Introduction
The origin and evolution of body plans lies in the heart of evolutionary and developmental biology studies. From an evolutionary perspective, the body plans serve as a canvas on which the hierarchical genetic programs are challenged by the most diverse ecological situations to bring about this great improvisation around a theme we see as morphological diversity. In other cases, a body plan is versatile enough so as to be observed performing the most diverse repertoire of behaviors in a lineage with minimal changes. In either case, in understanding the evolution of a Bauplan, the primary goal of the scientist is to be capable of discriminating phylogenetic inertia from adaptation and the latter from exaptation in the observed diversity. Whether traits have predated, accompanied, or followed the evolution of particular functions is the basic inference in order to establish the type of explanations required for morphological evolution.
Anuran amphibians show a distinctive Bauplan among tetrapods, with unique postcranial features that have usually been considered adaptations to a saltatory locomotion (Jenkins and Shubin Reference Jenkins and Shubin1998; Handrigan and Wassersug Reference Handrigan and Wassersug2007; Přikryl et al. Reference Přikryl, Aerts, Havelková, Herrel and Roček2009). This Bauplan is characterized by a short body with a reduced number of presacral vertebrae (6–10), a single bony postsacral element (urostyle) lying between elongated ilia, paired limbs with fused zeugopodian elements, and relatively long hindlimbs with elongated proximal tarsals (Handrigan and Wassersug Reference Handrigan and Wassersug2007). According to fossil evidence this Bauplan would have been achieved at least ca. 200 Ma before the crown-group anurans diversified, as is attested by the Jurassic salientians Prosalirus bitis (Shubin and Jenkins Reference Shubin and Jenkins1995) and Vieraella herbsti (Báez and Basso Reference Báez and Basso1996). Since then, this general body plan has been highly conserved, despite extant taxonomic and ecological diversity observed in frogs, including a variety of locomotor capabilities and specializations (Emerson Reference Emerson1978; Roček Reference Roček2000; Handrigan and Wassersug Reference Handrigan and Wassersug2007). The latter includes strong jumping, hopping, burrowing, walking, swimming, climbing, and even gliding (Emerson Reference Emerson1978, Reference Emerson1979; Emerson et al. Reference Emerson, Travis and Koehl1990), albeit a basic pattern of synchronous bilateral movements of the hindlimbs to propel a short, rigid body underlies several of these locomotor behaviors, both on land or underwater (Nauwelaerts and Aerts Reference Nauwelaerts and Aerts2002).
Locomotion in anurans has been investigated for decades, with several studies converging, in that different aspects of postcranial morphology, mainly limb proportions and features of the sacro-caudo-pelvic complex, correlate to a varying degree with locomotor modes (Zug Reference Zug1972, Reference Zug1978; Emerson Reference Emerson1976, Reference Emerson1979, Reference Emerson1988; Gomes et al. Reference Gomes, Rezende, Grizante and Navas2009; Essner et al. Reference Essner, Suffian, Bishop and Reilly2010; Reilly and Jorgensen Reference Reilly and Jorgensen2011; Jorgensen and Reilly Reference Jorgensen and Reilly2013; Enriquez-Urzelai et al. Reference Enriquez-Urzelai, Montori, Llorente and Kaliontzopoulou2015). Among locomotor modes, jumping has generally been considered to have evolved early in salientian history, together with most prominent features of the anuran Bauplan (e.g., Gans and Parsons Reference Gans and Parsons1966; Jenkins and Shubin Reference Jenkins and Shubin1998; Přikryl et al. Reference Přikryl, Aerts, Havelková, Herrel and Roček2009; Sigurdsen et al. Reference Sigurdsen, Green and Bishop2012; but see Reilly and Jorgensen Reference Reilly and Jorgensen2011 for an alternative view), although this hypothesis has derived from studies that did not incorporate an explicit phylogenetic framework. In any case, the anuran body plan and behavior depart considerably from an ancestral tetrapod condition with a locomotor pattern similar to that seen today in salamanders, which move with lateral bending of the spine and asymmetrical movements of their short limbs (Karakasiliotis et al. Reference Karakasiliotis, Schilling, Cabelguen and Ijspeert2013).
