INTRODUCTION
The National Comprehensive Cancer Network defines cancer-related fatigue (CRF) as a distressing, persistent, subjective sense of physical, emotional, and/or cognitive tiredness or exhaustion related to cancer or cancer treatment that is not proportional to recent activity and which interferes with usual functioning (Levy et al., Reference Levy, Adolph and Back2012). CRF is one of the most common and important issues in palliative medicine and has a significant impact on patient quality of life (QoL) (Campos et al., Reference Campos, Hassan and Riechelmann2011). It can occur due to the side effects of treatment or directly as a result of the disease. The prevalence of fatigue has been reported to range from 10 to 56% in a heterogeneous population of cancer patients (Cella et al., Reference Cella, Davis and Breitbart2001; Andrykowski et al., Reference Andrykowski, Schmidt and Salsman2005; Yeh et al., Reference Yeh, Lau and Su2011; Fernandes et al., Reference Fernandes, Stone and Andrews2006); however, the profile of CRF is unclear, as the frequency of the condition is likely to vary depending on the assessment method and threshold. Since the definition of “fatigue” is quite subjective, it is difficult to establish evidence supporting interventions for CRF from the viewpoint of outcome measurements. Another problem in conducting clinical trials to assess CRF is that clinicians do not wish to add the burden of participating in a clinical trial for patients who are undergoing palliative care. Reports of promising pharmacological treatments for CRF, such as methylphenidate (Bruera et al., Reference Bruera, Driver and Barnes2003; Reference Bruera, Yennurajalingam and Palmer2013; Johnson et al., Reference Johnson, Block and Gold2010; Sarhill et al., Reference Sarhill, Walsh and Nelson2001), erythropoietin, and darbepoetin (Esquerdo et al., Reference Esquerdo, Llorca and Cervera2011; Revicki et al., Reference Revicki, Stull and Vernon2012), have been published, but these studies included a small sample size from which it was impossible to draw any definitive conclusions. Minton and colleagues (Reference Minton, Richardson and Sharpe2010) also concluded that the safety concerns and side effects of these drugs prevent them from being recommended in the treatment of CRF. Larger randomized control trials are therefore needed before these drugs can be recommended for use in patients with CRF.
While there is little evidence regarding palliative care in CRF, administration of corticosteroids has empirically been used in patients with CRF, and it is taken for granted that most physicians believe in the effectiveness of steroid treatment. The results of a randomized clinical trial (RCT) evaluating the efficacy of dexamethasone for CRF were recently reported by Yennurajalingam et al. (Reference Yennurajalingam, Frisbee-Hume and Palmer2013) in which total Functional Assessment of Chronic Illness–Fatigue (FACIT–F) (Debb et al., Reference Debb, Arnold and Perez2011) scores were significantly improved in the dexamethasone group compared to placebo. These results are substantial and meaningful for the many clinicians who provide palliative care for cancer patients.
We previously conducted a similar RCT from 1999 to 2003 that was strictly designed to verify the effectiveness of methylprednisolone for CRF, the results of which have not yet been published. It was closed before patient registration was completed because the pace of registration was slower than expected. Detailed clinical data (including laboratory data, endocrine parameters, and patient-reported outcomes) were collected, despite the small number of patients enrolled. Though the sample size was too small and not sufficient to provide for definitive conclusions, we believe that reporting these results will be useful in order to prevent publication bias and demonstrate the need for more clinical trials and/or metaanalyses to investigate the effectiveness of corticosteroids in the treatment of CRF.
METHODS
Study Design
The study was a randomized, double-blind, multicenter, placebo-controlled trial. A total of 22 institutions and hospitals participated, and the study protocol received approval from the institutional review board of each institution under the principles of the Declaration of Helsinki. All patients provided written informed consent.
Inclusion Criteria
Eligible patients were those older than 18 years of age with a diagnosis of advanced cancer confirmed on a histological or cytological examination. The other inclusion criteria were as follows: (1) the patient had a life expectancy estimated to be longer than four months; (2) there were no future plans for chemotherapy, radiotherapy, or surgical treatment; (3) patients had CRF refractory to other treatments; (4) they were able to receive medications orally; (5) patients were being treated in a hospital; and (6) they had an ALT level ≤300 U/ml, an aspartate aminotransferase (AST) level £300 U/ml, a creatinine level ≤3.0 mg/dl, and a total bilirubin level ≤3.0 mg/dl.
