Introduction
Hominins, Hyenas, and Rodents
The relationship between hyenas and hominins has been considered by several authors as an important aspect of mutual evolution in the use of space, hunting resources, or scavenging strategies (Stiner Reference Stiner1991, 1994, Reference Stiner2004; Brantingham Reference Brantingham1998). There are also descriptions of frequent hominid–hyena associations during the Plio-Pleistocene (e.g., Bunn et al. Reference Bunn, Harris, Isaac, Kaufulu, Kroll, Schick, Toth and Behrensmeyer1980; Binford Reference Binford1981; Brain Reference Brain1981; Potts Reference Potts1989; Shipman and Walker Reference Shipman and Walker1989; Blumenschine et al. Reference Blumenschine, Cavallo and Capaldo1994). Stiner et al. (Reference Stiner, Munro and Surovell2000) postulated that humans could not have fed solely on large animals, given the high cost to obtain this food source, but must also have fed on small game and other animal protein sources (such as small mammals), which must have had an important role in their daily subsistence activity. We have found evidence of hyenas preying upon rodents in the Bois Roche Pleistocene site, as humans did in the past and even do today (Lupo and Schmitt Reference Lupo and Schmitt2005; Sealy Reference Sealy2006; Rodríguez-Hidalgo et al. Reference Rodríguez-Hidalgo, Saladié and Canals2011; Medina et al. Reference Medina, Teta and Rivero2012; Fernández-Jalvo and Andrews Reference Fernández-Jalvo and Andrews2016). The initial interpretation of Bois Roche was as a site where humans and hyenas coexisted due to the presence of stone artifacts and putative bone tools and ornaments. Microscopic analyses of the bone tools and stone artifacts showed that the former were modified by hyenas and the latter were carried into the site by gravity and slope wash (Villa and Bartram Reference Villa and Bartram1996; d’Errico and Villa Reference d’Errico and Villa1997; Villa and Soressi Reference Villa and Soressi2000; Villa and d’Errico Reference Villa and d’Errico2001).
Foraging habits have definitively changed in the human lineage (Diamond Reference Diamond2002). Evidence from the cave site of Bois Roche indicates that a change also occurred in hyenas, due in part to the environmental context and the nature of this hyena den. Pokines and Kerbis Peterhans (Reference Pokines and Kerbis Peterhans2007) showed strong differences between bone accumulations in burrows versus caves in African environments, due mainly to the small size and impermanence of the burrows. According to these authors, cave dens allow higher numbers of individuals to occupy a site with longer periods of occupation. Dens also lead to improved rates of bone preservation when compared with open-air sites. Bois Roche has been interpreted as a hyena den that functioned as a maternity cave site (Marra et al. Reference Marra, Villa, Beuval, Bonfiglio and Goldberg2004; Villa et al. Reference Villa, Castel, Bourdillat, Beauval and Goldberg2004).
Excavations at Bois Roche, (see map, Fig. 1) in 1995, 1997, and 1998 by P. Villa and L. Bartram and in 1999 and 2000 by Villa yielded high quantities of small mammal bones. The history of the excavations of the site and the general cave morphology are documented elsewhere by Bartram and Villa (Reference Bartram and Villa1998), Marra et al. (Reference Marra, Villa, Beuval, Bonfiglio and Goldberg2004), and Villa et al. (Reference Villa, Castel, Bourdillat, Beauval and Goldberg2004, Reference Villa, Sánchez-Goñi, Cuenca Bescós, Grün, Ajas, García Pimienta and Lees2010). Two major stratigraphic units have been distinguished in the site (units 1 and 2), with four subdivisions of the upper unit 1 (Goldberg Reference Goldberg2001; Marra et al. Reference Marra, Villa, Beuval, Bonfiglio and Goldberg2004). The electron spin resonance (ESR) averaged value of six samples from units 1c and 2 is 69.7 ± 4.1 Ka, placing the site at the beginning of MIS 4, that is, in the upper Pleistocene during a period of cold climate (Villa et al. Reference Villa, Sánchez-Goñi, Cuenca Bescós, Grün, Ajas, García Pimienta and Lees2010). With the exception of a high concentration of amphibians and squamate reptiles (minimum number of individuals [MNI]=4,851) from all levels (Blain and Villa Reference Blain and Villa2006), most small vertebrate bones were the remains of small mammals. These authors noted: “Herpetofauna suggests a very open environment, with damp meadows and small grove areas of broadleaved trees and conifers” (Blain and Villa Reference Blain and Villa2006: p. 30).
Figure 1 Location map of the site of Bois Roche in France.
Taxonomic analysis of the small mammals of the 1995 material was undertaken by C. Sesé at the Museo Nacional de Ciencias Naturales, Madrid (Sesé and Villa Reference Sesé and Villa2008). Villa et al. (Reference Villa, Sánchez-Goñi, Cuenca Bescós, Grün, Ajas, García Pimienta and Lees2010) analyzed subsequent material obtained from the site during the 1997 through 2000. Sesé and Villa (Reference Sesé and Villa2008) and G. Cuenca Bescós (in Villa et al. Reference Villa, Sánchez-Goñi, Cuenca Bescós, Grün, Ajas, García Pimienta and Lees2010) described an assemblage dominated by Microtus gregalis (94% and ~80 %, respectively), with Arvicola terrestris present in much lower abundance. In terms of paleoenvironmental reconstruction, the northern water vole, A. terrestris, is known to inhabit waterside environments and thus represents locally moist conditions, and M. gregalis is a typical inhabitant of the cold steppe, living today in arctic areas (Sesé and Villa Reference Sesé and Villa2008).
Preliminary taphonomic results were provided in the above-cited studies, suggesting the contribution of hyenas in introducing the small mammals to the site. Villa et al. (Reference Villa, Sánchez-Goñi, Cuenca Bescós, Grün, Ajas, García Pimienta and Lees2010) also suggested that nocturnal birds of prey were involved in the predation and deposition of small vertebrates in the cave. In this paper, we present a detailed taphonomic analysis of the small mammal fossil assemblage of Bois Roche in order to understand the action of predators and “any” natural forces involved in the deposition of the microfossil assemblage. Having identified the predator (or predators), it is then possible to consider how predation or other taphonomic bias might influence the reconstruction of past environments.
Small mammal Taphonomy
Bones and teeth of small mammals are regularly recovered from caves (Andrews Reference Andrews1990; Bramwell et al. Reference Bramwell, Yalden and Yalden1990; Avery Reference Avery1992; Fernández-Jalvo Reference Fernández-Jalvo1995) and open-air sites spanning wide geological and archaeological time periods (Buckland Reference Buckland1976; Mayhew Reference Mayhew1977; Andrews Reference Andrews1983; Wesselman Reference Wesselman1984; Maas Reference Maas1985; Denys Reference Denys1986; Dobney et al. Reference Dobney, Jaques, Carrott, Hail, Issitt and Large1996; Fernández-Jalvo et al. Reference Fernández-Jalvo, Denys, Andrews, Williams, Dauphin and Humphrey1998; Murphey et al. Reference Murphey, Torick, Bray, Chandler and Evanoff2001). At cave sites, the relative lack of active transportation and protection from aerial weathering processes combine to increase the possibility of deposited material being recovered during excavation. Caves also provide excellent locations for habitation and shelter for many birds and mammals (including humans), and over time, large deposits of dietary waste (and cultural material) can accumulate at these sites.
Taphonomic analysis of specific patterns of modification (such as breakage and digestion) of the small mammal bones and teeth from these sites can aid in the identification of the accumulation agent (e.g., Andrews Reference Andrews1990; Fernández-Jalvo and Andrews Reference Fernández-Jalvo and Andrews2016). These patterns are compared with those from actualistic studies of pellets or coprolites of modern avian and mammalian predators. The purpose of conducting a taphonomic analysis of small mammal deposits is to investigate how the fossil community represents the community from which it was originally drawn. Small mammals are sensitive to climatic and environmental constraints and are therefore good proxy indicators of past environments. Taphonomic analysis of small mammal accumulations increases the potential information on climate and environment by: (1) identifying a predator and its habits, (2) recognizing possible biases due to hunting methods and prey preferences (Andrews Reference Andrews1990; Fernández-Jalvo et al. Reference Fernández-Jalvo, Denys, Andrews, Williams, Dauphin and Humphrey1998), (3) detecting postdepositional hydrodynamic sorting and transport (Dodson and Wexlar Reference Dodson and Wexlar1979; Korth Reference Korth1979), and (4) by taking into account bone breakage or mixtures due to reworking processes (Fernández-Jalvo et al. Reference Fernández-Jalvo, Andrews, Sevilla and Requejo2014).
