Introduction
With more than 200 living species, Phyllostomidae is the family with the highest ecomorphological diversity in the order Chiroptera. The range of dietary niches that phyllostomid bats occupy is unparalleled across Mammalia (Fleming Reference Fleming, Estrada and Fleming1986; Aguirre et al. Reference Aguirre, Herrel, Van Damme and Matthysen2003; Dumont et al. Reference Dumont, Davalos, Goldberg, Santana, Rex and Voigt2012; Santana et al. Reference Santana, Grosse and Dumont2012; Arbour et al. Reference Arbour, Curtis and Santana2019). Studies have classified noctilionoid bats into seven dietary guilds: carnivory, frugivory, insectivory, nectarivory, omnivory, piscivory, and sanguivory, with insectivory being the most common (Norberg and Rayner Reference Norberg and Rayner1987; Dumont et al. Reference Dumont, Davalos, Goldberg, Santana, Rex and Voigt2012; Santana et al. Reference Santana, Grosse and Dumont2012; Denzinger and Schnitzler Reference Denzinger and Schnitzler2013). Reconstructions of dietary evolution in Phyllostomidae suggest that carnivory and nectarivory evolved independently multiple times in different lineages, diverging from an insectivorous common ancestor (Gunnell and Simmons Reference Gunnell and Simmons2005; Datzmann et al. Reference Datzmann, von Helversen and Mayer2010; Rojas et al. Reference Rojas, Vale, Ferrero and Navarro2012; Santana and Cheung Reference Santana and Cheung2016; Davies et al. Reference Davies, Yohe, Almonte, Sánchez, Rengifo, Dumont, Sears, Dávalos and Rossiter2020). Morphological variability associated with diet-based functional demands is thought to have facilitated the ecological radiation and taxonomic diversification in Phyllostomidae (Santana et al. Reference Santana, Grosse and Dumont2012; Shi and Rabosky Reference Shi and Rabosky2015; Arbour et al. Reference Arbour, Curtis and Santana2019; Morales et al. Reference Morales, Ruedi, Field and Carstens2019; Rossoni et al. Reference Rossoni, Costa, Giannini and Marroig2019; Giannini et al. Reference Giannini, Amador, Moyers-Arévalo, Fleming, Dávalos and Mello2020; Hedrick et al. Reference Hedrick, Mutumi, Munteanu, Sadier, Davies, Rossiter, Sears, Dávalos and Dumont2020). Traditionally, most studies on dietary ecomorphology in bats have focused on skull morphology to study the form–function link, providing crucial information to understand the range of morphological specializations (Aguirre et al. Reference Aguirre, Herrel, van Damme and Matthysen2002; Monteiro and Nogueira Reference Monteiro and Nogueira2011; Santana and Cheung Reference Santana and Cheung2016; Santana and Portugal Reference Santana and Portugal2016; Arbour et al. Reference Arbour, Curtis and Santana2019; Rossoni et al. Reference Rossoni, Costa, Giannini and Marroig2019). Some studies have also provided insights on the role of diet in the diversification of the postcranial skeleton (Vaughan Reference Vaughan1959; Norberg and Rayner Reference Norberg and Rayner1987; Louzada et al. Reference Louzada, Nogueira and Pessôa2019; Gaudioso et al. Reference Gaudioso, Martínez, Barquez and Díaz2020; López-Aguirre et al. Reference López-Aguirre, Hand, Koyabu, Tu and Wilson2021) and external sensory organs (Brokaw and Smotherman Reference Brokaw and Smotherman2020; Leiser-Miller and Santana Reference Leiser-Miller and Santana2020).
Despite the increasing evidence in support of diet as a key driver of bat evolution, reconstructing the macroevolutionary trajectories of dietary specializations in bats has been limited by the fossil record (Teeling et al. Reference Teeling, Springer, Madsen, Bates, O'Brien and Murphy2005). Compared with other tetrapod groups, the fossil record of Chiroptera has one of the lowest levels of taxonomic diversity and skeletal preservation, with an estimated 80% of the record missing (Eiting and Gunnell Reference Eiting and Gunnell2009; Brown et al. Reference Brown, Cashmore, Simmons and Butler2019). Geographic patterns in the completeness of the bat fossil record indicate that the Southern Hemisphere and Asia are especially underrepresented, obscuring important spatiotemporal information (Brown et al. Reference Brown, Cashmore, Simmons and Butler2019). In the Southern Hemisphere, two fossiliferous localities stand out in terms of bat fossil diversity: Riversleigh from the Oligocene to Pleistocene (ca. 25–2 Ma) of Australia with more than 40 species (Hand and Archer Reference Hand and Archer2005), and La Venta from the middle Miocene (ca. 13–12 Ma) of Colombia with at least 14 species (Czaplewski et al. Reference Czaplewski, Takai, Naeher, Shigehara, Setoguchi and Shigehara2003).
The La Venta fossil fauna, recovered from the Villavieja Formation in Colombia's Huila Department, is the richest Cenozoic vertebrate fossil community of northern South America (83 fossil mammal species) (Kay and Madden Reference Kay and Madden1997; Croft Reference Croft2016). The deposits span a poorly represented period in the middle Miocene, and its fossil mammals have helped define the Laventan South American Land Mammal Age (Madden et al. Reference Madden, Guerrero, Kay, Flynn, Iii, Walton, Kay, Madden, Cifelli and Flynn1997). The La Venta bat community includes representatives of six families, including extinct representatives of the family Phyllostomidae (Notonycteris magdalenensis, Notonycteris sucharadeus, Palynephyllum antimaster), and the oldest evidence of modern species of families Noctilionidae (Noctilio albiventris) and Thyropteridae (Thyroptera lavali) (Czaplewski Reference Czaplewski, Kay, Madden, Cifelli and Flynn1997; Czaplewski et al. Reference Czaplewski, Takai, Naeher, Shigehara, Setoguchi and Shigehara2003). Palynephyllum antimaster represents the earliest phytophagous bat in the New World, a dietary strategy that provided a key evolutionary innovation for the order (Czaplewski et al. Reference Czaplewski, Takai, Naeher, Shigehara, Setoguchi and Shigehara2003; Yohe et al. Reference Yohe, Velazco, Rojas, Gerstner, Simmons and Davalos2015).
La Venta's N. magdalenensis is known from a dentary fragment containing a complete m1 and the anterior portion of m2, as well as several other isolated teeth (Savage Reference Savage1951; Czaplewski Reference Czaplewski, Kay, Madden, Cifelli and Flynn1997). Postcranial remains referred to this species include distal and proximal humeral fragments (Savage Reference Savage1951; Czaplewski Reference Czaplewski, Kay, Madden, Cifelli and Flynn1997; Czaplewski et al. Reference Czaplewski, Takai, Naeher, Shigehara, Setoguchi and Shigehara2003). When Savage (Reference Savage1951) described the species, he placed N. magdalenensis in the phyllostomid subfamily Phyllostominae, a group of Neotropical animalivorous and omnivorous bats. Subsequent phylogenetic analyses have upheld that placement and grouped it consistently in a clade with Vampyrum spectrum and Chrotopterus auritus, both large-bodied carnivorous species (Czaplewski et al. Reference Czaplewski, Takai, Naeher, Shigehara, Setoguchi and Shigehara2003; Dávalos et al. Reference Dávalos, Velazco, Warsi, Smits and Simmons2014). Comparisons of dental features and body size indicate that N. magdalenensis was larger than C. auritus but smaller than V. spectrum, the largest bat in the New World. This fossil species is often included in phylogenetic analyses, as a calibration point for dated phylogenies of the bat superfamily Noctilionoidea (Rojas et al. Reference Rojas, Vale, Ferrero and Navarro2012; Dávalos et al. Reference Dávalos, Velazco, Warsi, Smits and Simmons2014; Hand et al. Reference Hand, Beck, Archer, Simmons, Gunnell, Scofield, Tennyson, De Pietri, Salisbury and Worthy2018). It also features in studies focused on the ecological history of phyllostomids, including the adoption of specialized ecological niches such as carnivory by the middle Miocene (e.g., Baker et al. Reference Baker, Bininda-Emonds, Mantilla-Meluk, Porter, Van Den Bussche, Gunnell and Simmons2012; Yohe et al. Reference Yohe, Velazco, Rojas, Gerstner, Simmons and Davalos2015; Simmons et al. Reference Simmons, Gunnell, Czaplewski, Fleming, Dávalos and Mello2020). Yet, despite its importance for the study of the evolution of Neotropical bats, studies focused on reconstructing the biology of N. magdalenensis are still needed.
Mammal teeth show highly specialized structures that vary in relation not only to phylogeny but also to dietary differences, providing informative and widely used proxies to identify niche partitioning and reconstruct diets of extinct taxa, including bats (e.g., Czaplewski et al. Reference Czaplewski, Takai, Naeher, Shigehara, Setoguchi and Shigehara2003; Simmons et al. Reference Simmons, Seymour, Habersetzer and Gunnell2008, Reference Simmons, Gunnell, Czaplewski, Fleming, Dávalos and Mello2020; Self Reference Self2015; Hand et al. Reference Hand, Sigé, Archer and Black2016). Studies of the dentition of N. magdalenensis by Savage (Reference Savage1951) and Czaplewski et al. (Reference Czaplewski, Takai, Naeher, Shigehara, Setoguchi and Shigehara2003) concluded it lacks the highly specialized dental traits observed in its living carnivorous relatives V. spectrum and C. auritus (Freeman Reference Freeman1988, Reference Freeman, Kunz and Racey1998), suggesting that N. magdalenensis probably had a less carnivorous diet. These dental features in N. magdalenensis, compared with those of its living carnivorous relatives, include relatively lower crown height, more robust crests and cusps, less reduced talonids in lower molars, less obliquely oriented ectoloph crests, and shorter postmetacrista in upper molars.