Exclusion Criteria
Patients were excluded if they (1) had severe heart disease, diabetes mellitus, active gastrointestinal ulcers, viral hepatitis, infectious disease, or tuberculosis; (2) received radiotherapy or chemotherapy in the prior four weeks; (3) had had surgery for their cancer in the previous two weeks; (4) had a history of corticosteroid allergy, (5) had been administered corticosteroids in the last two weeks, (6) required corticosteroids for other diseases; or (6) did not adequately understand their condition.
Random Allocation and Treatment
Patients were enrolled over the telephone or via fax to the data center and then randomly allocated to either a methylprednisolone (MP) group or a placebo group. Allocation of treatment groups was determined at the data center according to a dynamic randomization method in a double-blinded manner (i.e., both patients and physicians were blinded to treatment allocation). Patients received 16 mg of methylprednisolone (32 mg/day) or placebo by oral ingestion twice daily for seven days. The type and dose of steroid employed were determined by a pilot survey from palliative care physicians from 22 institutions. All physicians preferred to use prednisolone or methylprednisolone, except for two who preferred betamethasone and one who utilized dexamethasone. The mean maximum dose of MP that physicians were using for palliative patients at that time was chosen as the intervention for the study.
Efficacy and Safety Assessments
Our primary endpoint was an improvement in patient fatigue from baseline to day 7. The secondary endpoints were improvements in appetite loss and health-related QoL. We also investigated the safety of employing corticosteroids as palliative therapy. Data regarding fatigue, appetite loss, and QoL were collected as patient-reported outcomes using a questionnaire. Degree of fatigue and appetite loss were evaluated daily according to a visual analog scale (VAS); a 100-mm horizontal line was prepared, and the patient marked on that line the point they felt represented their current state. VAS score was determined by measuring in millimeters from the lefthand end of the line to the point that the patient marked. The VAS from before drug administration (day 0) to day 8 and QoL were assessed utilizing the Questionnaire for Cancer Patients Treated with Anticancer Drugs (QoL–ACD) (Kurihara et al., Reference Kurihara, Shimizu and Tsuboi1999), the most common cancer-specific QoL scale in use in Japan, on days 0, 3, and 8.
In order to assess the safety of treatments, we performed CBC, biochemical, and endocrine examinations on days 0 and 8. In addition, the attending physician recorded the incidence of adverse events based on daily interviews, rating the level of severity on a 5-point Likert-type scale after conducting the interview and an examination—0 (none), 1 (minimal), 2 (mild), 3 (moderate), and 4 (severe).
Statistical Analysis
The main efficacy analysis was performed based on an intention-to-treat population. The effectiveness of methylprednisolone was evaluated according to change in VAS scores for fatigue and appetite loss from baseline to day 7. Hence, the mean difference in VAS score from baseline to day 7 was compared using a t test with a 0.05 two-tailed α value between the two groups. For comparisons within groups, a paired t test was employed to compare differences from baseline to day 7.
Sample size was calculated based on an estimation of the expected effectiveness with respect to VAS score for fatigue (mean ± standard deviation):—
–40 ± 15 in the MP group and –30 ± 15 in the placebo group
—according to expected symptom improvement, with a two-tailed significant α value and statistical power set at 5 and 80%, respectively. We planned to enter 40 evaluable patients per group.
Exploratory Analysis for Future Studies
Regrettably, the pace of patient registration was insufficient to verify our primary endpoint. so we conducted the following post-hoc and exploratory evaluations in order to encourage further study. The mean difference in VAS score was compared based on daily points. For the sensitivity analysis, patients with a low baseline VAS score or poor performance status were excluded. In addition, participating physicians completed a survey immediately after the end of the study. We also investigated why patient registration was not accomplished using a questionnaire.
RESULTS
Patient Characteristics
Figure 1 presents a CONSORT flowchart of the study. We randomly assigned the 35 patients to an MP group (18 patients) and a placebo group (17 patients). One patient receiving placebo was excluded from the analysis because they withdrew consent to participate on day 1 and did not submit the questionnaire. In total, 18 patients in the MP group and 16 patients in the placebo group were included in the intention-to-treat analysis. The demographic and clinical characteristics of the two groups were not significantly different at baseline, though the number of patients with poor performance status was higher in the MP group (Table 1).

Fig. 1. CONSORT patient flowchart. Some 35 patients were randomly assigned to the methylprednisolone (MP) (18 patients) or placebo group (17 patients) from 22 participating hospitals and institutions.
