Published online by Cambridge University Press: 01 March 2004
Objectives: To validate the QUAL-E, a new measure of quality of life at the end of life.
Methods: We conducted a cross-sectional study to assess the instrument's psychometric properties, including the QUAL-E's associations with existing measures, evaluation of robustness across diverse sample groups, and stability over time. The study was conducted at the VA and Duke University Medical Centers, Durham, North Carolina, in 248 patients with stage IV cancer, congestive heart failure with ejection fraction ≤20%, chronic obstructive pulmonary disease with FEV1 ≤ 1.0 l, or dialysis-dependent end stage renal disease. The main outcome measures included QUAL-E and five comparison measures: FACIT quality of life measure, Missoula-VITAS Quality of Life Index, FACIT-SP spirituality measures, Participatory Decision Making Scale (MOS), and Duke EPESE social support scales.
Results: QUAL-E analyses confirmed a four-domain structure (25 items): life completion (α = 0.80), symptoms impact (α = 0.87), relationship with health care provider (α = 0.71), and preparation for end of life (α = 0.68). Convergent and discriminant validity were demonstrated with multiple comparison measures. Test–retest reliability assessment showed stable scores over a 1-week period.
Significance of results: The QUAL-E, a brief measure of quality of life at the end of life, demonstrates acceptable validity and reliability, is easy to administer, performs consistently across diverse demographic and disease groups, and is acceptable to seriously ill patients. It is offered as a new instrument to assist in the evaluation of the quality and effectiveness of interventions targeting improved care at the end of life.
Over the last decade, public and private organizations have devoted millions of dollars to fund research, education, and clinical interventions aimed at improving the experience of patients at the end of life (Council on Scientific Affairs, 1996; Field & Cassel, 1997; Gibson, 1998). The future success of these efforts depends, in part, on our ability to measure their effectiveness using appropriate and well-validated assessment tools. In fact, the Institute of Medicine's 2003 report, “Describing Death in America: What We Need to Know” argues that quality measures are an essential component in the quest for public accountability, internal quality improvements, and research evaluating the effectiveness of interventions aimed at improving outcomes for dying patients and their families (Lunney et al., 2003).
Adequate assessment is possible only when measurement tools match both the goals of palliative and end-of-life therapies and the needs of dying patients. Furthermore, one must specify the outcome of interest and select a suitably designed tool; for example, instrument developers tend to focus on measuring either of quality of care, quality of death and dying, or quality of life at the end of life. Each appraises a distinct aspect of end-of-life experience. For the purposes of this study, we seek to explore patients' direct subjective experience and, therefore, present a measure of the latter, quality of life at the end of life.
Measuring quality of life at the end of life presents a unique challenge to investigators because physical and functional decline are expected, and the goals of care are frequently different than with reversible, or even chronic, illness (Lev, 1991; Cohen & Mount, 1992; Byock, 1995; Cohen et al., 1995; Greisinger et al., 1997; Lynn, 1997; Byock & Merriman, 1998; Stewart et al., 1999; Waldron et al., 1999; Steinhauser et al., 2000a, 2000b; Cohen & Leis, 2002; Curtis et al., 2002). Scales developed for chronic but non-life-threatening conditions often are inadequate in capturing the unique experiences of dying patients.
In recent years, several quality of life measures have been developed specifically for use at the end of life, including the McGill Quality of Life Questionnaire, the Missoula-VITAS Quality of Life Index, McMaster Quality of Life Scale, the adapted EORTC QLQ30, the FACIT-Pal, and the SEIQoL (Cohen & Mount, 1992; Aaronson et al., 1993; Cohen et al., 1995; O'Boyle et al., 1995; Teno & Landrum, 1996; Byock & Merriman, 1998). They depart from traditional quality of life instruments by reducing the emphasis on physical functioning and including spiritual and transcendent concerns, allowing the opportunity for growth at the end of life and accommodating individual definitions of quality of life. The designers of the first generation of instruments reconceptualized measurement of quality of life at the end of life and provided empirical evidence that dying patients face unique challenges not captured by available measurement tools.
