Introduction
The number of hematopoietic stem cell transplants (HSCTs) performed annually has increased substantially. The Center for International Blood and Marrow Transplant Research reported that 43% of transplants performed since 1981 were conducted in the last 10 years (D'Souza and Zhu, Reference D'Souza and Zhu2016). In Mexico, the National Cancer Institute (INCan) reported 742 transplants from 1981 to 2016 (INCan, 2019).
For patients with hematologic cancers, the aim of HSCT is to control or cure the primary illness and achieve long-term quality of life (QOL) that is commensurate with general population QOL [European School of Haematology (ESH), 2008]. QOL is a dynamic, multifaceted concept related to physical, cognitive, emotional, and social functioning and well-being (Pidala et al., Reference Pidala, Anasetti and Jim2009). For this study, we define QOL as the subjective perception of physical, functional, social, and emotional well-being; such perceptions are likely to be influenced by the sociocultural context.
Achieving good QOL in patients treated by a transplant is more challenging than in patients with other type of cancer or treatment (Pidala et al., Reference Pidala, Anasetti and Jim2009; Kurosawa et al., Reference Kurosawa, Yamaguchi and Mori2015). For example, patients with acute leukemia treated only with chemotherapy reported statistically significantly better physical and social functionality in contrast with patients who received an HSCT (Kurosawa et al., Reference Kurosawa, Yamaguchi and Mori2015), probably as a result of the high doses of chemotherapy and long-term hospital stays.
Furthermore, patients may subsequently face long-time complications which could lead to low physical QOL. For example, 48–79% of patients suffer at least one nonmalignant late effect at 5 years after a transplant (Khera et al., Reference Khera, Storer and Flowers2012). Additionally, patients often present psychological reactions that interference with their emotional QOL. For example, up to half of patients experience clinical emotional distress (Trask et al., Reference Trask, Paterson and Riba2002), while 12–24% have depressive or anxious symptoms (Ranson et al., Reference Ranson, Jacobsen and Booth-Jones2006; Hoodin et al., Reference Hoodin, Zhao and Carey2013; Pillay et al., Reference Pillay, Lee and Katona2014) and 35% of survivors at 9 years report psychological or psychosomatic symptoms (Gielissen et al., Reference Gielissen, Schattenberg and Verhagen2007; Sun et al., Reference Sun, Francisco and Baker2011). As a consequence of immediate, medium-term, and long-term effects; patients who receive an HSCT show difficulties recovering the physical, emotional, social, and functional QOL levels reported before the treatment.
Because the impact of HSCT on patients’ QOL has been well documented, many hematology research groups have adopted QOL as a central treatment outcome (ESH, 2008; Janicsák et al., Reference Janicsák, Masszi and Reményi2013). However, most studies that have evaluated QOL in HSCT patients have focused on high-income Western and Asian populations (Janicsák et al., Reference Janicsák, Masszi and Reményi2013; Sun et al., Reference Sun, Kersey and Francisco2013), and few of them have included Latin American populations (Ocampo et al., Reference Ocampo, Zapata and Villa2007; Tostes dos Santos et al., Reference Tostes dos Santos, Okino and Ferreira dos Santos2011) much less Mexican populations.
One of the most widely used instruments for assessing QOL in cancer is the Functionality Assessment of Cancer Therapy (FACT) (Cella et al., Reference Cella, Tulsky and Gray1993; Cella and Bomani, Reference Cella and Bomani1995), which includes an HSCT specific scale [FACT-“Bone Marrow Transplantation (BMT)”] that has been validated in patients in different HSCT phases (candidate, hospitalized, and follow-up) (McQuellon et al., Reference McQuellon, Russell and Cella1997). The original FACT-BMT questionnaire was conducted with a sample from the USA (McQuellon et al., Reference McQuellon, Russell and Cella1997), although the instrument has been translated and validated in Chinese (Lau et al., Reference Lau, Chang and Tai2002), Korean (Yoo et al., Reference Yoo, Lee and Lee2006), and Brazilian (Mastropietro et al., Reference Mastropietro, Arantes de Oliveira and dos Santos2007) samples. These validations have demonstrated that the FACT-BMT versions have adequate internal consistency, acceptable concurrent validity (McQuellon et al., Reference McQuellon, Russell and Cella1997; Lau et al., Reference Lau, Chang and Tai2002; Yoo et al., Reference Yoo, Lee and Lee2006), and good sensitivity (McQuellon et al., Reference McQuellon, Russell and Cella1997; Yoo et al., Reference Yoo, Lee and Lee2006; Mastropietro et al., Reference Mastropietro, Arantes de Oliveira and dos Santos2007).