Table 1. Patient characteristics
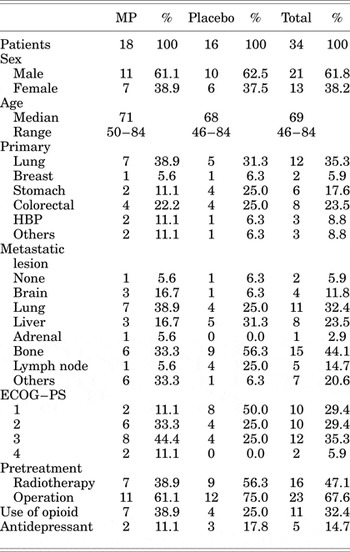
MP = methylprednisolone; PS = performance status; ECOG = Eastern Cooperative Oncology Group.
Assessment of Patients' Symptoms and QoL
As the primary endpoint, we evaluated mean difference in VAS score from baseline to day 7 (Table 2). The mean change (standard deviation) in the score for fatigue was –9.06 (27.2) in the placebo group and –1.56 (32.5) in the MP group (p = 0.484), while that for appetite loss was –6.44 (27.7) in the placebo group and –8.06 (38.3) in the MP group (p = 0.892). No significant changes were noted for either symptom. Total QoL scores at baseline and days 3 and 8 are depicted in Figure 2. A trend toward improvement in QoL score was evident in the MP group; however, there were no significant differences compared with the placebo group.

Fig. 2. Total quality-of-life (QoL) scores. Chronological changes in QoL scores and standard deviations at days 0, 3, and 8.
Table 2. The change of VAS score between day 0 and day 7

MP = methylprednisolone; VAS = visual analog scale; CI=confidence interval.
Laboratory Examinations
The results of comparison of the data obtained at baseline and on day 8 are shown in Table 3. The RBC, WBC, and platelet counts on day 8 were increased in the MP group. The serum AST, creatinine, bilirubin, protein, glucose, C-reactive protein (CRP), and electrolyte levels did not change significantly. Serum alanine aminotransferase (ALT) and total cholesterol levels were elevated in the MP group, though both parameters were within normal limits. There were no remarkable changes in any of the laboratory data in the placebo group.
Table 3. Laboratory findings in MP group

MP = methylprednisolone; RBC = red blood cell; WBC = white blood cell; AST = aspartate aminotransferase; ALT = alanine aminotransferase; CRP = C-reactive protein.
Adverse Events
The adverse events observed during our study are given in Table 4. Six adverse events of more than grade 3 were newly detected during the observation period. One case each of diarrhea, peripheral sensory neuropathy, and dyspnea were observed in the MP group and one each of dyspnea, headache, and fever in the placebo group, though there were no significant differences between the two groups. No allergic or fetal infectious side effects were documented.
Table 4. Adverse events

MP = methylprednisolone.
Grade 0 (none), 1 (minimal), 2 (mild), 3 (moderate), and 4 (severe).
Exploratory Analysis for Future Studies
The trends in daily VAS score are shown in Figure 3. Fatigue and appetite loss improved in both groups. A certain placebo or Hawthorne effect can be assumed. However, the fatigue scores on days 5 and 6 and those for appetite loss on day 5 were relatively better in the MP group. In particular, there was a significant improvement in appetite in the MP group on day 5 (p = 0.011). According to our sensitivity analysis, when patients with a low performance status at baseline were excluded, the fatigue scores on days 5 and 6 and those for appetite loss on days 4 to 6 were significantly improved in the MP group. However, neither symptom differed on days 7 or 8 between groups.

Fig. 3. Relationship among fatigue and appetite loss and visual analog scale score. Chronological changes in mean scores and standard deviations in the two groups.
Survey for Physicians Who Participated in the Study
The most common reason for delay in patient registration was that physicians hesitated in informing patients receiving palliative care about the study, though many patients in their hospital met the inclusion criteria. On the other hand, a few doctors who recruited patients found that some were quite cooperative and understood the importance of the study.
DISCUSSION
The results of this trial do not demonstrate any significant differences between the MP and placebo groups in alleviating CRF. Comparisons between the two treatment arms with respect to secondary endpoints—including other patient-reported outcomes, appetite loss, and total QoL score—also uncovered no significant differences. Concerning safety, the results were acceptable regarding the use of MP in palliative treatment.
We wish to discuss the reasons why our hypothesis that corticosteroids are effective in the treatment of CRF was not proven and evaluate the difficulty in performing clinical trials focused on CRF.
First, a fatal weakness of our study was its insufficient statistical power. While the sample size estimated in the preliminary protocol was 80, only 34 patients were enrolled for the analysis. As a result, the standard deviations for all data were large, and the statistical tests were not useful. The estimation of mean VAS score was not clearly different between the two groups. However, the expected value of investigators included a 10-point advantage in calculation of sample size. Though the registration of participants was implemented successfully, there was little chance that the hypothesis could be proved in an evidenced-based manner.