It is noteworthy that the fossil record shows meager evidence of this evolutionary transition, with only two notable exceptions. The earliest known salientians Czatkobatrachus polonicus (Evans and Borsuk-Białynicka Reference Evans and Borsuk-Białynicka1998) and Triadobatrachus massinoti (Piveteau Reference Piveteau1936), both from the Early Triassic, have been considered as representing an intermediate stage in the acquisition of the anuran Bauplan (Jenkins and Shubin Reference Jenkins and Shubin1998; Roček and Rage Reference Roček and Rage2000; Reilly and Jorgensen Reference Reilly and Jorgensen2011). This is most apparent in the peculiar combination of features of T. massinoti, which includes a high number of presacral vertebrae (14) compared with anurans, several discrete caudal vertebrae (at least 6) instead of the urostyle, a sacral vertebra bearing free ribs, moderately elongated ilia, hindlimbs slightly longer than the forelimb, and unfused elements of the zeugopodia (Rage and Roček Reference Rage and Roček1989). In this context, the origin of the anuran Bauplan and saltatory locomotion and the subsequent evolution in early anurans could not be fully understood without considering the basal locomotor capabilities of the earliest salientians in a phylogenetic context. Therefore, we aim to elucidate the main locomotor mode of the early salientian T. massinoti with a multivariate approach in an explicit evolutionary context, using linear morphometrics of the major skeletal elements of the paired limbs. Correlation of these morphometric traits with locomotion in amphibians was tested using multivariate statistics and comparative phylogenetic methods. A discriminant function analysis was performed to assess the ability of transformed morphometric variables to discriminate locomotor groups and to infer the locomotor capabilities of T. massinoti. Our findings, which contrast with some recent hypotheses (Jenkins and Shubin Reference Jenkins and Shubin1998; Přikryl et al. Reference Přikryl, Aerts, Havelková, Herrel and Roček2009; Sigurdsen et al. Reference Sigurdsen, Green and Bishop2012), indicate that some distinct features of the anuran Bauplan were not originally linked to a saltatory locomotion.
Materials and Methods
Taxonomic Sample
Observations of T. massinoti were based on a high-resolution mold of the holotype MNHN MAE126 kindly provided by Ana M. Báez (Fig. 1). The comparative sample (n=188 individuals) primarily included extant limbed amphibians (Anura + Caudata), representing 55 species and 21 families of frogs and 17 species and five families of salamanders (sensu Frost Reference Frost2015; Appendix S1). Additionally, nine species of lizards were sampled to avoid locomotor categories comprising a single taxonomic group (see “Locomotor Modes”) and to adequately polarize evolutionary changes in the phylogeny. This sampling aimed to represent taxonomic, morphological, and locomotor diversity among extant forms (i.e., swimming, lateral undulation, hopping, and jumping; Emerson Reference Emerson1979; O’Reilly et al. Reference O’Reilly, Summers and Ritter2000). Specimens were not sexed prior to measurement; thus we used a random mix of males and females on the premise that regressions of trait residuals on body length did not reveal any trends suggestive of sexual dimorphism in limb traits (Jorgensen and Reilly Reference Jorgensen and Reilly2013). The studied specimens belong to the permanent collections of the following institutions: American Museum of Natural History, New York, U.S.A. (AMNH); Facultad de Ciencias Exactas y Naturales, Universidad de Buenos Aires, Argentina (FCEN); Museo Argentino de Ciencias Naturales “Bernardino Rivadavia,” Buenos Aires, Argentina (MACN); Museo de Ciencias Naturales de la Universidad de Salta, Argentina (MCN); Museum of Comparative Zoology of Harvard, Cambridge, U.S.A. (MCZ); and Museo de Zoología de la Pontificia Universidad Católica del Ecuador, Ecuador (QCAZ).
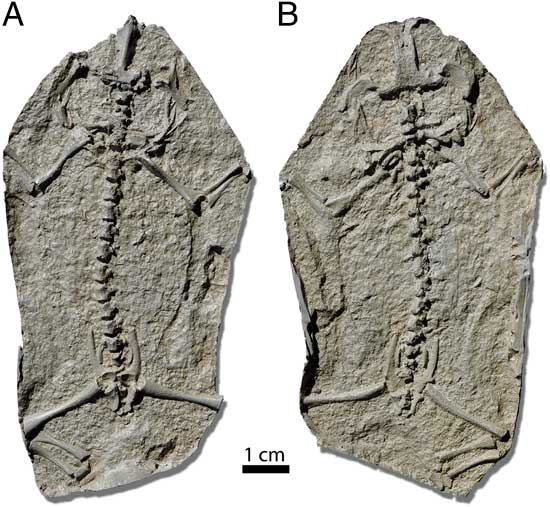
Figure 1 High-resolution cast of Triadobatrachus massinoti; stem Salientia from the Early Triassic of Madagascar. (A) Ventral view and (B) dorsal view. Cast kindly provided by Ana M. Báez.
