hume2017, Reference Hui, Hernandez and Larsson2019; Pinna et al., Reference Pinna, Bruera and Moralo2015; Simon et al., Reference Simon, Kloke and Alt-Epping2016; Yamaguchi et al., Reference Yamaguchi, Matsuda and Matsuoka2018) were included in this review. Eight articles (Allard et al., Reference Allard, Lamontagne and Bernard1999; Stone et al., Reference Stone, Rix and Kurowska2002; Bruera et al., Reference Bruera, Sala and Spruyt2005; Navigante et al., Reference Navigante, Cerchietti and Castro2006, Reference Navigante, Castro and Cerchietti2010; Wilcock et al., Reference Wilcock, Walton and Manderson2008; Peoples et al., Reference Peoples, Bushunow and Garland2016; Aabom et al., Reference Aabom, Laier and Christensen2020) were excluded on the basis of insufficient data for analysis (Figure 1).

Fig. 1. PRISMA flow diagram.
Study demographics are presented in Table 1. Two studies were open-label, while all others were double-blind studies. Five studies employed a crossover design. Five studies enrolled patients with any type and stage of cancer, five others enrolled only patients with advanced or incurable cancer, and two reported only on patients with lung cancer. Over half of the articles had an overall low risk of bias (Figure 2).

Fig. 2. Risk of bias assessment (a) summary and (b) by study.
Table 1. Study demographics

Legend: NR, not reported; NRS, numeric rating scale; VAS, visual analog scale.
Prophylaxis for exertional dyspnea
Six studies (Hui et al., Reference Hui, Xu and Frisbee-Hume2014, Reference Hui, Kilgore and Frisbee-Hume2016a, Reference Hui, Kilgore and Park2016b, Reference Hui, Kilgore and Frisbee-Hume2017, Reference Hui, Hernandez and Larsson2019; Pinna et al., Reference Pinna, Bruera and Moralo2015) reported on the prophylaxis for exertional dyspnea. Three studies (Pinna et al., Reference Pinna, Bruera and Moralo2015; Hui et al., Reference Hui, Kilgore and Park2016b, Reference Hui, Kilgore and Frisbee-Hume2017) compared rapid onset fentanyl relative to placebo, whereas one study each reported on parenteral fentanyl relative to placebo (Hui et al., Reference Hui, Xu and Frisbee-Hume2014), dexamethasone relative to placebo (Hui et al., Reference Hui, Kilgore and Frisbee-Hume2016a), and high-dose rapid onset fentanyl relative to rapid onset fentanyl (Hui et al., Reference Hui, Hernandez and Larsson2019) (Appendix 2a).
Rapid onset fentanyl had similar prophylactic effects on dyspnea compared with placebo (SMD 0.179; 95% CI: −0.495 to 0.853). Individual studies by Hui et al. in 2014 (Hui et al., Reference Hui, Xu and Frisbee-Hume2014), 2016 (Hui et al., Reference Hui, Kilgore and Frisbee-Hume2016a), and 2019 (Hui et al., Reference Hui, Hernandez and Larsson2019) reported no difference between parenteral fentanyl to placebo, dexamethasone to placebo, and high-dose rapid onset fentanyl compared with rapid onset fentanyl, respectively (Figure 3a).