Identifying Specific Predators
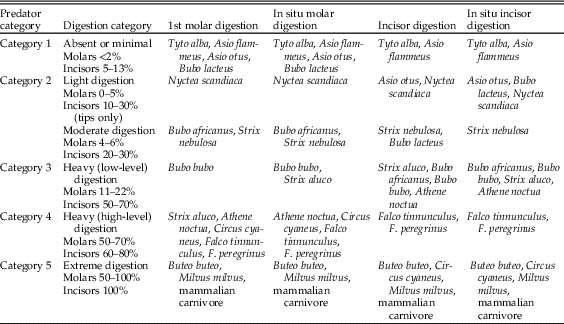
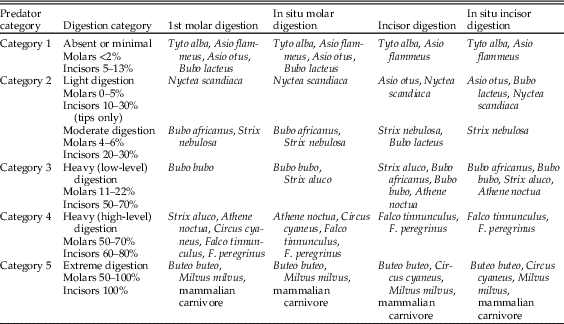
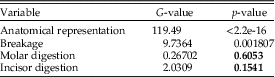
One of the most valuable pieces of information provided by taphonomic analyses of microfauna is the detection and identification of predators as sources of a bone assemblage. Analysis of actualistic data from dietary waste from avian and mammalian predators of small mammals was carried out by Andrews (Reference Andrews1990). Data were collected for breakage of long and cranial bones and digestion of molars, incisors, and some long-bone epiphyses. At a general level, Andrews showed that taphonomic differences exist among owls, diurnal raptors, and mammalian predators, and for the molar and incisor digestion data, it is possible to group specific species based on similarities in results. These groups and the respective levels of digestion are shown in Table 1. Statistical analysis of these data has been carried out, and it has been demonstrated that there is significantly more variation between these groups than within them (Williams Reference Williams2003).
Table 1 Summary of digestion category on molars (1st molar and in situ molars in jaws) and incisors (both isolated and preserved in jaws) of small-mammal prey (Fernández-Jalvo et al. [Reference Fernández-Jalvo, Andrews, Denys, Sesé, Stoetzel, Marin-Monfort and Pesquero2016], modified from Andrews [Reference Andrews1990] and Demirel et al. [Reference Demirel, Andrews, Yalçinkaya and Ersoy2011]).
There are not many small mammal taphonomic analyses from scats of modern large carnivores (above 30 kg), with some notable exceptions (Gómez Reference Gómez2003; Montalvo et al. Reference Montalvo, Pessino and González2007). Gómez’s (Reference Gómez2003) work was based on an experimental study of pumas fed on rodents in a zoo, but the sample of rodents recovered from the scats was too small for the taphonomic methodology to be fully applied. Montalvo et al. (Reference Montalvo, Pessino and González2007) collected 76 scats of pumas from the wild; they recovered enough small mammals to apply the methodology used in this paper (Andrews Reference Andrews1990). Montalvo et al. (Reference Montalvo, Bisceglia, Kin and Sosa2012) also studied 179 scats of Geoffroy’s cat (Leopardus geoffroyi), but the taphonomic results are similar to those of other small mammalian carnivores such as wild cats (López et al. Reference López, Rossi, Tabeni, Bender and Chiavazza2017), coyotes, and foxes (Andrews Reference Andrews1990), which are much more destructive than large carnivores.
The macrofaunal fossil bone assemblage at Bois Roche has been shown to have been accumulated by hyenas (Villa et al. Reference Villa, Castel, Bourdillat, Beauval and Goldberg2004, Reference Villa, Sánchez-Goñi, Cuenca Bescós, Grün, Ajas, García Pimienta and Lees2010; Blain and Villa Reference Blain and Villa2006). More specifically, it is suggested that the site functioned as a hyena maternity den (Marra et al. Reference Marra, Villa, Beuval, Bonfiglio and Goldberg2004; Sesé and Villa Reference Sesé and Villa2008). The interior of Bois Roche Cave should have been dry enough to allow the preservation of hundreds of complete or almost complete coprolites and their content. Among the bones, coprolites and fragments of coprolites have been recovered and plotted, although many were disintegrated into 1 cm or smaller pieces or can only be seen in micromorphological sections (Villa et al. Reference Villa, Castel, Bourdillat, Beauval and Goldberg2004). One of the complete coprolites from Bois Roche is illustrated in Villa et al. (Reference Villa, Sánchez-Goñi, Cuenca Bescós, Grün, Ajas, García Pimienta and Lees2010: Supplementary Fig. 5) and shows a microtine molar embedded within the coprolite.
It is unusual to find well-preserved coprolites or pellets of small mammal predators in the fossil record. Most degrade, and only the bones are left behind. Hyena coprolites survive at higher frequency than those of other mammalian predators (Horwitz and Goldberg Reference Horwitz and Goldberg1989; Harrison Reference Harrison2011; Bennett et al. Reference Bennett, Gorgé, Grange, Fernández-Jalvo and Geigl2016) due to the high mineral (calcium phosphate) content after bone digestion. However, we have not found any record of fossil hyena coprolites containing small mammal bones so far, other than from Bois Roche.
Most zoologists studying the ethology of modern hyenas have not observed them feeding on, or even being interested in, small mammals (I. Wiesel personal communication 2010; K. E. Holekamp personal communication 2011), although hungry hyena cubs may hunt insects or any other appropriately sized animals (K. E. Holekamp personal communication 2011). Apart from some rare cases of rodent remains recorded in modern hyena scats in South Africa (G. Avery personal communication 2015), there is little published information on this topic. Korb (Reference Korb2000), an ecologist and entomologist who studied the hyenas (C. crocuta) of the Comoe National Park (CNP, Ivory Coast), mentions that “it was rare to observe hunts, because faecal analysis demonstrated that small mammals such as rodents account for more than 60% of hyena diet in CNP” (Korb Reference Korb2000: p. 9). She described the rare solitary and shy behavior of these hyenas, which caused difficulties in applying standard methods used to locate and observe these populations. This solitary and shy behavior may account for the presence of rodents in their diet, which is absent in other communities (I. Wiesel personal communication 2010; K. E. Holekamp personal communication 2011). Unfortunately, the author did not report the number of scats analyzed, what taxa formed the other 40% nonrodent content (e.g., insects, worms, birds), or the procedure to calculate the percentage of each type of prey. No taphonomic analysis has been carried out on this modern reference collection yet. With the exception of the Ivory Coast hyenas, predation of microfauna by modern hyenas appears to be an irregular activity, which makes the high number of rodent individuals present in Bois Roche more outstanding.
Hyenas
Hyenas have a wide diet range, and they are considered one of the most generalist carnivores in the African ecosystems (Mills and Hofer Reference Mills and Hofer1998). Hyenas have been studied extensively in terms of their feeding and social habits for ecological purposes (Kruuk Reference Kruuk1972; Holekamp and Smale Reference Holekamp and Smale1990; Mills Reference Mills1990; Wiesel Reference Wiesel2006; Holekamp Reference Holekamp2007). Paleontological investigations have also been carried out to distinguish between the role of hyenas (especially in their maternity dens) and hominins as bone collectors in fossil sites (e.g., Brain Reference Brain1969; Sutcliffe Reference Sutcliffe1970; Haynes Reference Haynes1983; Hill Reference Hill1984; Skinner et al. Reference Skinner, Haupt, Hoffmann and Dott1998; Villa et al. Reference Villa, Castel, Bourdillat, Beauval and Goldberg2004, Reference Villa, Sánchez-Goñi, Cuenca Bescós, Grün, Ajas, García Pimienta and Lees2010; Pokines and Kerbis Peterhans Reference Pokines and Kerbis Peterhans2007; Prendergast and Dominguez-Rodrigo Reference Prendergast and Dominguez-Rodrigo2008; Kuhn Reference Kuhn2011).
The spotted hyena (C. crocuta) is the largest extant hyaenid, with a weight ranging between 45 and 85 kg. They are good hunters, versatile in their choice of prey (from large-sized [wildebeest and zebra] to small-sized [warthog and impala] mammals). They practice a diversity of hunting techniques and strategies (both solitary and in groups), usually hunting at night. Their roaming area varies greatly according to habitat and climatic region, from 28 to 80 km (Hofer Reference Hofer1998).