Limb Measurements
Measurements were selected based on the extensive evidence presented by various authors on the relationship between the morphology of limb elements and the locomotor habits in anurans (e.g., Zug Reference Zug1972; Emerson Reference Emerson1978, Reference Emerson1988; Nauwelaerts and Aerts Reference Nauwelaerts and Aerts2006; Gomes et al. Reference Gomes, Rezende, Grizante and Navas2009; Jorgensen and Reilly Reference Jorgensen and Reilly2013; Enriquez-Urzelai et al. Reference Enriquez-Urzelai, Montori, Llorente and Kaliontzopoulou2015), but also considering the preserved elements of T. massinoti. We measured the maximum length (without epiphyses) of the humerus (Hu), radio-ulna (RU), femur (Fe), tibiofibula (TF), and proximal tarsals (Tar; Fig. 2). For those elements with undistinguishable fused bony epiphyses, we corrected the measurement based on linear regressions (see Appendix S2). Linear measurements were collected from dry specimens, photographs, and radiographic images, depending on the availability of specimens (Appendix S1). We only used radiographic images of specimens in a dorsoventral position, with limbs and feet extended or neatly folded horizontally, in order to provide the same view of all specimens and reduce the effects of parallax. Measurements from radiographs of fixed material have been considered to equal measurements taken manually from the same skeletonized specimens (Jorgensen and Reilly Reference Jorgensen and Reilly2013). This also holds true for measurements taken digitally from photographs of dry skeletons, which has been tested through linear regression (r 2=0.998, p<0.001; see Appendix S2). Data from radiographs and photographs were taken digitally with ScreenCaliper, Version 4.0 (Iconico, New York), whereas those from dry specimens were taken with a manual digital caliper (0.01 mm error).
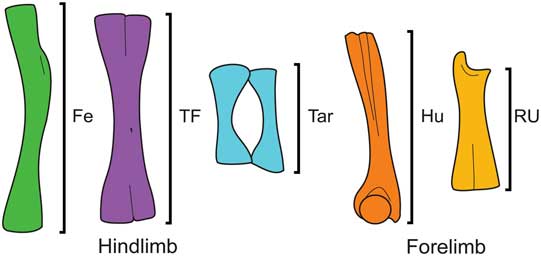
Figure 2 Linear measurements of forelimb and hindlimb elements, as exemplified by Odontophrynus americanus. Maximum lengths were taken without epiphyses. Abbreviations: Fe, femur; TF, tibiofibula; Tar, proximal tarsal; Hu, humerus; RU, radio-ulna.
Prior to statistical analyses we corrected morphometric data for global size differences by dividing each value by the geometric mean of the five linear measurements of each specimen. This transformation, which is derived from the nth root of the product of n measurements and the ratio of any particular measurement to the overall geometric mean (Mosimann and James Reference Mosimann and James1979), is robust in recovering similarities in shape or proportions if isometric size differences are disregarded (Jungers et al. Reference Jungers, Falsetti and Wall1995).
Locomotor Modes
All species were assigned a primary locomotor mode. Locomotor modes for extant species were obtained from the literature (e.g., Emerson Reference Emerson1978, Reference Emerson1979, Reference Emerson1988; Taigen et al. Reference Taigen, Emerson and Pough1982; Jorgensen and Reilly Reference Jorgensen and Reilly2013), Web resources (AmphibiaWeb 2015) or personal observations (see Appendix S1). As was done in most previous studies, we considered jumping performance of paramount importance in characterizing terrestrial locomotion of anurans. Species were scored as hoppers or jumpers based on the definition of Emerson (Reference Emerson1979) that regards jumpers (J) as those frogs that jump more than eight to nine body lengths in a single leap. Frogs that hop usually also walk (Emerson Reference Emerson1979), and hence were accordingly classified as hoppers/walkers (HW). This category also encompasses frogs that walk but typically do not hop, such as species of Pseudophryne. Even though burrowing has been typically considered a locomotor mode of many anurans (Emerson Reference Emerson1978, Reference Emerson1979, Reference Emerson1988; Taigen et al. Reference Taigen, Emerson and Pough1982; Jorgensen and Reilly Reference Jorgensen and Reilly2013), we consider this behavior as an activity pattern related to substrate use rather than a locomotor mode (a distinction that has previously been made for xenarthran mammals; Toledo et al. Reference Toledo, Bargo, Cassini and Vizcaíno2012). Similarly, although “arboreal” and “terrestrial” have previously been used as categories in ecomorphological studies of anuran locomotion (e.g., Emerson Reference Emerson1978, Reference Emerson1979, Reference Emerson1988; Zug Reference Zug1978; Gomes et al. Reference Gomes, Rezende, Grizante and Navas2009; Jorgensen and Reilly Reference Jorgensen and Reilly2013), we did not include those in our categorization, because we focused on locomotor modes and not on substrate preference or habitat. Swimming ability is almost ubiquitous among frogs, with most species making use of aquatic environments in the mating season and/or to escape from predators. Thus, we only classified as swimmers (Sw) those species that are almost totally aquatic (e.g., pipids, Pseudis spp., and some species of Telmatobius). It is noteworthy that in spite of their ecological diversity, salamanders exhibit the same overall pattern of limb kinematics whether in an aquatic, semiaquatic, or terrestrial environment (Ashley-Ross et al. Reference Ashley-Ross, Lundin and Johnson2009). Thus, we classified the “lateral undulatory” (LU) locomotion exhibited by salamanders and lizards as a single category.
