Introduction
Cancer is among the leading causes of death worldwide, but cancer survivorship in the United States is on the rise. It is estimated that the number of cancer survivors will increase by 31% by 2026. Cancer survivors are also living longer than before; in 2016, 67% of survivors had survived five years or more after their diagnosis (American Cancer Society, 2016). Despite these promising statistics, the cancer experience is still uniquely and profoundly stressful because of the multiple ambiguities that can accompany the disease, including uncertainty of treatment-related decisions, recurrence, when death will occur, and how the disease experience will affect survivor overall well-being and quality of life (Johnson et al., Reference Johnson, Butow and Kerridge2016; Li et al., Reference Li, Lin and Liu2014; Weeks et al., Reference Weeks, Catalano and Cronin2012; Winner et al., Reference Winner, Wilson and Ronnekleiv-Kelly2017). These biopsychosocial side effects can serve as a consistent and persistent reminder of cancer status for survivors across their lifespan (Kornblith & Ligibel, Reference Kornblith and Ligibe2003).
A cancer diagnosis not only affects the survivor; it can affect different social contexts, including spousal, familial, friendships, and work (Siminoff et al., Reference Siminoff, Zyzanski and Rose2008). Survivors have to adapt to physical and emotional changes at multiple points across the cancer experience, which may alter their relationships (Bellizzi et al., Reference Bellizzi, Smith and Reeve2010; Montazeri, Reference Montazeri2009; Sammarco, Reference Sammarco2001). These relationships and interpersonal contexts are crucial to cancer survivorship. Specifically, there is a strong body of research that social support is a significant factor for improved quality of life and overall well-being among cancer patients (Balogh et al., Reference Balogh, Ganz and Murphy2011; Ell et al., Reference Ell, Nishimoto and Mediansky1992; Roland et al., Reference Roland, Rodriguez and Patterson2013). Importantly, social support is not defined by the number of individuals in the social network of a cancer survivor, but a multifactorial concept that includes different types of support (e.g., emotional) at different levels (e.g., formal vs. informal). Previous research has demonstrated that survivor perceptions of the “right” type and level of support offered by the “right” person in their social network is associated with more optimal outcomes then simply measuring perception of support (Fife et al., Reference Fife, Weaver and Cook2013; Nausheen et al., Reference Nausheen, Gidron and Peveler2009). For example, a survivor may desire higher levels of emotional support from a spouse/partner, but prefer informational support from a member of their healthcare team.
A better understanding of who or what contexts affect cancer survivors may help researchers and clinicians provide better care for cancer survivors throughout the cancer experience. The Institute of Medicine advocates for a patient-centered approach to cancer care (McCormack et al., Reference McCormack, Treiman and Rupert2011). Patient-centered care promotes a more holistic view of the patient that includes consideration of health outcomes important to the patient, as well as consideration of their social contexts (Orom et al., Reference Orom, Underwood and Cheng2018). Measuring unilateral concepts such as “support” may fail to capture the complexity of individual-level variance in support preferences and, thus, inhibit the ability to tailor care to the needs and goals of the survivor. Indeed, research shows cancer patients perceive some support efforts as ineffective, excessive, or unwanted (Li et al., Reference Li, Mak and Loke2013; Shin et al., Reference Shin, Cho and Roter2013).
A novel approach to address this topic is to examine the bidirectional influence between a cancer survivor and their social context. Bidirectional, or mutual, influence is a recursive concept that cuts across multiple ecologically oriented theoretical approaches, including family systems theory, ecological systems theory, and the transactional model of development (Bowen, Reference Bowen1966; Bronfenbrenner, Reference Bronfenbrenner1992; Sameroff & Mackenzie, Reference Sameroff and Mackenzie2003). Bidirectional influence in the context of the current study suggests the experience of cancer influences the social contexts and relationships of the survivor and vice versa : the social contexts and relationships of the survivor influence the experience with cancer. The concept of bidirectional influence is present within the current body of published research on social contexts of cancer survivors. For example, Hagedoorn et al. (Reference Hagedoorn, Buunk and Kuijer2000) conducted a meta-analysis and reported a moderately strong association in emotional distress among cancer survivors and their spouse/partner (r = .29). These data suggested a more interdependent, mutually influential emotional reaction instead of two independent reactions. Most research fails, however, to directly examine the bidirectional relationship between social contexts and the effect of cancer. Focusing on these bidirectional relationships are important to understanding the social contexts that affect the adjustment to cancer across the illness trajectory and how the experience of cancer impacts social contexts and relationships of the survivor (Bellizzi et al., Reference Bellizzi, Miller and Arora2007).