FACT-BMT could be a useful tool for Mexican healthcare professionals to evaluate QOL in HSCT patients, but there has been no study of which we are aware that has validated this instrument for the Mexican population. The Functional Assessment of Chronic Illness Therapy organization (FACIT) translated into Spanish the FACT-BMT using a universal language approach that involved individual translators from multiple Spanish-speaking nations creating a single Spanish language translation. Although this is a well-established methodology (Eremenco et al., Reference Eremenco, Cella and Arnold2005), Mexican patients (as well as patients from other Spanish-speaking countries) use linguistic regionalisms that might impact understanding and interpretation of items. Therefore, in this study, we adapted linguistically this prior Spanish language version (the FACT-BMT Spanish Version 4) and evaluated its validity for the Mexican population. This study was approved by the ethics and research committees at the INCan.
Methods
Pilot and adaptation
The FACT-BMT Spanish version was pilot tested on 15 patients recruited at a single public hospital in Mexico City. Patients were at different stages of HSCT (2 in pre-transplant evaluations, 3 in hospitalizations for an HSCT, and 10 in follow-up medical consultations). The average age of pilot participants was 34.8 (SD = 13.14); 9(60.0%) participants were male; 4(26.7%) were single; 3(20.0%) were divorced; and 8(53.3%) were married. With regards to the diagnosis, 5(33.3%) participants had acute leukemia (AL), 4(26.7%) had Hodgkin's lymphoma (HL), 3(20.0%) had chronic leukemia (CL), 2(13.3%) had non-Hodgkin's lymphoma (NHL), and 1(6.7%) had aplastic anemia (AA). Finally, 9(60.0%) received an Allogeneic HSCT and 6(40.0%) an Autologous HSCT.
In order to evaluate the patients’ comprehension of each FACT-BMT item, they completed the questionnaire and were asked about its difficulty, clarity and use of complex, or potentially offensive words in each item. When at least 20% of the participants indicated difficulty in understanding an item, we asked the author of the original English version instrument to authorize a suggestion of linguistic adaptation. Subsequent instrument applications and psychometric analyses were performed with a new version of the FACT-BMT Spanish version that includes the changes proposed in this phase.
Validation
Sample
To estimate the psychometric properties of the FACT-BMT, this instrument and the European Organization for the Research and Treatment of Cancer's Quality of Life Questionnaire (EORTC QLQ-C30) (Cella and Bomani, Reference Cella and Bomani1995) were administered to 139 patients recruited at a single public hospital in Mexico City. The sample size was calculated based on two criteria: (a) sample sizes of previous studies (between 30 and 182 participants) (Cella et al., Reference Cella, Tulsky and Gray1993; McQuellon et al., Reference McQuellon, Russell and Cella1997; Lau et al., Reference Lau, Chang and Tai2002; Yoo et al., Reference Yoo, Lee and Lee2006; Mastropietro et al., Reference Mastropietro, Arantes de Oliveira and dos Santos2007) and (b) Hair's criteria (1998), which proposes a minimum of five participants per item; because the FACT has 27 items, we needed a minimum of 135 participants for the analysis. Previous studies have compared the psychometric properties of the instrument during hospitalization, at hospital discharge, and at 100-day follow-up; and while they have found mean differences in QOL among these three moments, the scale internal consistency and validity remained stable (McQuellon et al., Reference McQuellon, Russell and Cella1997; Tostes dos Santos et al., Reference Tostes dos Santos, Okino and Ferreira dos Santos2011). Therefore, we chose to enroll patients in these three different phases of HSCT and compare instrument performance between each phase.