Statistical Analyses
To evaluate the relationship between limb proportions and locomotor modes previously reported for anurans (e.g., Emerson Reference Emerson1988; Jorgensen and Reilly Reference Jorgensen and Reilly2013; Enriquez-Urzelai et al. Reference Enriquez-Urzelai, Montori, Llorente and Kaliontzopoulou2015), we first conducted a multivariate analysis of variance (MANOVA) to test whether the morphometric variables differ among locomotor groups in our sample. We used all five size-corrected linear measurements as the dependent variables and locomotor modes as the independent variable (four levels: J, Sw, HW, LU). We performed Unequal N HSD post hoc tests (p=0.05) to assess differences in group means. Assumptions of the model were checked before the analysis.
To properly test trait correlations independently of phylogenetic history, we conducted a phylogenetic generalized least-squares (PGLS) analysis (Grafen Reference Grafen1989; Martins and Hansen Reference Martins and Hansen1997) as implemented in NTSYS pc v. 2.11j (Rohlf Reference Rohlf2004), which has been recognized as a robust comparative phylogenetic method (Rohlf Reference Rohlf2001; Barr and Scott Reference Barr and Scott2014; Symonds and Blomberg Reference Symonds and Blomberg2014). An advantage of comparative methods over others also used in ecomorphological approaches, such as a discriminant analysis, is that lack of significance in the results would mean that trait correlation might be due to common ancestry alone (Barr and Scott Reference Barr and Scott2014). The PGLS analysis was performed on the size-corrected limb variables with respect to the locomotor categories, which were scored as dummy variables, with a phylogenetic covariance matrix to account for phylogenetic structure of the data set. The covariance matrix was constructed using a supertree (Supplementary Fig. 1) based on topologies derived from the two largest available molecular phylogenetic analyses, one of amphibians (Pyron and Wiens Reference Pyron and Wiens2011) and the other of Squamata (Pyron et al. Reference Pyron, Burbrink and Wiens2013); we trimmed the trees to match our taxonomic sample and arbitrarily ultrametricized them using Mesquite, Version 2.75 (Maddison and Maddison Reference Maddison and Maddison2011). Despite having branch lengths for Batrachia and Squamata, we arbitrarily constructed an ultrametric tree, because we lacked branch length data between these clades. For a few species that were not included by Pyron and Wiens (Reference Pyron and Wiens2011) and Pyron et al. (Reference Pyron, Burbrink and Wiens2013), we used a congeneric species from their tree.
Once we tested for trait correlation with PGLS, a discriminant function analysis (DFA) was performed with the aim of enhancing separation among locomotor categories and then obtaining functions that would allow us to classify T. massinoti. We built discriminant functions using the five size-corrected morphometric variables and the four already defined locomotor groups (J, HW, LU, and Sw). Discriminant functions were estimated without differential weighting of the variables and with probabilities independent of group size. We evaluated the effectiveness of our method (i.e., we tested the existence of significant differences between the groups) by applying the Wilks’s lambda test (Barr and Scott Reference Barr and Scott2014). In addition, we evaluated the accuracy of DFA by performing a jackknife cross-validation (Davis and McHorse Reference Davis and McHorse2013) in the MASS package in R (Venables and Ripley Reference Venables and Ripley2002; R Development Core Team 2012). The classification for each specimen was achieved by withholding the specimen, calculating a new DFA using the remaining individuals, and using the resulting DFA to classify this specimen (Lachenbruch and Mickey Reference Lachenbruch and Mickey1968). Both MANOVA and DFA were performed using Statistica, Version 8.0 (StatSoft 2007).
Results
Median values of the bony elements measured for each group are shown in Figure 3. According to the MANOVA they significantly differed among locomotor groups (F 15, 497.3=101.4; p<0.001; Fig. 3). Post hoc comparisons showed that all locomotor modes differ in at least one variable from the other habits. Despite these differences, jumpers and swimmers are the most statistically similar groups, being alike in all the variables (Fe, TF, Tar, and Hu) except for RU. Species considered jumpers and swimmers presented significant length differences from the HW and LU for all the elements considered.