The purpose of the current study therefore was to use a mixed-methods approach to assess the perspective of cancer survivors on the bidirectional impact between cancer and their social contexts. A fixed concurrent triangulation mixed-methods survey design was used with open- and closed-ended questions that were predetermined and administered to participants (Creswell, Reference Creswell2003). Quantitative questions were used to assess the magnitude of cancer impact in each context. Qualitative questions were designed to understand in greater depth the bidirectional effect between patients and their environment regarding specific contexts, specifically spirituality/faith, spousal/partner relationship (if applicable), and family. Qualitative and quantitative data were collected simultaneously, analyzed separately, and then merged for cross-validation and interpretation (Figure 1).

Fig. 1. Study design.
Methodology
To accomplish the goals of the current study, an online retrospective survey of cancer patients was conducted. Participants were recruited from ResearchMatch©, which is designed to match researchers and potential participants for study recruitment (Harris et al., Reference Harris, Scott and Lebo2012). Among the volunteers enrolled in ResearchMatch©, 71% are female, 29% are male, and 0.4% are transgender. Volunteers reside in all 50 states across the United States, with Ohio having the densest population of volunteers (~16,000) followed by New York (~10,300), California (~10,200), and Tennessee (~9,300). For purposes of the current study, volunteers who indicated a cancer diagnosis were recruited. Additionally, participants had to be older than 18 years of age, able to read and write English, more than four months postdiagnosis, and currently receiving treatment or follow-up care related to their cancer. This study was approved by the institutional review board (protocol #2017E0678).
Measurement
Demographics
Sociodemographic variables related to the individual participants were collected, including age, race, income, and current relationship status. Cancer demographics were also assessed, including diagnoses, treatment history, and current cancer status.
Bidirectional impact between cancer and social contexts
To assess the impact of cancer on participants, questions were adapted from the “Impact of Cancer” section of the Adolescent & Young Adult Health Outcomes and Patient Experience Study (Bellizzi et al., Reference Bellizzi, Smith and Schmidt2012). This section of the survey consisted of an 18-item Life Impact Checklist to assess the negative and positive effects of cancer across multiple social contexts (Bellizzi et al., Reference Bellizzi, Miller and Arora2007; Ganz et al., Reference Ganz, Desmond and Leedham2002). For each item, participants indicated the overall effect of their cancer diagnosis for each social context on a 5-point scale from 1 (very negative) to 5 (very positive). Participants could also select “not applicable” for social relationships and contexts that did not exist in their life.
Using a mixed-methods approach, the breadth of inquiry was expanded as quantitative methods only measured the linear relationship of cancer on social contexts. As such, qualitative questions were designed to explore in greater depth the bidirectional impact between the patient and specific contexts that were indicated as important in previous research including spirituality/faith (e.g., Cohen et al., Reference Cohen, Sawatzky and Russell2017; Holland et al., Reference Holland, Kash and Passik1998, Reference Holland, Passik and Kash1999), the spousal/partner relationship (e.g., Kayser, Watson, & Andrade, Reference Kayser, Watson and Andrade2007; Wintre & Gates, Reference Wintre and Gates2006), and the family (e.g., Edwards & Clarke, Reference Edwards and Clarke2004; Milberg, Wåhlberg, & Krevers, Reference Milberg, Wåhlberg and Krevers2014). Each topic area had two open-ended questions to assess the bidirectional impact of cancer. For example, the question “What impact did cancer have on your family?” was followed by “How did your family impact your cancer experience ?” Each participant had the opportunity to respond to all open-ended questions unless they were not in a committed relationship during their cancer experience. These participants were not presented with open-ended questions related to a partner/spouse.