A close evolutionary relationship between Eurasian Pleistocene hyenas (Crocuta crocuta spelaea) and modern Crocuta has been established based on nuclear genes, while the limited genetic diversity in striped and brown hyenas indicates population bottlenecks in these species during the Pleistocene (Bon et al. Reference Bon, Berthonaud, Maksud, Labadie, Poulain, Artiguenave, Wincker, Aury and Elalouf2012). An African origin during the Pleistocene has been established genetically, and dispersal to Eurasia of both spotted and striped hyenas must have been rapid during subsequent migration waves (Rohland et al. Reference Rohland, Pollack, Nagel, Beauval, Airvaux, Pääbo and Hofreiter2005). Some Plio-Pleistocene European fossil representatives (i.e., Pliocrocuta) have been proposed to be taxonomically conspecific with brown hyenas (Kurten Reference Kurten1968; Turner Reference Turner1990; Turner and Anton Reference Turner and Anton1996), although this is not a universally held view (Werdelin and Solounias Reference Werdelin and Solounias1991; Jenks and Werdelin Reference Jenks and Werdelin1998).
Hyenas mark their territory by leaving a secretion from the anal gland. Mills and Hofer (Reference Mills and Hofer1998) record scat territory marking, like canids, with defecation at latrine sites. Chemical analysis by X-ray diffraction of hyena coprolites (Lewis Reference Lewis2011; Pesquero et al. Reference Pesquero, Salesa, Espílez, Mampel, Siliceo and Alcalá2011) and modern scats (Horwitz and Goldberg Reference Horwitz and Goldberg1989; Larkin et al. Reference Larkin, Alexander and Lewis2000) yields a high abundance of hydroxylapatite, the mineral component of bones, compared with organic content. The enrichment in calcite phosphates is the result of the large amount of bone cracked and ingested by hyenas. The high mineral content in fresh hyena scats produces a sticky coating on the brown scats, which becomes white and hardened a few hours after exposure to the sun in an open environment. The high calcite phosphate content in hyena coprolites thus favors their preservation and high abundance in fossil sites, both in caves and open-air sites. By comparison, herbivore coprolites are more friable and less common in the fossil record (Harrison Reference Harrison2011).
Materials and Methods
The taphonomic analysis reported here was undertaken on samples recovered in the 1995 season, referred to in Table 2. The material was received already sorted into cranial and postcranial elements. All of the sediment from the 1995 excavation had been sieved through 5 mm and 2 mm mesh screens. The smallest-sized mesh was decreased in size to 1.4 mm in the 1997 and subsequent field seasons. From the 1998 season’s material, subsamples from the first 5 liters of sediment from each new 5 cm spit of each square were sieved through the 1.4 mm mesh. The remainder of the deposit was sieved through the 5 mm mesh and then a 2 mm mesh to ensure recovery of all coprolite fragments smaller than 5 mm. All mandibular first molars were removed for taxonomic analysis. The taphonomic analysis reported here was undertaken on the rest of the small mammal material. The taxonomic analysis of the 1995 season small mammal assemblages was carried out by Sesé and Villa (Reference Sesé and Villa2008), and material recovered from 1997 to 2000 was analyzed by Cuenca Bescós (Villa et al. Reference Villa, Sánchez-Goñi, Cuenca Bescós, Grün, Ajas, García Pimienta and Lees2010).
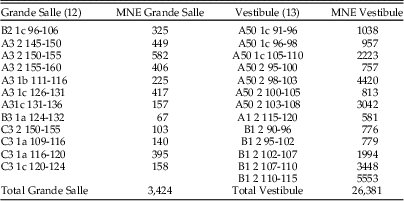
Table 2 Samples from the Bois Roche 1995 season taphonomically analyzed. The labels indicate the square (letter and number), followed by the stratigraphic unit (unit 1a, 1b, 1c, or 2), and then the depth range. MNE, minimum number of elements.
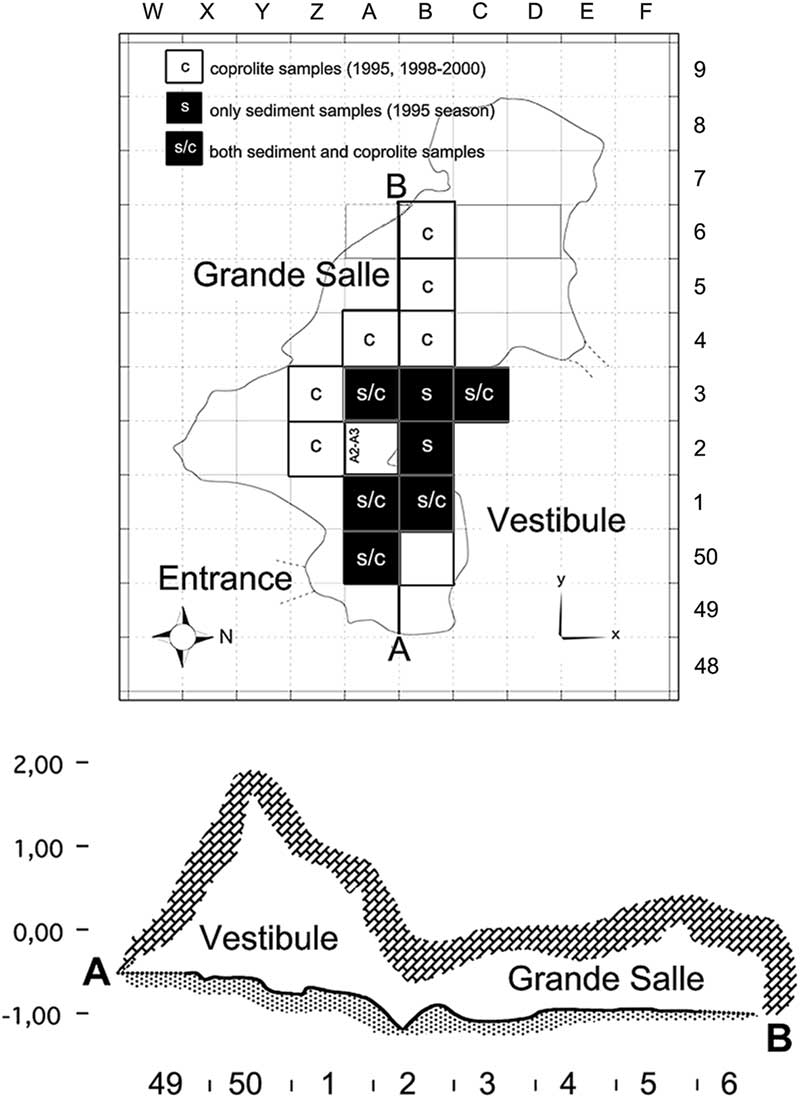
The Bois Roche site has two distinct areas, the so-called Vestibule (about 5 m2), giving access to the cave from the entrance, and the Grande Salle, a larger chamber (9 × 4 m) in the caves’s interior. The cave deposits slope away from the entrance toward the rear of the inner chamber (Villa et al. Reference Villa, Sánchez-Goñi, Cuenca Bescós, Grün, Ajas, García Pimienta and Lees2010). Samples come from two levels, unit 1, with at least three subunits (1a, 1b, and 1c), and a more massive unit 2. In this paper, we compare material from the two different areas of the cave and also from the two different stratigraphic units (Fig. 2).