Dental topography analysis (DTA) is an informative quantitative approach to study dental morphology within an ecological context and has the potential to be incorporated into modern phylogenetic comparative methods (Evans et al. Reference Evans, Wilson, Fortelius and Jernvall2007; Bunn and Ungar Reference Bunn and Ungar2009; Cooke Reference Cooke2011; Allen et al. Reference Allen, Cooke, Gonzales and Kay2015; Prufrock et al. Reference Prufrock, Boyer and Silcox2016a; Pineda-Munoz et al. Reference Pineda-Munoz, Lazagabaster, Alroy and Evans2017; López-Torres et al. Reference López-Torres, Selig, Prufrock, Lin and Silcox2018; Selig et al. Reference Selig, Sargis, Chester and Silcox2020). A battery of DTA metrics have been developed in recent decades (Evans et al. Reference Evans, Wilson, Fortelius and Jernvall2007; Boyer Reference Boyer2008; Bunn et al. Reference Bunn, Boyer, Lipman, St Clair, Jernvall and Daubechies2011; Berthaume et al. Reference Berthaume, Winchester and Kupczik2019a), further developing the capacity to apply multivariate analyses (Pineda-Munoz et al. Reference Pineda-Munoz, Lazagabaster, Alroy and Evans2017). DTA has been widely used to study dietary adaptations in mammals (Lazzari et al. Reference Lazzari, Charles, Tafforeau, Vianey-Liaud, Aguilar, Jaeger, Michaux and Viriot2008; Selig et al. Reference Selig, López-Torres, Sargis and Silcox2019, Reference Selig, Sargis, Chester and Silcox2020), especially in primates (Ungar Reference Ungar2004; Bunn and Ungar Reference Bunn and Ungar2009; Ledogar et al. Reference Ledogar, Winchester, St. Clair and Boyer2013; Winchester et al. Reference Winchester, Boyer, St Clair, Gosselin-Ildari, Cooke and Ledogar2014; Allen et al. Reference Allen, Cooke, Gonzales and Kay2015; López-Torres et al. Reference López-Torres, Selig, Prufrock, Lin and Silcox2018; Ungar et al. Reference Ungar, Healy, Karme, Teaford and Fortelius2018; Berthaume et al. Reference Berthaume, Lazzari and Guy2020), and to analyze morphofunctional specializations (Bunn and Ungar Reference Bunn and Ungar2009; Bunn et al. Reference Bunn, Boyer, Lipman, St Clair, Jernvall and Daubechies2011), elucidate macroevolutionary trajectories (López-Torres et al. Reference López-Torres, Selig, Prufrock, Lin and Silcox2018), resolve systematic arrangements (Selig et al. Reference Selig, Sargis, Chester and Silcox2020), and reconstruct ecological interactions (Prufrock et al. Reference Prufrock, Boyer and Silcox2016a) across mammalian taxa. In bats, Santana et al. (Reference Santana, Strait and Dumont2011) used DTA to explore diet-based adaptations in the occlusal surface of molars in Phyllostomidae, correlating plant-based diets with higher dental complexity.
We used 3D computational modeling, multivariate DTA, and phylogenetic comparative methods to reconstruct the diet of N. magdalenensis by comparing the dental complexity of the first lower molar of N. magdalenensis with 25 modern phyllostomid and noctilionid species, covering all major dietary categories. We examined three measures of dental complexity (Bunn et al. Reference Bunn, Boyer, Lipman, St Clair, Jernvall and Daubechies2011; Pineda-Munoz et al. Reference Pineda-Munoz, Lazagabaster, Alroy and Evans2017): Dirichlet normal energy (DNE), relief index (RFI), and orientation patch count rotated (OPCR). We also reconstructed the body mass (BM) of N. magdalenensis using equations developed for Chiroptera based on body-size measurements (Gunnell et al. Reference Gunnell, Worsham, Seiffert and Simons2009). Based on the phylogenetic relationships of N. magdalenensis and previous anatomic comparisons, we hypothesize that it was a large-bodied species, less specialized for carnivory than modern carnivorous phyllostomids. We predict sampled carnivores and N. magdalenensis to have high DNE and OPCR values and low RFI values, following general trends found in other mammals. Based on previous comparisons of body proportions with modern relatives, we predict N. magdalenensis's BM to be intermediate between those of C. auritus (~70 g; Vleut et al. Reference Vleut, Carter and Medellín2019) and V. spectrum (~150 g; Amador et al. Reference Amador, Simmons and Giannini2019).
Methods
The cast of a partial lower left jaw of Notonycteris magdalenensis with a complete first molar from the collection of the University of California Museum of Paleontology (UCMP39962) was scanned at the University of New South Wales using a U–CT (Milabs, Utrecht) with 55 kV and 0.17 mA, ultrafocused setting at a resolution of 30–50 μm (Fig. 1). Our comparative sample consisted of 25 modern noctilionoid bat species of known diet from two noctilionoid bat families (Phyllostomidae and Noctilionidae; Supplementary Table 1). Together, these species represent all major dietary guilds (i.e., carnivory, frugivory, insectivory, nectarivory, omnivory, piscivory, and sanguivory) recognized in bats (Table 1, Fig. 1). For each species, a single lower-left first molar (m1) from adult specimens that did not show excessive wear (no dentine exposed on occlusal view) was analyzed to avoid methodological artifacts in our DTA (Pampush et al. Reference Pampush, Spradley, Morse, Harrington, Allen, Boyer and Kay2016a). Lower molars have been proposed to better reflect food item breakdown, whereas upper molars better reflect biomechanical stabilization of food items (Berthaume et al. Reference Berthaume, Lazzari and Guy2020). The 3D models of modern species were retrieved from Shi et al. (Reference Shi, Westeen and Rabosky2018) based on μCT scans available on MorphoSource, except for an additional 3D model of a lower mandible of Vampyrum spectrum provided by S. Santana upon request. Individual m1s were segmented from adjacent teeth and bone using MIMICS v. 20 software (Materialise NV, Leuven, Belgium). Differences in imaging settings and intraspecific variation have been shown to have a negligible effect on DTA analyses (López-Torres et al. Reference López-Torres, Selig, Prufrock, Lin and Silcox2018; Berthaume et al. Reference Berthaume, Winchester and Kupczik2019b). Additionally, following standard protocols for DTA, individual m1s were cropped below the cingulid to isolate the crown (Prufrock et al. Reference Prufrock, Boyer and Silcox2016a; Ungar et al. Reference Ungar, Healy, Karme, Teaford and Fortelius2018). Dávalos et al.'s (Reference Dávalos, Velazco, Warsi, Smits and Simmons2014) phylogeny of the Phyllostomidae was pruned to match our sample and used as phylogenetic scaffold for phylogenetic comparative analyses.
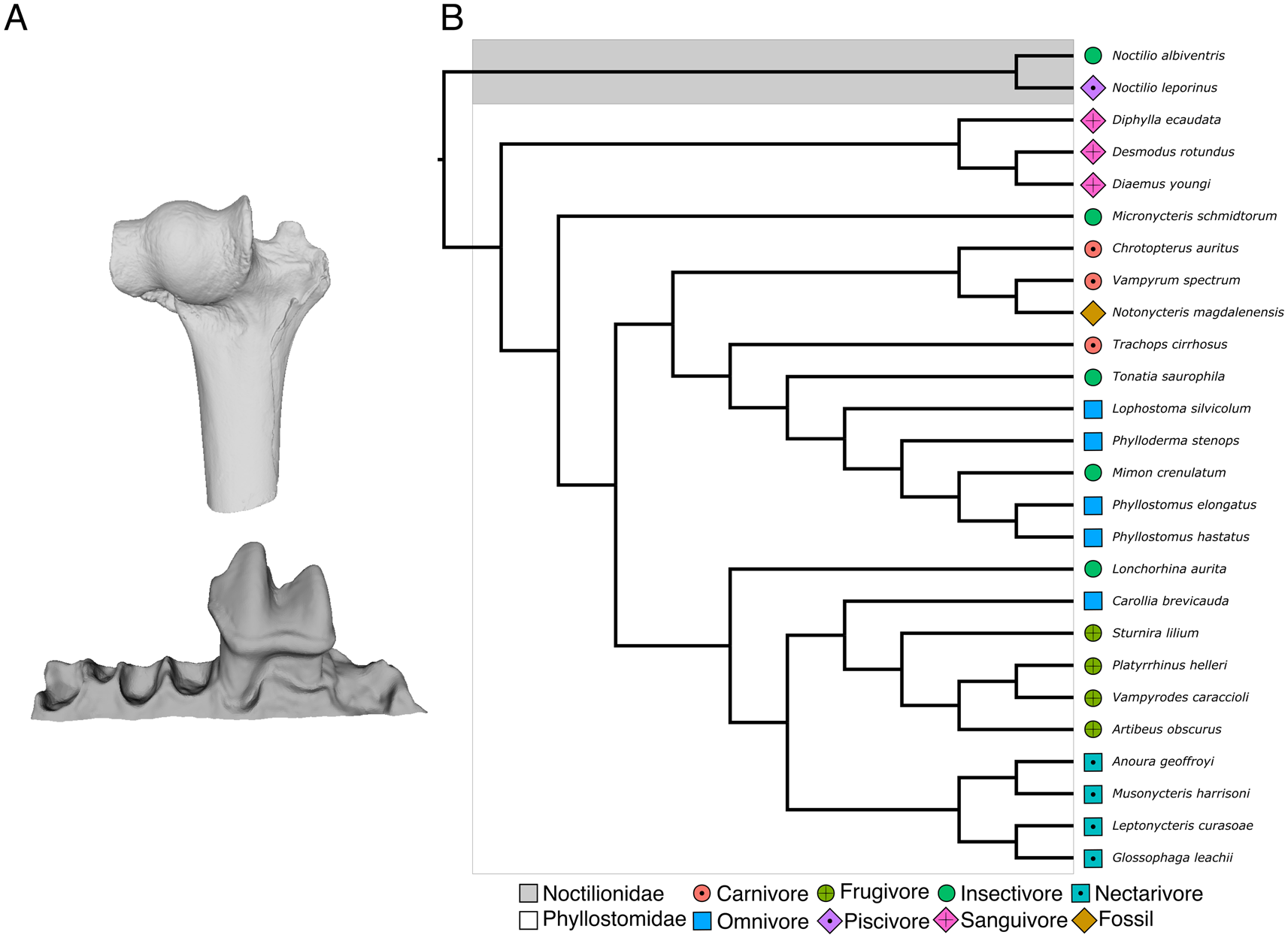
Figure 1. Fossil and modern species analyzed in this study. A, Partial humerus (UCMP 38990; left) and lower left jaw (UCMP 39962; right) of Notonycteris magdalenensis used in this study for body-mass (BM) reconstruction and dental topography analysis (DTA); B, evolutionary relationships of species analyzed in this study, modified from Dávalos et al. (Reference Dávalos, Velazco, Warsi, Smits and Simmons2014). Tip colors represent dietary guilds and the fossil taxon. (Color online.)
