Introduction
Six genera of crassatellid bivalves are known from the Cenozoic of New Zealand, and three of these, Spissatella Finlay, Reference Finlay1926, Eucrassatella Iredale, Reference Iredale1924, and Triplicitella Collins et al. Reference Collins, Crampton and Hannah2014, are closely related (see Fig. 1 for species included in this study). All three are extinct in New Zealand, although Eucrassatella has extant representatives in Australia (Darragh Reference Darragh1965). Bivalves preserve a record of their ontogeny via accretionary growth around their margin, making them amenable subjects for studies of heterochrony (e.g., Gryphaea [Jones and Gould, Reference Jones and Gould1999]; Crassostrea [Kirby, Reference Kirby2001]; Spisula [Ivany et al. Reference Ivany, Wilkinson and Jones2003]; Corbulidae [Goodwin et al. Reference Goodwin, Anderson and Roopnarine2008]). Heterochrony is the name given to “changes in the relative time of appearance and rate of development for characters already present in ancestors” (Gould Reference Gould1977: 482) and is considered to be a major mechanism of evolutionary change (Jaecks and Carlson Reference Jaecks and Carlson2001; Gould Reference Gould2002; McNamara and McKinney Reference McNamara and McKinney2005).

Figure 1 Species included in this study. A, Spissatella media. B, Spissatella maxwelli. C, Spissatella trailli. D, Eucrassatella subobesa. E, Spissatella poroleda. F, Spissatella clifdenensis. G, Spissatella acculta. H, Triplicitella australis. I, Eucrassatella ampla. J, Eucrassatella scopalveus. Scale bar, 50 mm.
Heterochrony operates in terms of acceleration or retardation of growth of somatic features relative to reproductive organs (Gould Reference Gould1977). The visible results of heterochrony are either peramorphosis, in which a descendant species has gone through the stages of its ancestor and developed further in some aspect, or paedomorphosis, in which the adult of the descendant species resembles a juvenile stage of its ancestor. Heterochrony occurs when life span, size, and shape are offset relative to one another in ancestral and descendant species. “Shape” is used here to mean any morphological feature unrelated to absolute size or age. Depending on the interplay between the timing of appearance of a morphological feature, rate of growth, and length of life span, there are four broad categories of heterochrony defined on the relative offset between developmental milestones and age: acceleration, progenesis, neoteny, and hypermorphosis. Offsets in developmental timing can also produce pre- and postdisplacement patterns (Fig. 2).

Figure 2 Summary of heterochronic and developmental displacement processes. (Modified after Alberch et al. Reference Alberch, Gould, Oster and Wake1979.)
Investigations of heterochrony require size, shape, and ontogenetic age data, and a phylogenetic framework within which ancestor–descendant relationships can be identified (Jaecks and Carlson Reference Jaecks and Carlson2001). However, age data can be problematic to gather, particularly for extinct species (Jones and Gould Reference Jones and Gould1999).
Crampton and Maxwell (Reference Crampton and Maxwell2000) investigated size-structured heterochrony in the genera Spissatella and Eucrassatella, using size as an approximate proxy for ontogenetic age. They took three outlines from each individual, spaced 10 mm apart along the axial length, and designated the resulting size classes as “juvenile,” “immature,” and “adult,” for convenience, while acknowledging that some size classes would not necessarily correspond to the same absolute age classes in different taxa. They compared these outlines using shape analysis based on the fast Fourier transform, which describes complex curves in terms of simpler, harmonically related, curves, and used principal components analysis (PCA) to construct a morphospace. PCA takes multivariate shape data (in this case, independent of size) and reorients the data set to find the dimension of maximum variance, designating this the first principal component (PC1), and the next-highest dimension of variance the second principal component (PC2), and so on. PC axes can be correlated to aspects of shape, making PCA plots useful for visualizing and interpreting shape variation, and PC scores convenient summaries of originally higher-dimensionality multivariate data. By this method, Crampton and Maxwell (Reference Crampton and Maxwell2000) were able to show shifts in average shape-at-size between species. By plotting their juvenile, immature, and adult size classes for each species in this morphospace, the authors were able to produce approximate ontogenetic trajectories for each species, which allowed visual comparison of the degree of size-structured allometry displayed by each species. They concluded that heterochrony was the dominant evolutionary mechanism in the study group, on the basis of correspondence between the orientations of trajectories. However, their observations of heterochronic processes were limited by a lack of ontogenetic age data for their specimens, and they were thus unable to identify which heterochronic processes were involved.