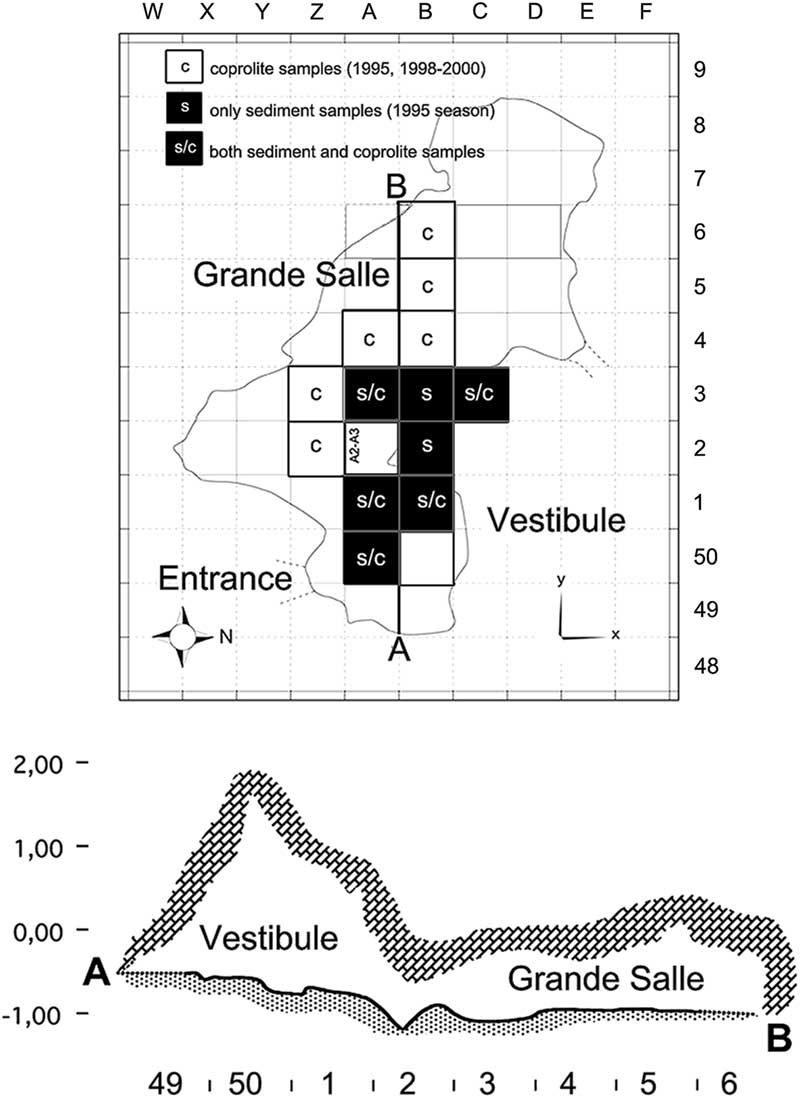
Figure 2 Bois Roche map. Top, map of the excavation. Letters at the top and numbers on the right correspond to the grid system used to label squares. The black squares refer to sediment (s) sieved during the 1995 season that contained sufficient cranial and postcranial small mammal skeletal elements to undertake the taphonomic analysis (25 samples). The white squares refer to squares that yielded coprolites (c); five of the black squares also yielded coprolites (s/c), making a total of 135 coprolites studied in this paper. Bottom, profile of the cave along the line A–B; numbers at the bottom refer to the square labels. The cross section indicates the Vestibule and the Grande Salle areas distinguished during excavations and described in this paper.
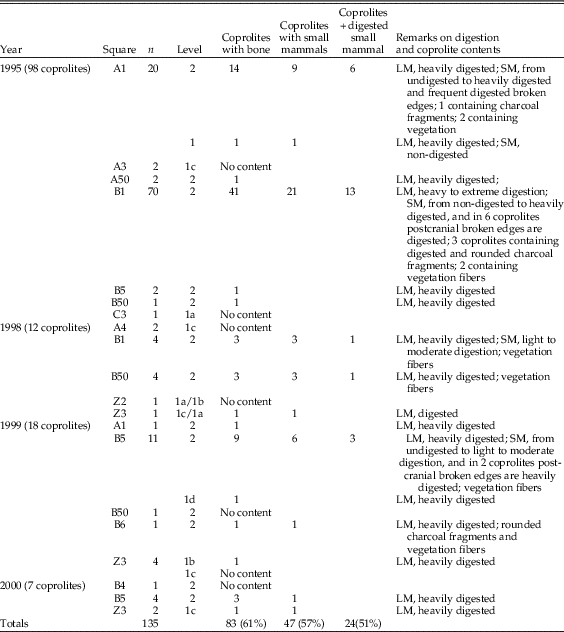
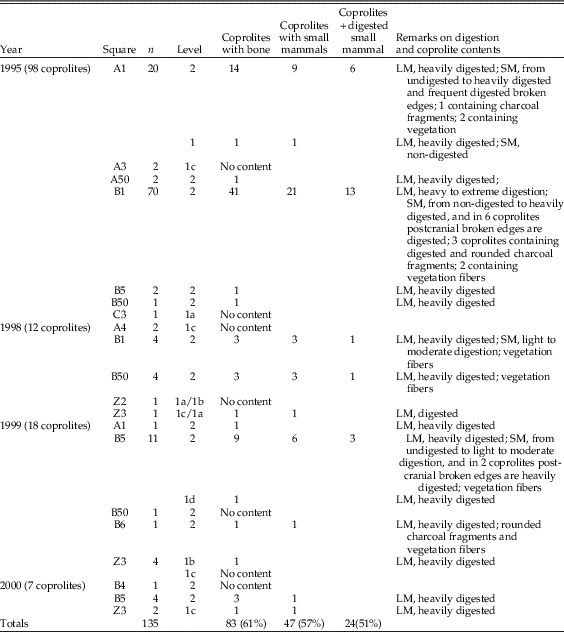
Our taphonomic analysis followed the methodology set out by Andrews (Reference Andrews1990) and Fernández-Jalvo and Andrews (Reference Fernández-Jalvo and Andrews1992). Twenty-five different samples containing both cranial and postcranial elements were analyzed from seven different squares of the two distinct areas (Vestibule and Grande Salle) (Table 2, Fig. 2). The samples were spatially distributed in different areas of the site and also from different depths within specific squares corresponding to the two stratigraphic units (11 samples from unit 1 and 14 from unit 2). Samples with large numbers of small mammals were preferentially selected to ensure that the information from the taphonomic analysis would be sufficiently reliable. In addition, a total of 135 coprolites from both units and areas were also studied. Eighty-three of these contained osseous remains (micromammal bones and small bone flakes of larger mammals) (see Table 3).
Table 3 Coprolites from Bois Roche containing osseous material: LM, large-mammal fossils; SM, small-mammal fossils. A total of 135 individual coprolites were analyzed, 39% of which had no fossil bone in the interior. Large mammal fossil fragments were present in 83 coprolites; almost 60% of these coprolites (57%) contained rodent remains, and almost half had no traces of digestion.
Cranial and postcranial material was analyzed using binocular light microscopes with variable magnification. Selected bones were also studied under an environmental scanning electron microscope (FEI-Quanta 200) hosted at the Museo Nacional de Ciencias Naturales (Madrid) using secondary and backscattered electron detectors.
A G-test of independence using R (R Core Team 2017) was applied to the results, with a significance level of 0.05. The tests compared anatomical representations (taking into consideration cranial vs. postcranial remains), bone breakage (considering broken vs. complete), and digestion (with digestion vs. without digestion).
This test compared unit 1 and unit 2 to investigate differences between the two time periods. The Grande Salle and Vestibule areas were compared separately, as was a single sample named “total” (which was the sum of data from either the Vestibule and Grande Salle or units 1 and 2). The total sample results were also compared with results obtained from the coprolites.
Results
Abundance, Completeness, and Distribution
The taphonomically studied samples of small mammal anatomical elements from the 1995 excavations are displayed in Table 4. Samples having high abundance of both cranial and postcranial elements were primarily chosen for this study. Figure 2 shows the squares from which samples were selected. Unit 2 has a higher fossil content than unit 1, and the Vestibule is richer than the Grande Salle (Table 2). Figure 2 also shows the squares from which coprolites studied here were recovered. The coprolites come from the 1995, 1998, 1999, and 2000 excavation seasons, as shown in Table 3.
Table 4 Survival rates of anatomical elements represented in the samples of Bois Roche. The “Total” column is the sum of data from either unit 1 and unit 2 or the Vestibule and the Grande Salle areas. Note that the “Molar” row includes the M1 teeth from the sieved samples recovered from the sediment (i.e., all samples except coprolites), which were removed for taxonomic analysis before this taphonomic study started).
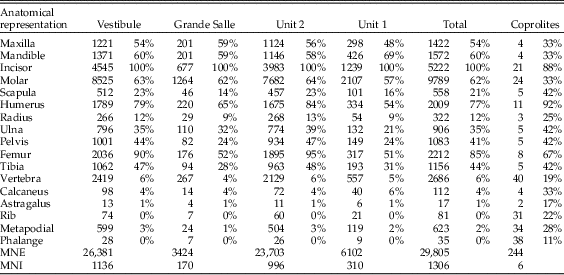
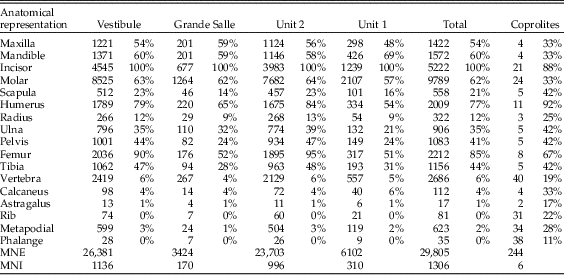
The completeness of the small mammal samples (and thus the anatomical representation, see following section) is difficult to assess, because there have been a number of taphonomic biases acting on this material. First, all elements that can pass through a 2 mm sieve may be underrepresented. For the postcranial material, the major limb bones are recorded, although small items such as ribs and bones of the extremities would not have been retained by the 2 mm sieve (Williams Reference Williams1997). The most common limb bones are the femur and the humerus. Small and fragile bones such as the radius, scapula, and pelvis are particularly underrepresented, as are distal portions of the ulna, which as a narrow bone is likely to have been lost through sieving.