Table 1. Description of dietary guild classifications used in this study.
Body-Mass Estimate
Based on a dataset of 1160 extant bats, Gunnell et al. (Reference Gunnell, Worsham, Seiffert and Simons2009) demonstrated that regression analysis of skeletal elements can be an accurate proxy to infer BM in extant and extinct bats, with humeral shaft diameter (R 2 = 0.869) and first upper/lower molar area (R 2 = 0.818 and 0.829) providing best estimates. We used lower first molar area and humeral shaft diameter to reconstruct the BM of N. magdalenensis (Gunnell et al. Reference Gunnell, Worsham, Seiffert and Simons2009; Hand et al. Reference Hand, Sigé, Archer, Gunnell and Simmons2015b, Reference Hand, Sigé, Archer and Black2016, Reference Hand, Beck, Archer, Simmons, Gunnell, Scofield, Tennyson, De Pietri, Salisbury and Worthy2018; Hand and Sigé Reference Hand and Sigé2018; Jones et al. Reference Jones, Coster, Licht, Métais, Ocakoğlu, Taylor and Beard2019). Lower first molar area was calculated from a 3D model of a complete m1 extracted from a fragmented lower left jaw (UCMP 39962). Only fragmented humeral elements have been reported for N. magdalenensis, limiting our capacity to obtain measures of mid-shaft diameter. The humeral dorsoventral diameter of the shaft at the distal end of the deltopectoral ridge of a proximal humerus fragment (UCMP 38990) was retrieved from Savage (Reference Savage1951), and the proximal-most shaft diameter of the humerus was extracted from the 3D model of the distal end of a partial humerus (UCMP 38990; Fig 1). A cast of UCMP 38990 was scanned at the University of New South Wales following the same protocol described earlier. The BM estimate based on m1 size was used to compare N. magdalenensis with bats from modern dietary guilds. The BM values for modern bat species were retrieved from the literature (Gunnell et al. Reference Gunnell, Worsham, Seiffert and Simons2009; Molinari et al. Reference Molinari, Bustos, Burneo, Camacho, Moreno and Fermín2017; Curtis and Santana Reference Curtis and Santana2018; Hand et al. Reference Hand, Beck, Archer, Simmons, Gunnell, Scofield, Tennyson, De Pietri, Salisbury and Worthy2018; Amador et al. Reference Amador, Simmons and Giannini2019; Jones et al. Reference Jones, Coster, Licht, Métais, Ocakoğlu, Taylor and Beard2019).
Dental Topographic Analysis
Segmentation and processing practices (e.g., cropping and smoothing) of 3D meshes can impact features of the models (e.g., triangle count and mesh resolution) that are determinant for DTA (Berthaume et al. Reference Berthaume, Winchester and Kupczik2019b). Hence, all meshes were standardized following recommendations for DTA; 3D models were simplified to 10,000 faces and smoothed using 30 iterations with lambda set at 0.6 (Pampush et al. Reference Pampush, Winchester, Morse, Vining, Boyer and Kay2016b; Berthaume et al. Reference Berthaume, Winchester and Kupczik2019b). After standardization of meshes, three widely used dental topography metrics were analyzed (Fig. 2): DNE (Bunn et al. Reference Bunn, Boyer, Lipman, St Clair, Jernvall and Daubechies2011), OPCR (Evans et al. Reference Evans, Wilson, Fortelius and Jernvall2007), and RFI (Boyer Reference Boyer2008). DNE is a measure of the degree of curvature of the crown (Fig. 2A); this correlates with shearing capacity and is usually higher in animalivores (Ledogar et al. Reference Ledogar, Winchester, St. Clair and Boyer2013, Reference Ledogar, Luk, Perry, Neaux and Wroe2018; Allen et al. Reference Allen, Cooke, Gonzales and Kay2015; López-Torres et al. Reference López-Torres, Selig, Prufrock, Lin and Silcox2018; Ungar et al. Reference Ungar, Healy, Karme, Teaford and Fortelius2018). OPCR is an estimate of surface complexity of the crown based on the number of patches facing in the same direction (Evans et al. Reference Evans, Wilson, Fortelius and Jernvall2007; Pineda-Munoz et al. Reference Pineda-Munoz, Lazagabaster, Alroy and Evans2017; López-Torres et al. Reference López-Torres, Selig, Prufrock, Lin and Silcox2018; Selig et al. Reference Selig, Sargis, Chester and Silcox2020). As for DNE, species with a higher OPCR have more complex tooth surfaces (Fig. 2B), usually associated with more biomechanically demanding diets such as folivory and animalivory (Evans et al. Reference Evans, Wilson, Fortelius and Jernvall2007; Cooke Reference Cooke2011; Santana et al. Reference Santana, Strait and Dumont2011). RFI is a ratio of the 3D and 2D areas of the tooth crown (Fig. 2C) and provides a measure of crown height and, effectively, hypsodonty (Boyer Reference Boyer2008; Cooke Reference Cooke2011; Allen et al. Reference Allen, Cooke, Gonzales and Kay2015); low RFI values reflect flat crown surfaces commonly found in mammalian frugivores, whereas high values represent tall cusps, such as those found in granivores and insectivores. Incorporating several metrics of dental complexity to inform dietary reconstructions has been suggested to provide more robust characterizations than studies based on a single metric (Pineda-Munoz et al. Reference Pineda-Munoz, Lazagabaster, Alroy and Evans2017). All DTAs were performed with the R package molaR (Pampush et al. Reference Pampush, Winchester, Morse, Vining, Boyer and Kay2016b).
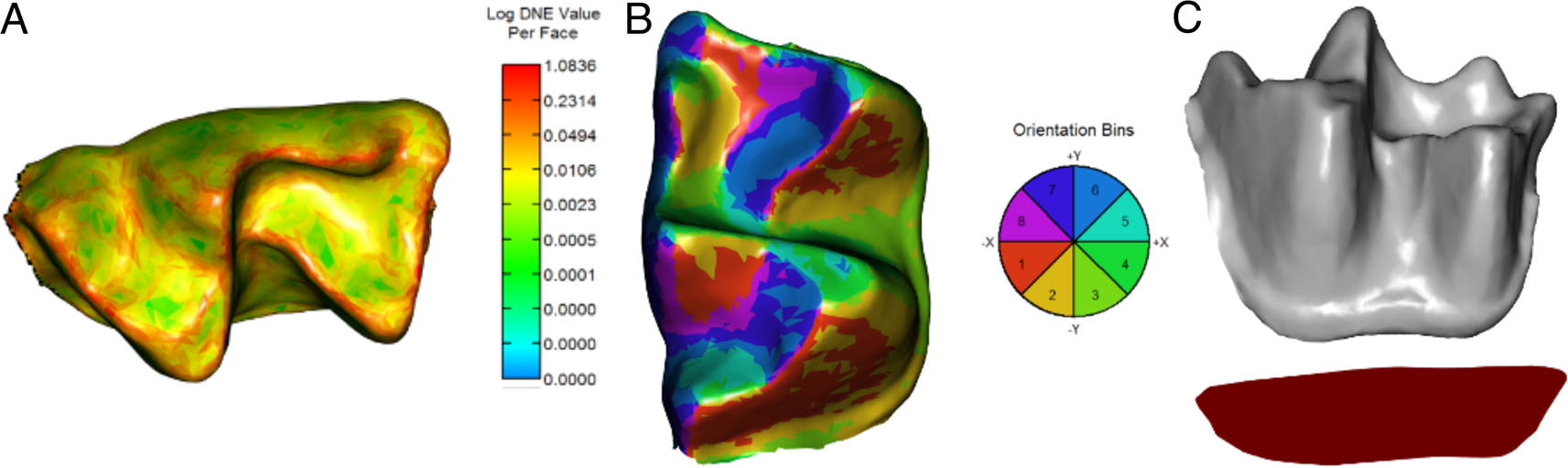
Figure 2. Reconstructed meshes showing topographic maps of Dirichlet normal energy (DNE; A), orientation patch count rotated (OPCR; B), and relief index (RFI; C) for the lower m1 of Notonycteris magdalenensis (UCMP 39962).