Analytic plan
A cross-sectional descriptive approach was used to evaluate the quantitative survey items. Data were analyzed using SPSS , version 24 (IBM, 2016). The listwise deletion procedure was used to manage missing data for the analyses. Data were collapsed and categorized as positive (responses of 4 , somewhat positive, and 5 , very positive), negative (ratings of 1, very negative, and 2, somewhat negative), and no impact (3) based on the literature (Bellizzi et al., Reference Bellizzi, Miller and Arora2007). Qualitative data from open-ended questions were imported into NVivo 11 (QSR International, 2012). The authors read responses to the open-ended questions multiple times to orient themselves to the data. Similar to the format of the quantitative measure, the data were first categorized as “positive,” “negative,” or “no impact.” These three categories served as the three major themes for each question. The authors used the constant comparative method to guide the development of subthemes under the positive and negative categories (Glaser & Strauss, Reference Glaser and Strauss1967). Similar codes were discussed until a consensus was reached and key subthemes were collectively determined. If there were no patterns in response or a small response size under a positive or negative theme, no subthemes were created.
Results
Approximately 2,500 ResearchMatch volunteers >18 years of age registered as having a cancer diagnosis and received an e-mail containing information related to the survey study. A subset of participants (N = 242) met inclusion criteria and expressed interest in participating in the survey and were contacted for more information. Potential participants received an anonymous link to complete the survey, with 119 individuals initiating the survey. After reviewing the data, three participants were excluded because of lack of consent (n = 1) or failure to complete the questions after consenting (n = 2). The final sample size for the study was 116 participants.
Sample characteristics
The average age of the study participants was 58.43 years (SD = 12.05, range 27.0–86.0). Approximately 32.4% were male and 66.7% were female; more than one-half of the sample had a college or postgraduate degree (60.0%) and made >$50,000 annually (78.3%). Among the 116 participants, 86% indicated a religious/spiritual belief, with the majority stating they were Christian (49%), followed by Catholic (19%). Other religious/spiritual practices reported included Judaism (9%), Buddhism (1%), and other (5%). Last, 16% of participants identified as atheist or agnostic. Table 1 summarizes participant demographic variables.
Table 1. Demographic variables

Many participants indicated a diagnosis of breast cancer (37.9%) or prostate cancer (16.5%). The average length of survivorship for participants was 8.36 years (SD = 6.65, range 1.0–42.0). Most participants reported receiving multiple cancer treatments, with the highest percentage undergoing surgery (81.1%), chemotherapy (54.7%), or radiation (56.8%). Of note, 73.9% of participants described themselves as currently cancer free at the time of data collection versus 13.8% and 6.9% of individuals who responded that they either had recurrent disease or were unsure, respectively.
Quantitative results
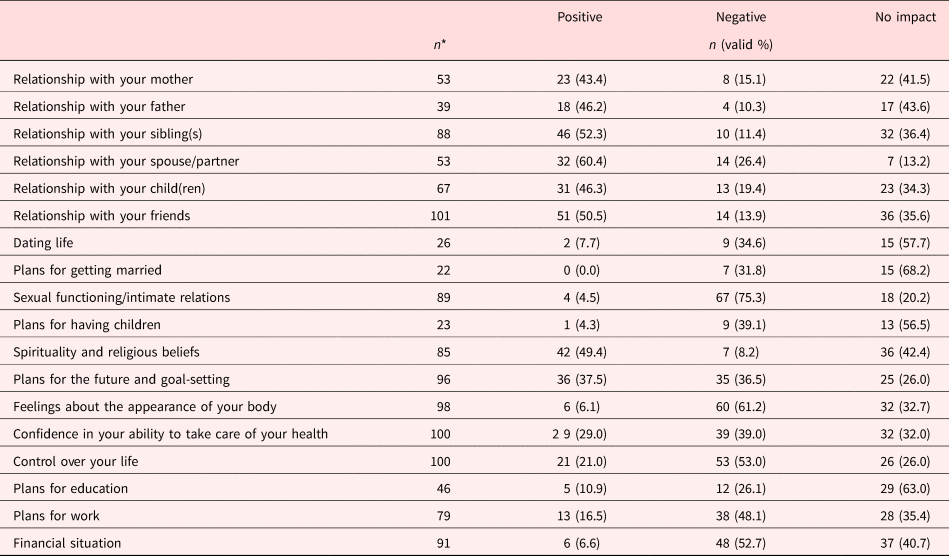
After combining and transforming the items on the Adolescent & Young Adult Health Outcomes and Patient Experience Study , the social context that participants perceived to be affected most positively by the experience of cancer was their relationship with their spouse partner (55% positive, 30% negative, and 13% no impact). The social context most frequently rated with a negative effect was sexual functioning and intimate relations (5% positive, 75% negative, 20% no impact). Table 2 summarizes the frequency statistics for each item. Descriptive statistics for each scale item are noted in supplemental Table 1.
