Introduction
“…to avoid the taint of theory in morphology is impossible, however much it may be wished. The whole science is riddled with theory. Not a specimen can be described without the use of a terminology coloured by theory, implying the acceptance of some one or other theory of homologies.” William Bateson (Reference Bateson1894: p. vii)
Paleobiologists study the rich, geologic history of fossilized morphologies to examine patterns and processes in macroevolution. The ~520 million year history of Phanerozoic metazoan evolution revealed by fossils provides an empirical record of mass extinctions, morphologic innovations, and adaptive radiations otherwise unknowable from observations on extant species alone (Sepkoski Reference Sepkoski1981; Alroy Reference Alroy2010). Such studies are of interest to ecologists, developmental, and evolutionary biologists because they offer insight into evolution on timescales inaccessible to direct observation or experimentation. Integration between paleobiology and other biologic disciplines frequently leads to a better understanding of evolutionary patterns and processes at multiple hierarchical levels. For example, combining the stratigraphic record of fossil occurrences with recent discoveries in evolutionary developmental biology (EDB, or “evo-devo”) has facilitated unprecedented insights into the causal mechanisms of morphologic diversity, body plan evolution, and developmental links between micro- and macroevolution (Shubin and Marshall Reference Shubin and Marshall2000; Raff Reference Raff2007; Wagner Reference Wagner2007; Carroll Reference Carroll2008; Erwin and Davidson Reference Erwin and Davidson2009).
Many studies combining fossils with other biologic disciplines, such as developmental biology, utilize phylogenetic information at coarse taxonomic levels (Raff Reference Raff2007). Unfortunately for paleobiologists, transformations between ancestor-descendant morphologies at low taxonomic levels are not always unambiguously arrayed in stratigraphic succession. The fossil record is notoriously incomplete and can obscure patterns of first and last appearances (Smith Reference Smith2001). In addition to paleontologic incompleteness, mosaic and/or heterogeneous rates of phenotypic evolution frequently confound accurate interpretations of morphologic disparity and taxonomic evolution (Erwin Reference Erwin2007). In an attempt to account for these difficulties, paleobiologists are increasingly using quantitative computational methods (e.g., maximum parsimony, Bayesian inference) to construct phylogenetic hypotheses for fossil taxa rather than relying on “expert” taxonomic opinion and/or the stratigraphic distribution of first and last appearances. Reconstructed phylogenies of fossil taxa are becoming commonplace for analyzing a broad swath of macroevolutionary topics ranging from extinction dynamics (Purvis 2008; Harnik et al. Reference Harnik, Fitzgerald, Payne and Carson2014) to phenotypic trait evolution (Wagner Reference Wagner1995; Bapst Reference Bapst2014) and paleobiogeography (Lieberman 2000; Wright and Stigall Reference Wright and Stigall2014). Importantly, phylogenetic information is necessary for fossil occurrences to be meaningfully applied with other kinds of evolutionary data such as in EDB (Raff Reference Raff2007).
The use of model phylogenies as a template for paleobiological studies is not without concern (Wagner Reference Wagner2000a). Setting aside methodological issues involving optimality criteria (Wright and Hillis Reference Wright and Hillis2014), the accuracy of recovered phylogenies are fundamentally limited by the information content of their underlying morphologic data and a researcher’s ability to codify fossil morphology into a set of a priori hypotheses of homology. Therefore, choosing a robust set of primary homologies (sensu de Pinna Reference de Pinna1991) is critical to reconstructing model phylogenies. Given their importance for accurately reconstructing phylogenetic trees, how should paleobiologists propose homologous features in extinct lineages? What criteria form a logical basis for choosing among hypotheses of homologies when alternatives are possible? How are these hypotheses tested to discover features that reflect “true” evolutionary homologies?
This paper presents a combined phylogenetic and “paleo” ontogenetic approach to these questions (cf. Mooi et al. Reference Mooi, David and Wray2005). A research program in Phylogenetic Paleo-ontogeny combines fossils, phylogenetic systematics, and evolutionary developmental biology to provide a logical basis for discovering homologies and discerning patterns of character evolution among fossil species. As an example, I present a character analysis of posterior plate homologies among fossil and living crinoids (Echinodermata, Crinoidea) to illustrate how combining fossil morphology with developmental data can help resolve homology schemes and provide an ontogenetic basis for generating phylogenetic hypotheses of fossil taxa. The reassessment presented herein not only provides a step towards building a phylogeny that links extant crinoids with their Paleozoic ancestors but may also be useful guide for other researchers seeking a logical rationale when proposing homology statements for fossil taxa. As a prelude to my discussion on crinoid plate homologies, I first present an overview of theoretical and epistemological aspects of homology and discuss their relationship with phylogenetic systematics. Throughout, I integrate concepts from EDB when discussing homology and phylogenetic systematics to develop a general framework for a research program in Phylogenetic Paleo-ontogeny.
Homology: Conceptual Basis and Operational Definition
The concept of homology is fundamental to evolution and permeates all aspects of biology (Laubichler Reference Laubichler2000). Despite its significance, homology is a seemingly elusive concept because different disciplines use the word in different ways (Brigandt Reference Brigandt2003). For example, an evolutionary (paleo)biologist might propose an historical definition of homology involving a comparison of morphologic structures among species to reveal a sequence of adaptive transformations. In contrast, an evolutionary developmental biologist may instead prefer a mechanistic definition where homologous features are described in terms of their underlying developmental genetics. Contrasts between perspectives are potentially problematic for relating fossilized morphologies to mechanistic definitions of homology because the overwhelming majority of species known from fossils are entirely extinct and therefore unavailable for study in an evo-devo laboratory. More worrisome for paleobiologists is the knowledge that paralogous genes may produce superficially “homologous” morphological structures, and truly homologous loci may control non-homologous structures (e.g., Bolker and Raff Reference Bolker and Raff1996).
In light of evolution, differing perspectives of homology (i.e., historical and mechanistic) among disciplines are united by a common theoretical basis: homology is best viewed as a concept reflecting the continuity of information in the context of phylogenetic history (van Valen Reference van Valen1982). Ever since Darwin (Reference Darwin1859) the concept of homology has been used intuitively among biologists to convey the “same” features in different species arising from shared common ancestry. Recent insights from EDB suggests that the “sameness” underlying complex morphologic structures result from the inheritance of gene regulatory networks (GRNs) with co-adapted transcription factors rather than the cumulative expression of individual “homologous” genes (Wagner Reference Wagner2007; Erwin and Davidson Reference Erwin and Davidson2009). Indeed, most morphologic evolution likely results from changes in cis regulatory elements rather changes in gene number or protein function (Carroll Reference Carroll2008). GRNs can be dissected into quasi independent “developmental modules” responsible for the occurrence, reoccurrence, and modification of a morphological character across a phylogeny (Wagner 1996; Arnone and Davidson Reference Arnone and Davidson1997). Thus, a unified definition of homology combines developmental causality with phylogenetic continuity (Hall Reference Hall2003).
The phylogenetic distribution of homologous information is hierarchically nested across multiple levels of biological organization—from genes to species (Hall Reference Hall2003). The notion of “deep” homology requires a decoupling between phylogenetic, phenotypic, and genetic levels of homology. Deep homology arises when identical sets of genes are shared among phylogenetically disparate taxa despite great morphological differences between them (Shubin and Marshall Reference Shubin and Marshall2000). For example, the last decade of research in EDB has discovered striking similarities among the gene families of analogous morphological structures (Carroll Reference Carroll2008; Shubin and Marshall Reference Shubin and Marshall2000). When considering morphological traits, instances of convergence or parallelism are not homologous at the phenotypic level even if such patterns are causally linked to “deep” homologies at the genetic level (Hall Reference Hall2003). Because this paper is about formulating homology statements for realized fossil morphology, it is useful herein for the concept of “homology” to refer to the subset of phylogenetic information expressed in the phenotypes of ancestor-descendant lineages while recognizing that deeper genetic homologs (or paralogs) play an important role in morphologic evolution.
The theory and practice of phylogenetic systematics unites ontological and epistemological aspects of homology by providing the concept with a set of procedures that lead to its discovery in empirical data (Wiley and Lieberman Reference Wiley and Lieberman2011). In fact, the advent of phylogenetic systematics has led many biologists to equate homology with synapomorphy (Hennig Reference Hennig1966; Wiley Reference Wiley1975; Patterson Reference Patterson1982; Wheeler Reference Wheeler2012). Any trait present in an ancestor and all of its descendants is by definition a homologous trait via continuity of descent (note that symplesiomorphies are synapomophies when considered at a more inclusive level). Thus, “a homology is always a synapomorphy and a synapomorphy is always a homology” (Wheeler Reference Wheeler2012: p. 117).
Phylogenetic Systematics and the Discovery of Homologous Characters
Knowledge of “true” evolutionary homology requires knowledge of absolute truth, which is of course nonexistent in science. Phylogenetic empiricism requires observational, a priori hypotheses of homology (Wiley and Lieberman Reference Wiley and Lieberman2011). Hypotheses of homology are repeatedly tested and ultimately either corroborated or falsified on the basis of available evidence (Hennig Reference Hennig1966). The “discovery” of homologous characters among fossil lineages is therefore anchored by empirical observations and best approached when multiple lines of independent evidence are considered.