Dietary Reconstruction
Box plots were used to visually compare values of DNE, OPCR, and RFI in N. magdalenensis with those of modern bats of known dietary guilds. A one-way analysis of variance (ANOVA) was used to test for differences in DNE, OPCR, and RFI across our groups. The presence of phylogenetic structuring in our dental complexity data was tested using a multivariate Kmult (K−) statistic implemented in the physignal function in Geomorph v. 3.2.1 (Adams Reference Adams2014), using a pruned version of Dávalos et al.'s (Reference Dávalos, Velazco, Warsi, Smits and Simmons2014) phylogeny. Given the strong phylogenetic signal in our dataset, a principal component analysis (PCA, herein morphospace) and a phylogenetic PCA (pPCA, herein phylogeny-corrected morphospace) were performed to reduce the dimensionality of our dental complexity data and visualize patterns of variation, using the phylomorphospace and phyl.pca functions of the R package phytools (Revell Reference Revell2012). A morphospace plot allowed us to elucidate general patterns of differentiation across dietary guilds, whereas the phylogeny-corrected morphospace allowed us to discern the influence of phylogenetic kinship in dental complexity variation. A linear discriminant analysis (LDA) was used to classify the diet of N. magdalenensis as one of seven dietary guilds, using the lda function in the R package MASS. Differences in phylogenetic kinship and patterns of morphological diversity were further explored with a tanglegram to: (1) identify dental complexity similarities between N. magdalenensis and other bats of known dietary guilds and (2) detect instances of morphological innovation and convergence across our sample. Loadings of the first two principal components (PC) of the pPCA (explaining >90% of phenotypic variation) were used as phylogeny-corrected phenotypic data. First, the phenotypic dendrogram was built with a hierarchical clustering analysis of the PC loadings, using the upgma function in the R package phangorn (Schliep Reference Schliep2011). Next, we compared the position of each species across the phylogeny and morphological dendrogram using the tanglegram produced by the cophylo function in the R package phytools (Revell Reference Revell2012). Parallel lines linking a species across the phylogeny and dendrogram indicate similarities, whereas diagonal links indicate a phylogenetic–phenotypic mismatch.
Results
Body-Mass Estimate
Depending on the proxy, BM estimates of Notonycteris magdalenensis ranged from 53.05 g (m1 area) to 103.39 g (humeral shaft diameter) to 133.35 g (humeral shaft diameter from Savage [Reference Savage1951]). However, a direct measure of humeral diameter at the mid-shaft could not be made (this point is not preserved in fossil material), and this is generally the narrowest point of the bat humerus. Hence, our BM estimates for N. magdalenensis based on humeral shaft diameter potentially misestimate BM, so all further analyses and discussion are based on the average of our three estimates (~95 g). Compared with modern species in our sample, the BM of N. magdalenensis was greater than the average for frugivores (32.18 g), insectivores (19.23 g), nectarivores (15.2 g), omnivores (46.42 g), and sanguivores (33.36 g), close to the piscivore Noctilio leporinus (61 g), and slightly smaller than the average for carnivores (97.74 g; Fig. 3). Compared with its modern relatives, N. magdalenensis was larger than Chrotopterus auritus (BM = 80 g) and smaller than Vampyrum spectrum (BM = 170 g).
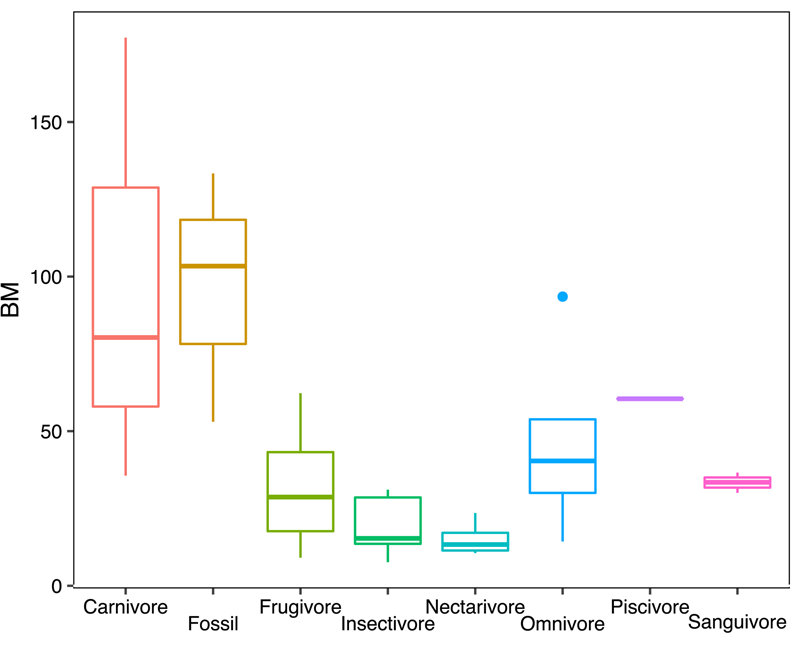
Figure 3. Box plots of body-mass (BM) distributions of modern taxa pooled by dietary guilds and BM reconstruction of Notonycteris magdalenensis based on m1 area.
Dietary Differences in Dental Complexity
In our sample, nectarivores and sanguivores consistently had the lowest DNE and OPCR values, whereas frugivores and sanguivores had the lowest RFI values (Fig. 4). Omnivores and piscivores had the highest DNE values, followed by frugivores and carnivores (Fig. 4A). Frugivores had the highest OPCR values, followed by carnivores, insectivores, omnivores, and piscivores with very similar values (Fig. 4B). Piscivores and sanguivores had the highest RFI values, followed by carnivores, whereas insectivores and omnivores showed intermediate values (Fig. 4C). Across the three metrics, N. magdalenensis overlapped with values for insectivores and omnivores and showed lower values than its carnivorous close relatives (Fig. 4). One-way ANOVA revealed statistically significant differences in dental complexity metrics across dietary guilds (F = 2.46, p = 0.05), and the K- statistic revealed a significant phylogenetic signal in our data (K = 0.686, Z = 2.273, p = 0.006).
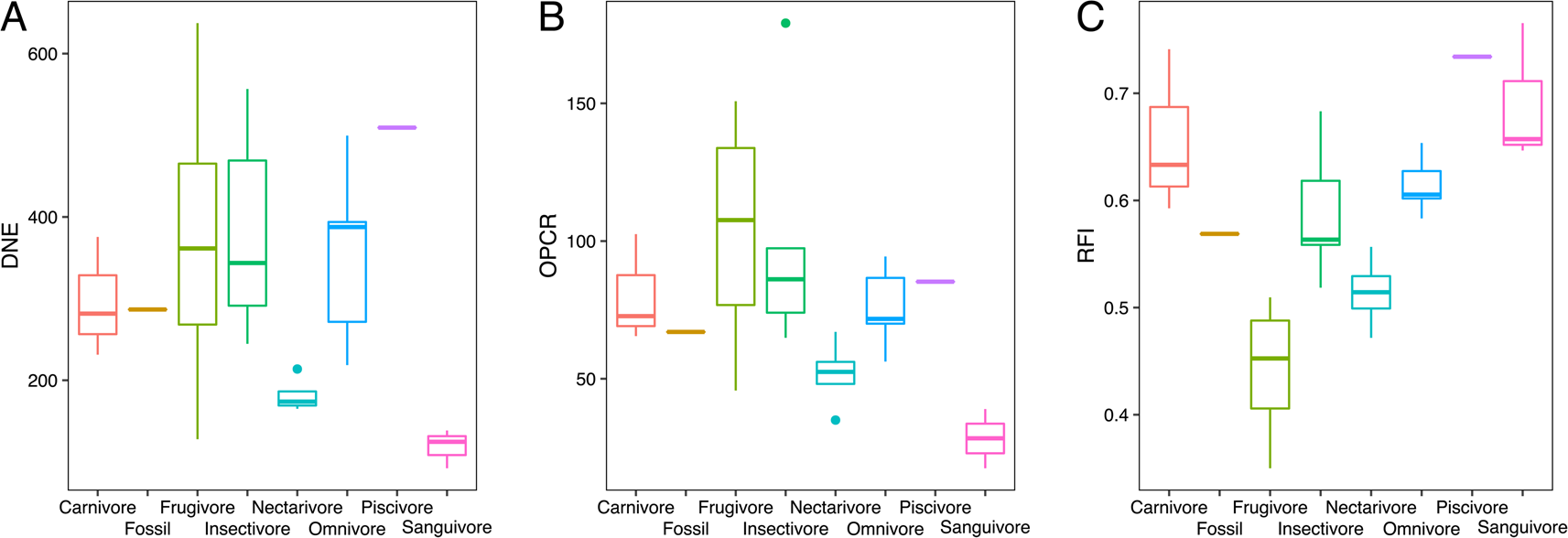
Figure 4. Box plots of dental topography analysis (DTA) results for Dirichlet normal energy (DNE; A), orientation patch count rotated (OPCR; B), and relief index (RFI; C) of modern taxa pooled by dietary guilds and Notonycteris magdalenensis.