However, some methodological limitations remained. Many of these instruments were validated among single-disease patient populations. For example, most end-of-life scales have been developed within cancer populations whose relatively predictable disease trajectory is markedly different from the acute episodic course of advance heart or lung disease (Padilla et al., 1983; Cella et al., 1993; Ahmedzai et al., 1994; Waldron et al., 1999). Additionally, instruments often were created for use in hospice or palliative care settings, where patients usually acknowledge the terminal nature of their illness (Byock & Merriman, 1998). Yet, many terminally ill patients do not elect such care and do not perceive themselves as dying. Instruments such as the MVQOL index were developed primarily as clinical feedback instruments rather than research evaluative tools (Byock & Merriman, 1998). As a result, instrument development focused on capturing information for immediate treatment plans; however, item content did not always work well as a scale, psychometrically. Finally, scale domains of the MVQOL, for example, were based on a priori choices of developers rather than systematic empirical evidence. Analyses reveal the domains have limited psychometric support.
Our goal, therefore, was to create and validate an instrument to assess quality of life at the end of life that would accommodate patients with a variety of advanced illness trajectories (cancer, congestive heart failure, chronic obstructive pulmonary disease), function across care settings (hospice, palliative or conventional), and apply to those who may or may not define themselves as terminally ill. Of note, we originally conceived of developing a quality of dying measure but in the course of data collection, learned patients most frequently referred to this crucial time as a period of life at the end of life. Finally, we sought to develop a tool that was inductively derived, and, where possible, extend the strengths of existing instruments.
Instrument development began with the collection of qualitative accounts from bereaved family members, health care providers (nurses, social workers, chaplains, and hospice volunteers), and seriously ill patients who were asked to identify important domains of a “good death” (Steinhauser et al., 2000b). These included pain and symptom management, clear decision making, preparation, life completion, being known as a whole person, and contributing to others. These qualitative findings were confirmed in subsequent studies by Curtis and others (Steinhauser et al., 2000a; Cohen & Leis, 2002; Curtis et al., 2002). Subsequently, we distributed a survey questionnaire to national samples of the aforementioned groups to affirm or reject, quantitatively, those themes (Steinhauser et al., 2000a). A detailed description of the methods can be found elsewhere (Steinhauser et al., 2000a, 2000b).
These qualitative and quantitative data formed the foundation for the QUAL-E, a measure quality of life at the end of life (Steinhauser et al., 2002; see the appendix). In a previous study, we evaluated the QUAL-E's initial factor structure. The purpose of this study was to confirm factor structure, assess construct validity and reliability by comparing it with existing measures, evaluate its performance across diverse sample groups, and preliminarily examine its sensitivity to change.
This was a cross-sectional study to ascertain the instrument's psychometric properties.
Patients with stage IV cancer, congestive heart failure with ejection fraction ≤20%, chronic obstructive pulmonary disease with FEV1 ≤ 1.0 l, or dialysis-dependent end stage renal disease were eligible for the study. To identify potential patients for instrument validation, we reviewed weekly rosters from oncology, heart failure, pulmonary, and dialysis clinics at the Durham Veterans Affairs and Duke University Medical Centers. We randomly assigned a recruitment order to all eligible patients and enrolled as many as time allowed for each clinic half-day. Written informed consent was obtained at the time of recruitment. We administered the Short Portable Mental Health Status Questionnaire (SPMSQ) at enrollment and excluded patients with scores less than 8/10 (Pfeiffer, 1975). We continued to recruit subjects until we accrued a total of 248 patients, a number considered sufficient for factor analysis (Fleiss, 1981; Kline, 1986; Streiner & Norman, 1995).
The study was approved by the institutional review boards of both the VA and Duke University Medical Centers.
In a previous study, we administered the initial version of the instrument (54 items) to 214 patients with advanced serious illness. Results from exploratory factor analyses yielded an initial factor structure and informed item reduction. The resulting instrument included 25 items representing five domains: life completion (α = 0.84), relationship with health care provider (α = 0.77), symptom management (α = 0.77), preparation (α = 0.77), and affective social support (α = 0.60) (Steinhauser et al., 2002). Following each multi-item domain, patients were asked to evaluate its overall importance to their quality of life. For example, after completing questions about the severity, frequency interference, and worry related to their symptoms, patients rated the overall importance of physical symptoms to their quality of life. Including importance items and a single overall quality of life item resulted in a questionnaire with 31 items. The instrument exhibited strong internal consistency and was acceptable to seriously ill patients.