Second, the problem of outcome measurements should be considered. Since “fatigue” is quite a subjective term, quantification methods for assessing it have not been established. VAS score, which was utilized in our study, has not been proven to be valid for assessing a patient's level of fatigue or appetite loss in the setting of palliative medicine. The demerits of using VAS score include the low reproducibility of measurements and larger standard deviations (Wewers & Lowe, Reference Wewers and Lowe1990). In particular, psychological outcomes can be greatly influenced by mental or physical condition at the moment of filling out a questionnaire. When our study was conducted, there were no appropriate scales translated into Japanese that had proven validity. At the present time, there are various assessment tools for evaluating CRF, including the European Organization for Research and Treatment of Cancer Quality-of-Life Questionnaire (EORTC–QLQ–C15–PAL) (Groenvold et al., Reference Groenvold, Petersen and Aaronson2006), the Edmonton Symptom Assessment System (ESAS) (Bruera et al., Reference Bruera, Kuehn and Miller1991; Chang et al., Reference Chang, Hwang and Feuerman2000), the Brief Fatigue Inventory (BFI) (Mendoza et al., Reference Mendoza, Wang and Cleeland1999), and the Functional Assessment of Chronic Illness–Fatigue (FACIT–F) (FACIT.org, 2010). In a recent RCT evaluating the effectiveness of dexamethasone for CRF reported by Yennurajalingam and colleagues (Reference Yennurajalingam, Frisbee-Hume and Palmer2013), total FACIT–F scores on day 15 were improved in the intervention group. The FACIT–F scale contains 13 items associated with fatigue in which the patient selects one of five degrees indicating their response as it applies to the preceding seven days. Different from VAS methods, which can only be employed to evaluate symptoms at one timepoint, the FACIT–F questionnaire provides a multifaceted and more detailed assessment of the overall trend during the previous week.
Third, as a matter of course, it should be noted that the effectiveness of corticosteroids for CRF may be more limited than that assumed by many physicians. Indeed, there is little scientific evidence regarding the effects of corticosteroids on fatigue and/or appetite loss, with the RCT reported by Yennurajalingam et al. (Reference Yennurajalingam, Frisbee-Hume and Palmer2013) being the first clinical trial. Additionally, a large amount of dexamethasone (8 mg/day for 14 days) was administered in the present study; however, this dose is not necessarily common in palliative care patients, and information regarding the efficacy of this treatment is limited. Further studies are therefore needed to identify more effective interventions for treating CRF in palliative patients. It should also be considered that other promising interventions can be combined to enhance effectiveness, including other drugs: hematopoietic growth factors (Revicki et al., Reference Revicki, Stull and Vernon2012) or methylphenidate (Sarhill et al., Reference Sarhill, Walsh and Nelson2001); exercise (Schneider et al., Reference Schneider, Hsieh and Sprod2007), and/or psychosocial interventions (Armes et al., Reference Armes, Chalder and Addington-Hall2007). In addition, it is important to focus on other treatable contributing factors (e.g., anemia, pain, insomnia, malnutrition, emotional distress) (Minton et al., Reference Minton, Richardson and Sharpe2010; Campos et al., Reference Campos, Hassan and Riechelmann2011).
Finally, when conducting future studies, it is important for researchers to recognize the difficulty in performing clinical trials focused on patients receiving palliative care. The main reason why the pace of patient registration was delayed in our study was hesitation on the part of physicians to inform palliative care patients about the trial. Most physicians avoided registering patients, though many in their care were eligible. It is therefore necessary to recognize the importance and need for clinical trials in order to advance palliative medicine, and investigators should be more careful in implementing a study protocol than when conducting studies outside of the palliative care milieu. Regarding informed consent, the method of obtaining informed consent should have been discussed more carefully within our study team. It is also important to prepare physicians for participation in such studies.
In conclusion, this randomized control trial was unable to prove the efficacy of methylprednisolone in improving cancer-related fatigue.
ACKNOWLEDGMENTS
Our study was supported in part by the Epidemiological and Clinical Research Information Network (ECRIN), a nonprofit organization. The authors thank Professor Tetuso Kashiwagi, Dr. Naohiro Nomura, Dr. Yoshihiro Noso, Dr. Michikazu Ono, Dr. Ichinosuke Hyodo, Dr. Kyuhei Koda, Dr. Yoshihiro Honda, Dr. Toumei Tsukamoto, Dr. Masayuki Ikenaga, and all the other physicians who participated, as well as the patients. Without their cooperation, this study would not have been possible.