Ernst Haeckel claimed to have employed the “threefold parallelisms” of Louis Agassiz to infer phylogeny: ontogenetic sequences, comparative anatomy, and the phyletic transformation of fossils in geologic succession (see Gould Reference Gould1973). Although theoretical and computational aspects of modern phylogenetic methods bear little resemblance to Haeckel’s, the components of Agassiz’s parallelisms still comprise the basic character data used to propose homologous characters and build phylogenies. The essence of Agassiz’s parallelisms can be combined with the ontological basis of homology provided by EDB and the epistemological principles of phylogenetic systematics to forge an interdisciplinary research program: Phylogenetic Paleo-ontogeny.
Characters and phylogenetic hypothesis testing
Homology statements in phylogenetics originate as character data. Any observable, heritable organismal feature is potentially a phylogenetically informative character (e.g., morphologic structures, a sequence of nucleotides, developmental traits, or behavioral data). Because morphologic characters are produced by developmental modules in the GRN, the phenotype of organisms can similarly be atomized into components of semi-autonomous “morphologic modules”; where each module has a degree of independence despite some integration with other modules (Wagner Reference Wagner2006). The quasi-independent nature of characters underscores a common (if not ubiquitous) assumption in mathematical phylogenetic methods: a character must operationally be expressed as an independent random variable X with N mutually exclusive transformational states (X 0, X 1, X 2…X N) in quantitative phylogenetic analysis (Sereno 2007).
De Pinna (Reference de Pinna1991) recognized two distinct levels of homology statements that arise in phylogenetic systematics: primary and secondary homologies. A primary homology refers to a proposition that two characters are homologous (de Pinna Reference de Pinna1991). Primary homologies are generated prior to phylogenetic analysis and therefore represent a priori hypotheses. In practice, primary homology statements for characters X 0, X 1, X 2…X N are given by the distributions of transformational states among the columns of a character by taxon matrix. Primary homology statements can be falsified as instances of homoplasy or corroborated by other characters via phylogenetic analysis. Patterson’s (Reference Patterson1982) suggestion to examine the degree of congruence among characters forms the most decisive test of homology for morphologic characters (de Pinna Reference de Pinna1991). Under Hennig’s (Reference Hennig1966) auxiliary principle, primary homologies represent inchoate hypotheses of common descent. If a primary homology passes Patterson’s congruence test, it matures into a secondary homology (de Pinna Reference de Pinna1991).
Quantitative phylogenetic methods form a natural test of Patterson’s (Reference Patterson1982) notion of character congruence because they utilize the covariance structure among characters simultaneously when searching for optimal tree topologies. Once an analysis results in a tree (or set of trees), characters can be mapped onto the tree(s) to delimit monophyletic groups and discover instances of homoplasy. Congruence obtains when multiple primary homology statements corroborate one another and diagnose the same monophyletic group, viz. primary homologies become secondary homologies. However, if a primary homology statement requires independent derivations in different clades, then that primary homology has been falsified by the available evidence and cannot be considered a homology at a hierarchical level inclusive of those clades. Note that homoplasies at one phylogenetic level may be considered synapomorphies when considered at a less inclusive level. Observations of character congruence in real datasets reflect a mixture of homology and homoplasy at different phylogenetic scales (Wagner Reference Wagner2000b).
Of course, erroneous assumptions about characters are likely to affect the accuracy of phylogenetic inferences (Wagner Reference Wagner2000b). Although the proposition of primary homology statements requires a priori assumptions about what constitutes the quality of sameness between characters, they do not require a priori assumptions regarding inferences of actual patterns of character evolution. (Those require an independent set of assumptions involving computational and/or optimality criteria in phylogenetic analysis, not discussed here.) The accuracy of all evolutionary inferences in phylogenetic systematics is constrained by what is herein termed the phylogenetic uncertainty principle. The phylogenetic uncertainty principle simply states that the certainty of any output tree topology recovered using phylogenetic methods is fundamentally limited by the inherent uncertainty in the original choice of primary homologies. Primary homologies may be falsified during phylogenetic inference (rendering phylogenetics an empirically rigorous and testable science), but the underlying character data must be assumed a priori to comprise comparable elements in the first place. The phylogenetic uncertainty principle is an epistemological constraint imposed by the nature of historical data in biology and cannot be circumvented by mathematical or other methodological techniques, although such procedures may provide useful heuristics. The identification of comparable elements in molecular phylogenetics has benefited greatly from the advent of alignment techniques for DNA sequences, but no analogous framework currently exists for morphology. Moreover, uncertainty in phylogeny reconstruction cannot be solved by continuously adding more characters, regardless of quality, to overwhelm the noise to signal ratio and increase clade resolution, particularly in morphologic systematics (Wagner Reference Wagner2000b; Bapst Reference Bapst2012). Ideally, all “errors” discovered among primary homology statements through phylogenetic analysis arise for biologic reasons and not from poor interpretations of morphology. Therefore, it is critical when making phylogenetic inferences that all a priori assumptions of primary homology have a logical, biologic basis supported by empirical observations via character analysis.
Character analysis: Remane’s criteria and developmental biology
Hypotheses of primary homology arise from character analysis, not phylogenetic analysis. These hypotheses are critical to accurately reconstruct evolutionary relationships because the outcome of a phylogenetic analysis is determined by the matrix analyzed (Bryant Reference Bryant1989). Given their fundamental importance, assessments of primary homology should be critically evaluated and only proposed after careful analysis and argumentation. Remane (Reference Remane1952) outlined three principles useful for recognizing potentially homologous features among organisms: (1) similarity in position, (2) similarity in structure, and (3) the existence of transitional forms.
Criterion (1) corresponds with Geoffroy Saint-Hilaire’s (Reference Saint-Hilaire1830) “principe des connexions” and refers to similarities in the topological position of a character and its relation to other characters; whereas criterion (2) refers to an “intrinsic” similarity where two or more features match in their structural details and complexity without reference to topological position (Wiley and Lieberman 2001). For a given character, an unambiguous match between criteria (1) and (2) would support the proposition of a primary homology statement. Unfortunately, it is not always clear what constitutes a “match”. Multiple hypotheses of primary homologies can arise when evolution has transformed one structure into another and/or a character has shifted in topological position. What if the evidence supporting alternative interpretations is equivocal? Criterion (3) offers a solution to this dilemma by incorporating information on the transitional forms of fossils and development.
Observations of the embryologic stages of development combined with the geologic succession of fossilized morphologies have long helped guide the recognition of homologous characters (von Baer Reference von Baer1828; Darwin Reference Darwin1859; Hall Reference Hall2002). Homologous characters need not be similar in structure or position if it can be shown they have common genealogical origins via the existence of transitional forms exhibited in developmental patterns or the fossil record. Moreover, the existence of intermediate forms in fossils and embryos provides a temporal axis to character transformations. Gilbert and Bolker (Reference Gilbert and Bolker2001) pointed out that a significant feature of embryological development is not necessarily the appearance (or disappearance) of individual transient morphologic structures, it is the temporal sequence of changes the embryo undergoes and their underlying genetic mechanisms. In other words, these temporal sequences (paleontologic or developmental) themselves can provide a logical basis for proposing primary homologies.
Conflicts Between Homology, Terminology, and Phylogenetics: Examples from the Echinodermata
“I salute the echinoderms as a noble group especially designed to puzzle the zoologist.” Libbie H. Hyman (Reference Hyman1955: p. vi)
A major source of character data in systematic studies come from taxonomic descriptions of morphology. This is particularly true in studies utilizing paleontological data because most fossils are limited to providing only morphologic information about extinct organisms. However, taxonomic descriptions and the terminology employed therein do not express unbiased observations of nature. All descriptive observations, including those in this paper, are colored by theories and expectations (Eldredge and Gould Reference Eldredge and Gould1972). Notably, detailed taxonomic descriptions are often entrenched in theoretical considerations of homology and rich in predictions of character evolution.
When theories of homology and character evolution change, the terminology used to name and/or identify a character may or may not. Obviously, problems arise in downstream comparative analyses if the descriptive terminology used to describe a morphologic feature does not reflect its evolutionary history. The existence (and persistence) of a poor descriptive nomenclature in taxonomic literature obfuscates hypotheses of primary homology when building a character matrix and results in specious topologies when such a matrix is analyzed using phylogenetic methods. The examples below taken from echinoderm studies demonstrate that hypotheses of primary homology should not be taken from taxonomic literature uncritically.
Echinoderm homologies and phylogeny
Echinoderms are a phylum of marine organisms represented by more than 7000 living species (Brusca and Brusca Reference Brusca and Brusca2003) distributed among five classes: Crinoidea (sea lilies and feather stars), Ophiuroidea (brittle stars), Asteroidea (sea stars), Echinoidea (urchins and sand dollars), and Holothuroidea (sea cucumbers). The apparent diversity of extant echinoderms masks their more prodigious geologic history. The half-billion year echinoderm fossil record is spectacularly complete and reveals approximately 30 clades distributed among 21 taxonomic classes spanning the entire Phanerozoic Eon (Sprinkle and Kier Reference Sprinkle and Kier1987). Moreover, the calcitic endoskeletons of fossil and living echinoderms showcase a bewildering array of disparate morphologies making them ideal for studying large scale evolutionary patterns (Foote Reference Foote1992; Sprinkle and Guensburg Reference Sprinkle and Guensburg1997).