Table 2. Frequencies for AYAHOPE/Life Impact Scale (N = 117)

*For each life impact item, there are different percentages of the sample for which the question does not apply. “Does not apply” was treated as missing.
Qualitative results
The qualitative analysis procedure revealed subthemes associated with both positive and negative effects for each topic area. Tables 3–5 summarize the qualitative results, including counts associated with the organizing themes related to each topic area, subthemes, and exemplar quotes. Subthemes were described and presented with quotations.
Table 3. Summary of themes and subthemes for the bidirectional impact between cancer and spirituality/faith

*Themes are organized by a positive, negative, no impact framework.
Table 4. Summary of themes and subthemes for the bidirectional impact between cancer and spouse/partner relationship

*Themes are organized by a positive, negative, no impact framework.
†Participants who did not indicate they were in a relationship during their cancer treatment did not receive open-ended questions about a spouse/partner relationship.
Table 5. Summary of themes and subthemes for the bidirectional impact between cancer and the family

*Themes are organized by a positive, negative, no impact framework.
Spirituality/ faith
Effect of spirituality/faith on the cancer experience
For participants who reported an impact of spirituality/faith beliefs on their experience with cancer (either positive or negative vs. no impact), the majority were positive. The most dominant subtheme was putting trust in God or a higher power.
“I knew God would not give me anything I could not handle…”
Participants that cited a negative impact on their cancer experience reported anger toward God or their higher power.
“Actually, I was pretty angry at my higher power when going through treatment…”
Effect of cancer on spirituality/faith
When participants reported a positive impact, they discussed a strengthening of their beliefs.
“I found myself praying more and thinking in a spiritual way more often.”
Similar to the previous question, participants who reported a negative impact discussed anger and doubts with God or a higher power.
“Took a long time… for my trust to come back.”
Spouse/ partner
Effect of spouse/partner on the cancer experience
Participants who reported that their spouse or partner had an impact on the cancer experience reported a positive impact only. Two themes emerged in this question that were classified as emotional and instrumental support. Descriptions of emotional support included love, encouragement, and being “there for one another.”
“He went with my [treatment] decision and stayed by my side.”
“The encouragement my partner gave me made me confident that the decisions were the best for me.”
Instrumental support included processing, researching, and gathering information related to cancer.
“She is a retired nursing professor and was able to read and understand the research…”
“We attend all medical appointments together. My wife is a better note-taker than I am. We discussed what we heard…”
Impact of cancer on the spouse/partner relationship
Unlike the previous question, the majority of participants who discussed an impact of cancer on the spouse/partner relationship more frequently reported negative effects, including relationship strain and sexuality/intimacy challenges.
“It stressed our relationship. We separated a few times in 10 years.”
“Pretty well ended our sex life.”
“We have had some issues being intimate because sometimes sex is painful.”
Participants did report some positive impacts of cancer on their relationship, including an improved relationship and closeness.
“Wrestling with all the complexities that attend cancer strengthened us as a couple… We value each other even more than before.”
“In many ways, it brought us closer together.”
Family
Effect of family on the cancer experience
Similar to spouse/partner, respondents who indicated that their family affected their cancer experience positively said that family supported their decisions through treatment and helped them process information (instrumental support).
“My children were supportive of whatever I decided.”
“They came to appointments, listened, asked good questions, and supported my decisions.”
An additional positive subtheme emerged; family members (often in association with children/grandchildren) served as motivation and inspiration to keep going through the cancer experience.
“I want to see my grandsons grow to adulthood.”
“Because I have a young daughter, I opted for the most aggressive treatment options.”
There were a few negative experiences around poor interactions with family members. Because of limited responses, there was no subtheme and all responses were classified as “negative experiences.”
“My daughter… is disappointed that I am doing the medications that the oncologist wants me to take.”
Although it was not considered a subtheme, participants who reported that their family had no effect on their cancer treatment did note that their spouse/partner was still an exception to that rule, reemphasizing the importance of the spousal role during the cancer experience.
Impact of cancer on the family
There was a mix of positive and negative responses for participants who discussed the impact of cancer on their family members. When discussing the negative impact, participants most often discussed the concern or worry (emotionally taxing) of their family members.