During the participant enrollment process, a psychologist reviewed patient medical records and identified patients who met the inclusion criteria (hemato-oncological patients aged 18 year or older who received or were scheduled to receive HSCT). Potential participants were recruited in the Bone Marrow Transplantation Unit (BMTU) or before their medical consultation. The psychologist introduced herself, explained the study's objective and what participation in the study would involve if he or she decided to participate. Patients who agreed to participate provided verbal informed consent and responded to the self-administered instruments. According to the hospital's human subjects research and ethics committees, this study was determined to be a low risk, and verbal consent was considered to be sufficient. Upon obtaining consent, the psychologist reviewed patients’ clinical information based on a form developed for this study that included diagnoses, clinical response, and type of transplant.
The 139 patients were enrolled between June 2016 and July 2017. One patient declined to enroll because she did not have time. Of the included participants, 53(37.3%) were enrolled during their pre-transplant evaluations, 29(20.4%) while they were in the BMTU and 60(42.3%) during their first 5 years of follow-up medical consultations. The final results were shared with the FACIT organization professionals, and they agreed with the publication of this report (Table 1).
Table 1. Socio-demographic characteristics of patients included in the validation phase (n = 139)

HSCT, Hematopoietic stem cell transplantation.
Measurement
Two instruments were used in this study. The first one was the FACT-BMT Spanish version which is composed of 27 items grouped into five domains: (a) Physical well-being (PWB) (7 items); (b) Functional well-being (FWB) (7 items); (c) Emotional well-being (EWB) (6 items); (d) Social and family well-being (SWB) (7 items) (Cella et al., Reference Cella, Tulsky and Gray1993; Cella and Bomani, Reference Cella and Bomani1995); and (e) BMT specific well-being scale that evaluates HSCT side effects (23 items but only 10 are considered in the score). This instrument uses a 5-point Likert scale in which higher scale scores mean better QOL. FACT-BMT has been validated in patients treated with different type of transplant, from different countries and in different HSCT phases with a mean internal consistency of α = 0.89, acceptable concurrent validity (McQuellon et al., Reference McQuellon, Russell and Cella1997; Lau et al., Reference Lau, Chang and Tai2002; Yoo et al., Reference Yoo, Lee and Lee2006), and good sensitivity (McQuellon et al., Reference McQuellon, Russell and Cella1997; Yoo et al., Reference Yoo, Lee and Lee2006; Mastropietro et al., Reference Mastropietro, Arantes de Oliveira and dos Santos2007).
The second instrument was administered in order to test for concurrent validity was the EORTC QOL-C30, which includes 30 items evaluating five functional scales (physical, cognitive, emotional, social, and role) and nine symptoms scale (fatigue, nausea and vomiting, pain, dyspnea, insomnia, appetite loss, constipation, diarrhea, financial difficulties) (Fayers et al., Reference Fayers and Bottomley2002). The EORTC QOL-C30 includes 28 items which use a 4-point Likert scale and 2 items with a 7-point Likert scale; higher scores indicating better QOL. While this instrument measures QOL in cancer patients, it is not specific to those undergoing HSCT. The EORTC QLQ-C30 has shown adequate internal consistency higher α ≥ 0.7 in each subscale and good convergent and discriminant validity in the Mexican population (Oñate-Ocaña et al., Reference Oñate-Ocaña, Alcántara-Pilar and Vilar-Compte2008).
Statistical analysis
First, each item's frequency was calculated to determine the distribution across response options. Second, we scored the total FACT-BMT and created two extreme groups according to the first and fourth quartiles of the total score. To identify each item's discrimination (p < 0.05), we compared these two groups’ scores using a Student's t-test. Items that did not discriminate were eliminated. Third, a cross-table analysis was performed to establish the correlation between items and to determine the type of exploratory factor analysis (EFA). The criteria to retain an item were (a) a factor loading >0.40 and (b) items grouped into a latent variable. When at least three items resulted were grouped, they were considered a factor; and when one or two items were grouped, they were considered an indicator. Finally, Cronbach's alpha of the total scale and subscales were computed to determine the instrument's internal consistency. Statistical analyses were performed through the SPSS v.21 software package.