Dietary groups showed similar patterns of phenotypic variation across morphospace and phylogeny-corrected morphospace (Fig. 5). In morphospace (the first two PCs explained 95.77% of variation), frugivores, nectarivores, and sanguivores occupied non-overlapping regions, whereas carnivores, insectivores, and omnivores overlapped, each showing different levels of dispersion across morphospace (Fig. 5A). Morphospace also indicates phylogenetic structuring in morphological variation and dietary specializations, with desmodontines (all sanguivores), glossophagines (all nectarivores), and stenodermatines (all frugivores) occupying exclusive areas in morphospace. Species showed a similar arrangement in phylogeny-corrected morphospace (Fig. 5B). Frugivores, nectarivores, and sanguivores occupied unique subspaces, and carnivores, insectivores, and omnivores overlapped around the origin. The specialized piscivore N. leporinus always separated from all other guilds, but the omnivore Noctilio albiventris overlapped in morphospace with other animalivore guilds. Across morphospaces, N. magdalenensis was always nested within the insectivore subspace, close to the omnivore subspace and outside the carnivore subspace.
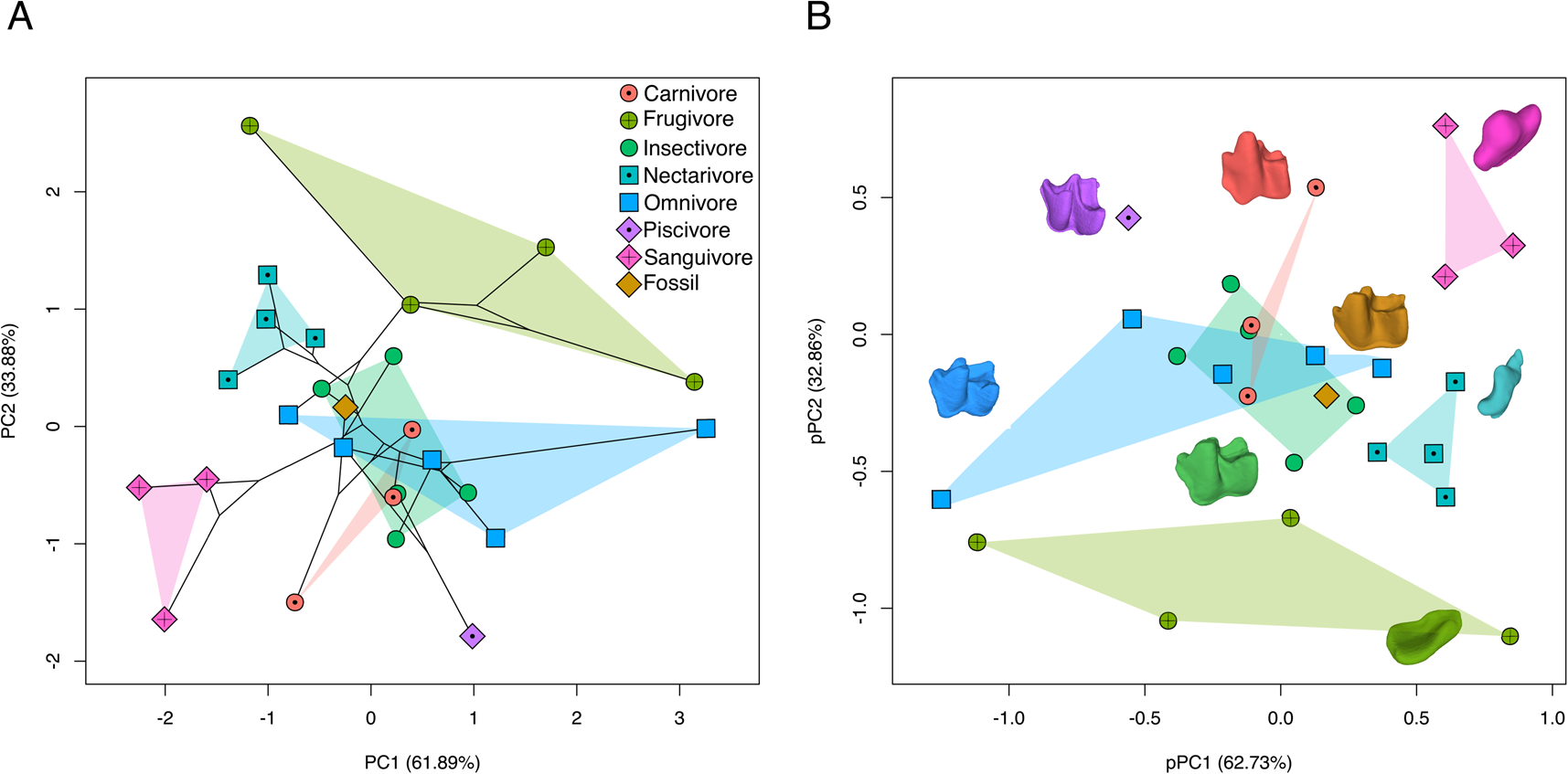
Figure 5. Morphospace (PCA; A) and phylogeny-corrected morphospace (pPCA; B) based on dental topography analysis (DTA: Dirichlet normal energy [DNE], orientation patch count rotated [OPCR], and relief index [RFI]). Dot colors represent dietary guild categories (carnivory, frugivory, insectivory, nectarivory, omnivory, piscivory, sanguivory) and fossil species (Notonycteris magdalenensis). The 3D models of m1 illustrate dental diversity in sampled taxa, colors matching dietary and fossil categories: carnivory (Phyllostomus hastatus), frugivory (Sturnira bogotensis), insectivory (Micronycteris schmidtorum), nectarivory (Musonycteris harrisoni), omnivory (Lophostoma silvicolum), piscivory (Noctilio leporinus), sanguivory (Desmodus rotundus), and fossil (N. magdalenensis). (Color online.)
Dietary Reconstruction
The tanglegram showed a similar placement of the piscivore, two carnivores, and one insectivorous species in the phylogeny and dendrogram and a consistent clustering of sanguivorous and nectarivorous taxa (Fig. 6). A mismatch between the placement of N. magdalenensis in the phylogeny and phenotypic dendrogram was also evident. The phenotypic dendrogram clustered N. magdalenensis with the insectivorous Lonchorhina aurita, and these two were clustered with the omnivorous Phylloderma stenops in our sample.
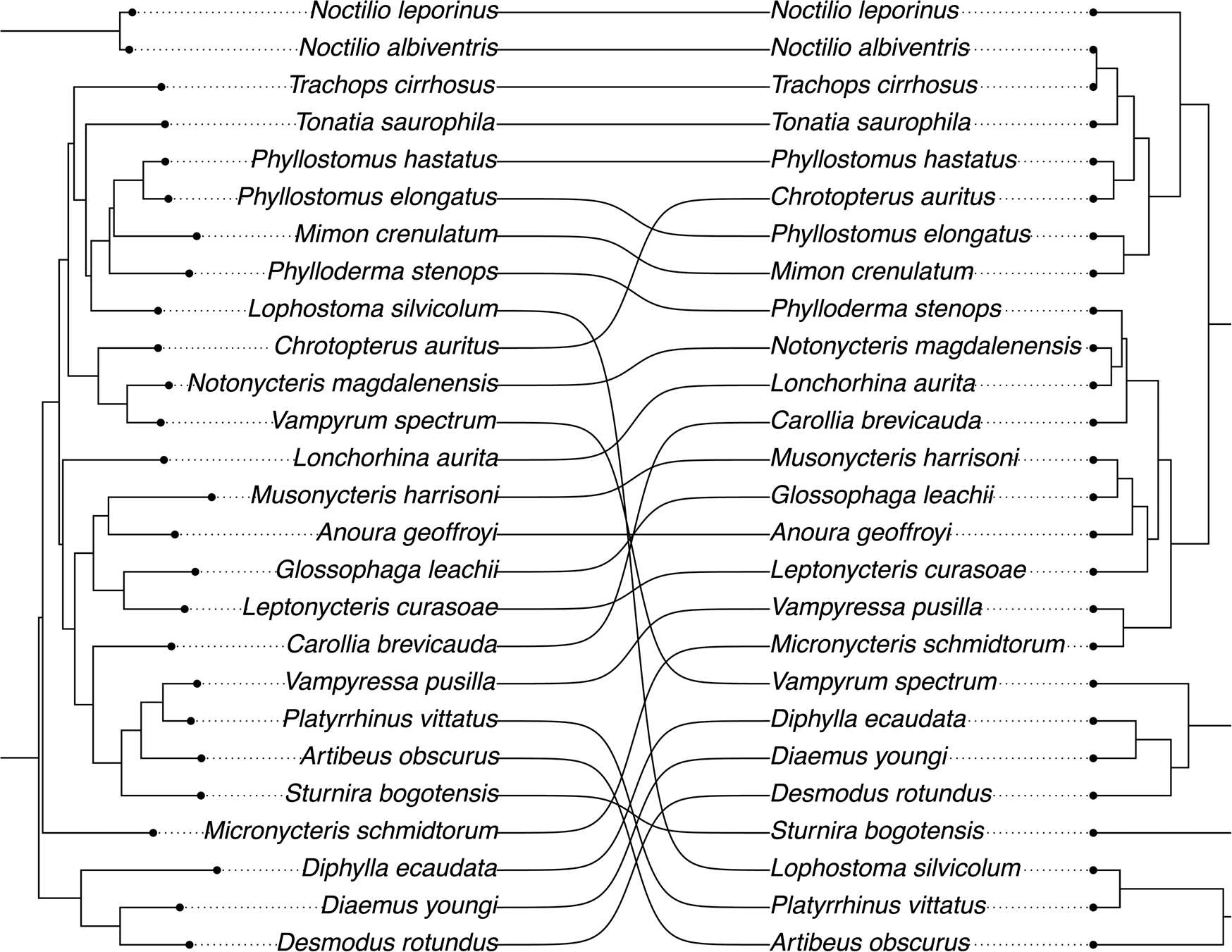
Figure 6. Tanglegram of evolutionary relatedness (left) and phenotypic dendrogram (right) of bat dental complexity. Lines indicate the change in position of the same species in both panels.