Sclerochronology, the study of physical and chemical variations in accretionary hard tissues and the temporal context in which those tissues are formed (Oschmann Reference Oschmann2009), can provide a solution to this problem by allowing the estimation of ontogenetic age at any given growth stage and/or at death. Growth lines of various periodicities (daily, monthly, annual, etc.) have been found in many bivalves and can be used to extrapolate growth rates (e.g., Schöne et al. Reference Schöne, Houk, Castro, Fiebig, Oschmann, Kröncke, Dreyer and Gosselck2005; Hallmann et al. Reference Hallmann, Burchell, Schöne, Irvine and Maxwell2009; Butler et al. Reference Butler, Wanamaker, Scourse, Richardson and Reynolds2013). Macroscopic banding in the Crassatellidae has been observed (Taylor et al. Reference Taylor, Kennedy and Hall1973), but its periodicity has not hitherto been known. Identification of the periodicity of crassatellid banding is one aim of the present study.
Oxygen isotope data (δ18O) from bivalve shells show seasonal fluctuations reflecting changes in temperature (e.g., Jones and Gould Reference Jones and Gould1999; Ivany et al. Reference Ivany, Wilkinson and Jones2003; Jones et al. Reference Jones, Quitmyer and Andrus2005). This allows (1) comparison of macroscopic growth lines with oxygen isotope curves to establish whether or not the growth lines are annual and (2) estimation of the ontogenetic age of the shell at death. Using sclerochronology to provide age data that are independent of size, relationships between taxa as identified by the most recent phylogeny from Collins et al. (Reference Collins, Crampton and Hannah2014) (Fig. 3), and the shape and size data from Crampton and Maxwell (Reference Crampton and Maxwell2000), we revisit the role of heterochrony in New Zealand crassatellid evolution.

Figure 3 Stratocladistic phylogeny of Triplicitella, Spissatella, and Eucrassatella from Collins et al. (Reference Collins, Crampton and Hannah2014). Broad bars indicate species ranges, thin lines indicate phylogenetic relationships. Nodes are representative, and their positions are not intended to indicate absolute times of lineage splitting. Species not included in this study are grayed out. Lineage segments for which heterochronic relationships have been considered (see Table 3) are indicated with curved arrows. Timescale produced using TSCreator, Version 6.4 (https://engineering.purdue.edu/Stratigraphy/tscreator/index/index.php) and latest calibration of the New Zealand geological timescale (Raine et al. Reference Raine, Beu, Boyes, Campbell, Cooper, Crampton, Crundwell, Hollis, Morgans and Mortimer2015).
Methods
It is hypothesized that the macroscopic shell bands visible in the crassatellid shells studied here are laid down annually. Correspondence between the δ18O pattern and the banding pattern through the shell would provide support for this inference. This approach was used by Jones and Gould (Reference Jones and Gould1999) to establish chronological age in Gryphaea spp. for the purpose of studying heterochrony, and it is emulated here. Nine specimens, which represent the majority of species included in recent morphometric and phylogenetic analyses of this group (Collins et al. Reference Collins, Crampton and Hannah2013, Reference Collins, Crampton and Hannah2014), were donated by GNS Science from their fossil reference collection (Table 1).
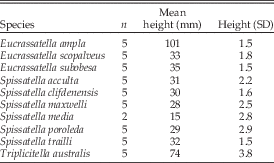
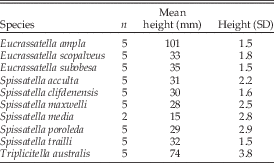
Table 1 Size data from this study, as measured and/or calculated for the largest specimens from various collections, including specimens measured by Crampton and Maxwell (Reference Crampton and Maxwell2000) wherever possible.