Confirmatory factor analyses (CFA) were performed in order to test the factor structures shown in the EFA. Latent variables’ variance loadings were set at 1.0. Variances of error terms were specified as free parameters, and maximum likelihood estimation methods were used. Three criteria were considered to determine if a factor structure showed good fit: (a) standardized root mean square residual (SRMR) ≤0.08, (b) root mean square error of approximation (RMSEA) ≤0.06, and (c) comparative fit index (CFI) and Tucker Lewis Index (TLI) values from 0.90 to 0.95. A model showed an adequate fit when (a) RMSEA <0.08 or (b) CFI and TLI were close to 0.90. A model showed a poor fit when (a) RMSEA ranged from 0.08 to 0.10 or (b) CFI and TLI were far from 0.90 (Brown, Reference Brown2006).
In order to compare the psychometric properties of the adapted FACT-BMT Spanish version in Mexican patients with those obtained in different countries and to determine its usefulness, three analyses were performed: (a) a correlation between the FACT-BMT scale and the EORTC scale was estimated to determine the concurrent validity of the former, (b) a confirmatory factor analysis was performed to determine the FACT-BMT's construct validity, and (c) the instrument's sensitivity was determined comparing the FACT-BMT scores between patients at different moments of treatment with a Kruskal–Wallis (p < 0.05) and by the type of HSCT (AuHSCT vs. AlloHSCT) with a Student's t-test (p < 0.05). These analyses were conducted with the original FACT-BMT structure because it was the version used in all the previous validations.
Results
Pilot and adaptation
Of the items on the FACT scale, 17(63.0%) were reported to be clear, easy, and did not use difficult words; 7(25.9%) were difficult for one participant, and 3(11.1%) were unclear for two participants, so none were adapted or excluded.
Of the items on the BMT scale, 15(62.2%) were clear for all participants, 6(26.1%) were unclear or difficult for one participant, 1(4.4%) was unclear for two subjects, and 1(4.4%) was unclear for 4(26.67%) participants. Consequently, BMT item 16 (“I have trouble with my bowels”) which was difficult for more than 20% of participants was changed from “Tengo problemas con mis deposiciones intestinales” to “Tengo problemas con mis deposiciones (evacuaciones) intestinales” with approval from the original author. The other 22 items were not edited or excluded.
Validation
The response options were adequately distributed in 58% of the items (i.e., each response option had <50% of participants’ responses). However, 14% of the items showed asymmetry or kurtosis >2 indicating the tendency to respond near the extremes.
The instrument was scored with all the FACT-BMT items. The total mean score was 145.35(SD = 17); the maximum value of the lower quartile was 136; and the minimum value of the upper quartile was 157. The global score showed a normal distribution (Kolmogorov–Smirnov = 0.772, p = 0.591), so a Student's t-test was performed to evaluate discrimination between extreme groups. All items of the FACT-BMT scale considered in the scoring manual showed adequate discrimination capacity (p < 0.05). However, the following three items from the BMT subscale did not discriminate between extreme groups, so they were eliminated for subsequent analyses: BMT7 “I have concerns about my ability to have children,” BMT9 “I regret having the bone marrow transplant,” and BMT11 “I have frequent colds/infections.”
In order to determine each item's contribution to the instrument's internal consistency, a Cronbach's alpha was computed. The analysis indicated adequate internal consistency (α = 0.906), and the elimination of any item would not significantly increase the instrument reliability.
The cross-table analysis among items showed that 45% of the possible crosses were not statistically significant (p > 0.05), 52% had low correlation (r < 0.50, p < 0.05), and 3% showed moderate correlations (r > 0.50 to <0.80, p < 0.05). Therefore, we performed EFA with varimax rotation to determine the instrument construct validity. Three independent EFAs were performed: (a) one with the total FACT items, (b) one with the 10 items from the BMT subscale considered in the scoring manual, and (c) one with all the items from the BMT subscale that showed the capacity to discriminate.
The general FACT analysis resulted in a Kaiser Meyer Olkin Test (KMO) = 0.744 and a Bartlett sphericity test (χ 2 = 1216.77, df = 351, p < 0.001). This analysis showed a factor structure with four factors and four indicators that explained 70.84% of the variance. Two items were eliminated: (a) GF4 “I have accepted my illness” because the factor loading was <0.40 for any factor and (b) GE3 “I am satisfied with how I am coping with my illness” because it loaded on a factor whose content was from a different subscale. The FACT's overall internal consistency was α = 0.867. Less than half of the patients were married, so the internal consistency of the SWB domain which includes items regarding partner/couple satisfaction might be impacted for this reason (Table 2). CFA indicated a good model fit when item GE3 was eliminated (χ 2 = 250.39, Cmin/df = 1.108, p < 0.001; RMSEA = 0.028, 90% CI, 0.000–0.046; RMR = 0.148; CFI = 0.865; TLI = 0.835).