Yet the phylum’s extreme morphologic disparity also presents difficulties for determining homologies between and among clades and obstructs accurate phylogenetic inferences (Paul and Smith Reference Paul and Smith1984; Sumrall Reference Sumrall1997). Research assembling a “complete” echinoderm phylogeny has been stymied for decades in part due to the lack of a unified set of morphologic terms representing homologous skeletal structures and much effort has recently been applied to the problem (Mooi et al. Reference Mooi, David and Marchland1994; Mooi and David Reference Mooi and David1997; Mooi et al. Reference Mooi, David and Wray2005; David et al. Reference David, Mooi and Parsley2000; Sumrall Reference Sumrall2008; Reference Sumrall2010; Sumrall and Waters Reference Sumrall and Waters2012; Zamora et al. Reference Zamora, Rahman and Smith2012; Kammer et al. Reference Kammer, Zamora, Ausich and Deline2013). For example, Sumrall and Waters (Reference Sumrall and Waters2012) examined thecal plate elements among four clades of fossil blastozoan (i.e., stalked, non crinoid) echinoderms and discovered that homologous plates in closely related clades often had different names and that some nonhomologous plates had the same name. Egregious terminology is not limited to fossil echinoderms. Mooi and David (Reference Mooi and David1997) pointed out that the five living classes also have major terminological problems that obfuscate common features across clades, such as the name applied to the various expansions of the oral ring (Hyman Reference Hyman1955). Thus, using the traditional names of plates to construct hypotheses of primary homology would produce spurious topologies in phylogenetic analysis because these plates are only “homologous” in the sense that they share the same name but do not share evolutionary origins. Clearly, character analyses and revisions of morphologic terms must accompany efforts to reconstruct echinoderm phylogeny.
Crinoids as a fractal analog of a phylum
Difficulties determining skeletal homologies are pervasive within as well as between echinoderm clades. Within the Crinoidea, the 600 or so living species constitute a fractal analog of homology problems outlined above characteristic of the phylum and offer the opportunity for a smaller scale case study detailing a Phylogenetic Paleo-ontogenetic approach to discovering homology (Simms Reference Simms1993; Ausich Reference Ausich1996). Below, I resolve contention surrounding primary homologies for posterior plates of “pan-cladid” crinoids by combining data from fossil morphology and developmental patterns in living crinoids. Although conducting a comprehensive phylogenetic analysis on pan-cladid crinoids is beyond the scope of this paper, the analysis presented here provides a logical basis for choosing hypotheses of primary homologies for posterior plate characters in future phylogenetic analyses. Moreover, it is hoped that the argumentation used herein will serve as general framework for others seeking a logical basis for proposing primary homologies to test phylogenetic hypotheses.
Terminological Antinomies and Transitional Homologies: Parallels Between Crinoid Ontogeny and Fossilized Evolutionary History
“The mystery of this controversy is curiously full of misunderstandings and misrepresentations.” Francis A. Bather (Reference Bather1891: p. 480)
Natural history of the pan-cladid Crinoidea
The Pan-Cladida are a long-lived clade of crinoids spanning the Ordovician Period (~485.4 Ma to 443.4 Ma) (Cohen et al. Reference Cohen, Finney, Gibbard and Fan2013) to the present and include all extant species of Crinoidea as well as fossil forms. Together, the pan-cladids are the most diverse clade of crinoids and comprise three of the fived named subclasses: Cladida, Flexibilia, and Articulata (Ausich et al. Reference Ausich, Kammer, Wright, Cole, Peter and Rhenberg2015; Wright and Ausich Reference Wright and Ausich2015). The subclass Cladida is paraphyletic and gave rise to both the Flexibilia and Articulata (Springer Reference Springer1920; Simms and Sevastopulo Reference Simms and Sevastopulo1993). Thus, the name “Pan-Cladida” used herein refers to the common ancestor of all species included within the subclass Cladida and all of its descendants, regardless of taxonomic rank in the Linnaean hierarchy (Wright and Reference Wright and AusichAusich 2015). The flexible crinoids split from the cladids during the Late Ordovician, diversified, and went extinct at the end of the Paleozoic Era. The Articulata, of which all living crinoid species are grouped, originated from cladid ancestors either during the Late Paleozoic or earliest post-Paleozoic (Simms and Sevastopulo Reference Simms and Sevastopulo1993; Webster and Jell Reference Webster and Jell1999) (Fig. 1). Recent phylogenies of living crinoids indicate a monophyletic Crinoidea, but the monophyly of Articulata has been questioned and awaits further analysis (Rouse et al. Reference Rouse, Jermiin, Wilson, Eeckhaut, Lanterbecq, Oji, Young, Browning, Cisternas, Helgen, Stuckey and Messing2013; Roux et al. Reference Roux, Hemery and Ameziane2013). Because the greatest diversity of pan-cladid crinoids are known only as fossils, tracing the ancestry of living crinoids and discovering their phylogenetic affinities with extinct fossil lineages requires a detailed understanding of crinoid comparative morphology to generate hypotheses of homology.

Figure 1 Summary of previously proposed phylogenetic relationships of pan-cladid crinoids. A, Phylogeny and classification from the Treatise of Invertebrate Paleontology based on Moore and Teichert (Reference Moore and Teichert1978) and Moore et al. (Reference Moore, Lane and Strimple1978). B, A revised phylogeny and classification based on Simms and Sevastopulo (Reference Simms and Sevastopulo1993) and Ausich (Reference Ausich1998). C, Phylogenetic relationships according to Webster and Jell (Reference Webster and Jell1999).
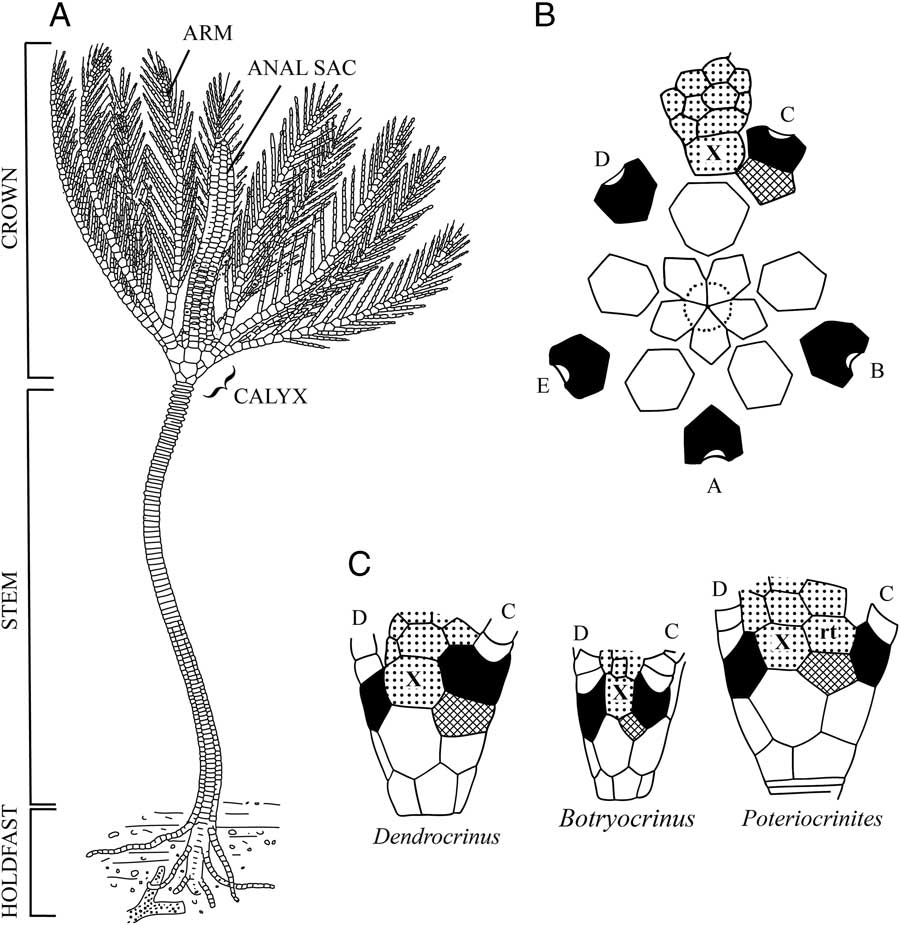
The skeletal morphology of a crinoid is highly complex and consists of several morphologic modules: the size and shape of the calyx, number and arrangement of posterior plates, arm morphology and branching pattern, and stem (Fig. 2). The combinatorial nature of module configurations has given rise to a staggering diversity of pan-cladid morphologies and nearly 1000 named genera. In Paleozoic cladids, the pentaradial symmetry of the calyx is interrupted by one to three plates located in the posterior CD interray of the cup. These additional, so called “anal” (sensu Ubaghs Reference Ubaghs1978) plates, in the posterior region are referred as the radianal, anal X, and right tube plate (listed from the aboral to oral direction) (Fig. 2B–C). Fossil forms display temporal variation in the number, shape, and positional relations of posterior plates (Moore et al. Reference Moore, Lane and Strimple1978; Webster and Maples Reference Webster and Maples2006). In extant crinoids, a posterior plate is present in juvenile stages but absent in adults (Clark Reference Clark1915; Amemiya et al. Reference Amemiya, Tsurugaya, Hibino, Yamaguchi, Kuraishi, Kiyomoto and Minokawa2014). Because posterior plating patterns are an important module of morphologic differentiation, they have been extensively used as taxonomically significant characters for delimiting crinoid species and higher taxa (Moore et al., Reference Moore, Lane and Strimple1978; Webster and Maples Reference Webster and Maples2006). Thus, previous attempts at testing phylogenetic hypotheses and evolutionary patterns among pan-cladids included posterior plate characters despite substantial uncertainty underlying their primary homology statements (Brower Reference Brower1995; Ausich Reference Ausich1998; Gahn and Kammer Reference Gahn and Kammer2002; Kammer Reference Kammer2008).