Anatomical Representation
The mandibular first molars (2592 in total) had been removed for taxonomic analysis and were not physically available at the time when the taphonomic analysis took place. Thus, evidence of digestion on the M1 teeth could not be studied, but the database of excavated M1 teeth was provided by C. Sesé and the number of M1s was included in the analysis of skeletal element abundance (Table 4).
Around 30,000 skeletal elements (minimum number of elements [MNE]) were available for taphonomic analysis. No postcranial material was available from samples labeled as B2/1c, 96–106, and A50/1c, 91–96, in Table 2. There are lower numbers of calcanei, astragali, ribs, metapodials, and phalanges observed in all samples relative to the MNI calculated on the number of the most common element (incisors). Larger skeletal elements, such as pelvises, scapulae, radii, or vertebrae also have a reduced relative abundance (Table 4). The most abundant postcranial elements are usually femurs and humeri, with a lower MNE of tibiae and ulnae.
Indices
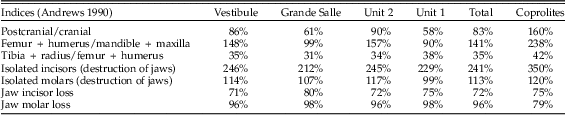
The postcranial/cranial indices, isolated teeth, and incisor and molar loss are shown in Table 5. Postcranial/cranial indices are usually below 100% when isolated teeth are included (pc/c) and slightly above 100% when isolated molars are not included (e.g., see the femur + humerus/mandible + maxilla index in Table 5). Femurs and humeri are always more common than distal long bones (tibiae and radii). The index of isolated molars (which indicates jaw destruction when values are above 100%) yielded 113%. This would indicate that mandibles and maxillae are not highly destroyed. However, the isolated incisor indices reach values around 241%, indicating there are more incisors present in the sample than jaws from which they have been lost. In situ teeth are rare, as is also indicated by high tooth-loss indices (rate of empty alveoli), mostly above 90%, sometimes 100%. The most commonly retained tooth is the mandibular incisor, which is retained in about half of the mandibles recovered. The value of this index, together with the difference between indices of isolated molars (below 100%) and incisors (well above 100%) suggests a relatively high loss of molars during sieving.
Table 5 Postcranial/cranial indices, isolated teeth (including the M1 removed for taxonomic studies) and incisor and molar loss from the mandible and maxilla.

Breakage
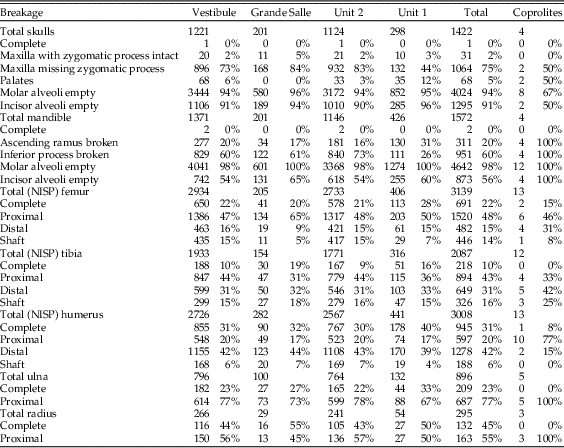
Cranial breakage is high (Table 6), with almost no intact skulls found. Very few skull fragments have a zygomatic process still attached. Mandible breakage is also high, and almost all samples contain more than 60% frequency of breakage of the inferior border. Teeth are broken but most of this breakage appears to have occurred after deposition, as teeth that are broken and then digested are found in lower frequency to the total proportion of digested teeth; less than 20% of broken incisors have evidence of digestion, and less than 7% of broken molars had been digested (Table 7). Fragments of mandibles and maxillae are present in all samples, with many empty molar alveoli (above 90% in almost all samples). The number of incisors removed from jaws is more variable, with values between 55% in mandibles and around 90% in maxillae.
Table 6 Breakage in cranial and postcranial elements. Note totals of femur, tibia, and humerus are here the number of identified specimens (NISP), while Table 4 provides the minimum number of elements (MNE) of these anatomical elements.
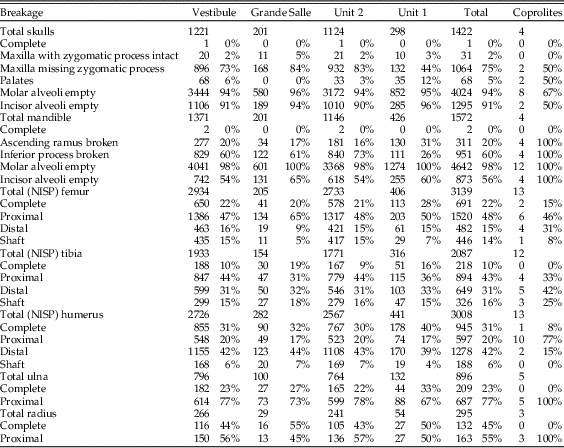
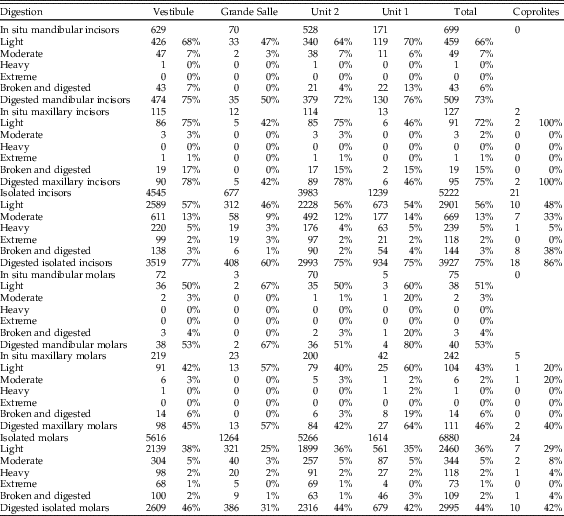
Table 7 Digestion in cranial elements. Note that these figures do not include the M1 teeth removed for taxonomic analysis, except in the “Coprolites” column.
Breakage of limb bones is relatively low, with several long-bone categories containing more than 20% complete bones. The most commonly recorded elements are distal humerus, proximal ulna, and proximal femur. Tibia breakage appears to be more random, with variable recovery of both proximal and distal ends. Breakage in radii is the most variable across the different parts and depths of the cave (Table 6).
Digestion
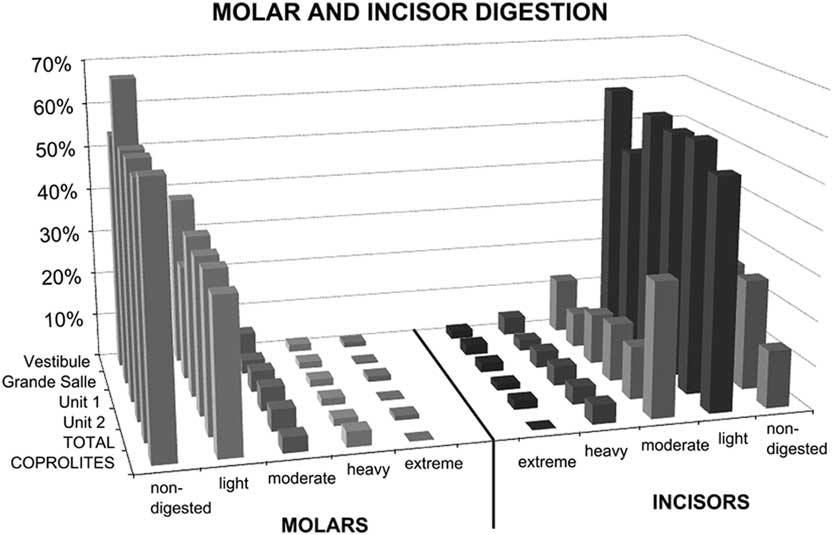
Digestion was analyzed for molars, incisors, distal humeri, and proximal femurs. The frequency of digestion for incisors corresponds to around 75% (Table 7), most frequently affecting the tip of the incisor. The low values of breakage before digestion indicate that small mammal individuals were not heavily broken during ingestion and were most probably swallowed complete. Molar digestion is similar between samples, around 45%, as can be seen in Table 7, exhibiting low breakage before digestion. Both incisors and molars, in situ or isolated, are lightly digested in general, followed at a lower percentage with moderate degrees of digestion, while heavy and extreme degrees are rare, although present in most samples.
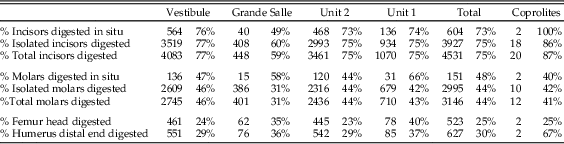
Postcranial digestion affects about 25% of proximal femurs and 30% of distal humeri (Table 8). The higher figures reaching 40% in unit 1 and Grande Salle are likely to be a product of small sample size. Digestion ranges from a mild pitting of the epiphyses to total digestion of these areas and loss of bone. Digestion is also observed on the proximal ulna, in some cases resulting in significant loss of bone.