Only one specimen per species was available for destructive analyses due to the limited number of specimens held in the collection. Spissatella media, Eucrassatella marshalli, E. maudensis, and E. kingicola were omitted entirely from oxygen isotope analysis for this reason. Each specimen selected for analysis was embedded in epoxy resin and sectioned along the direction of maximum growth, referred to as the axial length by Crampton and Maxwell (Reference Crampton and Maxwell2000), which is marked by a broadly rounded posterior ridge. The posterior block was retained for preparation of thin sections. The larger shell block was cut 5–10 mm anterior to the axial length to produce a thick section, and the axial-length face was polished to produce a smooth, flat surface.
Polished slabs were microsampled at the National Institute of Water and Atmospheric Research (NIWA) in Wellington, New Zealand, using a New Wave micromill with a 0.2 mm endmill. A micromill is an xyz microsampling device that has been designed for accurate high-resolution milling to obtain samples for chemical and isotopic analysis. Approximately 20 µg of material was removed from a series of continuous swaths at regularly spaced intervals that followed, where possible, growth lines through the shell, avoiding the outer sculpture-bearing layer, the discontinuity that marks the pallial myostracum (where visible), and the flexure of the hinge plate where banding becomes thin and hard to discern (Fig. 4). Multiple samples were taken through each growth band in every instance that this was possible. A total of 336 samples were milled from the nine specimens available.

Figure 4 Typical crassatellid shell (Spissatella maxwelli) showing features pertinent to sampling procedures. Samples were milled from the thickest part of the shell, following the growth lines and avoiding the pallial myostracum, sculpture, and flexure.
Crassatellid bivalves are originally aragonitic but may be partially or wholly altered to calcite during diagenesis. All shell material was assessed for diagenetic effects in thin section at Victoria University of Wellington, and then using X-ray diffraction analysis (XRD) and scanning electron microscope (SEM) examinations at CSIRO, Australian Resource Research Centre (ARRC) in Perth, Western Australia. The XRD data were collected on the powdered samples for modal mineralogy using a Bruker D4 Endeavor instrument fitted with a Co tube, Fe filter, and a Lynxeye position-sensitive detector. SEM imaging was performed on a Philips XL40 controlled-pressure SEM operating with a chamber pressure of about 0.5 mBar (also at ARRC).
Stable oxygen isotope analyses were undertaken at NIWA. Samples were reacted with three drops of H3PO4 at 75°C in an automated individual-carbonate reaction (Kiel III) device coupled to a Finnigan MAT252 mass spectrometer. All values are reported relative to vPDB (Vienna Pee Dee Belemnite), where δ18O has a value of −2.20‰ for NBS19 calcite. Internal precision of measurements is 0.02–0.08‰; external precision is 0.03‰ relative to vPDB. All δ18O data are available in the supporting supplemental material.
To test the hypothesis that the growth lines in crassatellid shells are laid down annually, two separate estimates of age for each specimen were established. First, using thin sections, growth lines were counted through the shell, taking both minimum and maximum estimates, as growth lines were often so closely spaced as to make counting difficult. Second, peaks in oxygen isotope transects were counted, both conservatively (counting only the highest peaks) and comprehensively (counting every high point in the curve), to provide minimum and maximum estimates. Due to damage and natural discontinuities in shell material, oxygen isotope transects never spanned the entire thickness of the shell. If a shell was twice as thick as the length of the transect, for instance, the number of peaks was doubled to derive an estimate of the age of the shell at the time of death. This was considered conservative, because as bivalves age, the growth lines of shell material they lay down become narrower (Richardson Reference Richardson2001). Total estimates of age from oxygen isotopes were then obtained by taking the number of peaks counted over a known shell thickness and scaling to obtain a minimum estimate of number of peaks for the total shell thickness.