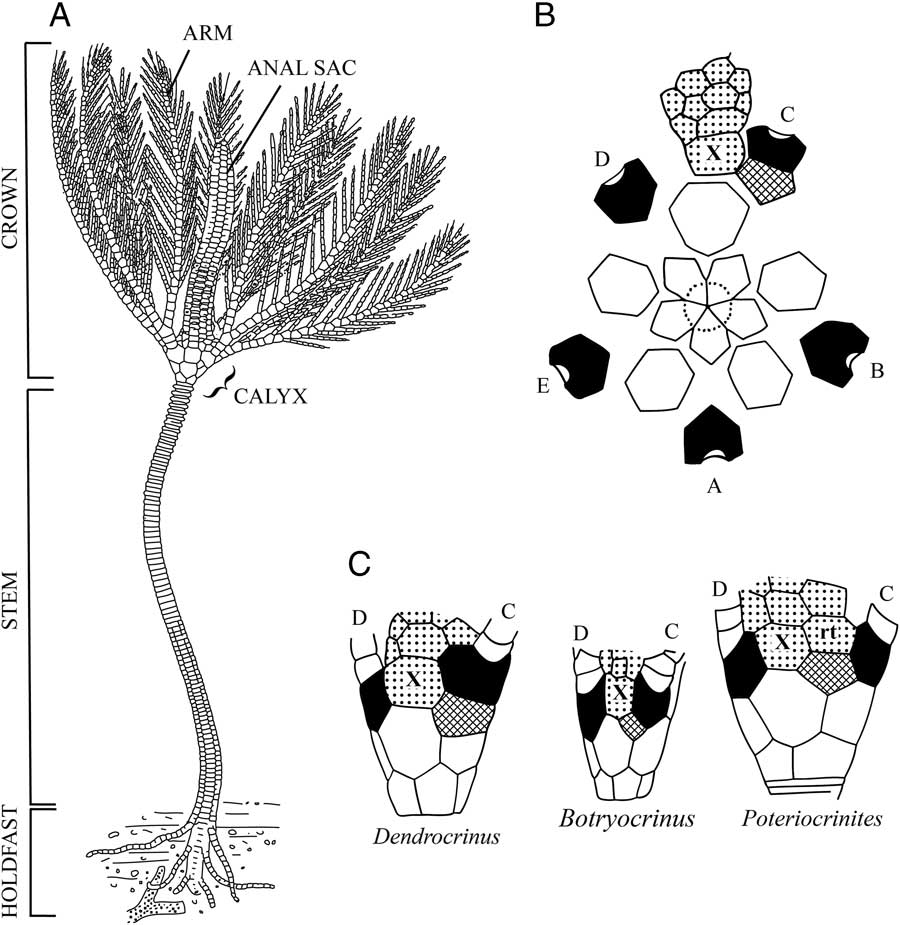
Figure 2 A, Reconstruction of the Silurian crinoid Dictenocrinus decadactylus depicting the morphologic features described in the text. Note that a modern reconstruction would place the crown in a down current position with the arms in an outstretched position to form a rheophilic filtration fan (modified from Bather 1900: Fig. 3). B, Plate diagram of Dendrocrinus longidactylus showing the orientation of rays and interrays in pan-cladid crinoids. The crinoidal plane of symmetry is interrupted in the posterior region by the addition of plates in the CD interray (modified from Moore et al. Reference Moore, Lane and Strimple1978: Fig. 395). C, The CD interray of three genera depicting common positions and arrangements of posterior plates (modified from Moore et al. Reference Moore, Lane and Strimple1978: Fig. 394). (A–E ray designations in Carpenter’s [Reference Carpenter1884] system, radials black, radianal cross ruled, anal X, and right tube plate [rt] stippled.)
Posterior plates: temporal trends and contentious homologies
Patterns of posterior plate evolution in fossil pan-cladids have been characterized as exhibiting a “progressive change toward increased simplicity” (Moore and Laudon Reference Moore and Laudon1943: p. 34). The oldest known pan-cladids have multi-plated posterior interrays (Sprinkle and Wahlman Reference Sprinkle and Wahlman1994; Guensburg and Sprinkle Reference Guensburg and Sprinkle2009) and many lineages subsequently exhibit a general trend to reduce the number of posterior plates in the cup throughout the Paleozoic (Moore and Teichert Reference Moore and Teichert1978). Temporal changes in the number of posterior plates broadly correspond with concomitant shifts in ecologic abundance and taxonomic diversity (Webster and Maples Reference Webster and Maples2006). Complex, multi-plated morphologies were the most diverse during the Ordovician and subsequently disappear from the fossil record; whereas crinoids with three posterior plates were most dominant throughout the Silurian to Pennsylvanian (Webster and Maples Reference Webster and Maples2006). Crinoids with a single posterior plate rapidly diversified during the Pennsylvanian and increased in frequency to become the most common morphology during the Permian (Webster and Maples Reference Webster and Maples2006). Older taxonomic literature capture these so called progressive changes by describing a plate arrangement as having either a “primitive” or “advanced” condition, where primitive refers to stratigraphically older multi-plated morphologies and advanced refers to younger two or single-plated forms (Moore et al. Reference Moore, Lane and Strimple1978).
Homology schemes for posterior plates among fossil lineages and between fossil and living crinoids have been debated, somewhat inimically, for more than a century (Carpenter Reference Carpenter1882; Wachsmuth and Springer 1879; Bather 1980; Reference Bather1891; Reference Bather1918; Clark Reference Clark1915; Mortensen Reference Mortensen1920; Springer Reference Springer1920; Ubaghs Reference Ubaghs1953; Moore Reference Moore1962; Phillip Reference Philip1964; Moore and Teichert Reference Moore and Teichert1978; Webster and Maples Reference Webster and Maples2006). The overwhelming majority of named fossil genera have three posterior plates in the cup, including putative Paleozoic ancestors of living crinoids and fossil flexibles (Webster and Jell Reference Webster and Jell1999; Webster and Maples Reference Webster and Maples2006). Using Remane’s (1) criterion described above, proposing primary plate homologies for crinoids with three posterior plates is straightforward. The radianal is the most proximal posterior plate to (and always in contact with) the C radial, typically occupying a position beneath or to the left of the C radial. The anal X is interradial in position and may be in lateral contact with the radianal, C radial, BC and CD basals, or the CD basal, and occupies a position above and/or to the left of the radianal (Ubaghs Reference Ubaghs1978). The right tube plate rests either above the radianal or both the radianal and anal X and typically provides support for other plates in the anal sac (Fig. 2). Where only two plates are present in the cup, Remane’s (1) criterion can once again be used to propose primary homologies for the two remaining more proximal plates: the radianal and anal X (Moore and Teichert Reference Moore and Teichert1978). However, any further reduction in the number of posterior plates renders Remane’s (1) criterion inapplicable because the single posterior plate does not occupy a position more similar to either the radianal or anal X where two or more posterior plates are present. In other words, one of the posterior plates migrated from its ancestral position and the other is absent.
An antinomy can be characterized as a kind of paradox that describes two equally compelling but mutually incompatible explanations and is a useful term to describe the contention that arises when only a single posterior plate is in the cup. Choosing whether a single plate in the posterior interradius is the radianal or anal X presents an antinomy of alternative homology schemes (Fig. 3). If there is only one posterior plate in a fossil pan-cladid, is it the radianal or anal X? Is this fossilized single posterior plate homologous with the single plate present in the juvenile stages of extant crinoids? If so, which posterior plate is it? Unfortunately, Remane’s (2) criterion cannot help because the shape of a single posterior plate is constrained to accommodate changes in the size and shape of the calyx and therefore does not retain the shape of either the radianal or anal X when alone in the cup.

Figure 3 An antinomy of alternative primary homology schemes for a single posterior plate in the Mississippian genus Phanocrinus. A, Possible homology scheme depicting an evolutionary trend in posterior plate reduction leaving the anal X in the cup. B, An alternative homology scheme depicting the radianal as the single posterior plate. (Redrawn from Strimple [Reference Strimple1948]. Radials black, radianal cross ruled, anal X, and right tube plate [rt] stippled.)
The problem is further confounded by a history of problematic terminology favoring plate topologies over plate homologies. Remarkably, a perusal of the taxonomic literature of fossil crinoids reveals the presence of a single posterior plate in a fossil cladid is frequently termed an anal X in taxonomic descriptions and figured specimens even when an author considered the plate homologous to the ancestral radianal or the evidence equivocal (cf. Moore and Laudon Reference Moore and Laudon1943; Ubaghs Reference Ubaghs1953; Reference Ubaghs1978; Moore Reference Moore1962; Philip Reference Philip1964). Kirk’s (Reference Kirk1944: p. 234) description of the single posterior plate in the Mississippian genus Cymbiocrinus epitomizes this dubious practice: “it is doubtful if this plate is homologous to [the] anal X, but we may so denominate it for convenience”. These misnomers obfuscate any notion of evolutionary continuity among characters. It is not surprising there has been much confusion given that different sections of the crinoid Treatise of Invertebrate Paleontology (Moore and Teichert Reference Moore and Teichert1978) disagree with one another with respect to posterior plate homologies and terms for fossil and living pan cladids (cf. Breimer Reference Breimer1978, Brower Reference Brower1978, Moore et al. Reference Moore, Lane and Strimple1978; Strimple Reference Strimple1978; and Ubaghs Reference Ubaghs1978). Thus, crinoid paleobiologists should not take Treatise descriptions of posterior plate characters at face value when proposing primary homologies to make phylogenetic inferences.