Table 8 Summary of digestion rates. Note that these figures do not include the M1 teeth removed for taxonomic analysis, except in the “Coprolites” column. The number of distal elements of the humerus and proximal end of femurs are given in Table 6 by the complete and distal/proximal end of the humerus/femur (NISP).
Coprolites
Coprolites were treated separately, as they have a reduced fossil content and because they are a reference for the traits of predation that should be diagnostic of hyena predation. In total, 135 coprolites were studied, 83 of which contained bones. Charcoal and plant remains are also present in a number of coprolites. About 57% of the 83 coprolites contain small mammal bones, also with evidence of digestion (Table 3).
Breakage is high, although femurs and humeri include complete elements (Table 6), as observed in samples recovered from the sediment.
Digestion of teeth is frequently light (Table 7), but moderate and heavy digestion is also recorded. Twenty-three coprolites contain small mammal bones showing no signs of digestion. The low number of microfaunal skeletal elements recovered from the coprolites (n=244; Tables 4–7) does affect the resulting percentages of breakage, postcranial versus cranial indices, relative abundance of skeletal elements, and especially the digestion of distal humeri (Table 8). Nonetheless, digestion of femurs and teeth from coprolites seems to show similar digestion traits and percentages compared with samples obtained from the sediment.
Statistical Treatment
The p-values obtained for each of the variables analyzed are shown in Table 9. Anatomical elements and breakage show differences between Grande Salle and Vestibule and for units 1 and 2. Nonetheless, for digestion of molars and incisors, a comparison of both stratigraphic units shows a p-value higher than 0.05, which indicates that there are no differences with respect to the total percentage of digested remains. In addition, if we compare the distribution of the degrees of digestion in both units, no differences are observed either in molars (p=0.09455) or in incisors (p=0.4053).
Table 9 The p-values obtained for each of the variables analyzed in relation to the cave areas and the stratigraphic units studied. Numbers shown in bold are those p-values that are not significant and therefore indicate similarities. df=1.

Statistical results obtained when comparing taphonomic variables of fossil assemblages present in the coprolites and those from the total sample (the sum of the two stratigraphic units: unit 1 and unit 2) show values above p=0.05. The p-values obtained for each variable show that there are no significant differences between total and coprolites for the percentage of digested dental remains (Table 10).
Table 10 The p-values obtained for each of the variables analyzed in relation to coprolites and total. Numbers shown in bold are the p-values that do not indicate differences between the samples. df=1.
Postdepositional Modifications
In addition to predepositional bone modification related to predator action, a number of postdepositional impacts on the bones can be recognized. These include puncture marks, which are most frequent on flakes of large mammal bones, and breakage (most likely as a result of trampling). One further peculiar phenomenon seen in these samples is the presence on some molars of tubular formations, composed mainly of calcite. These may be root casts, although there is no evidence of root marking on bones or teeth. No manganese or any other postdepositional mineral staining is observed.
Discussion
The small mammal assemblage from Bois Roche is characterized by an extremely high abundance of individuals that were relatively poor in species richness. Most small mammals were identified to the genera Microtus and Arvicola (Sesé and Villa Reference Sesé and Villa2008; Villa et al. Reference Villa, Sánchez-Goñi, Cuenca Bescós, Grün, Ajas, García Pimienta and Lees2010). The lack of prey diversity is one indication that an assemblage has been accumulated by a specialist predator that has adapted a prey acquisition strategy specific to a particular prey species (discussed in Andrews Reference Andrews1990). The large mammal fauna and the presence of numerous coprolites indicate that the cave was a hyena maternity den, as shown in previous taphonomic studies of the large mammal remains by Villa and d’Errico (d’Errico and Villa Reference d’Errico and Villa1997; Villa and d’Errico Reference Villa and d’Errico2001). Other Pleistocene sites may have a predominance of a single rodent species or genus, but these usually fall within the range of 45% to 70% of the assemblage. The hyperabundance of a single species seen in Bois Roche (Microtus gregalis 80–94%) is unusual. Given that hyena predation of large mammals is usually characterized as being opportunistic , it seems somewhat contradictory that hyenas should become specialized predators of rodents. The overrepresentation of Microtus, most especially, Microtus gregalis, was therefore most likely caused by periodic population outbreaks among this prey species.
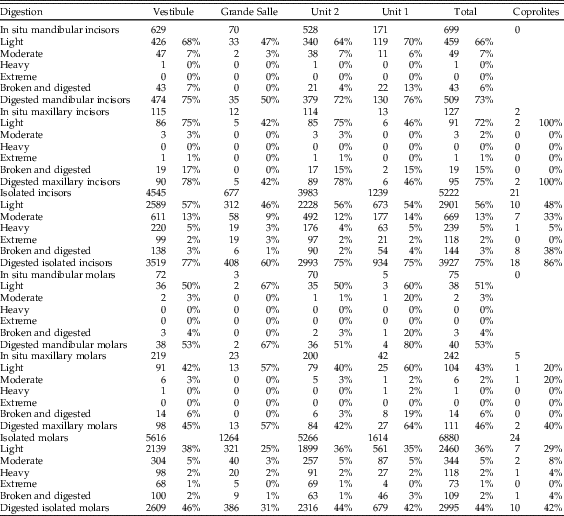
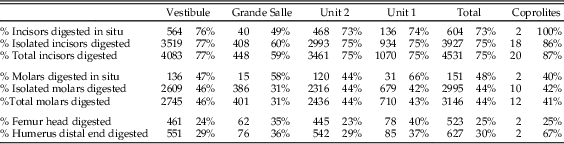
There are no taphonomically analyzed modern hyena scat samples containing rodents currently available for comparison with the Bois Roche fauna. Many of the 135 hyena coprolites from Bois Roche contained bone remains (64%), and almost 60% of the bone content recovered from the coprolites corresponds to rodents. As 52% of the small mammal remains display evidence of having been digested, it is clear that hyenas consumed rodents. The 86 coprolites containing bone always yielded heavily digested large mammal bone flakes, but the small mammal teeth and bones in these same coprolites exhibited a variable degree of digestion, from none to heavily digested specimens (see Fig. 3). The apparent discrepancy between the high levels of digestion seen on bone flakes of large mammals in the coprolites and the lower degree of digestion of small mammal remains in the same coprolite (Fig. 3) may be due to the presence of the soft tissue and fur covering of the small mammals when they were ingested (which helps to protect the bones from the corrosive gastric juices), whereas the larger bone flakes most likely resulted from gnawing and breaking larger (mainly defleshed) bones, which then entered the digestive system without additional protection. It is not surprising, therefore, that the most-exposed skeletal element, the incisor, is also the most frequently digested. The pattern obtained in Bois Roche suggests that rodents were barely chewed (i.e., swallowed whole) and were digested complete. A similar trait has been observed in modern puma scats described by Montalvo et al. (Reference Montalvo, Pessino and González2007); in part, this reflects the large difference in relative size between the prey and its predator (Mondini Reference Mondini2000).
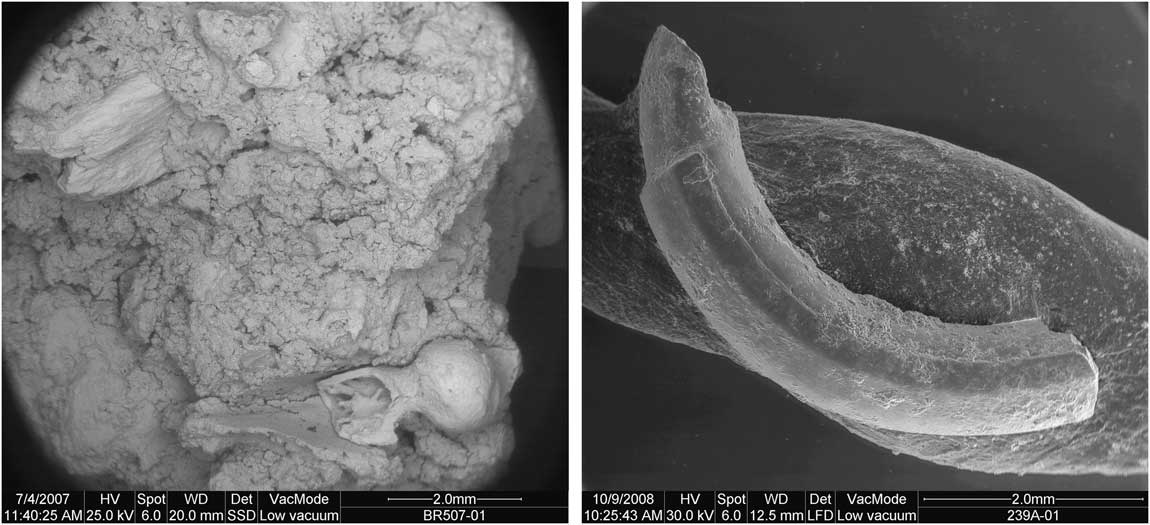
Figure 3 Left, Scanning electron microscope of a Bois Roche coprolite, with nondigested proximal end of femur (bottom right) of a small mammal (unfortunately, the shaft was broken during sample preparation) next to a heavily digested large mammal bone flake (top left). Right, small mammal incisor from a coprolite showing digestion concentrated on the tip, frequent in the Bois Roche fossil assemblage.