Figure 3 Box plot of the MANOVA comparing limb variables against locomotor modes. Boxes represent the 25%/75% quartiles, and the median is shown with a horizontal line. The minimal and maximal values are shown with short horizontal lines. Variables marked with the same letter are not significantly different (Unequal N HSD post hoc tests, p>0.05). Abbreviations: Fe, femur; TF, tibiofibula; Tar, proximal tarsal; Hu, humerus; RU, radio-ulna.
The PGLS analysis showed a significant association between the length of limb elements and the locomotor modes in our sample (Wilks’s λ=0.372; F 20, 243.1=4.213; p<0.001). This result would imply that trait correlation exists beyond shared evolutionary history, and therefore limb morphometrics would serve as a reliable proxy to infer locomotor capabilities.
The DFA was statistically significant (Wilks’s λ=0.02093; approximate F 15, 497=101.4; p<0.001), indicating substantial variation on element proportions to permit discrimination of locomotor groups. The analysis correctly identified more than 82% of the 188 specimens analyzed (Table 1), with very similar classification values after jackknifing the data set (Table 1; Fig. 4). Locomotor groups showed high reclassification success rates (>75%) except for swimmers that were correctly classified in only half of the cases. It has to be noted that most of the highly specialized swimmers included, namely pipids and species of Pseudis, were ascribed to their locomotor group, and this low classification value is due to misclassification of less specialized swimming forms either as J or HW (Appendix S3). However, the majority of these misclassifications also showed relatively low posterior probabilities (<70%), with the Sw category always being the second most probable choice in those cases (Appendix S3). Among frogs, jumpers and hopper/walkers were virtually never classified as the other, but jumpers were misclassified as Sw in 24% of the cases (Appendix S3). It is noteworthy that all the species of frogs analyzed here showed almost 0% of posterior probability of being classified as LU, a category with a 100% reclassification success rate. Applying the computed classification functions to T. massinoti resulted in this specimen being classified as an LU with a high posterior probability of over 99.5% (Fig. 4; see Appendix S3).

Figure 4 First two canonical axes (Root 1, Root 2) from the discriminant analysis. The different locomotor categories are indicated by different colors. Triadobatrachus massinoti is represented as a black cross, and some representative taxa of each locomotor group are shown.
Table 1 Reclassification matrix of the discriminant function analysis. Results of the cross-validation and the leave-one-out jackknife approaches are shown before and after the slash (/), respectively. Correct classifications are marked in bold. Locomotor modes: J, jumper; Sw, swimmers; HW, hopper/walker; LU, lateral undulatory.

Locomotor modes: J=jumper; Sw=swimmers; HW=hopper/walker; LU=lateral undulatory.
Discussion
The anuran Bauplan is frequently depicted as both highly conserved across the order and morphologically divergent from other tetrapods (Handrigan and Wassersug Reference Handrigan and Wassersug2007). This has often been interpreted in adaptive explanations as being of functional significance, with a direct link to locomotion (saltatory, more precisely; Jenkins and Shubin Reference Jenkins and Shubin1998). To test this assumption of concurrence of form and function in anurans, we first need accurate reconstructions of primitive morphologies and locomotor habits on stem salientians.
Correlation Between Limb Morphology and Locomotion
Our study shows that limb proportions of extant amphibians correlate with locomotor modes, a pattern that has previously been described for anurans by various authors (Zug Reference Zug1972, Reference Zug1978; Emerson Reference Emerson1978, Reference Emerson1988; Gomes et al. Reference Gomes, Rezende, Grizante and Navas2009; Jorgensen and Reilly Reference Jorgensen and Reilly2013; Enriquez-Urzelai et al. Reference Enriquez-Urzelai, Montori, Llorente and Kaliontzopoulou2015). This was evidenced in different ways by the results of the MANOVA and DFA (Figs. 3 and 4), as well as those of the PGLS, which also indicated that limb traits correlate with locomotor modes when the phylogenetic structure of the data is considered. Among frogs, those that usually hop and/or walk (HW) are characterized by relatively short limbs with femora longer than tibiofibulae and shorter and stouter proximal tarsals than other anurans. Jumping frogs (J) typically have comparatively longer hindlimbs in which the tibiofibulae are the major limb bone and the proximal tarsals are long and slender. These proportions would improve jumping performance by increasing the time in which the muscular force applied against the substrate acts (Zug Reference Zug1972; Emerson Reference Emerson1978). Swimming frogs (Sw), like jumpers, have long and muscular hindlimbs and comparatively short forelimbs, but many of them have an Fe/TF ratio more similar to that of HW. Globally, anurans are readily distinguishable from animals with lateral undulatory locomotion in their limb proportions. Additionally, the different locomotor modes of anurans considered here are also discernible to some extent based on a few linear measurements, particularly when comparing jumping with nonjumping anurans, disregarding their evolutionary history. Compared with anurans, salamanders have a relatively long body with forelimbs and hindlimbs of similar length, in which the femur is always longer than the unfused tibia and fibula and the proximal tarsals are short. They also contrast with anurans in their gait pattern, which involves lateral body undulation and asynchronous limb movements (Ashley-Ross et al. Reference Ashley-Ross, Lundin and Johnson2009).