Potentially more confusing for a phylogeneticist is the terminological scheme proposed by Webster and Maples (Reference Webster and Maples2006). In an attempt to rectify terminological misnomers, Webster and Maples (Reference Webster and Maples2006) proposed to abolish all implications of homology from morphologic nomenclature by renaming the posterior plates the primanal, secundanal, and tertanal. Under this terminology, the primanal is always the most proximal plate in the cup, regardless of whether it is the radianal or anal X. Thus, Webster and Maples (Reference Webster and Maples2006) “solved” the epistemological dilemma of proposing homology statements by avoiding them altogether. This so called solution is problematic for two reasons. First, because the terminology of Webster and Maples (Reference Webster and Maples2006) is based purely on topology without reference to any ontological theory of homology, it is useless for proposing primary homologies to test phylogenetic hypotheses. Second, the proposed terms are already in use to describe an unrelated, non-homologous set of plates in a phylogenetically distant clade of crinoids, the subclass Camerata (Moore and Teichert Reference Moore and Teichert1978; Ausich Reference Ausich1998). For these reasons, Webster and Maples’ (Reference Webster and Maples2006) terminology should not be followed. Instead, we should confront the problem directly by seeking a logical basis for choosing between alternative primary homology schemes. This approach has the advantage of combining posterior plate characters with other modular complexes of crinoid morphology in a phylogenetic analysis. Thus, characters can be tested against one another when inferring phylogenetic relationships and any parallel instances of plate reduction (or addition) can be determined empirically.
In the next two sections, I apply Remane’s (3) criterion to examine potential transitional forms in two independent sources of data: the ontogeny of extant crinoids and paleontologic studies on individual lineages. The recognition of intermediate morphologies present in developmental patterns of extant representatives and/or high resolution paleontologic sequences may provide paleo-ontogenetic evidence supporting one set of primary homologies over another and dissolve antinomies arising from examining comparative morphology alone.
Developmental patterns in living crinoids
Numerous observations of embryologic stages in extant crinoids have been described, particularly for the stalkless comatulid crinoids (Thomson, Reference Thomson1865; Carpenter Reference Carpenter1866; Clark Reference Clark1915; Springer Reference Springer1920; Mortensen Reference Mortensen1920; Mladenov and Chia Reference Mladenov and Chia1983; Lahaye and Jangoux Reference Lahaye and Jangoux1987; Shibata et al. Reference Shibata, Oji and Akasaka2008). Developmental patterns in living species of stalked crinoids were largely unknown until recently (Nakano et al. Reference Nakano, Hibino, Oji, Hara and Amemiya2003; Amemiya et al. Reference Amemiya, Tsurugaya, Hibino, Yamaguchi, Kuraishi, Kiyomoto and Minokawa2014). Because stalked crinoids and comatulids share many similarities with respect to the skeletal development of the calyx and posterior plates, the distinction between the two adult forms is not pertinent for the purposes of this paper.
Kammer (Reference Kammer2008) lucidly described common themes of crinoid development, from which I base the following conspectus. Crinoid ontogeny consists of five successive life stages: the embryo, doliolaria, cystidean, pentacrinoid, and (in comatulids) the comatulid stage (Mladenov and Chia Reference Mladenov and Chia1983; Lahaye and Jangoux Reference Lahaye and Jangoux1987). The doliolaria is an endotrophic, free swimming larva that emerges from the embryonic membrane. Once the doliolaria settles on a suitable substrate, it metamorphoses into the stalked cystidean stage. The skeleton at the cystidean stage consists of paired, interradially oriented basals and primary peristomial cover plates (i.e., oral plates, see Kammer et al. Reference Kammer, Zamora, Ausich and Deline2013), a terminal stem plate, and a few columnals. Infrabasal plates, common in fossil pan-cladids, do not usually develop in living articulates but are known to occur in some species (Rasmussen 1978). Next, more skeletal plates are added including an “anal” plate, radials, and numerous additional plates relating to the construction of the arms and pinnules initiating the exotrophic pentacrinoid stage. Comatulids become adults when they excise the stem; whereas stalked crinoids grow into larger “adult” pentacrinoids. Although variation in the details of crinoid ontogeny exists, all crinoid species exhibit the general growth sequence and patterns of skeletal plate addition outlined above.
This single posterior plate was originally called an “anal” plate in living species because it was first discovered in the pentacrinoid stage of comatulids to directly overlie the CD basal between the radials, and therefore assumed on the basis of topological position to be homologous with the anal X in fossil crinoids (Thomson Reference Thomson1865; Carpenter Reference Carpenter1866; Bather Reference Bather1890). However, development is not a static process and characters cannot be accurately traced by giving special consideration to a single ontogenetic stage. Skeletal plates within the calyx grow at different rates and change in size and shape over the course of ontogeny (Lahaye and Jangoux Reference Lahaye and Jangoux1987). In addition, the topological arrangement of plates may vary in different growth stages. These changes must be accounted for when tracing parallels between development and evolution. Comparisons between a single ontogenetic stage and adult morphology can lead to specious proposals of primary homology because the developmental origin of a character may not correspond to its position during a later time in ontogeny. Instead, the entire sequence of changes must be considered when connecting the developmental origin of a morphologic character with its ontogenetic history and ultimate fate in the adult.
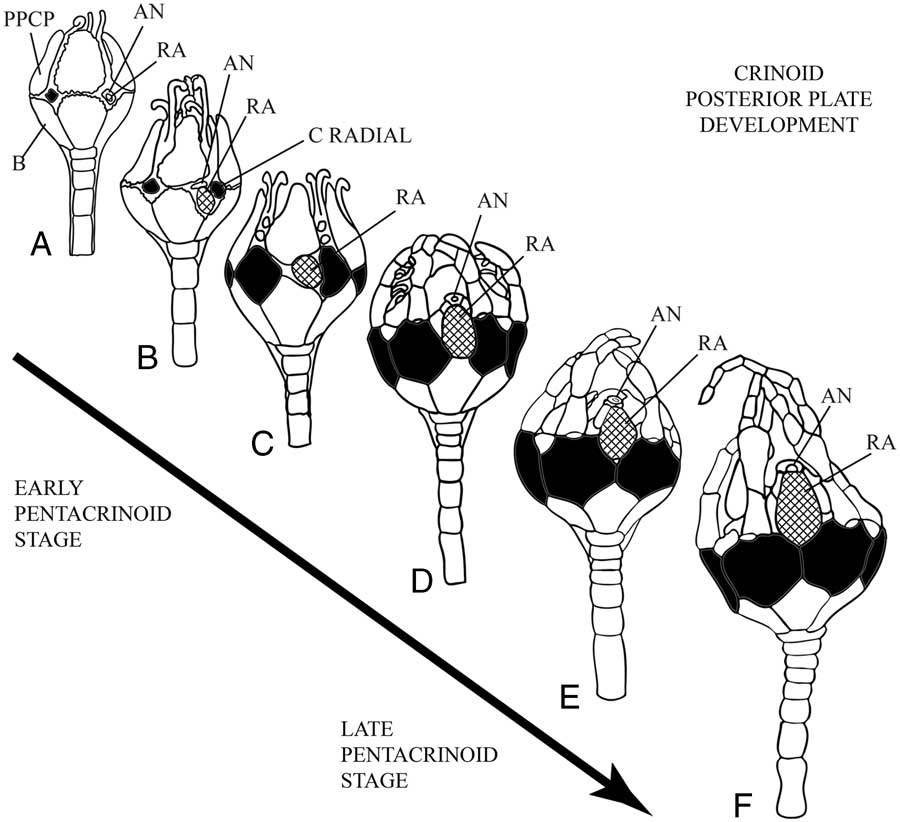
The developmental origin, migration, and eventual resorption of the single posterior plate in juvenile crinoids have been well characterized throughout the succession of ontogenetic stages by Clark (Reference Clark1915), Springer (Reference Springer1920), and Lahaye and Jangoux (Reference Lahaye and Jangoux1987) (Fig. 4). Detailed observations by these authors demonstrate that the development of the posterior “anal” plate originates in a radial position (with respect to the basals and primary peristomial cover plates) prior to the radials during the cystidean stage, and occurs within the same radius as the C radial. When the radial plates begin to grow, the larger posterior plate occupies a position beneath and to the left of the C radial, maintaining a close affinity with the developing gut tract (Springer Reference Springer1920). As the radials grow larger, the posterior plate occupies a position on the right hand side of the CD interray and is accommodated within a concavity in the C radial plate. Eventually the radials push the posterior plate into an inter radial position (i.e., the CD interray) where it supports a lappet that protects the developing anal cone (Lahaye and Jangoux Reference Lahaye and Jangoux1987). The posterior plate subsequently migrates out of the cup and is resorbed once the anal cone has formed. In summary, the posterior plate originates in a radial position, maintains lateral continuity with the C radial, and later moves into the CD interray and out of the cup. In fossil pan-cladids with multiple posterior plates, it is the radianal that has affinities with the C radial and maintains lateral contact with it; whereas the anal X is an interradial plate (Ubaghs Reference Ubaghs1978). Thus, the evidence from crinoid ontogeny indicates that the single, prominent posterior plate found in living crinoids is homologous with the radianal in fossil pan-cladids, not the anal X.