It is hypothesized that both bands and isotope peaks in crassatellid bivalves result from annual growth and annual environmental change, respectively. Both quantities are uncertain. The average of the minimum and the maximum of each of the band and isotope peak counts is taken as an estimate of the mean for that specimen, and the separation between the minimum and the maximum is taken to be 4 standard deviations. Nine specimens had their growth lines and oxygen isotope peaks tallied. We can express our hypothesis as:
where G is the number of growth lines, P is the number of peaks in δ18O, j represents the j th specimen and the possibility of a systematic variation between peaks and bands is allowed for with the factor λ.
These equations are nonlinear, but can be solved by linear least squares after log transformation:
where the set of log agej and log λ are the parameters to be solved for.
One thousand random sets of G and P were made using the assumed means and standard deviations of the specimens and assuming Gaussian distributions. Equations 2a and b were then solved by least squares for the log ages and log λ. A best estimate of log agej was obtained by averaging the 1000 individual estimates, and 95% confidence intervals were calculated from the spread of the log ages. The means and 95% limits were then exponentiated to give best ages and λ, with corresponding 95% confidence intervals.
Average heights in millimeters from the largest specimens available provide the size information (Table 1). In the absence of a morphological indicator of maturity, we have taken maximum size to indicate adulthood. As the intent of the study is to examine shape independent of size, we prefer a measure of size that is separate from that inherent in the shape data. Shell height is a functionally meaningful measure of size in the present context, as it is the maximum dimension of the shell profile orthogonal to the direction of burrowing, separate from the dimension of maximum growth, which functions as a crude measure of posterior elongation and is thus duplicated in the shape data explained below.
The shape data used are the scores for the first PC axis of all adult growth stages from the Crampton and Maxwell (Reference Crampton and Maxwell2000) data set. The Fourier analysis was conducted using the fast Fourier transform method of Haines and Crampton (Reference Haines and Crampton2000). The first PC captures shape variation related to posterior elongation and explains 51% of the variance in the data set, including the majority of all ontogenetic variation (Crampton and Maxwell, Reference Crampton and Maxwell2000).
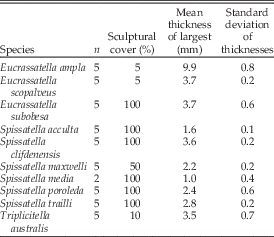
Crassatellids are very conservative in their shell characters (Collins et al. Reference Collins, Crampton and Hannah2014), but two other morphological parameters that vary between species are (1) extent of sculptural coverage and (2) shell thickness (Table 2). Sculpture and thickness, like shape, are of functional significance to infaunal bivalves, and the degree of this significance is known to be at least partially related to the stage of ontogeny and/or adult size of the animal (Stanley Reference Stanley1970, Reference Stanley1981). After identification of heterochronic processes across the Triplicitella + Spissatella + Eucrassatella phylogeny, we have compared shell thickness and sculptural coverage changes along lineage segments to gain further understanding of the possible functional drivers of heterochronic change.
Table 2 Sculptural and thickness measurements from crassatellid bivalves.

Results
In thin section, only GS2155 (Eucrassatella ampla) displayed evidence of recrystallization, and this was only on the sculpture-bearing layer of the shell, which was avoided for sampling purposes. The XRD analyses did not detect calcite, and SEM examination revealed no evidence of any diagenetic alteration in all samples, except for minor silicate phases present in Spissatella acculta (Fig. 5).

Figure 5 SEM mapping of Spissatella acculta. A, Backscatter electron image (dotted line demarcates milling pit from oxygen isotope sampling (as per Fig. 4). Curved growth lines can be seen running parallel to the long axis of the pit, roughly from top to bottom of the image). B, Si concentration (color intensity is scaled to the maximum and minimum values measured in a map). C, Ca concentration. D, Mixed Ca and Si concentration, showing some irregular zones of silicate contamination, mostly outside the area milled for isotope samples.