In the current study, we administered the reduced 31-item version of the QUAL-E and a battery of comparison measures to a second sample of seriously ill patients and conducted confirmatory factor analyses to assess structural validity. The QUAL-E was readministered one week hence to evaluate test–retest reliability. At that time, we also recorded whether individuals experienced any significant health events during the intervening week.
To assess convergent and discriminant validity of the QUAL-E subscales, we administered a series of comparison measures. We considered weak associations to be 0.1–0.3; moderate, 0.4–0.6; and strong, >0.6.
The FACIT-SP is an expanded version of the FACT-G, a 27-item four-domain quality of life scale, and an additional 12-item, spiritual well-being subscale (Brady & Cella, 1999). The FACT-G subscales include physical well-being, functional well-being, social/family well-being, and emotional well-being (Cella et al., 1993). It was not designed, specifically, for use in terminally ill populations but remains a broadly used, well-validated, and reliable general quality of life tool. The spirituality well-being subscale has been validated in patients with cancer and HIV. We compare the full scale broadly with the QUAL-E and expected strong and moderate associations, respectively, between the spiritual well-being subscale and the QUAL-E's completion and preparation subscales. Because the FACIT-SP was not designed specifically for the end of life we expected moderate associations with QUAL-E subscales in the overall comparison.
The Missoula-VITAS Quality of Life Index is a 15-item measure specifically designed to assess quality of life of dying patients, whose five domains include transcendence, function, symptoms, emotional well-being, and interpersonal concerns (Byock & Merriman, 1998). It was developed as a clinical feedback tool for palliative care settings. This instrument was administered at retest to reduce patient burden at initial instrument administration. We compared it broadly to the QUAL-E, and due to the MVQOL's clinical versus psychometric focus, expected moderate associations across similar domains.
Three items, developed for the Medical Outcomes Study, ask patients to rate how much control they feel in decision making, how much involvement, and the extent to which their physician asks them to take responsibility for their own health. We expected a moderate correlation between the first two PDM items and the QUAL-E healthcare subscale (Kaplan et al., 1991, 1995). The third PDM item is not covered, substantively, in the QUAL-E, and therefore, should not correlate.
We conducted a range of analyses to examine reliability and validity.
We evaluated the previously identified factor structure using maximum likelihood estimation (Joreskog & Sorbom, 1981). Though the initial factor structure included five domains, the fifth, affective social support, only included two items, which also loaded well on factor one, life completion. One goal of these analyses was to assess the fit of both five- and four-factor model solutions.
We assessed model fit using several criteria: chi-square; Bentler's comparative fit index (CFI), a robust measure based on the concentrality of the chi-square index; and the goodness of fit index (GFI), a maximum likelihood estimation of the relative amount of variance and covariance accounted for by the model (Joreskog & Sorbom, 1981; Bentler, 1990). CFI and GFI values range from 0 to 1.0, with values closest to 1.0 suggesting a better fit. Values above 0.90 are considered adequate for trait measures, such as personality or intelligence scales. With a state measure such as the QUAL-E and a highly heterogeneous sample (i.e., varying diagnoses and disease states), values approaching 0.90 indicate an adequate fit (Bentler & Bonett, 1980). Finally, the root mean square approximation of error (RMSEA) was examined as a measure of model discrepancy per degrees of freedom (Steiger & Lind, 1980). Values from 0.06 to 0.08 indicate a reasonable fit; those at 0.05 or below indicate a good fit (Browne & Cudeck, 1993).
We examined correlations among QUAL-E subscales, hypothesizing no relationships among unrelated subscales (e.g., symptoms and completion) and weak to moderate correlations (r = 0.1–0.3) among related scales or those with conceptual overlap (e.g., completion and preparation).
After the final model was obtained, reliability first was assessed with Cronbach's alpha coefficient of internal consistency. Second, we evaluated test–retest reliability using intraclass correlation coefficients, comparing scores from the first administration and those obtained one week later. Ideally, reproducibility should be assessed by serial administration of an instrument to a group of subjects believed to be stable (Tulsky, 1990). However, individuals with progressive illness nearing the end of life cannot be considered stable. Under such conditions, test–retest coefficients may be low because of true change in the patient's condition and his or her perceptions of quality, yet interpreted as unreliability of the instrument. Because the sample showed strong variation in illness status between instrument administrations, we compared correlations between patients who had reported interim illness events and those who did not. Illness events included a change in medication or treatment regimen, an emergency room visit, or hospitalization.
