Introduction
Most terminally ill patients experience delirium (Breitbart and Strout Reference Breitbart and Strout2000; Hosie et al. Reference Hosie, Davidson and Agar2013; Lawlor and Bush Reference Lawlor and Bush2015; Lawlor et al. Reference Lawlor, Gagnon and Mancini2000; Morita et al. Reference Morita, Tei and Tsunoda2001), and the delirium often does not improve before death (de la Cruz et al. Reference de la Cruz, Ransing and Yennu2015; Leonard et al. Reference Leonard, Raju and Conroy2008). The irreversible delirium during the dying process, referred to as terminal delirium (Bush et al. Reference Bush, Leonard and Agar2014), places a heavy burden on patients, health-care providers, and patients’ families (Cohen et al. Reference Cohen, Pace and Kaur2009; Finucane et al. Reference Finucane, Lugton and Kennedy2017; Kerr et al. Reference Kerr, Donnelly and Wright2013; Partridge et al. Reference Partridge, Martin and Harari2013). Family caregivers often experience distress in the face of terminal delirium, and the emotional care of family members should be emphasized (Agar Reference Agar2020). In addition, the standard treatment of terminal delirium has not been established, and family members experience a considerable burden as surrogate decision-makers. A relationship between terminal delirium in patients and depression in bereaved family members was recently demonstrated (Hatano et al. Reference Hatano, Morita and Mori2022).
Many rating scales are used for delirium (Breitbart et al. Reference Breitbart, Rosenfeld and Roth1997; Gaudreau et al. Reference Gaudreau, Gagnon and Harel2005; Inouye et al. Reference Inouye, van Dyck Ch and Alessi1990; Thurber et al. Reference Thurber, Kishi and Trzepacz2015). However, these scales do not work for patients with terminal delirium who are in poor physical condition and require medication to relieve their distress. In addition, to evaluate terminal delirium, it is necessary to consider a balance between distress relief and communication, between distress relief and the family’ s mental preparation forthe patient’ s death, and between treatment of delirium itself and physical treatment is needed (Uchida et al. Reference Uchida, Morita and Ito2018). Therefore, specific scales are needed to assess patient and family distress related to terminal delirium.
Based on a previous qualitative analysis (Uchida et al. Reference Uchida, Morita and Ito2018) and systematic literature search, we conducted a survey regarding the views of bereaved families and developed a questionnaire. Items that bereaved families regarded as important were extracted and an evaluation scale of terminal delirium was developed. This questionnaire was used in a cross-sectional survey of bereaved relatives of cancer patients who were admitted to hospice or palliative care units. Based on this survey, a 24-item Terminal Delirium-Related Distress Scale (TDDS) was developed and validated (Uchida et al. Reference Uchida, Akechi and Morita2021). The TDDS consists of 5 subscales, including support for families and respect for the patient, ability to communicate, hallucinations and delusions, adequate information about the treatment of delirium, and agitation and restlessness.
To reduce the physical and psychological burden of responding to questionnaires on family members and patients, we developed a shortform of the TDDS (TDDS-SF), consisting of 15 items that were most important to bereaved families. The aim of this study was to evaluate the validity and reliability of the TDDS-SF.
Methods
A nationwide self-administered questionnaire survey of the families of cancer patients who died in hospice/palliative care units certified by the Japan Hospice and Palliative Care Association was conducted. The survey was mailed to the families of patients from the participating facility in August 2018, along with a document explaining the survey. The participants were asked to return the completed questionnaire within 2 weeks. In September 2018, a postcard reminding participants to return the completed questionnaire was sent.
Participants
Adults from bereaved families of adult patients with cancer who died at participating hospices/palliative care units from February 2014 to January 2018 were included in the study. The exclusion criteria were as follows: (1) family members could not be identified; (2) patient died due to treatment-related death or in the ICU ward; (3) patient used hospices/palliative care unit for <3 days; (4) potential participant was incapable of responding to the self-completed questionnaire due to cognitive impairment, mental disorder, or visual disability; (5) potential participant was not mentally stable; (6) potential participant did not consent to participation; and (7) potential participant’s involvement in the study was undesirable based on a comprehensive judgment made by multiple professionals due to a strong dissatisfaction with or misunderstanding of the medical care received or a poor relationship with medical staff.