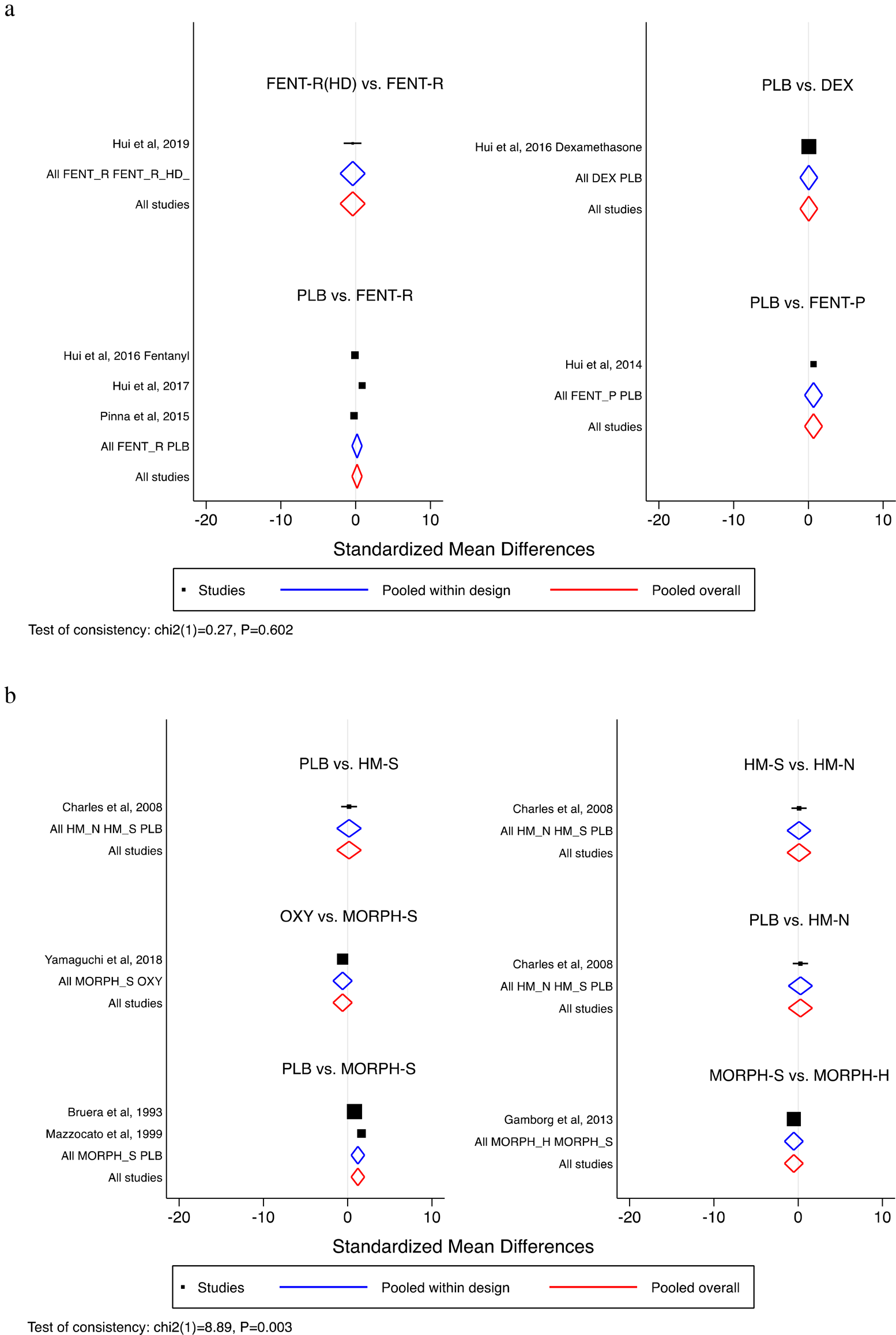
Fig. 3. Comparative efficacy of pharmacologic intervention. By study (a) prophylaxis for exertional dyspnea and (b) treatment of everyday dyspnea. Legend: DEX, dexamethasone; FENT-P, fentanyl, parenteral; FENT-R, fentanyl, rapid onset; FENT-R(HD), fentanyl, rapid onset, high dose; HM-N, hydromorphone, nebulized; HM-S, hydromorphone, systemic; MORPH-H, morphine, hydrochlorate; MORPH-S, morphine, sulfate; OXY, oxycodone, oral; PLB, placebo.