When calculated for the crassatellid data set, the scale factor between the counts of δ18O peaks and the counts of bands is close to 1 (1.05±0.16, 95% CI), which strongly suggests that they are equivalent, that is, that the bands are annual. Age estimates for each species at maximum size were calculated using the growth rates obtained for each species from the sclerochronological data (Fig. 6).

Figure 6 The pattern of paedomorphosis and peramorphosis across the Triplicitella + Spissatella + Eucrassatella clade. Lineages are shown as gray arrows, with ontogenetic trajectory plots for each pair of species along the lineage segment superimposed. The x-axis on each trajectory plot is in years (starting at 0 in all cases and with each tick mark representing 10 years), the y-axis is the first PC axis from Crampton and Maxwell (Reference Crampton and Maxwell2000), scaled in units of standard deviation from the mean shape (see scale legend). Abbreviations for types of heterochrony follow those given in Fig. 2. Shell images are included to show shape changes through time; the descendant shell is illustrated to the right of each trajectory plot (except for Triplicitella australis, the oldest member of the clade, illustrated at the bottom left). Scale bar, 50 mm (top right), applies to all shells. The time axis (extreme left) is indicative only.
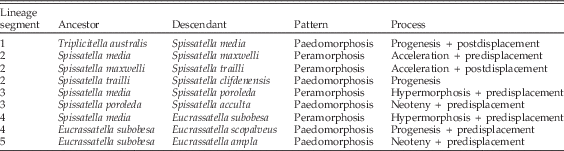
The age of Spissatella media, the earliest member of its genus, had to be estimated, as the scarcity of specimens in collections meant that we were unable to sample for oxygen isotopes and derive a life span. Given this restriction, we have used the available age and size data for the entire clade and averaged a growth rate across the entire clade, from which we have extrapolated a conservative age estimate of 6 years at maximum size for S. media. As heterochronic relationships are constructed from relative advancement and retardation along the age axis, rather than absolute age values, our results are not likely to be significantly affected by the uncertainty involved in S. media’s estimated age. Heterochronic processes between pairs of species are identified by plotting ontogenetic trajectories for each species (as in Alberch et al. Reference Alberch, Gould, Oster and Wake1979) (Fig. 2). Crampton and Maxwell (Reference Crampton and Maxwell2000) plotted trajectories in pure shape space, using size as an analogue for age. Here, we have replotted their ontogenetic trajectories in terms of shape against age (Fig. 6), to identify true heterochronic patterns, summarized in Table 3.
Table 3 Summary of heterochronic processes along crassatellid lineages.

The most striking feature of these results is that no lineage is either entirely peramorphic or entirely paedomorphic, but rather, that paedomorphosis and peramorphosis, and pre- and postdisplacement, come close to alternating along lineages (Fig. 6). The effect of this chopping and changing is to produce a range of adult sizes and uncouple shell shape from absolute shell size.
When sculptural coverage and shell thickness are mapped onto the clade as well, two distinct groupings of the different states of key shell characters (posterior shape/elongation and sculpture) can be discerned in the data set. Sculpture extending to the ventral margins (Table 2) is generally associated with inequilateral shells that are squarely truncated (e.g., S. media, S. clifdenensis) and/or very elongate (e.g., E. subobesa). We term this association of characters the “snorkel” form group, in reference to their morphology allowing them to bury themselves deeply and yet have their inhalant and exhalant currents still reach the sediment/water interface. Conversely, shells such as S. maxwelli, T. australis, and E. ampla, which have very rounded posteriors and are generally more equilateral, bear sculpture typically only on the juvenile portions of the shell, at the beak. We term this association of characters the “anchor” form group, as their bulk is inferred to be anchoring them in their substrate.