All analyses were conducted using SAS version 8.2.
A total of 325 potential subjects were approached. We enrolled 248 patients from the Durham VA (n = 100) and Duke University Medical Centers (n = 148). Fifty-three refused, 5 were ineligible due to phase I participation, and 19 demonstrated significant cognitive impairment, yielding a response rate of 76%. All 248 patients completed the interview. Twenty-two did not report any symptoms. As compared with the full sample, they were composed of more females (50%), slightly older (mean age 64), not married (55%), with a higher percentage of congestive heart failure (27%).
Participants had at least one of four life-threatening conditions: stage IV cancer (56%), congestive heart failure (21%), END stage renal disease (14%), and chronic obstructive pulmonary disease (8%; see Table 1). Approximately 59% of subjects were male, 59% Caucasian, and 34% African-American. The sample showed a broad educational distribution, and a majority (62%) were married. The median age of patients was 61 (range 28–88).
Sample profile (n = 248)a

Of the 31 items in the QUAL-E, 23 were appropriate for factor analysis; we excluded domain importance ratings (7) and the single-item quality of life question.
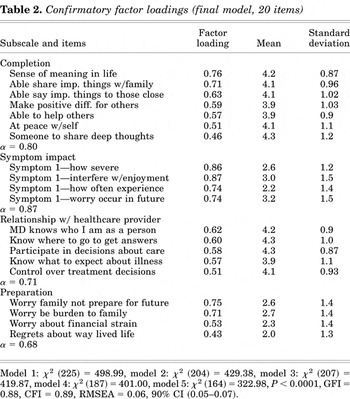
We tested several confirmatory models that included both five- and four-factor solutions: Model 1 included five factors and all variables originally entered in exploratory analyses, χ2 (225) = 498.99; model 2 was similar to model 1 but excluded the item “thoughts of dying frighten me” because it loaded negatively on the preparation subscale, χ2 (204) = 429.38; model 3 placed two items formerly constituting a fifth factor on factor 1, thus resulting in four factors, χ2 (207) = 419.87; model 4 removed one of those items, “time spent with family,” which did not load above 0.40, χ2 (187) = 401; and finally, model 5 removed “I have enough information about my illness” from the healthcare subscale due to insufficient loading, χ2 (167) = 348.6. Each model showed significantly improved fit as indicated by a significant change in chi-square. Model 5, our final model, comprised of 20 items and four factors, demonstrated acceptable levels of fit (see Table 2): CFI = 0.89, GFI = 0.88, and RMSEA = 0.06 (90% C.I. 0.05–0.07).
Confirmatory factor loadings (final model, 20 items)
Within QUAL-E subscale comparisons showed a moderate association between completion and healthcare (0.40, p < 0.001; see Table 3). Preparation showed a weaker but negative correlation with each of the other subscales, indicating the possibility of a paradoxical relationship between various aspects of quality of life. We believe this is an important finding and will explore it more fully in the discussion section. In general, the strength of correlations suggests these subscales have some conceptual association, yet also measure distinct concepts.
Correlations among QUAL-E subscales

Cronbach's alpha, a measure of internal consistency, was above 0.70 for three of the four scales and approached that value for the fourth: life completion (α = 0.80), symptom impact (α = 0.87), relationship with health care provider (α = 0.71), and preparation (α = 0.68). The lower value on the preparation subscale may be due, in part, to the small number of items (i.e., three).
As a second check on reliability, we examined intraclass correlations between QUAL-E responses from the initial administration and those collected 1 week later. Results indicate significant change in health events during the intervening week (see Table 4). Twenty-nine percent of subjects reported experiencing a change in medication, 13% a change in treatment regimen, 6% an ER visit, and 6% a hospitalization. The full sample results revealed moderate to strong correlations in three of the four domains. Symptom impact scores, as expected, emphasized the health status fluctuation of this population by showing the least stability, with an intraclass correlation of 0.23 for the full sample. Subgroups experiencing illness events showed more stability of symptom impact (r = 0.58 and 0.63, respectively), likely due to a more homogeneous but sicker subsample. Other correlations were fairly consistent across groups. However, those who had been to the ER or had a hospitalization showed less consistency on the preparation scale, which may be related to heightened sensitivity regarding issues of burden or financial strain caused by disease exacerbation.