Table 2. Factor analysis of the adapted version of the FACT
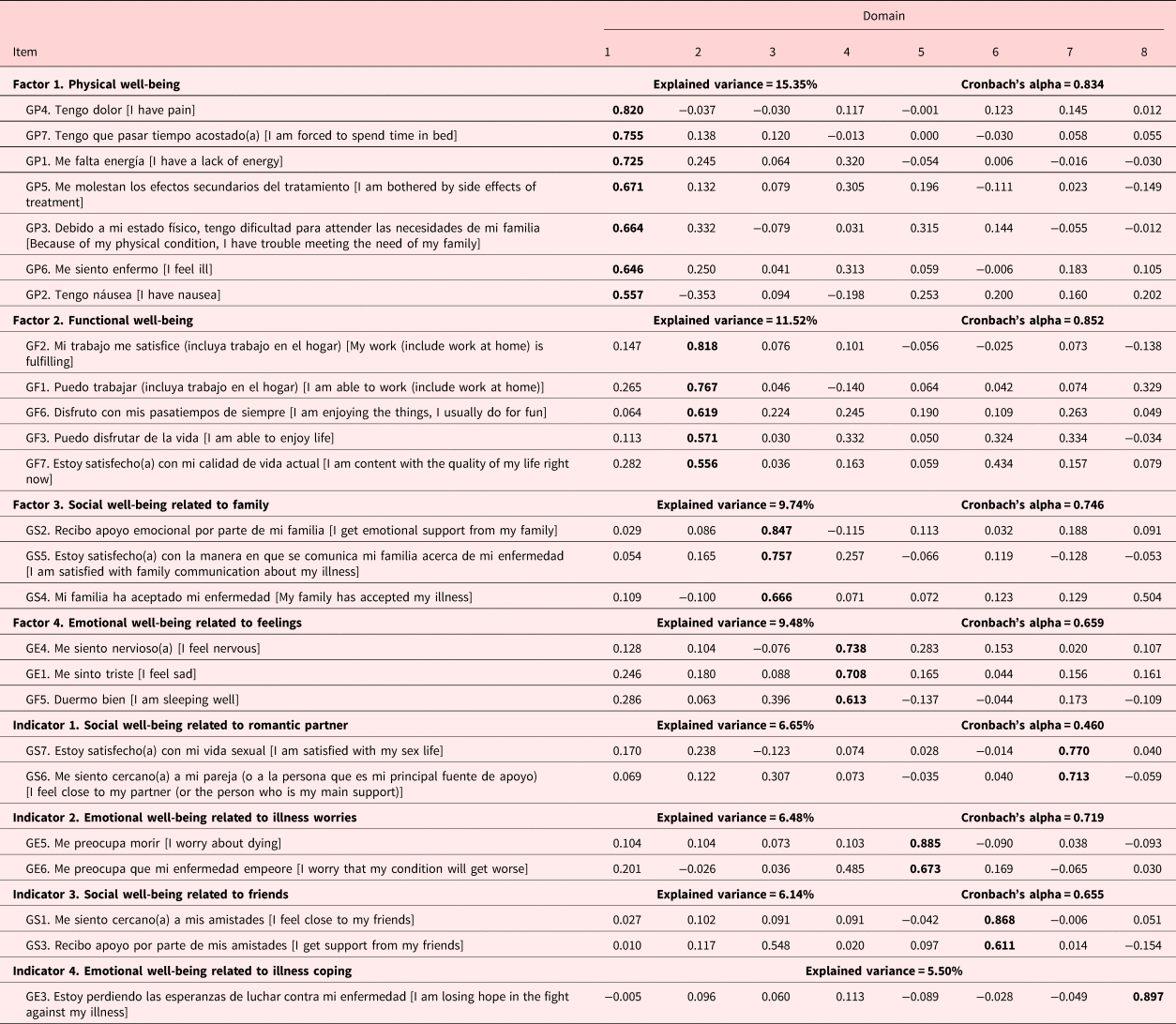
The EFA of the BMT subscale (10 items) produced a KMO = 0.734 and a Bartlett sphericity test (χ 2 = 246.97, df = 45, p < 0.001). The factor structure explained 55.08% of the variance in two factors and one indicator. The internal consistency of this subscale was α = 0.703 (Table 3). CFA indicated a good model fit when item BMT8 was eliminated (χ 2 = 33.85, Cmin/df = 1.410, p < 0.087; RMSEA = 0.055, 90% CI, 0.000–0.094; RMR = 0.097; CFI = 0.952; TLI = 0.927).