Discussion
The two genera Spissatella and Eucrassatella originated in the Eocene from Triplicitella or a Triplicitella-like ancestor and have coexisted for much of their time range, following a radiation in the latest Oligocene–earliest Miocene (Collins et al. Reference Collins, Crampton and Hannah2014). The earliest members of the clade (the late Eocene Triplicitella australis and Spissatella media) are both equilateral—S. media as a paedomorphic descendant of T. australis is essentially just a sexually mature juvenile, with progenesis meaning there is no shape or size change relative to ontogenetic age. However, in the Oligocene the descendants of S. media all evolved by peramorphosis, by which mechanism they became larger and more elongate: S. poroleda and E. subobesa by hypermorphic extension of the posterior compared to S. media, S. maxwelli simply by growing larger overall, a trend continued as it gave rise to S. trailli. The younger species in these lineages, however, are paedomorphic again, producing short, thick, well-sculptured (S. clifdenensis), thin and strongly inequilateral (S. acculta), small but massively overthickened (E. scopalveus), and giant, nonsculptured (E. ampla) variations on the general crassatellid theme.
Like all crassatellids, Triplicitella, Spissatella, and Eucrassatella were nonsiphonate infaunal suspension feeders, similar to astartids, trigoniids, and some carditiids (Stanley Reference Stanley1968). This lack of siphons meant that their posterior margin had to remain in contact with the sediment/water interface to allow feeding, respiration, and excretion. Stanley (Reference Stanley1968, Reference Stanley1970) noted that nonsiphonate bivalves are, in general, sluggish burrowers because of their nonfused mantle. Therefore, they are ill equipped to live in high-energy environments with the threat of disinterment by wave action. Spissatella species are known from shelf and upper-slope environments, Eucrassatella ampla is inferred as being primarily an inner-shelf dweller, and Triplicitella australis is known from inner- or middle-shelf assemblages (as “Eucrassatella” australis [Beu and Maxwell, Reference Beu and Maxwell1990]).
Adaptations of shell form that confer greater stability within the sediment are likely to be selected for in a group with these constraints. Stanley (Reference Stanley1970, Reference Stanley1981) identified asymmetrical commarginal ribbed sculpture (Fig. 7) as stabilizing the shell in soft substrates and reducing risk of disinterment in juveniles, noting that in some cases the sculpture may become obsolete as the animal ages and the shell grows larger. Ribbed sculpture on the posterior of a burrowing shell has also been shown to reduce scour of surrounding sediment at the expense of burrowing speed (Stanley Reference Stanley1981). Thick, heavy shells in infaunal bivalves have also been observed by Stanley (Reference Stanley1970) to increase the stability of the shell within the substrate and prevent disinterment. Thicker-shelled bivalves are also less vulnerable to predation by both crabs (Boulding Reference Boulding1984) and naticid gastropods (Kitchell et al. Reference Kitchell, Boggs, Kitchell and Rice1981).

Figure 7 Spissatella maxwelli, illustrating the transition between ribbed commarginal sculpture and smooth shell only, showing growth lines.
In Spissatella, the species of which are small to moderately sized (up to 40 mm height), ribbed commarginal sculpture typically (with only one exception) extends to the ventral margin. In the stratigraphically older Eucrassatella (comparable in shell height to Spissatella), the same type of sculpture likewise extends to the ventral margin but is obsolete to varying degrees on the posterior area; whereas in the stratigraphically younger Eucrassatella (which can be up to 100 mm height), sculpture is often restricted to the beaks or extends only to the center of the disk. Thicker-shelled species typically have less sculpture and are more inequilateral and rounded posteriorly, regardless of size.
The net result of all the patterns described above is that there are no particular phylogenetic trends of size, shape, sculpture, or thickness, but rather, these characters, with their tight functional controls, are easily modified by changing the timing of development. We suggest that the pressures on crassatellid bivalves can be best avoided in two ways; either by physically resisting disinterment and predation by being a thick-shelled, heavy anchor, or by avoiding disinterment and predation by being an elongate, highly sculptured snorkel. For anchors, extensive sculpture over the disk is rendered obsolete by their weight, and for snorkels, the overthickened shell is an unnecessary metabolic cost. These groupings of shell characters produce species with counterintuitive shell morphologies—for example, S. acculta is anomalously thin for its size; E. scopalveus is entirely without sculpture and massively thick, despite being small.