This correlation between limb morphology and locomotion allowed us to infer locomotor abilities in fossil taxa like T. massinoti. This, in turn, may contribute to unraveling the origin of the anuran Bauplan and locomotion during early salientian evolution.
Phylogenetic Position and Ontogenetic Stage of Triadobatrachus
Several authors have tried to infer the modes of locomotion of T. massinoti and determine its phylogenetic relationships and ontogenetic stage (Piveteau Reference Piveteau1937; Hecht Reference Hecht1962; Griffiths Reference Griffiths1963; Estes and Reig Reference Estes and Reig1973; Roček and Rage Reference Roček and Rage2000). Currently, there is broad consensus that T. massinoti is more related to frogs than to salamanders, being recovered as the sister group of the remaining Salientia (Báez and Basso Reference Báez and Basso1996; Gao and Wang Reference Gao and Wang2001; Gao and Chen Reference Gao and Chen2004). However, the ontogenetic stage of the holotype, which has even been interpreted as larval, has largely been debated (Griffiths Reference Griffiths1956, Reference Griffiths1963; Hecht Reference Hecht1962; Estes and Reig Reference Estes and Reig1973; Rage and Roček Reference Rage and Roček1986). In agreement with Hecht (Reference Hecht1962) and Rage and Roček (Reference Rage and Roček1986), we consider that the latter interpretation is unfounded and that the holotype would instead represent a postmetamorphic individual. This is evidenced by the extent of cranial bones that begin to ossify late in amphibian metamorphosis, or even after metamorphosis, such as the sphenethmoid and some dermal bones of the suspensorium (Maglia et al. Reference Maglia, Pugener and Mueller2007; Weisbecker and Mitgutsch Reference Weisbecker and Mitgutsch2010), together with the well-ossified posteromedial processes of the hyoid (Rage and Roček Reference Rage and Roček1986; R. O. Gómez personal observation). Also, well-ossified carpals and tarsals of the holotype contrast with the cartilaginous condition of anuran larvae (Rage and Roček Reference Rage and Roček1986), which is also that of many juvenile frogs and adult salamanders (Marjanović and Witzmann Reference Marjanović and Witzmann2015; R. O. Gómez personal observation).
On the other hand, unossified long bone epiphyses, as in the holotype of T. massinoti, have previously been considered indicative of immaturity (Rage and Roček Reference Rage and Roček1986). Nevertheless, basal salientians such as Notobatrachus degiustoi and species of Liaobatrachus are represented by articulated and well-preserved specimens also lacking ossified epiphyses that have been interpreted as mature individuals (Báez and Basso Reference Báez and Basso1996; Gao and Wang Reference Gao and Wang2001; Gao and Chen Reference Gao and Chen2004; Dong et al. Reference Dong, Roček, Wang and Jones2013). Additionally, in several extant amphibian species, particularly salamanders, the epiphyses ossify late during ontogeny or even remain cartilaginous throughout most of their life (Marjanović and Witzmann Reference Marjanović and Witzmann2015; R. O. Gómez personal observation). Therefore, morphology of the holotype of T. massinoti is consistent with that of adults of many amphibian species, and interpreting it as an immature individual requires additional ad hoc assumptions.
Triadobatrachus Morphology and Locomotion
Our results show that the limb proportions of T. massinoti, though somewhat intermediate, are more similar to those of salamanders than of frogs, which is also reflected in the high posterior probability with which it was assigned to the LU group. These findings would indicate that salamander-like asynchronous lateral undulatory movements were an important part of the locomotor skill repertoire of this taxon. This hypothesis is consistent with that of previous authors, who considered that T. massinoti’s axial and appendicular morphology “could have allowed quick crawling … but certainly not effective jumping” (Rage and Roček Reference Rage and Roček1989: p. 15).
In contrast, it has recently been suggested, based primarily on the morphology of the humeral deltopectoral crest, allegedly similar to that of jumping frogs, “that hopping or jumping was an important form of locomotion” of T. massinoti, “perhaps combined with salamander-like crawling” (Sigurdsen et al. Reference Sigurdsen, Green and Bishop2012: p. 87). Nevertheless, this morphology (Sigurdsen et al. Reference Sigurdsen, Green and Bishop2012: Fig. 4) is also present in nearly all anurans, regardless of their locomotor mode. Besides, humeral morphology might also be related to other aspects of anuran behavior, including feeding and mating (Duellman Reference Duellman1992; Grey et al. Reference Grey, O’Reilly and Nishikawa1997; Sigurdsen et al. Reference Sigurdsen, Green and Bishop2012). In this regard, the use of forelimbs during amplexus is ubiquitous among anurans and unique within amphibians (Wells Reference Wells2007). If this mating behavior evolved early in salientian history, this might provide an alternative scenario for the humeral morphology seen in T. massinoti.