Table 3. Factor analysis of the BMT scale with the 10 items considered in the scoring manual

The EFA with the 23 BMT subscale items produced a KMO = 0.730 and a Bartlett sphericity test (χ 2 = 497.55, df = 136, p < 0.001). The analysis showed a factor structure with four factors and two indicators that explained 61.65% of the variance. The internal consistency of this subscale was α = 0.769 (Table 4). CFA indicated a good model fit when item BMT8 was eliminated (χ 2 = 149.48, Cmin/df = 1.451, p < 0.002; RMSEA = 0.057, 90% CI, 0.035–0.076; RMR = 0.080; CFI = 0.880; TLI = 0.841).
Table 4. Factor analysis of the BMT subscale with all the items

We explored the factor structure of the original FACT-BMT items in order to compare with other studies. CFA of the original FACT scale indicated an acceptable model fit (χ 2 = 432.69, Cmin/df = 1.433, p < 0.001; RMSEA = 0.05, 90% CI, 0.044–0.068; RMR = 0.106; CFI = 0.897; TLI = 0.881). CFA of the BMT scale (10 items) indicated a good model fit (χ 2 = 45.413, Cmin/df = 1.514, p = 0.035; RMSEA = 0.06, 90% CI, 0.017–0.095; RMR = 0.09; CFI = 0.926; TLI = 0.890). Figure 1 compares the original FACT-BMT structure with the factor structure identified in this study. The Mexican structure has subscales that can more specifically assess SWB, EWB, and BMT into further subdimensions.

Fig. 1. Confirmatory factor analysis.
To compare our results with the values found in previous studies, the internal consistency of the original FACT-BMT subscales was estimated (Table 5). The subscales’ internal consistency was within the range of other studies, and in all the subscales, the internal consistency was adequate.
Table 5. Internal consistency (Cronbach's alpha coefficients) of the FACT-BMT subscales for the validation samples and values reported in previous studies

PWB, Physical well-being; RWB, Relation with the doctor well-being; SWB, Social well-being; EWB, Emotional well-being; FWB, Functional well-being; BMTS, Bone marrow transplant subscale; FACT-G, Functional assessment of cancer therapy-general (PWB + FWB + EWB + SWB); FACT-BMT, Functional assessment of cancer therapy-bone marrow transplant (FACT-G + BMTS); TOI, Trial Outcomes Index (PWB + FWB + 10-items BMTS).
1McQuellon et al., Reference McQuellon, Russell and Cella1997; 2Lau et al., Reference Lau, Chang and Tai2002; 3Yoo et al., Reference Yoo, Lee and Lee2006; 4Mastropietro et al., Reference Mastropietro, Arantes de Oliveira and dos Santos2007.
a Version 4.
b Version 3.
Construct validity was evaluated by FACT-BMT's inter-scale correlation; almost all scales correlated (Table 5). The concurrent validity was estimated by a correlation among the FACT-BMT domains and the EORTC-QLQ domains; 85.71% of the possible crosses reported significant correlations (p < 0.05), and most of them were from moderate to high correlations (r > 0.50). These results are similar to the FACT-BMT's concurrent validity demonstrated in other studies (Table 6). Multiple correlations between the FACT-BMT and other scales indicated a high construct validity of the FACT-BMT.
Table 6. Construct and concurrent validity coefficients of the FACT-BMT showed in different studies

PWB, Physical well-being; SWB, Social well-being; EWB, Emotional well-being; FWB, Functional well-being; BMTS, Bone marrow transplant subscale; TOI, Trial Outcome Index (PWB + FWB + 10-items BMTS); FACT-G, Functional assessment of cancer therapy-general (PWB + FWB + EWB + SWB); FACT-BMT, Functional assessment of cancer therapy-bone marrow transplant (FACT-G + BMTS); RP, Role limitations due to physical health problems; GH, General health; RE, Role limitations due to emotional problems.