The greatest diversity of crassatellid bivalves in New Zealand is observed in the Duntroonian stage (late Oligocene, 27.2–25.3 Ma), coinciding with the time of greatest inundation of the Zealandia landmass (the Oligocene, 33.7–23.8 Ma; King et al. Reference King, Naish, Browne, Field and Edbrooke1999), which presumably provided the greatest amount of shelf habitat for exploitation. Two species of Spissatella and two species of Eucrassatella coexisted during this time period. Diversity decreased over the early Miocene, and by the late Miocene, only Eucrassatella ampla survived, to later be succeeded by Eucrassatella marshalli (not included in this study, but very similar in form).
Tashiro and Matsuda (Reference Tashiro and Matsuda1988), in their study of trigoniids, suggest that the diversity of that group in the Cretaceous may have been triggered by competition with siphonate bivalves. Trigoniids developed extremely elongate posteriors, with a median “siphon groove” that separated their inhalant and exhalant currents and acted as a snorkel, similar to the siphons of other bivalves. The inference is that their low diversity today (a single genus of trigoniids—Neotrigonia—still inhabits Australian waters) is due in large part to inability to compete successfully with truly siphonate bivalves such as venerids. Crassatellids would have been subject to similar competition pressure from coexisting burrowing bivalves such as Neilo (Malletiidae), Dosinia (Veneridae: Dosiniinae), Circomphalus (Veneridae: Chioninae), Notocallista (Veneridae: Pitarinae), and Mactra (Mactridae), which would have benefited as much as the crassatellids from the expansion of available habitat caused by the flooding of Zealandia.
In general, crassatellids seem to have had little morphological latitude to diversify into novel regions of morphospace. Compared with other nonsiphonate bivalves such as trigoniids and carditiids, they are morphologically and sculpturally conservative. Despite this, the interplay between evolutionary pressures on shape, size, and sculpture has produced two different solutions to the problems of life as a nonsiphonate bivalve from this restricted anatomical tool kit—anchors and snorkels. These constitute two different successful body plans for the New Zealand crassatellids, with snorkels relying on elongation to avoid predation and the stability provided by the sculpture to minimize sand scour, and anchors relying on size and weight to keep them buried and protected from boring or crushing predators. The dissociation of size from thickness and sculptural cover, and shape from age, which allowed these two form groups to arise, is the result of repeated heterochronic shifts along lineages.
Overall, the low morphological diversity of New Zealand’s larger crassatellid genera would seem to be due, in some part, to the strong anatomical “starting condition” constraints on their development, including most notably their nonsiphonate condition and lack of sculptural variation. Despite these tight controls, New Zealand crassatellids appear to have had plastic developmental mechanisms, leading to the ability for lineages to “switch” between anchor and snorkel characteristics, presumably in response to environmental change.
Conclusions
Shell form in New Zealand crassatellid bivalves is constrained tightly by the combination of anatomical starting conditions and environmental pressures. Two associations of shell characters (form groups) can be recognized as alternate successful nonsiphonate body plans that can be “swapped” between by heterochronic means. The restricted habitat available to these bivalves has channeled and limited their occupancy of morphospace without restricting the developmental processes involved.
Acknowledgments
This work would not have been possible without the loan for photography and analysis of a great many specimens held in collections around the country. We would like to thank J. Simes, M. Terezow, and A. Beu at GNS Science; N. Hudson at the University of Auckland; T. Trnski, W. Blom, and S. Hannam at Auckland War Memorial Museum; B. Marshall at Te Papa Tongarewa; E. Fordyce and the late A. Grebneff at the University of Otago; and N. Hiller at Canterbury Museum for their generosity and patience. Thanks also to P. Marriott at NIWA for help with photography and micromilling equipment. CSIRO Mineral Resources Flagship is acknowledged for access to SEM and XRD facilities. The authors acknowledge the use of information contained in the New Zealand Fossil Record File (www.fred.org.nz). We would like to thank Bernd Schöne and an anonymous referee for their helpful reviews.
Supplementary Material
Supplemental materials deposited at Dryad: doi:10.5061/dryad.pm5rt