Treatment of everyday dyspnea
Five studies (Bruera et al., Reference Bruera, MacEachern and Ripamonti1993; Mazzocato et al., Reference Mazzocato, Buclin and Rapin1999; Charles et al., Reference Charles, Reymond and Israel2008; Gamborg et al., Reference Gamborg, Riis and Christrup2013; Yamaguchi et al., Reference Yamaguchi, Matsuda and Matsuoka2018) reported on the treatment of everyday dyspnea. Morphine sulfate was compared to placebo in two studies (Bruera et al., Reference Bruera, MacEachern and Ripamonti1993; Mazzocato et al., Reference Mazzocato, Buclin and Rapin1999) and to morphine hydrochlorate in one study (Gamborg et al., Reference Gamborg, Riis and Christrup2013). Charles et al. (Reference Charles, Reymond and Israel2008) conducted a three-arm studying comparing morphine sulfate to morphine hydrochlorate to placebo. One study (Yamaguchi et al., Reference Yamaguchi, Matsuda and Matsuoka2018) compared morphine sulfate to oral oxycodone (Appendix 2b).
Morphine sulfate is better at controlling everyday dyspnea than placebo (SMD 1.210; 95% CI: 0.415–2.005). As reported by individual studies, no other significant pairwise comparisons were observed (Figure 3b).
Treatment of episodic dyspnea
One study by Simon et al. (Reference Simon, Kloke and Alt-Epping2016) reported on the treatment of exertional dyspnea. No difference was reported between rapid onset fentanyl and placebo for the treatment of exertional dyspnea.
Discussion
To our knowledge, this is the first network meta-analysis reporting on pharmacologic treatments of cancer-related dyspnea. We report on 12 studies, with a total sample size of 232 patients. Given this small sample size, and the lack of common comparisons (i.e., many pairwise comparisons are only reported in one to three studies), indirect comparisons were ill-powered or not possible. In fact, the consistency assumption is not upheld in the analysis of studies reporting on treatment of everyday dyspnea.
We report no difference between nearly all pairwise comparisons, except for morphine sulfate to placebo in the setting of treatment of everyday dyspnea. Based on the results of two studies by Bruera et al. (Reference Bruera, MacEachern and Ripamonti1993) and Mazzocato et al. (Reference Mazzocato, Buclin and Rapin1999), morphine sulfate may be superior to placebo in the treatment of everyday dyspnea. However, caution is needed when interpreting these findings, as these two studies only had a combined sample size of 17 patients; further investigation is needed to determine if this is a true effect of superiority.
The aforementioned results, however, need to be interpreted in lieu of the strength of the literature base. Although there is a generally low risk of bias across studies, 9 of 11 pairwise comparisons reported in this meta-analysis have only one study reporting on the said comparison. As well, several notable limitations exist in the literature base — many studies are heterogeneous in design and pharmacologic agents, have a small sample size that is not powered for between-group comparison, are preliminary in nature albeit showing interesting within-group effect, were single dose and/or single-center studies, and had multiple outcomes with the risk of false positives. Given these concerns with the literature base and the limited statistical power, a conventional network meta-analysis is not appropriate at this time. We, therefore, conclude that there currently is insufficient data to recommend any one treatment rather than conclude that there is no one superior treatment. This conclusion is in line with the latest clinical guidelines by the American Society of Clinical Oncology (Hui et al., Reference Hui, Bohlke and Bao2021), which reported that the strength of evidence is weak at this time. As some of these medications may have adverse effects, such as mental sedation or respiratory compromise, use in routine management should be tempered by the knowledge that the proven evidence of benefit relative to placebo is limited.
A recent review by Feliciano et al. (Reference Feliciano, Waldfogel and Sharma2021) meta-analyzed studies irrespective of the type of agent and its intent. While this may seemingly overcome the concerns around statistical power, it is important to differentiate between different intents for agents — use of opioids in the prophylaxis of exertional dyspnea is noticeably different from the use of opioids for the treatment of acute dyspnea in hospitalized patients. Combined analysis may lead to an imprecise and inaccurate effect estimate.