Test–retest reliability—Interclass correlation coefficients by illness events

As expected, QUAL-E subscales showed mostly moderate correlation with subscales of comparison measures (see Table 5). Life completion, which includes issues of transcendence, related most strongly to the FACIT-SP spiritual well-being scale (0.62); it was moderately correlated with social, emotional, and functional domains. It also showed moderate correlation with the MVQOL index interpersonal, emotional, and transcendent subscales. Relationship with health care provider showed weaker but consistent associations with FACIT-SP subscales. As expected, it correlated weakly with two Participatory Decision Making items, control over decisions and involvement in care. It showed weak association with MVQOL symptom, emotion, and interpersonal subscales. Symptom impact was significantly but weakly associated with the FACIT-SP physical domain, reflecting the QUAL-E's distinct emphasis on the impact of symptoms versus tallying the presence of pain or fatigue, for example. It also was associated with the emotional subscale. Finally, as with other QUAL-E subscales, preparation was negatively correlated with all FACIT-SP subscales. It was weakly associated with the MVQOL emotion, function subscales and the FACIT-SP social subscale. For correlations between QUAL-E and MVQOL subscales, the negative directionality reflects opposite item anchoring.
Construct validity—Correlations between QUAL-E subscales and comparison measures

We also examined mean differences on all subscale scores by demographics, including gender, education, ethnicity, marital status, recruitment site, and primary diagnosis. Women reported higher levels of preparation (M = 2.51, SD = 0.99, versus 2.25 [0.96], p < 0.05). Patients with congestive heart failure had significantly lower scores on the relationship with health care subscale, perhaps reflecting the uncertainty that often accompanies such an episodic illness trajectory (M = 3.95, SD = 0.71, p < 0.05). Given the diversity of the sample, these results indicate instrument stability across multiple demographic categories and illness groups.
During the course of a career, a physician may care for more dying patients than those suffering from any single disease, yet only recently has the broader medical community brought attention to the need to systematically evaluate the quality of patients' experiences at the end of life. This article presents the psychometric validation of the QUAL-E, a four-domain measure of quality of life at the end of life. The instrument includes 20 individual items, four subscale importance ratings, and one overall quality of life question. It exhibits sound psychometric properties, is acceptable in populations of patients with different disease trajectories, and offers an evaluation tool for patients with advance cancer, heart, lung, and renal disease. It is designed for application in palliative care, hospice, or conventional medical settings.
QUAL-E subscales demonstrated expected associations with measures assessing similar constructs. Yet, the moderate size of correlations suggests that QUAL-E subscales measure constructs distinct from those assessed in other currently available measures. For example, though completion is associated strongly with the FACIT-SP subscale, the association is modest enough to suggest measurement of distinct constructs. Both subscales include items tapping issues of meaning and peace; however, the QUAL-E life completion subscale also assesses interpersonal connection and the ability to help others. Human development theorists refer to the latter as “generativity,” a key developmental growth task of late adulthood and time nearing the end of life (Erikson, 1982; Steinhauser et al., 2002).
The QUAL-E symptom impact subscale was designed to assess both symptom severity and symptom concern; as a result, we observed moderate correlations between it and both the FACIT physical and emotional well-being subscales. The relationship with health care provider subscale includes items associated with participating in decisions about care, knowing what to expect about the course of illness, knowing where to get answers, feeling control over treatment decisions, and feeling known as a whole person. Its structure as one domain reinforces the concept that knowledge and health decisions are integrally related to patients being known as whole persons in the context of their lives. In sum, we believe the QUAL-E domains relate appropriately to substantively similar domains within existing instruments and offer additional constructs such as preparation, relationship with health care provider, and completion not currently measured when assessing quality of life among dying patients.