The distribution of digestion of small mammal molars is consistent for both stratigraphic units (unit 1 and unit 2) in the Grande Salle and Vestibule and for all samples (Fig. 4, Tables 7 and 8). All individual samples have a high abundance of non-digested incisors and molars, with most molars showing light digestion. Variation from this pattern only occurs in samples with a low frequency of bones, where small changes can produce large percentage differences. Moderate digestion was seen in about 3% of molars, heavy digestion in 2%, and extreme digestion in 1%, again with large differences seen only in small samples.
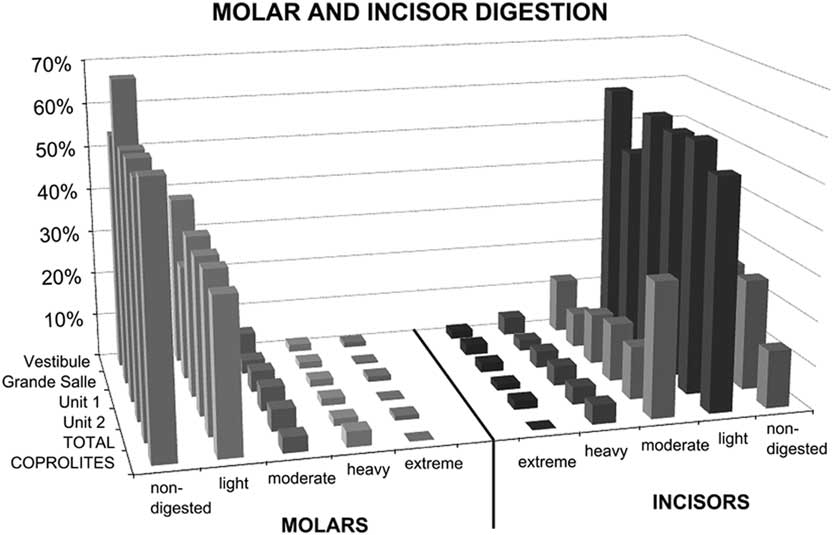
Figure 4 Molar and incisor digestion according to the excavation area (Vestibule, Grande Salle) and the stratigraphic level (unit 1, unit 2). “TOTAL” refers to all samples combined, i.e., either stratigraphic units (unit 1 and unit 2) or excavation areas (Vestibule and Grande Salle). Digestion grades from teeth (6048 incisors and 7197 molars) recovered from sediment samples are compared with dental remains (23 incisors and 29 molars) recovered from the interior of 135 individual coprolites. Most digestion is light. See Tables 7 and 8 for digestion levels for individual samples.
Most digested incisors are lightly digested (56–72%). Moderate to heavy digestion is present on incisors, both isolated incisors and those retained in the jaws. There is a high abundance of incisors digested at the tip, indicating they were still in their alveoli when digested. The low rate of broken edges on the teeth affected by digestion also suggests a low rate of breakage during ingestion, further confirming the probability that these animals were ingested complete. Montalvo et al. (Reference Montalvo, Pessino and González2007) described a much lower degree of breakage than referred to by Andrews (Reference Andrews1990) for small- to medium-sized carnivores and have proposed that this is a characteristic taphonomic pattern for large-sized mammalian carnivores such as pumas consuming small prey items.
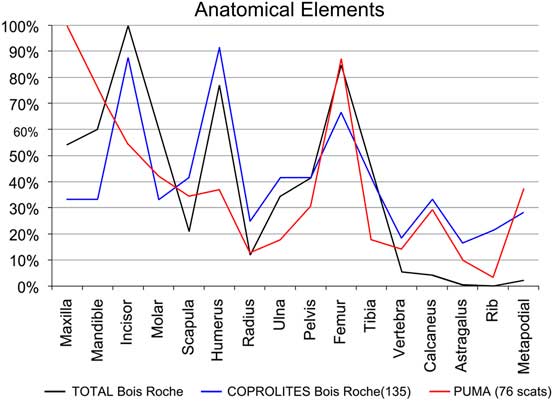
With regard to the anatomical elements recorded (Fig. 5), the Bois Roche assemblage shows a fairly equal representation of postcranial and cranial elements (pc/c). There is also a good representation of mandibles and maxillae compared with the main long bones (femurs and humeri). Both of these indices add further to the suggestion that prey were swallowed whole. Femurs, humeri, tibiae, radii, and ulnae appear complete (10% to ~30%). However, damage to the skull and lower jaws is evident in the high indices of incisor and molar tooth loss (with the notable exception of mandibular incisors, of which 50% are still preserved in their mandibular alveoli). On that basis, the high frequency of isolated teeth and thus relative absence of jaws cannot be entirely explained by destruction by chewing during ingestion and digestion of the prey, as this occurred when the prey was still largely intact.
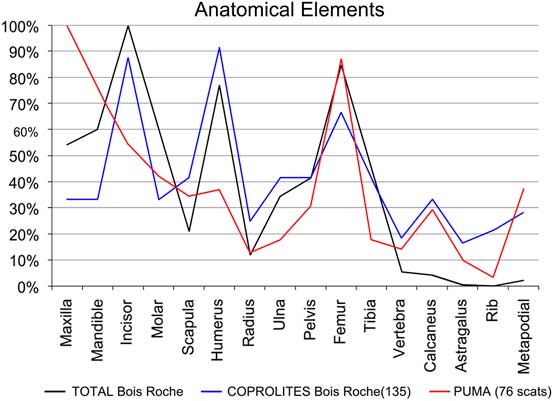
Figure 5 Relative abundances of anatomical elements recovered from the sediment (TOTAL Bois Roche). “COPROLITES Bois Roche” refers to small mammal anatomical elements recovered from the 135 individual coprolites compared with the relative abundance obtained from 76 “PUMA” scats (Puma concolor, according to Montalvo et al. Reference Montalvo, Pessino and González2007).