* p < 0.05; **p < 0.01.
Significant differences were found in PWB scores among patients in different stages of treatment with better scores in follow-up patients. Additionally, most of the FACT-BMT scores revealed a tendency to increase with stage of treatment (Table 7). Significant differences were found in FWB, FACT, and FACT-BMT scores between patients treated by an AuHSCT vs. an AlloHSCT with higher levels of QOL in the AlloHSCT group (Table 8). This indicates the instrument's sensitivity.
Table 7. Differences in FACT-BMT scores among patients in different phases of an HSCT: results of Kruskal–Wallis tests

PWB, Physical well-being; SWB, Social well-being; EWB, Emotional well-being; FWB, Functional well-being; BMTS, Bone marrow transplant subscale; TOI, Trial Outcome Index (PWB + FWB + 10-items BMTS); FACT-G, Functional assessment of cancer therapy-general (PWB + FWB + EWB + SWB); FACT-BMT, Functional assessment of cancer therapy-bone marrow transplant (FACT-G + BMTS).
Table 8. Differences in FACT-BMT scores between patients treated by autologous versus allogeneic HSCT: results of Student's t-tests

PWB, Physical well-being; SWB, Social well-being; EWB, Emotional well-being; FWB, Functional well-being; BMTS, Bone marrow transplant subscale; TOI, Trial Outcome Index (PWB + FWB + 10-items BMTS); FACT-G, Functional assessment of cancer therapy-general (PWB + FWB + EWB + SWB); FACT-BMT, Functional assessment of cancer therapy-bone marrow transplant (FACT-G + BMTS).
Discussion
The objective of the present study was to adapt and validate the Spanish language FACT-BMT questionnaire for the Mexican population. The linguistic adaptation process showed that only change in one item was necessary to improve participants’ understanding of the FACT-BMT items.
The FACT-BMT EFA suggests a little different structure than the original instrument. The SWB factor was divided into three subscales that represent different support sources; there is evidence that each one of these support sources provides separate satisfaction levels and accomplish diverse objectives (Polizzi and Arias, Reference Polizzi and Arias2014). This is a key finding because the prior literature has shown Mexican cancer patients experience different expectations of their family, friends, and partners that might impact their social support perceptions. For example, patients expect “personalismo” from health professionals which is characterized by a warm, personal, and empathetic way of relating to others; on the other hand, expect “familismo” from their family which is a strong identification and attachment (Lopez-Class et al., Reference Lopez-Class, Perret-Gentil and Kreling2011). So, the opportunity to identify different support sources may be helpful for a more specific and relevant patient evaluation.
Five items of the FWB factor were preserved from the original domain, as well as those evaluating patients’ capacity to enjoy their work and hobbies and their QOL satisfaction. Additionally, item GF5 “Duermo bien” (I sleep well) loaded with two EWB items (GE4 “Me siento nervioso” (I feel nervous) and GE1 “Me siento triste” (I feel sad). According to the Diagnostic and Statistical Manual of Mental Disorders (DSM 5), sleep disturbance is a criterion of mood and anxiety disorders [American Psychiatric Association (APA), 2014]; in addition, there is evidence that HSCT patients who reported greater depression and anxiety suffer poorer sleep quality (Nelson et al., Reference Nelson, Coe and Juckett2014). Our Mexican patients that experience sleep problems may be better understood by their emotional concerns than by their physical discomfort. As many as 80% of patients hospitalized for an HSCT report difficulty maintaining sleep. Sleep deprivation could increase the probability of cognitive disorder and maladaptive behaviors like spending prolonged time in bed (Jim et al., Reference Jim, Evans and Jeong2014); therefore, the presence of these symptoms could magnify the psychological impact and, for these reasons, be a useful indicator of the patients’ emotional status.