Other authors, based on sacro-caudo-pelvic complex morphology, have proposed that T. massinoti was able to move through frog-like synchronous limb movements, either by jumping on land (Shubin and Jenkins Reference Shubin and Jenkins1995; Jenkins and Shubin Reference Jenkins and Shubin1998) or swimming in an aquatic environment (Hecht Reference Hecht1962; Estes and Reig Reference Estes and Reig1973). It has to be noted that the sacro-caudo-pelvic complex of T. massinoti shows a distinctive mosaic of features not seen in any other amphibian (Fig. 5C,D), which makes it difficult to interpret this structure functionally. This complex includes a pelvis with a derived iliac morphology similar to that of frogs, but unlike in frogs, the ischia are not fused. In turn, sacral morphology exhibits the plesiomorphic condition today seen in salamanders, with recurved ribs articulating with robust transverse processes (Rage and Roček Reference Rage and Roček1989; Fig. 5A,B). This configuration suggests that the sacroiliac joint would be like that of salamanders, in which the sacral rib laterally contacts the medial surface of the iliac shaft via a ligamentous connection (Rage and Roček Reference Rage and Roček1989; Jenkins and Shubin Reference Jenkins and Shubin1998; Gardner et al. Reference Gardner, Roček, Přikryl, Eaton, Blob and Sankey2010). This condition markedly contrasts with that of frogs (Fig. 5E,F), in which the sacral diapophyses contact the dorsal surface of the iliac shaft ventrally (Reilly and Jorgensen Reference Reilly and Jorgensen2011).
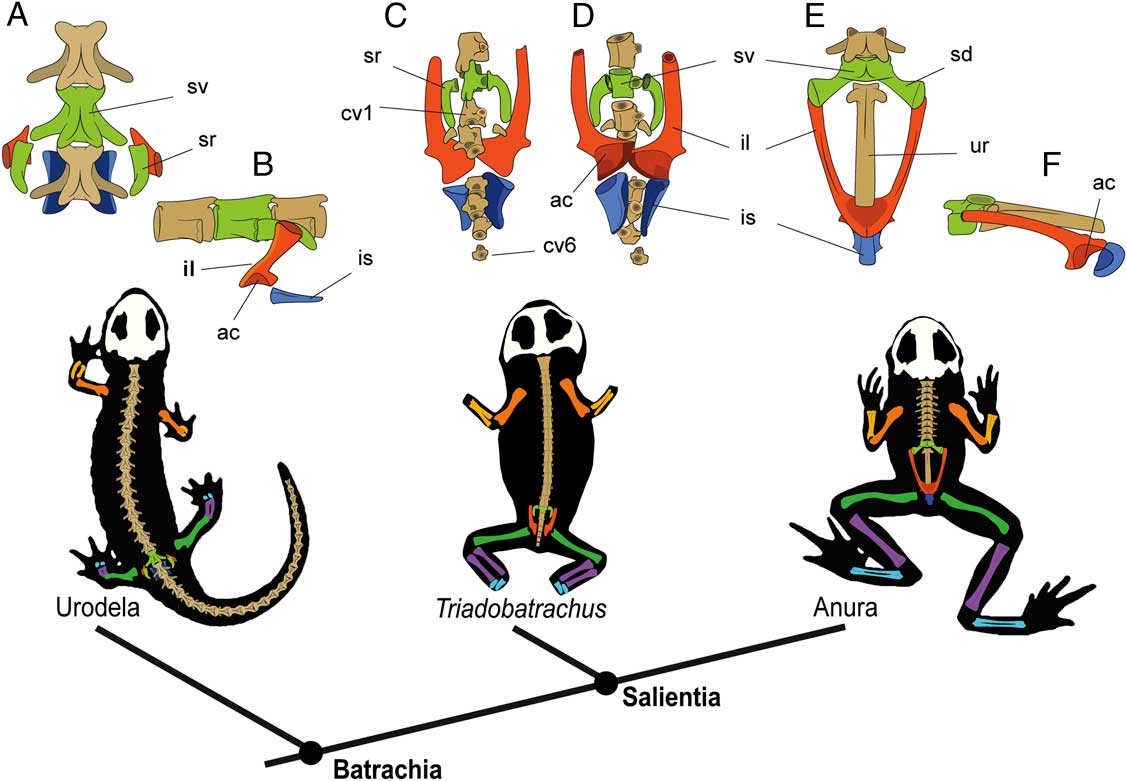
Figure 5 Stylized diagrams of representative batrachian pelvic girdles and limb proportion in a phylogenetic context. Urodele sacro-caudo-pelvic complex in dorsal (A) and left lateral (B) view. Triadobatrachus massinoti sacro-caudo-pelvic complex in dorsal (C) and ventral (D) view. Anura sacro-caudo-pelvic complex in dorsal (E) and left lateral (F) view. Abbreviations: sv, sacral vertebra; sr, sacral rib; sd, sacral diapophyses; il, ilium; is, ischium; cv, caudal vertebra; ac, acetabulum.