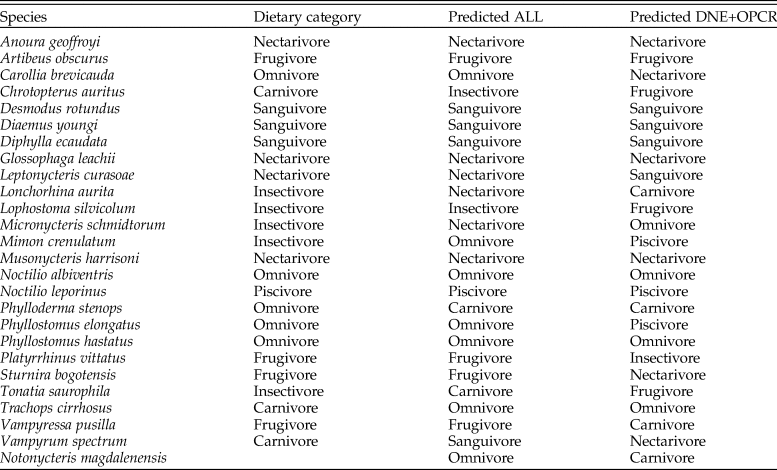
LDA correctly classified 68% of modern species in our sample into their dietary guilds when all three DTA metrics were analyzed, and 40% of modern species when only DNE and OPCR were analyzed (Table 2). When DNE, OPCR, and RFI were combined, 100% of frugivores, nectarivores, piscivores, and sanguivores were correctly classified, followed by omnivores (80%) and insectivores (20%). Excluding RFI from the LDA decreased the accurate classification of frugivores by 75%, of omnivores by 40%, of nectarivores by 25%, and of insectivores by 20%. Both iterations of LDA classified N. magdalenensis as an omnivore–insectivore, with a posterior probability of 34.46% and 27.57% (using all metrics) and 21.92% and 14.75% (excluding RFI). LDA excluding RFI recovered a 21.99% posterior probability of N. magdalenensis being a carnivore. Both iterations of LDA failed to correctly classify all modern carnivores, with 33.33% classified as omnivores, 33.33% as insectivores, and 33.33% as sanguivores. Nevertheless, carnivory had the second-highest posterior probability (>15%) across all modern carnivores (Supplementary Table 2). In contrast, carnivory had the fourth-highest LDA posterior probability (10.04%) when classifying N. magdalenensis's diet.
Table 2. Predicted dietary guild classification based on linear discriminant analyses (LDAs) analyzing Dirichlet normal energy (DNE), orientation patch count rotated (OPCR), and relief index (RFI) (ALL) and excluding RFI (DNE+OPCR).
Discussion
DTA reconstructed the diet of Notonycteris magdalenensis as an omnivorous or insectivorous species and distinct from its modern sister taxa Chrotopterus auritus and Vampyrum spectrum, both of which are carnivores. BM estimates suggest that N. magdalenensis was a large-bodied bat (~95 g), larger than most modern phyllostomids and bats in general (Gunnell et al. Reference Gunnell, Worsham, Seiffert and Simons2009), but smaller than its largest modern relative V. spectrum. The reconstructed diet and BM of N. magdalenensis suggest this species could represent a transitional stage between small-bodied ancestral insectivorous phyllostomids (Simmons et al. Reference Simmons, Gunnell, Czaplewski, Fleming, Dávalos and Mello2020) and large, specialized modern carnivores, illustrating the importance of body-size increases for the evolution of carnivory in Chiroptera (Santana and Cheung Reference Santana and Cheung2016; Giannini et al. Reference Giannini, Amador, Moyers-Arévalo, Fleming, Dávalos and Mello2020). DTA offers an additional tool to help reconstruct and differentiate dietary niches in bats.
Dental Topography Analysis and Dietary Specializations in Bats
Functional demands associated with the biomechanical processing of food have been linked to morphological adaptations of the feeding apparatus and sensory organs in bats (Nogueira et al. Reference Nogueira, Peracchi and Monteiro2009; Santana et al. Reference Santana, Dumont and Davis2010, Reference Santana, Grosse and Dumont2012; Jacobs et al. Reference Jacobs, Bastian and Bam2014; Arbour et al. Reference Arbour, Curtis and Santana2019; Rossoni et al. Reference Rossoni, Costa, Giannini and Marroig2019; Brokaw and Smotherman Reference Brokaw and Smotherman2020; Leiser-Miller and Santana Reference Leiser-Miller and Santana2020). Food item hardness has been linked with the robustness of the jaw and the length of the rostrum (Freeman Reference Freeman1981, Reference Freeman, Kunz and Racey1998, Reference Freeman2000; Arbour et al. Reference Arbour, Curtis and Santana2019). Studies have also found evidence suggesting dietary adaptations correlate with postcranial morphological differences, revealing the interaction between diet (i.e., food processing) and locomotion (i.e., foraging strategies) has an overarching effect on the ecomorphology of bats (Norberg and Rayner Reference Norberg and Rayner1987; Morales et al. Reference Morales, Ruedi, Field and Carstens2019; Gaudioso et al. Reference Gaudioso, Martínez, Barquez and Díaz2020; López-Aguirre et al. Reference López-Aguirre, Hand, Koyabu, Tu and Wilson2021). Scapular morphology in bats has been correlated with the convergent evolution of dietary adaptations (Gaudioso et al. Reference Gaudioso, Martínez, Barquez and Díaz2020). Dental morphology has been shown to have both a phylogenetic signal and an ecological signal, reflecting evolutionary relatedness as well as ecological differences within Chiroptera (e.g., Hand Reference Hand1985, Reference Hand1996, Reference Hand1998; Freeman Reference Freeman1988, Reference Freeman1995, Reference Freeman2000; Santana et al. Reference Santana, Strait and Dumont2011; Self Reference Self2015; Simmons et al. Reference Simmons, Seiffert and Gunnell2016; Zuercher et al. Reference Zuercher, Monson, Dvoretzky, Ravindramurthy and Hlusko2020). Our results also detected a dual phylogenetic and ecological signal in dental complexity variation, which highlights DTA as a potential tool in the study of dental ecology and evolution in bats. Santana et al. (Reference Santana, Strait and Dumont2011) used orientation patch count to assess diet-based ecomorphological differences between noctilionoid bats. By applying DNE, OPCR, and RFI, ours is the first study to apply multivariate DTA to investigate bats, fossil and living. There is potential for future studies to explore a variety of ecological and evolutionary questions, including the many cases of convergent diets between different bat clades (e.g., frugivory, nectarivory, and carnivory) and the reconstruction of ancestral states to investigate dietary diversification in Chiroptera. Our results of DTA-based LDA for carnivorous bats contrast with previous studies analyzing carnivorous crocodylomorphs (Melstrom and Irmis Reference Melstrom and Irmis2019) and non-volant mammals (Pineda-Munoz et al. Reference Pineda-Munoz, Lazagabaster, Alroy and Evans2017) that revealed a greater differentiation of this diet. One possible explanation is that our approach was based on a single tooth, due to the nature of the N. magdalenensis fossil material, whereas previous studies analyzed whole toothrows, possibly capturing a greater suite of dental specializations. Also, it is possible that the presence of other non-dental adaptations for carnivory in bats (e.g., cranial specializations and foraging strategies) are significant, relaxing the selective pressures on dental complexity (Norberg and Fenton Reference Norberg and Fenton1988; Santana and Cheung Reference Santana and Cheung2016).