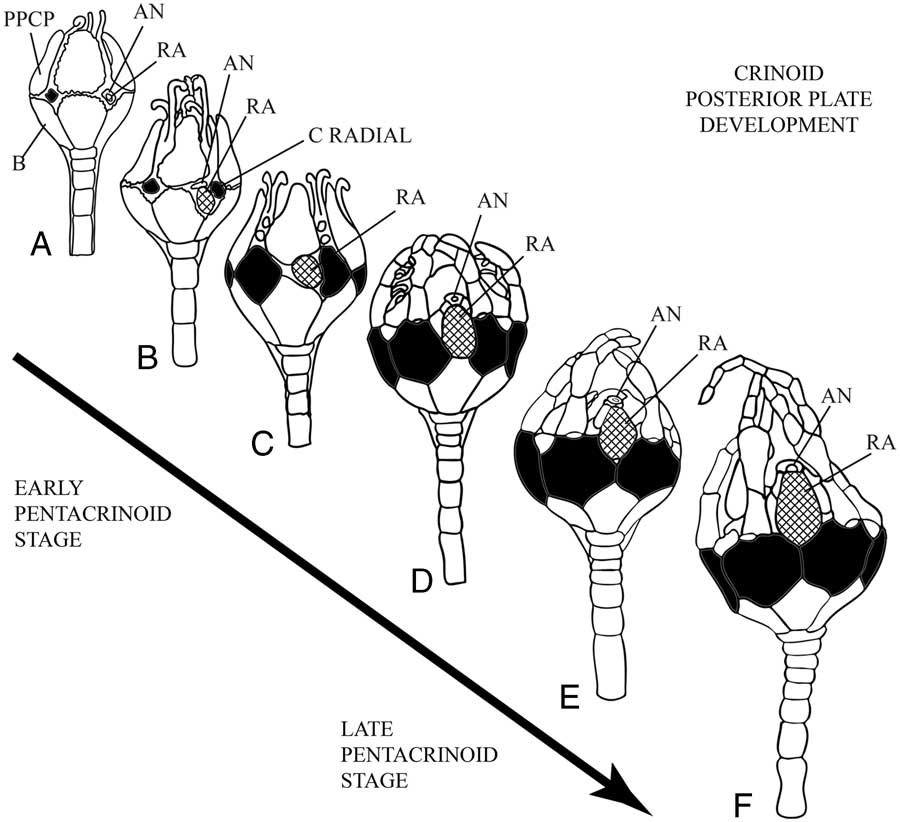
Figure 4 Pentacrinoid stage development and morphogenesis of the extant comatulid crinoid Comactinia meridionalis. A, Early pentacrinoid stage showing the radianal in the C ray with close affinities to the gut tract. B, The radianal is larger than the newly formed C radial and lying obliquely below it. C, Radial plates have increased in size. The radianal has migrated toward the middle of the underlying CD basal while still occupying the inner margin of the C radial plate. D, Radial plates are in in lateral contact except in the CD interray, where the radianal occupies a medial position. E, Radial plates are now in complete lateral contact. The radianal has been lifted up within the cup with the growth of the anal tube. F, The radianal rests on the shoulders of the C and D radial plates and continues its upward migration with the anal tube. The radianal is resorbed shortly after this stage. (Stages redrawn from Springer [Reference Springer1920: Plate B]. Radials black, radianal cross ruled, AN=anus, B=basal plate, PPCP=primary peristomial cover plate, RA=radianal plate).
If the single posterior plate in the juvenile stages of living species is the radianal, is there any ontogenetic evidence for the existence of an anal X? Recall that the anal X is an interradial plate within the CD interray. Interradial plates are known to occur in several species of living comatulids and appear late in development during the pentacrinoid stage (Clark Reference Clark1915; Breimer Reference Breimer1978). They either occur in all five interrays with the same degree of development or they are absent entirely (Breimer Reference Breimer1978). The early mid-pentacrinoid stage of Comactinia meridionalis displays posterior plating strikingly similar to the arrangement in many fossil cladid and flexible species, with the radianal side by side with the additional posterior plate (Springer Reference Springer1920: Plate B, Fig. 5a). Later, the interradials are resorbed along with the radianal leading to their absence in the adult phenotype (Springer Reference Springer1920; Breimer Reference Breimer1978). If the posterior interradial is homologous with the anal X in fossil crinoids, than the right tube plate (and any other posterior plates) in fossil pan-cladids may have developed from additional posterior interray plates. In this scenario, the interradial plates outside the CD interray either did not develop or were resorbed prior to adulthood, as they are unknown in fossil species. Alternatively, the posterior interradial plate may not be homologous with the anal X. The insertion of interradial plates may instead be a novel feature present among a subset of living comatulids. Interradial plates are presently unknown in juvenile stalked crinoids, but this may simply reflect the paucity of developmental studies on stalked crinoids (Amemiya et al. Reference Amemiya, Tsurugaya, Hibino, Yamaguchi, Kuraishi, Kiyomoto and Minokawa2014).
These observations have significant implications for ascertaining evidence-based primary homology statements if one is willing to assume geology’s useful aphorism regarding the present as the key to the past: the morphologic transitions unfolding during crinoid ontogeny are developmental relics of their Paleozoic precursors. The migration pathway of the radianal during crinoid development (viz. originating radially in the C ray followed by movement to the posterior interradius and out of the cup) parallels temporal trends in the paleontologic succession of posterior plate morphologies (Fig. 4). Therefore, a uniformitarian perspective supports the hypothesis that the radianal plate, not the anal X, is homologous with the single posterior plate in Paleozoic pan-cladids. Thus, the single posterior plate in the cup of a fossil pan-cladid should be coded as a primary homolog of the radianal plate occurring in crinoids with multi-plated posterior interrays. The implications of this hypothesis are not conditional on the monophyly of the Articulata because the pageant of ancestral morphologies echoed in crinoid development empirically demonstrates that the single posterior plate in at least one lineage is equivalent to the radianal, not the anal X. Given the similarities in posterior plate development among extant crinoids, a polyphyletic Articulata would only strengthen the argument above because it would suggest that the same pattern occurred independently among multiple Paleozoic ancestors.
Phyletic evolution in Paleozoic pan-cladids: a test of relative frequencies
The argumentation above concerning posterior plate homologies rests upon the assumption that patterns of character change exhibited by extant crinoids can be extended to apply to all pan-cladid lineages known only as fossils. When conducting a phylogenetic analysis incorporating extinct pan-cladid lineages, is such an extrapolation from living representatives justified? Large-scale temporal trends in evolution need not be invariant among lineages and do not necessarily correspond to phylogenetic trends. Individual clades may exhibit idiosyncratic trends to reduce (and/or subsequently add) posterior plates iteratively over time. Nevertheless, character coding decisions must be made if one seeks to conduct a phylogenetic analysis. Moreover, the question is not whether or not extant crinoids are closely related to Paleozoic pan-cladids. All evidence indicates they are closely related and share an overlapping distribution of taxonomically significant traits (Moore and Teichert Reference Moore and Teichert1978; Roux et al. Reference Roux, Hemery and Ameziane2013). In the absence of any additional information, basing primary homologies on ontogenetic sequence data present in living representatives is justified because empirical science must proceed in the direction of available evidence. However, one is faced with a seemingly impossible problem of assessing how often such a hypothesis is objectively accurate.
Null hypotheses in phylogenetics are constructed by making a priori assumptions of homology among comparable characters and using Hennig’s (Reference Hennig1966) auxiliary principle. What if a mistake is made in determining what constitutes a “comparable character” (or character state) in the first place? In other words, if the presence of a single posterior plate is determined to be equivalent to a radianal in pan-cladids during character analysis (H0=a single posterior plates is homologous with the radianal), how often has a researcher committed a type 2 error? Although such mistakes may, in principle, be unknowable; it is humbling to consider that a type 2 error during character analysis may result in type 1 errors during phylogenetic analysis and obstruct the recognition of “true” (i.e., empirical) evolutionary homologies and patterns of character evolution.
Central tendencies in historical science must be determined by examining relative frequencies (Gould Reference Gould1989). Although single or isolated occurrences of a phenomenon may be interesting and/or otherwise worthy of study, they do not provide insight into building general expectations of a theory. Luckily, the pan-cladid fossil record supplies several key examples relevant to assessing the relative frequency in which the fossil record supports or refutes available evidence from ontogeny. If examples of morphologic transitions from the fossil record predominantly corroborate the ontogeny-based solution to the posterior plate antinomy, then one can be more confident in the results of a phylogenetic analysis assuming those primary homologies to reconstruct evolutionary relationships and patterns of character evolution.