As mentioned, early quality of life item and subscale composition often reflected an a priori structure based on researchers' perspectives and disciplinary divisions rather than patients' and families' accounts of the dying experience. For example, it is common to see a physical–social–emotional–spiritual domain structure in quality of life measures. However, data from this study's foundational focus groups and national survey as well as studies by Singer et al. (1999), Curtis et al. (2002), and others generated a divergent conceptual model. Physical, psychosocial, and spiritual concerns are, of course, present, but undergird other domains. Among seriously ill patients and long-term care residents, Singer et al. (1999) identified receiving adequate pain and symptom management, avoiding inappropriate prolongation of dying, achieving a sense of control, relieving burden, and strengthening relationships as important components of a good death. Construct validation of the QUAL-E suggests we must go beyond traditional domain structure and include the more recently developed patient-driven constructs.
Data analyses revealed information not only about scale development, but also regarding the process of dying. For example, confirmatory factor loadings showed preparation, as a domain, was associated negatively with other subscales but still contributed to a representation of quality of life at the end of life. Preparation includes questions regarding patient concern over becoming a burden, reflection on life regrets, and perception of the extent of one's family's preparation for the patient's end of life. Initially, the results seemed contradictory. However, related research clearly shows the multidimensionality of the dying experience, a complex time in which patients can hold multiple “conflicting” points of view simultaneously (Cohen & Mount, 1992; Staton et al., 2001). Patients may be concerned about their family's level of preparation for the future, yet still believe life has meaning. In fact, others have argued the transcendent dimension may counteract the negative impact of functional decline that is common as death approaches (Cohen & Mount, 1992). This may explain why dying patients sometimes report higher quality of life in the face of what others consider dire circumstances (Cohen & Mount, 1992). In clinical activities, preparation questions may be used to probe specific concerns that dying patients may harbor about their loved ones' well-being following their death. An overall report of good quality of life at the end of life may not preclude worry, particularly related to family well-being.
In addition, the preparation subscale originally included the item “thoughts of dying frighten me,” which was shown to load opposite the other preparation items. These data reinforce previous focus group findings that patients' fears related to well-being of family are often greater than concern about dying (Steinhauser et al., 2000b). Though the item was not viable in the QUAL-E, psychometrically, its responses were instructive. Though not included in subscale scores, we have chosen to retain the item in the current version of the instrument and continue to test its statistical association with the existing factor structure.
This study has several limitations. The instrument was validated in one geographic region and, because 100 of the participants were recruited from the VA, the sample included more men than women. However, Duke University Medical Center draws from both a regional and national patient pool, and at this non-VA site, we oversampled women. The instrument has been developed and validated among patients with one of four life-threatening illnesses. However, sample size reported in this article is not sufficient to compare factor models stratified by disease type. These analyses, requiring approximately 1000 patients, may be done in the future. Current analyses did, however, show similar scoring by diagnosis category.
Future work on the QUAL-E includes at least two other tasks. First, because participants were asked to rate the importance of individual domains to overall quality of life, further analyses will guide the most appropriate use of these items and address other longitudinal methodological issues such as response shift. Second, we will develop a family version of the QUAL-E, designed to capture the experiences of those closest to the dying patient, who often serve as health care proxies. At the very end of life, approximately one-third of patients are too ill to complete a questionnaire. Health care institutions may wish to evaluate families' perceptions of patient quality of life when families assume responsibility for patient care.
Dying is an inevitable part of the life cycle. The 2003 IOM report argues that patients and families should be able to expect quality in their experience and care throughout the entire course of their illness and up to the moment of death; end of life is simply one phase in a lifetime of health needs (Lunney et al., 2003). The instrument presented in this article is offered as a way to assess the quality of experience for patients at this challenging time.
The QUAL-E demonstrates acceptable psychometric properties, including structural validity, internal consistency, test–retest reliability, and construct validity. Though designed primarily as an evaluation tool to benchmark the impact of interventions to improve care, it also may be useful clinically to highlight patient concerns and remind us of the paradoxical nature of the end of life that holds potential for both decline and growth. Ultimately, we hope this instrument, emerging from efforts to understand the meaning of a good death, will lead to improved quality of life for dying patients and their families.
This material was based on work supported by the Office of Research and Development Health Services Research and Development Service, Department of Veterans Affairs (IIR #98-162.) Dr. Steinhauser is a VA Career Development Awardee. The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs.


Sample profile (n = 248)a

Confirmatory factor loadings (final model, 20 items)

Correlations among QUAL-E subscales

Test–retest reliability—Interclass correlation coefficients by illness events

Construct validity—Correlations between QUAL-E subscales and comparison measures