Our study focused on reconstructing the likely diet of the extinct phyllostomid N. magdalenensis by comparing its dental morphology with that of modern members of this family. Phyllostomidae is recognized as the greatest adaptive radiation of any mammalian family (Monteiro and Nogueira Reference Monteiro and Nogueira2011; Rossoni et al. Reference Rossoni, Assis, Giannini and Marroig2017; Fleming et al. Reference Fleming, Dávalos and Mello2020). Adoption of novel dietary niches and morphological innovation have been linked to the diversification of this family (Freeman Reference Freeman2000; Dumont et al. Reference Dumont, Davalos, Goldberg, Santana, Rex and Voigt2012; Rossoni et al. Reference Rossoni, Costa, Giannini and Marroig2019; Hedrick et al. Reference Hedrick, Mutumi, Munteanu, Sadier, Davies, Rossiter, Sears, Dávalos and Dumont2020). Representing less than 15% of modern global bat diversity (~200 species), phyllostomid bats have successfully colonized almost every dietary niche found in the entire chiropteran order, with the exception of piscivory (Monteiro and Nogueira Reference Monteiro and Nogueira2011; Rossoni et al. Reference Rossoni, Assis, Giannini and Marroig2017; Fleming et al. Reference Fleming, Dávalos and Mello2020). Previous studies have suggested that different phyllostomid clades adapted to nectarivory convergently, reflecting the ecomorphological evolvability in phyllostomids (Datzmann et al. Reference Datzmann, von Helversen and Mayer2010; Rojas et al. Reference Rojas, Warsi and Dávalos2016). Low DNE and OPCR values found in our sanguivorous and nectarivorous bats indicate evolutionary convergence of reduced occlusal curvature in species with liquid diets. Loss or reduction of incisors and cheek teeth have been identified as convergent adaptations to liquid diets in Chiroptera (e.g., Freeman Reference Freeman1988, Reference Freeman, Kunz and Racey1998; Bolzan et al. Reference Bolzan, Pessôa, Peracchi and Strauss2015; Berkovitz and Shellis Reference Berkovitz, Shellis, Berkovitz and Shellis2018). Nevertheless, sanguivorous and nectarivorous bats also show divergent specialized cranial morphologies adapted to each diet, with these dietary innovations following different evolutionary trajectories (Rossoni et al. Reference Rossoni, Assis, Giannini and Marroig2017, Reference Rossoni, Costa, Giannini and Marroig2019). Sanguivorous bats have a short rostrum, enlarged and procumbent upper incisors adapted for slicing, and reduced cheek teeth and lower incisors (Berkovitz and Shellis Reference Berkovitz, Shellis, Berkovitz and Shellis2018). Nectarivorous bats exhibit elongated rostra and reduction or loss of incisors and modified cheek teeth (Freeman Reference Freeman1988, Reference Freeman1995). This divergence is also reflected in marked differences in RFI values between sanguivores and nectarivores and non-overlapping regions occupied in morphospace (Fig. 5). High RFI values found in sanguivorous bats reflect the reduction of molar width during the evolution of liquid diets in bats.
A previous study of dental complexity in bats found higher orientation patch count (an index of topographic complexity prior to OPCR) in frugivores compared with insectivores and omnivores (Santana et al. Reference Santana, Strait and Dumont2011), unlike the pattern recorded in rodents and primates (Evans et al. Reference Evans, Wilson, Fortelius and Jernvall2007; Winchester et al. Reference Winchester, Boyer, St Clair, Gosselin-Ildari, Cooke and Ledogar2014; López-Torres et al. Reference López-Torres, Selig, Prufrock, Lin and Silcox2018; Selig et al. Reference Selig, López-Torres, Sargis and Silcox2019, Reference Selig, Sargis, Chester and Silcox2020). Our results (i.e., high OPCR in frugivores compared with animalivores and omnivores) capture the uniquely complex teeth of frugivorous phyllostomid bats compared with other frugivorous mammals, including pteropodid bats. Non-phyllostomid frugivorous mammals tend to have flatter occlusal surfaces due to reduced shearing surfaces (e.g., cusps and crests), whereas the lower molars of phyllostomid frugivores retain a labial crest formed by the metaconid and paraconid (Berkovitz and Shellis Reference Berkovitz, Shellis, Berkovitz and Shellis2018). Frugivorous species in our sample had the lowest RFI values of any dietary group, showing low-crowned teeth similar to those in other mammalian groups (Boyer Reference Boyer2008; Bunn and Ungar Reference Bunn and Ungar2009; Prufrock et al. Reference Prufrock, López-Torres, Silcox and Boyer2016b). Carnivorous and piscivorous species occupied different subregions of morphospace, indicating demands of feeding on terrestrial and aquatic vertebrates shaped dental complexity differentially in each group. Cranial morphology of piscivorous bats has been found to be significantly different from other animalivorous bats, possibly due to differences in the masticatory and foraging biomechanics between both groups (Santana and Cheung Reference Santana and Cheung2016). Future studies could expand to include a wider range of bat species and families to test convergent evolution between more distantly related taxa.
Dietary and Body-Mass Reconstruction of Notonycteris magdalenensis
Our inferences of the diet and BM of N. magdalenensis may have bearing on broader understanding of the evolution of carnivory in Phyllostomidae. Some studies have estimated that carnivory had evolved in Phyllostomidae by at least the middle Miocene, based on the dental morphology of N. magdalenensis (Savage Reference Savage1951; Czaplewski et al. Reference Czaplewski, Takai, Naeher, Shigehara, Setoguchi and Shigehara2003; Simmons et al. Reference Simmons, Gunnell, Czaplewski, Fleming, Dávalos and Mello2020). Further comparisons of measurements of cranial and postcranial remains have been used to suggest this species was larger than C. auritus but smaller than V. spectrum (Savage Reference Savage1951; Czaplewski et al. Reference Czaplewski, Takai, Naeher, Shigehara, Setoguchi and Shigehara2003; Simmons et al. Reference Simmons, Gunnell, Czaplewski, Fleming, Dávalos and Mello2020). Giannini et al. (Reference Giannini, Amador, Moyers-Arévalo, Fleming, Dávalos and Mello2020) explored the evolution of BM in extant noctilionoid bats, including all of the phyletic lineages and dietary modes exhibited by them. These authors hypothesized that body-size stability early in the evolution of phyllostomids provided great potential for size increases and decreases. They suggest that the increase in BM that enabled specialized carnivory, ultimately resulting in the very large bodied Vampyrini (C. auritus and V. spectrum), probably occurred relatively late (late Miocene rather than the middle Miocene; molecular divergence dating of Amador et al. [Reference Amador, Moyers Arévalo, Almeida, Catalano and Giannini2016]), which is after the time of the La Venta fauna and of N. magdalenensis. Our results suggest that N. magdalenensis may have been a large insectivore or omnivore, with an intermediate BM between insectivores and carnivores in our sample, and are concordant with (or at least, do not refute) a later appearance of specialized carnivorous phyllostomids.
In our DTA study, N. magdalenensis had lower values of DNE, OPCR, and RFI than the modern carnivores and piscivores in our sample, indicating lower occlusal curvature, dental complexity, and crown height (i.e., lesser capacity to slice vertebrate prey). LDA classified N. magdalenensis primarily as an omnivore (posterior probability of 34.46%), and secondarily as an omnivore (posterior probability of 27.57%), whereas there was only a 10.04% posterior probability of carnivory. Our tanglegram based on the same data also suggests an omnivorous or insectivorous diet for N. magdalenensis, which groups within a cluster of omnivorous and insectivorous species, distant from carnivores or its sister taxa. Based on our results, we hypothesize that N. magdalenensis represents a transitional form between modern carnivory and the ancestral insectivore state.
Moreover, an omnivorous or insectivorous diet for N. magdalenensis also supports the observations by Savage (Reference Savage1951) and Czaplewski et al. (Reference Czaplewski, Takai, Naeher, Shigehara, Setoguchi and Shigehara2003). Their qualitative studies of the dentition of N. magdalenensis found that dental features in N. magdalenensis typically observed in carnivorous bats (Freeman Reference Freeman1988, Reference Freeman, Kunz and Racey1998) were not as highly developed as those same features in its living relatives V. spectrum and C. auritus, suggesting that N. magdalenensis probably had a less carnivorous diet than its modern relatives. These features include relatively lower crown height in N. magdalenensis, more robust crests and cusps, less reduced talonids in lower molars, less obliquely oriented ectoloph crests, and shorter postmetacristae in upper molars (Czaplewski et al. Reference Czaplewski, Takai, Naeher, Shigehara, Setoguchi and Shigehara2003).
With the exception of Trachops cirrhosus (~35 g), all carnivorous phyllostomids have average BMs higher than 80 g, well above the average for any other diet (Norberg and Fenton Reference Norberg and Fenton1988; Santana and Cheung Reference Santana and Cheung2016; Moyers Arévalo et al. Reference Moyers Arévalo, Amador, Almeida and Giannini2020). Vampyrum spectrum, thought to be the closest living relative of N. magdalenensis, is the largest bat in the New World (BM ~170 g) and is regarded as an example of the coevolution of increased body size and carnivory in Phyllostomidae (Arévalo Reference Arévalo, Fleming, Dávalos and Mello2020; Moyers Arévalo et al. Reference Moyers Arévalo, Amador, Almeida and Giannini2020). Our estimate of BM for N. magdalenensis (~95 g) based on m1 area indicates that this species was larger than most insectivores and within the range of carnivorous phyllostomids. In agreement with our DTA, this could indicate that N. magdalenensis was not a specialized carnivore and could have instead had an insectivorous or omnivorous diet, similar to other omnivorous, large-bodied Miocene noctilionoid species (Hand et al. Reference Hand, Lee, Worthy, Archer, Worthy, Tennyson, Salisbury, Scofield, Mildenhall, Kennedy and Linqvist2015a, Reference Hand, Beck, Archer, Simmons, Gunnell, Scofield, Tennyson, De Pietri, Salisbury and Worthy2018). Other studies have estimated phyllostomid BM ancestral state range as between 11–12 g, close to the ancestral state for Noctilionoidea (9–12 g) and modern Chiroptera (12–14 g) (Giannini et al. Reference Giannini, Gunnell, Habersetzer, Simmons, Gunnell and Simmons2012, Reference Giannini, Amador, Moyers-Arévalo, Fleming, Dávalos and Mello2020; Arévalo Reference Arévalo, Fleming, Dávalos and Mello2020; Moyers Arévalo et al. Reference Moyers Arévalo, Amador, Almeida and Giannini2020). This suggests relative evolutionary stasis in body size during the early evolution of modern bat families, most of which had evolved and/or had appeared in the fossil record before the early Oligocene (~35 Ma) (Giannini et al. Reference Giannini, Gunnell, Habersetzer, Simmons, Gunnell and Simmons2012; Arévalo Reference Arévalo, Fleming, Dávalos and Mello2020; Moyers Arévalo et al. Reference Moyers Arévalo, Amador, Almeida and Giannini2020). Within Phyllostominae, a significant shift to an increased size of more than 50 g has been recovered for the clade including C. auritus and V. spectrum (divergence dated at around 16 Ma), highlighting a rapid gain of >30 g in the first 10 Myr following the origin of Phyllostomidae (Arévalo Reference Arévalo, Fleming, Dávalos and Mello2020). Based on the age of the La Venta bat fauna (13–12 Ma), N. magdalenensis can be interpreted as fossil evidence for this evolutionary increase in body size within Phyllostominae (Arévalo Reference Arévalo, Fleming, Dávalos and Mello2020), and we propose it represents a transitional stage of body size between the small insectivorous phyllostomid ancestor and the large modern carnivorous phyllostomids (Datzmann et al. Reference Datzmann, von Helversen and Mayer2010; Baker et al. Reference Baker, Bininda-Emonds, Mantilla-Meluk, Porter, Van Den Bussche, Gunnell and Simmons2012). Taking our DTA and BM reconstruction together, we hypothesize that during the evolution of carnivory in Phyllostomidae, increased body size predated dental specializations.