Although the fossil record of crinoid genera is well sampled (Foote and Raup Reference Foote and Raup1996), many pan-cladid species are based on only one or a few specimens (Webster and Maples Reference Webster and Maples2006). All macroevolutionary studies must consider the species level, even when using higher taxa as proxies, because species are the fundamental units that preserve phenotypic change among populations (Hendricks et al. Reference Hendricks, Saupe, Myers, Hermsen and Allmon2014). Unfortunately, there is a dearth of pan-cladid species level phylogenies available (Kammer and Ausich Reference Kammer and Ausich2007; Gahn and Kammer 2012), and none contain a set of species relevant to the problem addressed here. Moreover, conducting such a species-level analysis itself may require addressing the antinomies this paper is attempting to resolve: is the single posterior plate homologous with the radianal or anal X? A comparison of fossilized ontogenetic sequences among pan-cladids might be helpful, but there are currently no known fossils preserving the larval and early developmental stages of posterior plates. At this point, an interesting light may be thrown on the problem by examining variations in posterior plate conditions among species with transitional morphologies. The collection of a large number of specimens allows one to examine a distribution of transitional morphologies and make comparisons combining all three of Remane’s (Reference Remane1952) criteria.
Webster and Maples (Reference Webster and Maples2006) noted numerous instances where intraspecific variations in posterior plate conditions occur when a large number of specimens were collected. These examples are particularly informative because they come from paleontologically well sampled stratigraphic sections representing short time intervals (Webster and Maples Reference Webster and Maples2006). Thus, it is likely they represent paleontologic sampling taking place over short enough time scales to sample transitional morphologies. Any bed scale time averaging and/or taphonomic discrepancies among first and last appearances between stratigraphic sections do not affect the inferences obtained herein because it is the overall distribution of sampled morphologies, not their sampled paleontologic sequence, that are used to make comparisons and examine intermediate forms.
For example, Wanner (Reference Wanner1916) recognized the presence of a single, large interradial posterior plate in his original description of the Permian pan-cladid Hydreionocrinus variabilis (=Cadocrinus variabilis), but called this plate the radianal rather than anal X because in some specimens the position of the plate was lying at an angle to the CD basal and in a more proximal position with the C radial. Wanner (Reference Wanner1916) noted that among the 197 specimens in his collection, a subset possessed proximal tips of one to two small plates above the radial summit, which he termed the anal X and right tube plate. Thus, Wanner (Reference Wanner1916) concluded that the large posterior plate must actually be a large radianal that migrated to an interradial position, supporting the anal X and right tube plate above. Webster and Maples (Reference Webster and Maples2006) suggested that if Wanner (Reference Wanner1916) had a smaller sample size typical of fossil pan-cladid species, he would likely have committed a misnomer by calling it an anal X. Given the inconsistent treatment in the Treatise and the standard portrayal of posterior plates in crinoid plate diagrams (Moore and Teichert Reference Moore and Teichert1978), it is probable that any crinoid worker since the 1800s would have nearly committed the same misnomer.
Similarly, Wright (Reference Wright1920; 1926; 1927) examined posterior plate conditions in a large number of Upper Paleozoic specimens of Eupachycrinus calyx (n=1000), Zeacrinus? konincki (n=342), Ulocrinus globosus (n=480), and Hydreionocrinus sp. (n=130) (=Phanocrinus calyx, Parazeacrinites konicki, and Ureocrinus globosus, respectively). Wright (Reference Wright1926, p. 149) noted that “extreme” variations in posterior plates are apparent in these species when samples become sufficiently large. With such a large sample size displaying a semi-continuous distribution of intermediate posterior plate conditions, Wright (Reference Wright1920; 1926; 1927) applied the logic of Remane’s criteria to the distribution of transitional morphologies. In all cases, the radianal can be seen to have increased in size and subsequently moved into an interradial position, pushing the other posterior plates out of the cup (e.g., Wright Reference Wright1926: Figs. 1–59). Intermediate morphologies display small remnants of an anal X and right tube plate above the interradially positioned radianal plate. Thus, the paleontologic evidence indicates that the posterior plate most proximal to the C ray is the last to migrate from the cup in a phyletic sequence. Another example supporting the conclusions of Wanner (Reference Wanner1916) and Wright (Reference Wright1926) was discovered by Webster and Lane (Reference Webster and Lane1967) for Arroyocrinus popenoei (n=83).
The examples above should not be interpreted as suggesting that all (or most) species of pan-cladids exhibit variation in posterior plate position and arrangement when large samples are collected. Webster and Lane (Reference Webster and Lane1967) also examined Moapacrinus rotundus (n=137) and Erisocrinus longwelli (n=59) and found no variation in the posterior plating in either species, therefore supporting the conclusion that the above examples represent special cases where an exceptionally abundant number of specimens in an evolving lineage were sampled over a short temporal duration. Further work is needed to address whether any of the observed posterior plate variations corresponded with other characters and/or were related to speciation.
Webster and Maples (Reference Webster and Maples2006) compiled a reference list for the number and arrangement of posterior plates for the type species of 378 pan-cladid genera from which relative frequency of the radianal as the single posterior plate may be inferred. Their dataset includes 152 genera with three posterior plates and therefore can be unambiguously homologized between species using procedures outlined earlier in this paper. Of these, 96% have the radianal “moderately or mostly underlying the right side” of the anal X (Webster and Maples Reference Webster and Maples2006: p. 200). This condition was also found to be the most common arrangement for pan-cladids with two plates in the posterior interray (Webster and Maples Reference Webster and Maples2006). These paleontological observations support the hypothesis that the single posterior plate in fossil pan-cladids is predominantly the radianal, not the anal X. Therefore, the relative frequency of morphologic transitions recovered from the fossil record overwhelmingly (≥96%) agrees with and corroborates the ontogenetic evidence provided by extant crinoids. This discovery is significant because it overturns more than 120 years of the status quo in crinoid paleontology.
Of course, it is possible that a small number of lineages with no living representatives lost the radianal plate yet retained the anal X. Both Kammer and Ausich (Reference Kammer and Ausich1996) and Webster and Maples (Reference Webster and Maples2006) suggested that this may have happened among species of the Mississippian genus Barycrinus. Some species of Barycrinus possess small radianal plates below and to the right of the much larger anal X, where others have only a single, large visible plate interpreted as an anal X. However, as noted by Gahn and Kammer (Reference Gahn and Kammer2002), it is possible that the anal X has overgrown the radianal in these species and is lamentably invisible on the calyx exterior. Although this condition is presently unknown in Barycrinus, it is known to occur among other Late Paleozoic pan-cladids. For example, the Pennsylvanian pan-cladid Perimestocrinus calyculus (=Vertigocrinus calyculus) described by Moore and Plummer (Reference Moore and Plummer1940) has only two plates visible on the exterior surface of the calyx. However, three posterior plates are visible when examined from inside of the cup. When viewed from the inside of the calyx, these plates are readily interpreted as the radianal, anal X, and right tube plate occupying an otherwise ordinary arrangement (Moore and Plummer Reference Moore and Plummer1940). Without examining the calyx interior, one may have inferred from topology that the radianal was absent and/or incorrectly coded the exterior plates in a character matrix. Additional taxa with cryptic plates within the calyx interior include species placed in the Pennsylvanian crinoid genera Arkacrinus, Paradelocrinus, Plaxocrinus, Vertigocrinus, and the Mississippian to Permian genus Erisocrinus (Strimple, Reference Strimple1978; Webster and Maples Reference Webster and Maples2006). Based on these considerations, further collection, preparation, and careful inspection of aberrant fossil specimens is necessary to better understand morphologies that fall outside of general expectations (see Rozhnov and Mirantsev Reference Rozhnov and Mirantsev2014).
Crinoid Ontogeny and Phylogeny: Implications for Evolutionary Studies
“Ontogeny does not recapitulate phylogeny: it creates it” –Walter Garstang (Reference Garstang1921: p. 82)
The existence of parallels between ontogeny and phylogeny is one of the most pervasive and influential concepts in evolutionary biology and relates directly to the discovery of homologous characters (Gould Reference Gould1977). Evolutionary developmental biology has recently refurbished von Baer’s law to study parallels between evolution and development (Abzhanov Reference Abzhanov2013). Von Baer’s law states that more generalized characters appear earlier in ontogeny than specialized characters, with specialized characters developing from generalized characters (Gould Reference Gould1977). For example, ontogenetic sequences for species within a clade may share generalized developmental features, but subclades may share additional more specialized features that reflect more recent evolutionary changes not shared with other subclades. Because developmental programs in the GRN have phylogenetic memory, they retain aspects of phylogenetic history and may “recapitulate” sensu von Baer Reference von Baer1828 (non Haeckel Reference Haeckel1866) ancestral morphologies in the juvenile forms of descendants (Abzhanov Reference Abzhanov2013). Parallels between evolution and development predict a significant degree of recapitulation between cladistically significant traits nested at different phylogenetic levels (Abzhanov Reference Abzhanov2013).
Kammer (Reference Kammer2008) suggested that the appearance and position of plates during crinoid development are likely controlled by genetic switches, such as Hox genes, that regulate proximal-distal morphogenesis and other aspects of skeletal development. Although developmental genetic studies on living crinoids have discovered the expression of Hox genes in the earliest developmental stages of the sea lily Metacrinus rotundus (Hara et al. Reference Hara, Yamaguchi, Akasaka, Nakano, Nonaka and Amemiya2006), it is for the moment an understatement to suggest that much work needs to be done to discover how these genes interact with other aspects of the GRN (such as cis regulatory elements) to direct downstream development and morphogenesis in crinoids. Nevertheless, the near uniform predictability in the developmental timing of skeletal elements among extant comatulid and stalked species suggests they are controlled by highly conserved developmental modules.