Triadobatrachus is also unique among salientians in lacking a urostyle and having instead at least six caudal vertebrae, of which the most anterior articulates with the sacral neural arch through well-developed zygapophyses (Rage and Roček Reference Rage and Roček1989: Fig. 3). These caudal vertebrae should not be considered as completely homologous to the frog urostyle, since the latter is formed by the fusion of a coccyx composed of a few neural arches and the ossified hypochord (Ročková and Roček Reference Ročková and Roček2005; Pugener and Maglia Reference Pugener and Maglia2009). The condition of T. massinoti may also not be functionally analogous to the urostyle of jumping frogs, which allows substantial dorsoventral excursion at the sacro-urostylic joint and remains stiffened, linked to the pelvis during jumping by means of the coccygeoiliacus muscle (Shubin and Jenkins Reference Jenkins and Shubin1998). In brief, sacro-caudo-pelvic complex morphology does not provide unequivocal evidence of the jumping capability of this basal salientian. In line with previous work in extant anurans (e.g., Emerson Reference Emerson1988; Enriquez-Urzelai et al. Reference Enriquez-Urzelai, Montori, Llorente and Kaliontzopoulou2015), limb proportions might be a better indicator of locomotor mode than the sacro-caudo-pelvic complex, at least in the present case.
Did Triadobatrachus Jump?
Taking into consideration all the above-discussed evidence, we conclude that T. massinoti traveled mainly by undulations of the body with asynchronous movements of the limbs and would have been unable to leap or jump like living frogs. Saltatory anuran locomotion has usually been associated with features of a morphofunctional complex formed by the ilia, sacrum, urostyle, and hindlimbs (Emerson and De Jongh Reference Emerson and De Jongh1980; Jenkins and Shubin Reference Jenkins and Shubin1998; Přikryl et al. Reference Přikryl, Aerts, Havelková, Herrel and Roček2009; Reilly and Jorgensen Reference Reilly and Jorgensen2011; Sigurdsen et al. Reference Sigurdsen, Green and Bishop2012; Jorgensen and Reilly Reference Jorgensen and Reilly2013). However, when the morphology of T. massinoti is studied in a phylogenetic context, it appears that elements of this complex did not evolve in a concerted fashion (Fig. 5). This is evident in the early appearance of a frog-like ilium in salientian evolution, as shown by T. massinoti, preceding that of other distinct features of the anuran Bauplan, such as fused zeugopodial bones and urostyle (Fig. 5). This evolutionary decoupling is partially mirrored in the ontogeny of extant frogs, which achieve adult locomotor behavior before the end of metamorphosis, when the limbs are already functional but the sacrum, urostyle, and pelvis are still disarticulated (Fabrezi et al. Reference Fabrezi, Manzano, Abdala and Lobo2014). In this context, derived postcranial features that T. massinoti shares with frogs, like iliac morphology, would not have originally been linked to a saltatory locomotion and, thus, they might have been co-opted as exaptations in jumping anurans. These interpretations widen the morphological gap between T. massinoti and the earliest frog-like salientians, leaving open questions about the role of locomotion in the origin of the anuran Bauplan.
Acknowledgments
We are grateful to A. Báez (Universidad de Buenos Aires), who kindly provided the high-resolution mold of the holotype of Triadobatrachus massinoti. Thanks are extended to M. Fabrezi (Museo de Ciencias Naturales, Salta) and J. Faivovich (Museo Argentino de Ciencias Naturales) for access to materials under their care. Thanks to P. Milla Carmona (Universidad de Buenos Aires) for his help with the statistical data analyses. This work was supported by grants from Agencia Nacional de Promoción Científica y Tecnológica, and the Universidad de Buenos Aires (UBACyT). A.I.L. is a postgraduate fellow of Consejo Nacional de Investigaciones Cientificas y Tecnológicas (CONICET). R.O.G. and I.M.S. are members of Carrera del Investigador Científico (CONICET).
Supplementary Material
Supplemental materials deposited at Dryad: http://dx.doi.org/10.5061/dryad.55c3d