We, therefore, encourage further trials investigating pharmacologic interventions for cancer-related dyspnea. Specifically, more high-quality studies are needed that have a larger sample size, carefully defined roles of pharmacologic agent and patient population (i.e., separating opioids for prophylaxis of exertional dyspnea from treatment of acute dyspnea in hospitalized patients), identified patient subgroups that may be more likely to experience benefit, and multicentered in study design. Greater funding in this field will likely be needed to support the undertaking of these trials.
This study has several major limitations. As previously mentioned, limited statistical power led to no indirect comparisons. This study, therefore, adopts a network meta-analysis methodology, but it cannot deliver all the results typically associated with a network meta-analysis. Furthermore, as is the nature with systematic reviews, the strength of the review's conclusion relies on the strengths and any limitations reported within the individual studies. Given the small literature base, definitive conclusions cannot be drawn from this review at this time. This review should serve as motivation for larger trials to provide a better understanding of the efficacy of pharmacologic treatments in the setting of cancer-related dyspnea.
In summary, no conclusions can be drawn from the current limited literature base on cancer-related dyspnea. The use of pharmacologic interventions that may have important adverse effects relative to placebo should be used cautiously or within the context of a trial. Morphine sulfate may be better at controlling everyday dyspnea than placebo. Further trials are needed to report on the efficacy of pharmacologic interventions for the prophylaxis and treatment of cancer-related dyspnea.
Funding
This research was funded, in part, through the NIH/NCI Cancer Center Support Grant P30 CA008748.
Conflicts of interest
The authors declare that they have no conflict of interest.
Appendix 1. Search Strategy
PubMed (769 results)
(dyspnea[mh] OR dyspnea[tw] OR dyspnoea[tw])
AND
(cancer[sb] OR neoplasms[mh])
AND
(pharmacologic*[tw] OR pharmacologic actions[mh] OR corticosteroid*[tw] OR benzodiazepine[mh] OR benzodiazepine*[tw] OR opioid*[tw] OR analgesics, opioid[mh] OR puffer*[tw] OR atrovent[tw] OR chlorpromazine[mh] OR chlorpromazine[tw] OR phenothiazines[mh] OR drug therapy[sh])
AND
(randomized[tw] OR randomised[tw] OR randomized controlled trial[pt] OR cohort[tw] OR case-control*[tw] OR controlled clinical trial[pt])
Embase (636 results) and Cochrane (39 results)
(exp dyspnea/ or dyspnea.mp. or dyspnoea.mp.)
and
(cancer.mp. or exp malignant neoplasm/ or exp neoplasm/)
and
(pharmacologic*.mp. or exp drug mechanism/ or exp corticosteroid/ or corticosteroid*.mp. or exp benzodiazepine/ or benzodiazepine*.mp. or opioid*.mp. or exp opiate/ or puffer*.mp. or atrovent.mp. or exp chlorpromazine/ or chlorpromazine.mp. or phenothiazines.mp. or exp phenothiazine derivative/ or exp drug therapy/)
and
(exp randomized controlled trial/ or randomized.mp. or randomised.mp. or exp cohort analysis/ or exp controlled study/ or cohort.mp. or exp case control study/ or case-control*.mp. or controlled clinical trial.mp. or exp controlled clinical trial/)
Limits: Limit to Cochrane Library, and Exclude Medline journals
Appendix 2. Network Meta-Analysis Map
(a) Prophylaxis for Exertional Dyspnea

(b) Treatment of Everyday Dyspnea

Legend:
DEX, dexamethasone;
FENT-P, fentanyl, parenteral;
FENT-R, fentanyl, rapid onset;
FENT-R(HD), fentanyl, rapid onset, high dose;
HM-N, hydromorphone, nebulized;
HM-S, hydromorphone, systemic;
MORPH-H, morphine, hydrochlorate;
MORPH-S, morphine, sulfate;
OXY, oxycodone, oral;
PLB, placebo.