Such evo-devo perspectives serve to strengthen the paleo-ontogenetic character analysis above. In living crinoids, the radianal appears much earlier in ontogeny than other additional plates in the posterior interray (i.e., as in Comactinia meridionalis) and exhibits the same degree of developmental canalization as other calyx plates (Lahaye and Jangoux Reference Lahaye and Jangoux1987). Parallels between crinoid development and fossilized morphology, as interpreted using von Baer’s refurbished law in EDB, indicate that living crinoids recapitulate the morphologies of Paleozoic ancestors because they inherited a common pathway of development (Abzhanov Reference Abzhanov2013). Thus, the regulatory machinery in the GRN controlling posterior plate development may not have substantively changed since the Late Paleozoic. This result corroborates studies by Foote (Reference Foote1995; Reference Foote1999) examining morphologic disparity in Paleozoic and post-Paleozoic crinoids. Foote (Reference Foote1995) found that pan-cladids steadily increase in disparity throughout the Paleozoic despite considerable volatility in generic richness. However, pan-cladid disparity dropped after the end Permian extinction and post-Paleozoic forms do not broadly overlap with their Paleozoic representatives in morphospace (Foote Reference Foote1999). Differences in both overall disparity and morphospace occupation led Foote (Reference Foote1999) to conclude that genetic and/or developmental constraints may have been responsible for substantive differences between Paleozoic and post-Paleozoic crinoids. Given that posterior plate characters show considerable variation throughout the Paleozoic and are correlated with other evolutionary changes occurring in the size and shape of the calyx (Moore et al. Reference Moore, Lane and Strimple1978; Webster and Maples Reference Webster and Maples2006), it is likely that developmental modules within the GRN of the lineage(s) that survived the end-Permian extinction became rigidly constrained. Such constraints would limit developmental variation in calyx design and decrease the propensity for lineages to expand into unoccupied regions of crinoid morphospace.
The notion of deep homology provides a potential explanation for putative parallel (i.e., homoplasious) trends in posterior plate characters occurring in different lineages of Late Paleozoic cladids (Moore et al. Reference Moore, Lane and Strimple1978; Webster and Maples Reference Webster and Maples2006). Given the numerous paleontologic species that display a net reduction in the number of posterior plates, it is possible that different lineages lost posterior plates independently through instances of parallel evolution. Parallel morphologic evolution can occur among distantly related lineages arising from selection acting on variation within shared developmental toolkits (Hall Reference Hall2003). Because most Paleozoic pan-cladids have three posterior plates, the tendency to evolve less complex morphologies via plate reduction is interpreted as a paedomorphic trend reflecting arrested development or progenesis (Kammer Reference Kammer2008). Precocious maturation can result from an adaptive response to pressures for small body size and/or as an r selection strategy for rapid growth rates for taxa inhabiting unstable environments (Gould Reference Gould1977). All putative ancestors of extant crinoids are members of the Late Paleozoic Crinoid Macroevolutionary Fauna (LPCMF) and broadly overlap in niche space (Kammer and Ausich 1987; Ausich et al. Reference Ausich, Kammer and Baumiller1994). The transition to a cladid dominated LPCMF was concomitant with considerable environmental changes, such as an increase in the abundance and distribution of siliciclastic habitats (relative to carbonate platforms) preferred by Late Paleozoic cladids (Kammer and Ausich Reference Kammer and Ausich2006). This increase in siliciclastic environments is related to orogenic activity and an increase in the frequency of sediment disturbance (Walker et al. Reference Walker, Wilkinson and Ivany2002). Kammer (Reference Kammer2008) noted that pan-cladids in unstable environments commonly have smaller body sizes. Thus, decreased environmental stability in substrate conditions may have led to an increase in frequency of pan-cladids with rapid growth rates and small body sizes exhibiting paedomorphic morphologies. Given that Late Paleozoic pan-cladids likely shared many developmental modules at “deep” genetic levels, different species facing similar selection pressures may have independently evolved paedomorphic morphologies as a response to similar environmental changes. Such selection could arise either through adaptive evolution within populations or at the species level if posterior plate characters are strongly correlated with diversification rates (Rabosky and McCune Reference Rabosky and McCune2010). Thus, a combination of selective trends and developmental constraints are hypothesized herein to have produced parallel evolution of paedomorphic morphologies in different lineages of Late Paleozoic pan-cladids. Further explorations of these observations await the construction of model phylogenies to test the relationship between the evolutionary acquisition and environmental context of paedomorphic morphologies in Late Paleozoic pan-cladid crinoids.
The discussion above suggests homoplasy may be common for posterior plate characters in pan-cladid crinoids. An a priori belief of homoplasy is not grounds for excluding posterior plate characters from future phylogenetic analyses because not all instances of homoplasy are artifactual results of poor character state interpretations (Wagner Reference Wagner2000b). Character reversals and instances of parallel evolution are real events in life’s history and must be mapped onto a model phylogeny to gain insight into historical patterns of character evolution. Moreover, numerous other morphologic characters are correlated with changes in posterior plates including the presence of muscular articulations, zyzygial sutures, structure and branching of the arms, and the development of pinnules (Kammer Reference Kammer2008; Webster and Maples 2008). Moreover, if one were to a priori disregard from phylogenetic analysis any character with the propensity for homoplasy, there would be no characters left to study or much point to conduct a quantitative phylogenetic analysis because such a proposal assumes one already knows the “true” phylogeny and is merely cutting out “noise” among characters to generate a more well resolved and/or well supported tree. To be clear, I am not advocating that homoplasious characters are desirable in phylogenetic analysis. I am only advocating that features revealed to be comparable elements through paleo-ontogenetic character analysis, as described in this paper, constitute ‘real’, empirical data that need to be rigorously tested against other characters in a phylogenetic context. Given the finite set of morphologic characters available and the important role of posterior plate characters in pan-cladid morphology, I suggest researchers employ a limited “prior belief” in the available paleo-ontogenetic evidence and therefore propose that future phylogenetic analyses consider the single posterior plate in fossil pan-cladids as a primary homolog with the radianal in multi-plated taxa.
Phylogenetic Paleo-ontogeny: A Multidisciplinary Approach to Discovering Homology
Paleobiology, phylogenetic systematics, and EDB play complementary roles in evolutionary biology because they provide the theoretical basis and basic historical data for understanding patterns and processes of macroevolution. Phylogenetic Paleo-ontogeny constitutes a total evidence approach to homology recognition by unifying these seemingly disparate fields to propose, test, and empirically “discover” homologous characters in fossil taxa. A case study in homologizing posterior plates among pan-cladid crinoids was presented to illustrate how a research program in Phylogenetic Paleo-ontogeny can provide insight into how developmental and fossil data can be combined to dissolve morphologic antinomies and ameliorate terminological difficulties obstructing clarity in homology schemes. It is hoped that this paper will serve as a template for other researchers seeking an ontological basis and epistemological framework for discovering homologies of fossil and living species.
For example, within the Echinodermata, two models of character analysis have been proposed to resolve homologies among classes: Extraxial Axial theory (EAT) (Mooi et al. Reference Mooi, David and Marchland1994) and Universal Element Homology (UEH) (Sumrall Reference Sumrall2010). The EAT model uses embryological and ontogenetic criteria and designates different kinds of skeletal regions in the echinoderm body as homologous based on developmental and growth patterns; whereas the UEH model attempts to identify individual skeletal plates across clades without reference to regional skeletal patterns. Interestingly, EAT and UEH make different predictions regarding the branching order of major clades in echinoderm phylogeny (cf. David et al. Reference David, Mooi and Parsley2000; Kammer et al. 2014) and are sometimes contrasted as alternatives in the literature (Zamora and Rahman Reference Zamora and Rahman2014). Under the umbrella of Phylogenetic Paleo-ontogeny, these two competing approaches to echinoderm homology become unified. Data supporting EAT and UEH are not mutually exclusive and likely reflect phylogenetic information nested within different hierarchical levels of body plan organization. Paleo-ontogenetic hypotheses for both skeletal regions and individual elemental homologies can be combined into a set of primary homology statements and tested against one another in a future phylogenetic analysis of fossil and living Echinodermata using all available evidence: embryology, ontogeny, genes, and morphology.
I do not propose that combining paleo-ontogenetic character analysis with phylogenetic systematics leads to a more “objective” framework for discovering homology. All propositions of primary homology are necessarily subjective. Indeed, there is no such thing as an assumption free phylogenetic analysis. In science, theories explaining the natural world arise from hypotheses that have survived repeated subjection to a battery of rigorous testing and empirical corroboration. Should we not subject our a priori assumptions behind such “testing” to even greater scrutiny? Phylogenetic empiricism is the basis of evolutionary inference in systematic biology (Wiley and Lieberman Reference Wiley and Lieberman2011). Phylogenetic information is necessarily obscured when a theoretical concept (such as homology) is empirically approximated (phylogenetic analysis), yet how else should science proceed towards asymptotically ascertaining “truth” if not by successive approximation? A research program in Phylogenetic Paleo-ontogeny may help pave the way towards such a future.
Acknowledgements
This manuscript was completed as an unintended (but necessary) part of my dissertation work on pan-cladid crinoid evolution. I would like to thank W. I. Ausich, J. R. Thompson, and S. R. Cole for insightful (and delightful) discussions on crinoid morphology, development, and evolution. S. R. Cole is thanked for generously providing assistance with figures. Tom Kammer and George Sevastopulo are thanked for kindly reviewing the manuscript. This research was supported by Sigma Xi.