Carnivory in phyllostomids is thought to have evolved convergently at least twice from an insectivorous common ancestor (Wetterer et al. Reference Wetterer, Rockman and Simmons2000; Hoffmann et al. Reference Hoffmann, Hoofer and Baker2008; Baker et al. Reference Baker, Bininda-Emonds, Mantilla-Meluk, Porter, Van Den Bussche, Gunnell and Simmons2012, Reference Baker, Solari, Cirranello and Simmons2016). The convergent evolution of carnivory in Chiroptera has been studied at the ordinal level (evolving at least six times; e.g., Norberg and Fenton Reference Norberg and Fenton1988; Datzmann et al. Reference Datzmann, von Helversen and Mayer2010; Santana and Cheung Reference Santana and Cheung2016) and the family level (evolving at least twice in Megadermatidae; e.g., Hand Reference Hand1985, Reference Hand1996). Independent evolutionary shifts to increasing body size in Vampyrini and Phyllostomini from a smaller phyllostomid ancestor also support two instances of convergent evolution of carnivory (Arévalo Reference Arévalo, Fleming, Dávalos and Mello2020). Similarities in DTA between carnivorous species of both tribes in our study indicate they converged in the same morphospace.
La Venta Bat Community
Authors of paleoecological reconstructions have inferred the La Venta paleoenvironment to have been a warm, wet, and aseasonal mosaic woodland, possibly with patches of open grasslands and riverine systems (Kay and Madden Reference Kay and Madden1997; Croft Reference Croft2001; Spradley et al. Reference Spradley, Glazer and Kay2019). Paleogeographic models of the eastern Andean cordillera suggest La Venta had a paleoelevation of <200 m, reflecting a lowland trans-Andean ecosystem that acted as a portal between the Andes and Central Andes (Hoorn Reference Hoorn1994; Hoorn et al. Reference Hoorn, Guerrero, Sarmiento and Lorente1995; Guerrero Reference Guerrero, Kay, Madden, Cifelli and Flynn1997; Montes et al. Reference Montes, Silva, Bayona, Villamil, Stiles, Rodriguez-Corcho, Beltran-Triviño, Lamus, Muñoz-Granados, Perez-Angel, Hoyos, Gomez, Galeano, Romero, Baquero, Cardenas-Rozo and von Quadt2021). Precipitation has been estimated in the range of 1500–2000 mm and temperatures around 20°C–25°C, similar to the conditions found in modern forests of the Andes–Amazonian transition of tropical South America (Kay and Madden Reference Kay and Madden1997; Spradley et al. Reference Spradley, Glazer and Kay2019). This type of transitional ecosystem is characterized by rich taxonomic diversity and high endemism, possibly due to reciprocal faunal exchange between highland and lowland tropical faunas since the Miocene (Upham et al. Reference Upham, Ojala-Barbour, Brito, Velazco and Patterson2013).
The fossil bat community is also informative for paleohabitat reconstructions of La Venta and supports the presence of mosaic woodlands. Two modern species of foliage-roosting species (Thyroptera spp.) typical of lowland tropical forests have been reported from La Venta, indicating the presence of Heliconia-like vegetation (Czaplewski et al. Reference Czaplewski, Takai, Naeher, Shigehara, Setoguchi and Shigehara2003). The presence of the trawling insectivore N. albiventris in the La Venta fossil fauna/community also indicates the presence of bodies of water in the ecosystem (Kalko et al. Reference Kalko, Schnitzler, Kaipf and Grinnell1998). Although this species is mainly an insectivore, some of its features (enlarged feet and constant frequency echolocation) have been interpreted as pre-adaptations for piscivory (Kalko et al. Reference Kalko, Schnitzler, Kaipf and Grinnell1998), supported by different studies reporting the consumption of insects, fruit, and pollen by N. albiventris (Fernando et al. Reference Fernando, Roberto, Priscila and Erich2007). Dietary reconstruction of La Venta's Palynephyllum antimaster indicates it was an omnivore that included nectar in its diet (Yohe et al. Reference Yohe, Velazco, Rojas, Gerstner, Simmons and Davalos2015). Our dietary reconstruction for N. magdalenensis and a previous reconstruction for P. antimaster (Yohe et al. Reference Yohe, Velazco, Rojas, Gerstner, Simmons and Davalos2015) corroborate the hypothesized mosaic woodland ecosystem of La Venta, with a mixture of high-canopy trees and understory flowering vegetation (Kay and Madden Reference Kay and Madden1997).
The 14 bat species recorded from La Venta would have occupied at least three dietary niches (insectivory, omnivory, and nectarivory), occupying the trawling, gleaning, and hawking aerial guilds. They occupied a wide body-size range (4–90 g), implying an ecologically diverse community (Czaplewski et al. Reference Czaplewski, Takai, Naeher, Shigehara, Setoguchi and Shigehara2003). Our results indicate N. magdalenensis was the largest known noctilionoid bat in the La Venta fauna, although BM reconstructions of the other extinct species would better elucidate the trophic structure of the bat community. The interpretation of Noctilio leporinus, P. antimaster, and N. magdalenensis as transitional forms (Paván et al. Reference Pavan, Martins and Morgante2013; Yohe et al. Reference Yohe, Velazco, Rojas, Gerstner, Simmons and Davalos2015; Simmons et al. Reference Simmons, Gunnell, Czaplewski, Fleming, Dávalos and Mello2020) suggests ecosystems like La Venta may have been important for the evolution of dietary innovations (i.e., carnivory, insectivory, and nectarivory) in Phyllostomidae (Kalko et al. Reference Kalko, Schnitzler, Kaipf and Grinnell1998; Yohe et al. Reference Yohe, Velazco, Rojas, Gerstner, Simmons and Davalos2015). Additional study of the La Venta bat fauna is expected to further inform hypotheses regarding the evolution of Phyllostomidae, the most ecologically diverse family of mammals.
Conclusions
We used DTA and a quantitative comparative approach to reconstruct the diet of the phyllostomine Notonycteris magdalenensis from the middle Miocene La Venta fauna (tropical South America), the most diverse fossil bat community in South America. Our study investigated patterns of dental complexity (DNE, OPCR, and RFI) variation across seven dietary guilds and provided the first multivariate DTA in Chiroptera. We found strong differentiation in dental complexity between dietary guilds, indicating that DTA is an informative tool to study ecomorphology in bats. Applying phylogenetic comparative methods, we found statistical support for the presence of both ecological and phylogenetic signal in the variation of molar complexity. Our results suggest N. magdalenensis was an omnivore or insectivore, rather than a carnivore like its extant sister taxa Chrotopterus auritus and Vampyrum spectrum. Based on our BM reconstructions of N. magdalenensis (~95 g), we infer it was the largest predatory bat in La Venta, being larger than most modern insectivorous bats and within range of modern carnivorous bats. Combining our diet and BM reconstructions, we interpret N. magdalenensis's BM and dental topography to represent pre-adaptations associated with the colonization of a carnivorous niche. Our results confirm that N. magdalenensis was probably not a specialized carnivore. Evidence that modern carnivory (typically associated with a large BM and specialized cranial morphology) had evolved in Phyllostomidae by the middle Miocene remains to be demonstrated.
Acknowledgments
We would like to thank T. Hung and the facilities and technical assistance of the National Imaging Facility, a National Collaborative Research Infrastructure Strategy (NCRIS) capability, at the Mark Wainwright Analytical Centre, UNSW Sydney; and S. Santana for granting access to the Vampyrum spectrum scans. C.L.-A. is supported by a Research Training Program (RTP) scholarship from the Australian Department of Education and a Science PhD Writing Scholarship from the University of New South Wales, and S.J.H. is supported by the Australian Research Council. Casts of UCMP specimens were provided by the late D. E. Savage.
Data Availability Statement
Data available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.pnvx0k6mt.