“My son was very upset and did not tell me. He ended up with a [driving under the influence violation] three days after my surgery ; he has never been in trouble.”
Participants who reflected on the positive impact of cancer discussed the concept of support and many respondents also noted increased feelings of closeness and communication throughout the cancer experience.
“It brought us all closer. We communicate better. I see some of my family much more often than before.”
Discussion
The current study used a mixed-methods approach to assess the effect of cancer across multiple social contexts (who/what) and explore the bidirectional impact of cancer on specific contexts in greater depth (how). The specific contexts that were examined included spirituality/faith, spousal/partner relationship (if applicable), and family. Herein, we provide an integration, triangulation, and interpretation of the quantitative and qualitative results. Specifically, within the quantitative part of the survey, almost one-half of respondents endorsed the positive impact of cancer on their spirituality/faith; in contrast, within the qualitative responses, most participants suggested that there was no impact of spirituality/faith on their cancer experience (n = 43) or that the cancer diagnosis had an effect on their spirituality/faith (n = 39; Table 3). To this point, there is a growing body of evidence in the literature that has suggested a relationship between religious/spiritual beliefs and the way patients experience illness and disease (Koenig, Reference Koenig2012; Puchalski, Reference Puchalski2012 , Reference Puchalski2013; Savel & Munro, Reference Savel and Munro2014). Within the open-ended responses, participants did not mention finding or abandoning a spiritual/religious practice as a result of their cancer experience; thus, a cancer diagnosis may deepen the importance, through bidirectional influence, of spirituality/faith among patients who are already possess these beliefs, spirituality/faith may not become an important factor in the cancer experience of patients who did not identify with a particular spiritual/faith practice.
Within the subset of respondents who did report an effect (either positive or negative) between cancer and spirituality/faith, the majority said the effect was positive. A positive subtheme that emerged from the question examining the impact of faith spirituality on the cancer journey was putting trust in a higher power. Putting trust in God or a higher power may have supported positive coping and adjustment that strengthened the patient's belief system, which was a positive subtheme that emerged from the effect of cancer on spirituality/faith. Indeed, spirituality/faith can be an important resource for patients diagnosed with cancer that facilitate positive coping, psychosocial adjustment, acceptance of prognosis, and meaning-making across the cancer trajectory (Pargament et al., Reference Pargament, Koenig and Tarakeshwar2004; Peteet & Balboni, Reference Peteet and Balboni2013; Wright et al., Reference Wright, Zhang and Ray2008). Defining mechanisms by which spirituality/faith influences the cancer experience and how healthcare providers can engage with patients to assess the importance spirituality/faith may facilitate optimal, supportive patient care.
Another interesting finding of the current study was the positive effect of a spouse/partner on the cancer experience relative to emotional and instrumental support. Within the quantitative data, survivor perceptions of the impact of cancer on the spouse/partnership were rated the most positive among all items. Cancer did have a negative impact on some aspects of the spouse/partnership. For example, respondents noted that cancer had a negative impact on sexual functioning and intimate relations. In fact, in the qualitative results, challenges with sexual functioning and intimacy was a substantial subtheme. Previous data have noted that most cancer diagnoses can affect sexual functioning via biological and/or psychological pathways (Christie et al., Reference Christie, Sharpley and Bitsika2015; Kornblith & Ligibel, Reference Kornblith and Ligibe2003; Li & Loke, Reference Li and Loke2014; Roland et al., Reference Roland, Rodriguez and Patterson2013); this effect can be particularly pronounced when the malignancy affects areas related to sexual functioning (Morreale, Reference Morreale2011). Within the current study, breast and prostate cancer survivors were overrepresented, so it is possible there were physical/anatomic challenges related to intimacy in addition to the negative biological and psychosexual side effects of cancer. The data serve to highlight that healthcare providers should not overlook emotional, informational, and sexual needs/concerns of the spouse/partner.