TDDS-SF development
This study was conducted in 2 steps: scale development and scale validation. The TDDS-SF was developed to reduce the burden of answering questionnaires on terminally ill patients and their families. Therefore, the items with insufficient loading (<0.6) in the TDDS were removed based on factor analysis. Palliative care experts, including cancer nurses, palliative care physicians, psycho-oncologists, and clinical psychologists, reviewed each item and checked the structure of the scale. The TDDS-SF consists of 15 items and 4 subscales, including “Care for the family,” “Ability to communicate,” “Psychiatric symptoms,” and “Adequate information and discussion about treatment for delirium.”
Scale validation
To assess the validity and reliability of the TDDS-SF, a cross-sectional, self-completed questionnaire survey of bereaved families of cancer patients who were admitted to a hospice/palliative care unit was conducted. The validity assessment included construct validity, convergent validity, and discriminant validity, and the reliability was assessed using Cronbach’s alpha coefficient for internal consistency.
Good Death Inventory – Short Version
The distress caused by terminal delirium is related to achieving a good death (Uchida et al. Reference Uchida, Akechi and Morita2021). Thus, the convergent validity between the Good Death Inventory (GDI) and TDDS-SF was assessed. The short version of the previously validated GDI was used to evaluate the patient’s achievement of a good death from the perspective of the bereaved family (Miyashita et al. Reference Miyashita, Morita and Sato2008). The short version of the GDI includes 18 representative items, which are evaluated using a 7-point Likert scale. The total score is calculated by summing the scores for all attributes; a high total score indicates the achievement of a good death.
Care Evaluation Scale – Short Version
The distress caused by terminal delirium is related to end-of-life care (Wright et al. Reference Wright, Brajtman and Macdonald2014). Therefore, we examined the convergent validity between the end-of-life care and the TDDS-SF. End-of-life care was assessed using the revised short version of the Care Evaluation Scale (CES2) (Miyashita et al. Reference Miyashita, Aoyama and Nakahata2017). The CES2 evaluates end-of-life care from the perspective of bereaved family members, focusing on the structure and process of care. The validity and reliability of the CES2e has been confirmed. The CES2 consists of 10 representative items. The questions allow respondents to evaluate the necessity for improvement for each item on a 6-point Likert-type scale. The total score was 100 points, with higher scores indicating good structure or process of care.
Delirium Experience Questionnaire
The Delirium Experience Questionnaire (DEQ) (Breitbart et al. Reference Breitbart, Gibson and Tremblay2002) was developed to assess recall of the delirium experience and the degree of distress related to the delirium episode experienced by patients, spouses/caregivers, and nurses. The DEQ includes 6 questions for patients who have recovered from an episode of delirium. In addition, 1 question each is directed to the spouses/caregivers and nurses. The following question for the bereaved family members was used in our assessment: “How distressed were you during the patient’s delirium?” Answers were given on a 5-point scale from 0 to 4. Because terminal delirium cannot be adequately evaluated using general delirium assessment scales (Leonard et al. Reference Leonard, Agar and Spiller2014), the 2 were compared for discriminant validity.
Statistical methods
The TDDS-SF was developed using factor analysis followed by varimax rotation. The number of factors was determined using the Scree test. The assessment of construct validity was based on how well a repetition of the factor analysis reproduced the factor loading pattern in the phase of scale development. Convergent validity was examined by calculating Pearson’s correlations between the TDDS-SF and the CES and GDI scores. Discriminant validity was examined using Pearson’s correlations between the TDDS scores and the DEQ distress score of the bereaved family. The internal consistency was assessed using the Cronbach’s alpha coefficient. All statistical analyses were conducted using SPSS statistical software (version 24; IBM Corporation, Armonk, NY, USA).
Results
Development of the Terminal Delirium-Related Distress Scale – Short Form
The 15-item draft questionnaire was based on the 24-item TDDS. The responses were evaluated by factor analysis followed by varimax rotation. Four factors were identified by the Scree test. The first subscale consisted of 5 items related to care and support for the family (“Care for the family” subscale). The second subscale consisted of 3 items related to communication (“Ability to communicate” subscale). The third subscale consisted of 4 items related to hallucinations, delusions, and agitation (“Psychiatric symptoms” subscale), and the fourth subscale consisted of 3 items related to the explanation and discussion about terminal delirium (“Adequate information and discussion about the treatment of delirium” subscale). An English version of the complete scale is presented in Appendix 1.