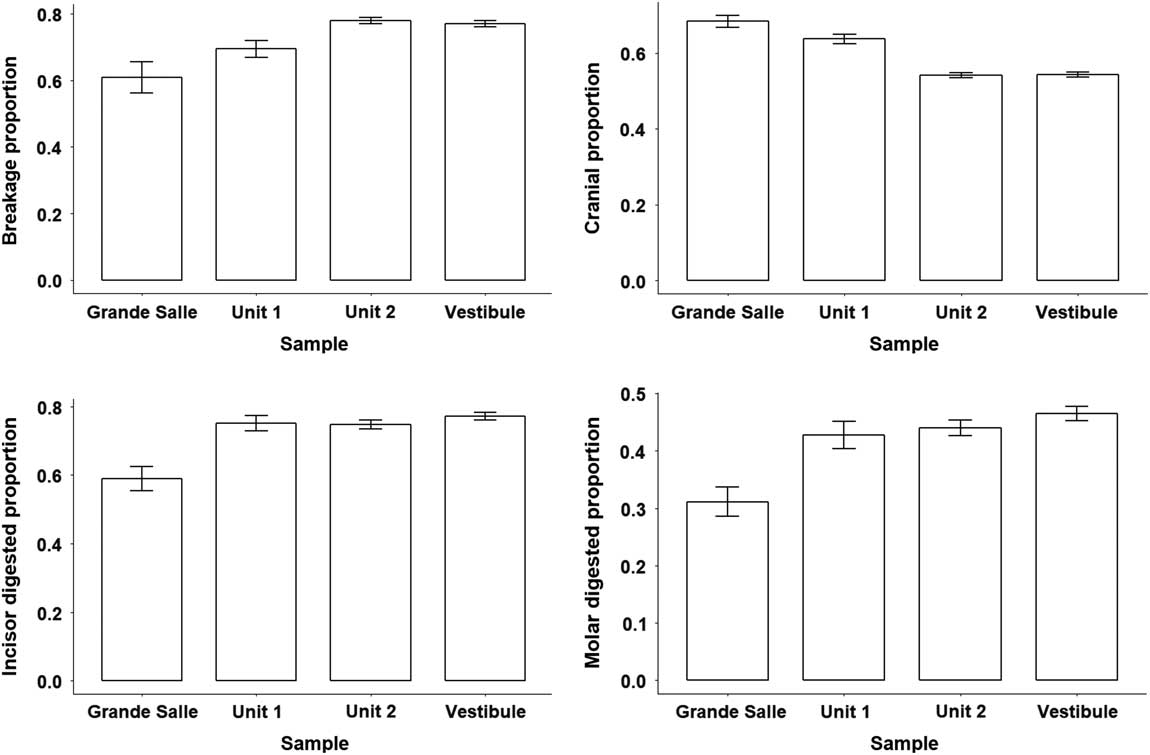
In general terms, the digestion pattern observed in the coprolites, that is, abundance of light digestion grades both in molars and incisors and a high number of non-digested molars (Fig. 4), is similar to that observed for the small mammal assemblages obtained from the sediment in both units (in the coprolite sample, molar digestion is 41% and incisor digestion is 87%; in the total sample, molar digestion is 44% and incisor digestion is 75%) The coprolite sample size is small compared with the rest of the samples recovered from the sediment, and this size difference may yield percentages different from those observed in sediment samples. Statistical analysis of tooth digestion indicates similarities between unit 1 and unit 2 (Table 9) and between coprolite and total (Table 10). The Vestibule and unit 2 are very similar, because almost all samples of this area are from unit 2. The Grande Salle sample, however, contains both unit 1 and unit 2 (Table 2) and has the lowest fossil content among samples recovered from the sediment (Table 4). This may be the cause of the significant differences observed between the Grande Salle and the rest of the samples (Fig. 6). With respect to fragmentation and anatomical representation, the differences may depend not only on predation, but also on different processes linked to postdepositional factors. Nonetheless, the fact that units 1 and 2 are statistically similar, as are the coprolite and the total samples (Tables 9, 10), does suggest that a single predator was involved in the predation and accumulation of rodents in Bois Roche during the time period covered by units 1 and 2. We have not been able to identify any other predator that might have contributed prey remains to the microfauna accumulations at Bois Roche through our taphonomic analysis of the bones and teeth and statistical treatment of the results.
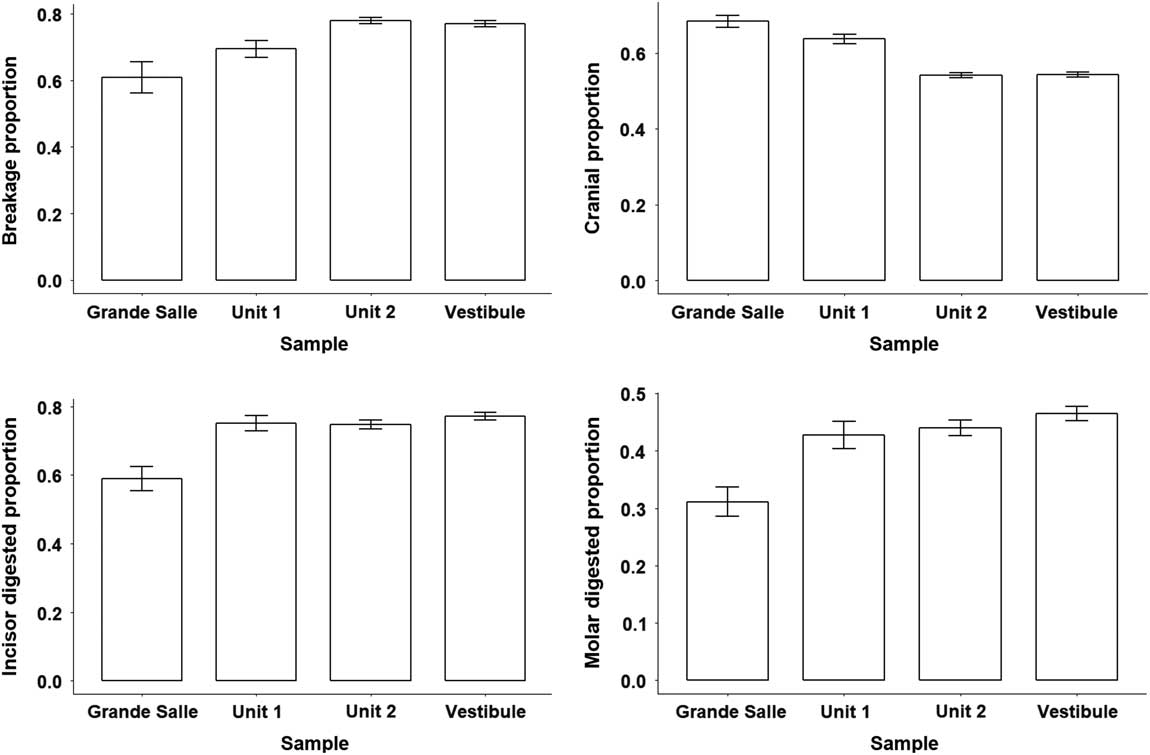
Figure 6 Bar charts and error bars of breakage, cranial remains, and incisor and molar digestion obtained from Grande Salle, Vestibule, unit 1, and unit 2.
Given that the cave was repeatedly used as a hyena maternity den, the most parsimonious hypothesis is that hyenas were the only predator that produced this almost monospecific small mammal assemblage. As hyenas, in common with most mammalian predators, are opportunistic hunters, we can conclude that Microtus gregalis was periodically abundant due to population outbreaks or behaved in a manner that made it particularly susceptible to hyena predation.
Hyena movement into, out of, and around the cave caused the coprolites to disintegrate, although some coprolites were deposited in parts of the cave where they were protected from trampling, hardening over time and surviving whole. The low frequency survival of some skeletal elements (e.g., vertebrae, ribs, metapodials, phalanges) is probably due to loss during sieving (2 mm mesh) and subsequent human bias in selection of bone material for analysis.
Conclusions
An exceptional abundance of microfauna was found at Bois Roche in association with large mammal faunas bearing clear indication of hyena breakage, chewing, and digestion. This type of damage on large mammals suggests that these bones were deposited at Bois Roche within a maternity hyena den. In this context, our working hypothesis is that the rodents also had entered the assemblage through hyena predation.
Although many coprolites disaggregated during recovery, some were complete and compact and had avoided obvious damage by trampling. It is also interesting to note the overall low number of small mammal bones in each coprolite analyzed, suggesting that survival of complete coprolites was a fairly rare occurrence, since a substantial number would have had to disaggregate to produce the quantities of small mammals recorded.
The absence of taphonomic studies of modern hyenas feeding on small mammals makes the taphonomic pattern of small mammals from Bois Roche a useful source of reference for other researchers studying hyena predation of fossil micromammals. The general pattern of postcranial versus cranial elements and the relatively high percentage of complete long bones indicates that small mammals were ingested complete. This pattern of swallowing prey whole without chewing has also been observed in modern large predators feeding upon rodents, such as pumas. The fact that the bones and teeth survived within the aggressive digestive tract of hyenas is probably due to protection provided by indigestible elements such as hair, skin, hoofs, and other bone fragments of large and small mammals already within the stomach. Nevertheless, the frequency of digestion of teeth is high (75% incisors, 44% molars), but with light degrees of digestion and long-bone epiphyses also affected by digestion.
The presence of small mammal bones within hyena coprolites indicates beyond doubt that the hyenas were feeding on small mammals. A consistent pattern emerges in which some micromammal bones show no signs of digestion or were lightly digested while others were very heavily digested. Statistical similarities between unit 1 and unit 2 and between the coprolite and the total samples analyzed suggest that only one predator was involved in the fossil assemblage of Bois Roche, and this predator was hyena. The presence of a second predator bringing rodent remains to Bois Roche has been discarded.
Acknowledgments
This investigation has benefited from projects CGL2007-66231 and CGL2016-79334P of the Spanish Ministry of Research. The Bois Roche excavations were funded by the French Ministry of Culture, the Leakey Foundation, the National Science Foundation, the General Council of the Charente Region, the Association of Archeologists of the Poitou-Charente Region, and Franklin and Marshall College. We thank the nondestructive techniques technicians of the Museo Nacional de Ciencias Naturales for their professional work. We also acknowledge the participation of Darío Herranz and Florentin Cailleux, students of the University Complutense of Madrid (Spain) and the University of Poitiers (France) who analyzed isolated samples (one from the sediment and one coprolite, respectively), thus helping to complete this large set of samples from Bois Roche. We are grateful to the editor, Catherine Badgley, to Claudia Montalvo, Norah Moloney, and two anonymous reviewers whose comments and suggestions have greatly improved this paper.