The integration of the qualitative and quantitative data further illustrated the importance of the spouse/partner during the cancer experience. Subthemes within the qualitative data delineated how the spouse/partner was important, including emotional and instrumental support. Although this subtheme was reflected in the qualitative data for the effect of the family, “no impact” was more frequently reported by participants, suggesting that family was not as regularly or directly involved with the cancer experience as a spouse/partner. In fact, many participants who indicated “no impact” of the family added the exception of their spouse/partner. Although family very often is important to patients diagnosed with cancer, there may be differences in family involvement resulting from differences in the developmental stages of the family members or patient, as well as varying cancer treatment factors (Laidsaar-Powell et al., Reference Laidsaar-Powell, Butow and Bu2013; Northouse, Reference Northouse1984; Rolland, Reference Rolland2005; Weihs et al., Reference Weihs, Reiss and Baider1996). For example, the spouse/partner may be highly involved in treatment decision-making, whereas young children may not; thus, the positive effect of cancer on these relationships may be occurring through different processes. The positive impact on the relationship with family members (e.g., children) may be explained by a more passive role, such as motivation and inspiration, whereas the spouse/partner has a more active role in the cancer experience through emotional and instrumental support. Overall, it is important for providers to assess patient preferences for family involvement across the cancer experience.
Limitations
The mixed-methods survey design allowed for the contextualization of results and improved validity. There were limitations, however, that should be considered when interpreting the results (Creswell, Reference Creswell2003). Volunteer bias may be evident in the sample, because individuals who participated in the study needed to willingly sign up for ResearchMatch© and agree to participate in the study. Social desirability bias may also have affected participant survey responses, particularly about negative feelings within familial relationships (Krumpal, Reference Krumpal2013). Retrospective perspectives are subject to bias for many reasons, including difficulty of recall, acquired meaning-making of memories, and the current mood of the participant (Hassan, Reference Hassan2005). The mix of open- and closed-ended questions affected the response rate among the different question types, because participants in web-based surveys may answer closed-ended questions more frequently than open-ended questions (Reja et al., Reference Reja, Manfreda and Hlebec2003).
Finally, the sample was also not nationally representative; thus, the results of this study may not be generalizable. For example, the majority of the sample identified as breast and prostate cancer survivors, which have higher five-year survival rates in the general population (89.7% and 98.6%, respectively; National Cancer Institute, 2016). Additionally, almost three-fourths of the sample identified as being currently cancer free, so the data may more accurately represent long-term cancer survivors. The homogeneity of the participant sample limited the ability to examine the influence other contextual variables, such as race and socioeconomic status, which can affect the cancer experience (Williams et al., Reference Williams, Hanson and Boyd2008).
Clinical implications
The current study highlights the important role of partner/spouse relationships to cancer survivors. By integrating a spouse/partner in the medical decision-making process, providers can minimize conflict and emotional distress associated with the cancer experience, but providers often neglect the influence of the spouse/partner in treatment-related conversations (Shin et al., Reference Shin, Cho and Roter2013). Failure to recognize the bidirectional influence between the spouse/partner and the cancer experience may lead to delay in identifying unmet biopsychosocial needs of survivors. In addition, caregivers may be an important source of information for treating physicians regarding patients’ day-to-day symptom levels (Shin et al., Reference Shin, Cho and Roter2013).
Similarly, current research shows that both patient and caregiver participants perceived family involvement in medical decision-making as helpful without compromising patient autonomy, whereas physicians were only comfortable with family involvement until caregiver influence appeared to conflict with the physician perception of patient values (Laidsaar-Powell et al., Reference Laidsaar-Powell, Butow and Charles2017). Healthcare providers should intentionally incorporate spouse/partners and other family members in different stages of the cancer experience. For example, goal setting can be guided by an awareness of the components of family functioning most relevant to particular phases of an illness. Sharing this information with the family and deciding on specific goals can provide a better sense of control and hope for the family. Enabling healthcare providers to think about the patient within the context of their family system and the bidirectional impact of a cancer diagnosis will be an important skill to examine patterns and anticipate psychosocial needs with patients over the course the of the cancer experience (Rolland, Reference Rolland2005).
In conclusion, cancer is a unique experience and understanding the bidirectional impact between cancer and social contexts of the survivor will promote patient-centered care throughout the cancer experience. Relationships and social contexts are important to patients diagnosed with cancer and the central role of relationships will only grow as the survivorship continues to lengthen. More research is needed to better understand what relationship or social context is important and how understanding these processes may assist in providing more optimal patient-center care.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S1478951519000038
Author ORCIDs
Timothy M. Pawlik, 0000-0002-7994-9870
Conflicts of interest
The authors do not have any relevant financial relationships or conflicts of interest to disclose.