Validation of the TDDS-SF
Of the 1,710 families receiving surveys, 1,112 (66%) bereaved family members returned the surveys, including 867 (51%) completed responses, and 366 bereaved family members (42% of the responders) indicated that their loved one had experienced delirium. Participant characteristics are shown in Table 1.
Table 1. Bereaved families’ and patients’ characteristics (N = 366)

The results of the factor analysis are shown in Table 2. Repeated factor analysis revealed that the construct validity was good.
Table 2. Factor validity of the terminal delirium related distress scale-short form, optimal 4 domains (N = 358)

a Reverse item.
The convergent validity, assessed by the correlation between the TDDS-SF and the CES2 (r = −0.54, P < 0.001) and GDI (r = −0.54, P < 0.001), was good. Discriminant validity was confirmed by the poor correlation between the TDDS-SF and the distress scores of the bereaved family members on the DEQ (r = 0.23, P < 0.001) (Table 3).
Table 3. Correlation between Terminal Delirium-Related Distress Scale – Short Form and CES・DEQ
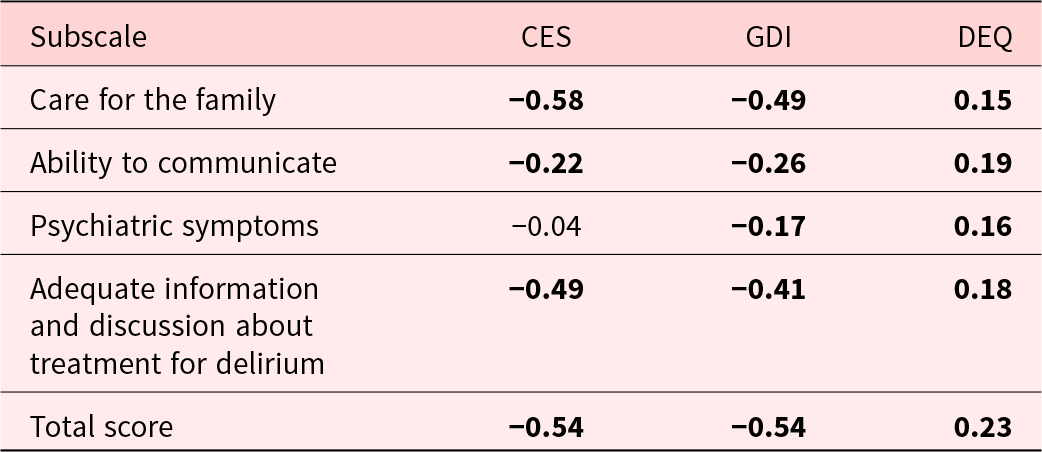
CES = Care Evaluation Scale; GDS = Good Death Inventory; DEQ: Distress score of bereaved family in Delirium Experience Questionnaire.
Bold: P < 0.001.
The TDDS-SF showed good internal consistency (Cronbach’s alpha coefficient for all 15 items = 0.82) (Table 4).
Table 4. Reliability of Terminal Delirium-Related Distress Scale – Short Form
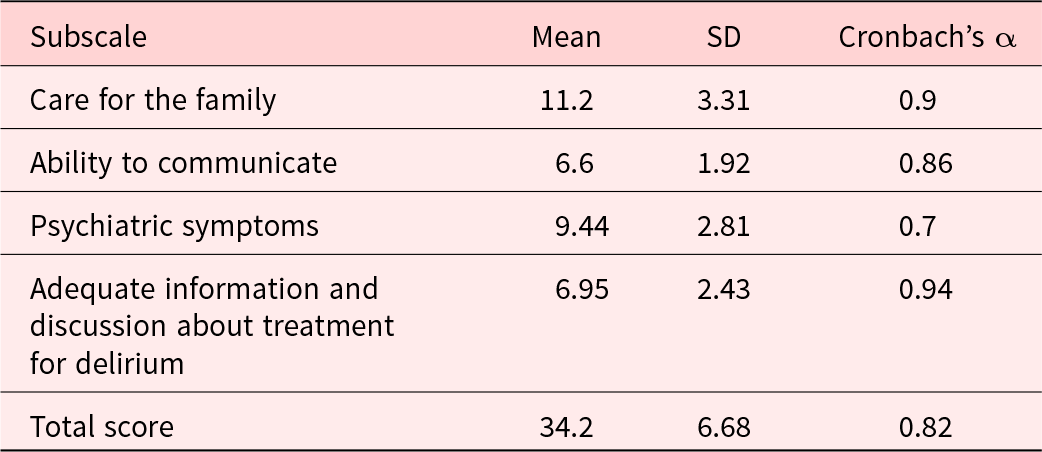
SD = standard deviation.