The EWB subscale represents emotional well-being related to illness concerns and is composed of items GE5 and GE6. This subscale evaluates one of the most intense and frequent concerns of HSCT patients, termed “shadow of death.” This phenomenon was previously described as patients’ thoughts of the continuous risk and threats posed by either the illness or the treatment's side effects (Zamanzadeh et al., Reference Zamanzadeh, Valizadeh and Sayadi2013). The literature indicates that the proportion of patients who require mental health services increases 23% after HSCT (Hoodin et al., Reference Hoodin, Zhao and Carey2013) and is frequently associated with physical concerns and fear or relapse, so items GE5 and GE6 could be a good indicator for evaluating this phenomenon in follow-up patients.
The BMT scale with the 10 items included in the scoring manual explained an adequate proportion of the variance, but the inclusion of seven “other worries” items increased explained variance by 6%. Items added were grouped in two indicators: (a) concerns related to transplant or illness requirements, this factor included worries about money and personal hardship for the close family. There are frequent problems for patients enrolled in the study because they do not have health insurance, so they and their family have to pay for treatment; and (b) side-effects discomfort, which evaluates acute chemotherapy side effects. Chemotherapy adverse effects have been related to nonadherence to treatment in patients with oral chemotherapy (Krikorian et al., Reference Krikorian, Pories and Tatatonis2019) and patients who need chronic treatment (Kleinsinger, Reference Kleinsinger2018). These two factors yield essential information to understand patients’ context, concerns, and physical condition, so we decided to include them in an additional score for the Mexican population.
On the other hand, the CFA and the reliability analysis showed that the original FACT-BMT structure is adequate for the Mexican population. The internal consistency of the EWB subscale was lower than other subscales, this could be because this is a more subjective construct, so it is more difficult to evaluate. The total scale had high internal consistency (α = 0.90), similar to those reported in other studies which have ranged from α = 0.85 to α = 0.92 (McQuellon et al., Reference McQuellon, Russell and Cella1997; Lau et al., Reference Lau, Chang and Tai2002; Yoo et al., Reference Yoo, Lee and Lee2006; Mastropietro et al., Reference Mastropietro, Arantes de Oliveira and dos Santos2007) indicating a robust and reliable instrument. We found evidence of concurrent validity comparing the FACT-BMT to the EORTC-QLQ similar to a study with a Brazilian sample that found cross-correlations in all the possible crosses between the FACT-BMT and the SF-36 (Mastropietro et al., Reference Mastropietro, Arantes de Oliveira and dos Santos2007).
The global psychometric properties observed suggest that the FACT-BMT is a valid instrument, which can be used to evaluate QOL in Mexican patients undergoing HSCT. The instrument validation also provides the opportunity to compare Mexican patients with other samples (McQuellon et al., Reference McQuellon, Russell and Cella1997; Lau et al., Reference Lau, Chang and Tai2002; Yoo et al., Reference Yoo, Lee and Lee2006; Mastropietro et al., Reference Mastropietro, Arantes de Oliveira and dos Santos2007).
The present study evaluated the instrument's basic psychometric properties, and it was carried out with a non-probabilistic sample; thus, it does not guarantee the generalization of results to all Mexicans patients. Additionally, while methodological precautions during the adaptation and validation procedures sought to include widely understood wording, further studies to evaluate other psychometric characteristics (such as temporal stability and sensitivity to changes after specific interventions are needed). Specifically about the instrument, in this and other studies, the EWB factor showed lower internal consistency in contrast with the other subscales; therefore, we consider this factor could be improved with the addition of more items for specific psychological symptoms for this population like loneliness or hopelessness feeling. In relation of the Mexican population, patients enrolled in this study showed lower educational in contrast with other studies samples. However, this socio-demographic characteristic did not have impact on patients’ comprehension of FACT-BMT neither in the instrument's validity. Nonetheless, further studies should evaluate how specific clinical and socio-demographic variables in the Mexican population could impact on patients’ QOL.
Despite these limitations, the study provides empirical evidence supporting the use of this instrument in clinical and research contexts, which could contribute to understanding factors that influence individuals’ experience with cancer and the HSCT procedure (Cella et al., Reference Cella, Tulsky and Gray1993; Imataki et al., Reference Imataki, Nakajima and Inoue2010; El-Jawahri et al., Reference El-Jawahri, Vandusen and Traeger2016). A clearer understanding of the patient's QOL can guide the development of specialized psychological interventions to promote and improve patients’ well-being.
Acknowledgment
This research report derived from the PhD thesis of the first author under supervision of the last author.