Discussion
To reduce the burden on terminally ill patients and their families, a shortened version of the TDDS was developed. The original version of the TDDS consists of 24 items. To make this scale easier to use in clinical practice, a 15-item version of the TDDS was created by extracting important items based on the perspective of the bereaved family.
Factor analysis confirmed the scale’s structure. The analysis also indicated that appropriate treatment and care for terminal delirium consists of 4 dimensions: care for the family, ability to communicate, psychiatric symptoms, and adequate information and discussion about the treatment of delirium.
Convergent validity was confirmed by the significant correlations between the 3 subscales of the TDDS-SF and the CES. The subscales of “Care for the family” and “Adequate information and discussion about treatment for delirium” moderately correlated with the total CES score because these 2 subscales are related to the structure and process of care, which are assessed by the CES. The “Psychiatric symptom” subscale did not significantly correlate with the CES because this subscale pertains to symptoms rather than the structure and process of care.
Convergent validity was also demonstrated by the significant correlations between the 4 subscales of the TDS and the GDI. The “Care for the family” and “Adequate information and discussion about treatment for delirium” subscales moderately correlated with the total GDI scores because the content assessed by these 2 subscales is necessary for a good death. The “Ability to communicate” subscale only slightly correlated with the total GDI score because the GDI-short version excluded items on communication. The “Psychiatric symptoms” subscale did not closely correlate with the total GDI score because the short version of the GDI excludes psychiatric symptoms.
Discriminant validity was shown by significant correlations between the 2 subscales of the TDDS and the DEQ.
All subscales of the TDDS-SF slightly correlated with the DEQ. The DEQ assesses the degree of distress related to a reversible delirium episode and includes only the distress of family caregivers. The TDDS-SF specifically targets terminal delirium and evaluates the overall severity of distress caused by terminal delirium, including family caregivers and the patient. Therefore, all of the TDDS-SF subscales significantly correlated with the DEQ. However, the association was weak (Table 3).
Each of the 4 subscales and the total score of the TDDS-SF showed high internal consistency. Due to the specific nature of the target population, we did not assess test–retest reliability.
The development of this scale began with an exploration of the best treatment and care for patients experiencing terminal delirium and their families. The TDDS-SF was developed with the help of family members who were bereaved after their loved ones experienced terminal delirium, not terminal delirium patients and their families who were already in a state of extreme distress.
There are several limitations to this study. First, the questionnaire was not administered immediately after the patient’s death but after some time had passed. Thus, recall bias may have occurred, and the level of distress may vary depending on the time since the bereavement. Second, these results may be difficult to generalize because the study targeted families of patients who died in hospice or palliative care wards. Third, the response rate was 66%. However, only 42% of the respondents represented bereaved families who had experienced terminal delirium. The study was not considered fatally flawed for this reason. Fourth, the subjects were all Japanese, and cross-cultural validation has not been conducted. Fifth, criterion-related validity could not be assessed because no gold standard for terminal delirium has been established.
In conclusion, our results show that the TDDS-SF is a valid and reliable scale for assessing the 4 aspects of terminal delirium care and treatment for bereaved family members. This scale, similar to the original version, may help healthcare providers to provide patients and families with appropriate care and treatment for terminal delirium.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S1478951525000227.
Acknowledgments
This study was carried out by J-HOPE-4 study group. We would like to thank all research collaborators.
Funding
This study was part of the Japan Hospice and Palliative Care Evaluation 2018 (J-HOPE4) Study. This study was funded by the Hospice Palliative Care Japan and JSPS KAKENHI Grant Number 22K10814.
Competing interests
The authors declare that there is no conflict of interest.
Ethical approval
This study was approved by all participating institutional review boards and the Ethics Committee of Tohoku University. It was conducted in accordance with the principles of the Declaration of Helsinki. When the subjects voluntarily completed and returned their questionnaire, we took this to mean they consented to this study.







