Introduction
Commonly cited as nature’s perfect food(Reference Nagpal, Behare and Kumar1), milk is the foundation of life for all mammalian neonates(Reference Ebringer, Ferenčík and Krajčovič2,Reference Nongonierma and FitzGerald3) . Representing a complex and unique liquid, milk (which is produced by the mammary glands(Reference Ferreira, Bislev and Bendixen4)) is the only foodstuff designed by nature to serve as a complete food, at least during the pre-weaning period. In this sense, milk contains numerous biological constituents and an assortment of nutrients necessary for immunological protection and initial growth(Reference Ebringer, Ferenčík and Krajčovič2,Reference Rezaei, Wu and Hou5) . Shortly after weaning, most mammals stop consuming milk(Reference Givens, Livingstone and Pickering6). With domestication and milking of various animals, however, some humans continue to drink milk throughout life(Reference Velten7), mostly cow’s milk. The value of cow’s milk in the human diet has been heavily debated for many years(Reference Ludwig and Willett8) and has been portrayed by some as ‘white poison’, a health hazard and promoter of Western chronic diseases(Reference Melnik9). This position is based on numerous hypotheses including that cow’s milk contains blood, pus, hormones and antibiotics, causes acne and cancer and has a high cholesterol and fat/saturated fat content collectively contributing to cardiovascular disease risk, weight gain and obesity. Nevertheless, such perceptions of cow’s milk being harmful to health are not supported by evidence. Conversely, cow’s milk has often been signified as the white elixir(Reference Givens, Livingstone and Pickering6).
Childhood is a key stage for the development of healthy eating patterns(Reference Morgan, Warren and Lubans10), and the school environment provides a valuable setting to develop such behaviours. This is particularly true considering children spend much of their time at school. Indeed, dietary habits shaped throughout the childhood years might carry forth and track into adulthood. Between the ages of 4 and 11 years (primary-school age), children grow and mature at a rapid rate preceding the onset of puberty(Reference Patton and Viner11). Childhood is therefore a critical transition period preceding adolescence, characterised by growing independence and marked physical development. Good nutrition during the childhood period is therefore particularly important(Reference Wilson and Temple12). Not only may good nutrition support proper growth and maturation, but it may therefore act as a base for immediate and lasting health, wellbeing and disease prevention. This is best achieved by consuming a balanced and varied diet that provides all the nutrients needed. The inclusion of cow’s milk as a staple component of a healthy balanced diet has been long recognised and is central in most public dietary recommendations(Reference Rozenberg, Body and Bruyère13). Milk is naturally nutrient-dense and is a significant source of many essential macro and micronutrients. Indeed, cow’s milk may confer nutrition-, physical- and health-related benefits beyond that of helping children to simply meet nutrient targets. Based on the available literature, it appears that many of these effects are a product of milk’s nutritional composition. It therefore seems relevant to provide readers with a brief description of the nutritional composition of cow’s milk.
Nutritional Composition of Cow’s Milk and Patterns of Consumption
The nutritional composition of cow’s milk is influenced by factors including genes (species and breed), physical state (age and stage of lactation) and environment (available nutrition and climatic conditions)(Reference Clare and Swaisgood14). The composition of whole cow’s milk is approximately 87 % water and 13 % solids(Reference Nagpal, Behare and Kumar1). The percentage split of water and solids of cow’s milk, as well as energy, is primarily determined by the amount of fat(Reference Parodi15) but is also influenced by added sugars and sweeteners. Cow’s milk is approximately 4·9 % carbohydrate. The primary carbohydrate portion of milk is lactose, a disaccharide comprising glucose and galactose. The lipid component of cow’s milk and other milk-based dairy foods contributes numerous properties including the provision of fat-soluble micronutrients and essential fatty acids, as well as influencing flavour, texture and appearance(Reference Parodi15). Of the lipid content within cow’s milk, roughly 64 % is saturated fatty acids, with a considerable amount (∼26 %) from monounsaturated fatty acids and a small contribution from trans- and polyunsaturated fatty acids (both ∼3 %)(Reference MacGibbon and Taylor16). Additionally, cow’s milk provides high-quality proteins, namely casein and whey. Casein and whey constitute approximately 82 % and 18 % of the total protein found in cow’s milk and provide an abundance of essential amino acids(Reference Wilkinson, Tarnopolsky and MacDonald17). Aside from the macronutrient content, cow’s milk contains essential micronutrients that contribute to dietary quality and overall nutritional status. As presented in Table 1, with the exception of vitamin C (which is broken down during pasteurisation) and vitamin D (unless fortified), cow’s milk is a good source of all vitamins(Reference Tunick and Van Hekken18). Calcium and phosphorus, crucial to healthy growth and maturation, as well as other biological processes, are the most prominent minerals present in cow’s milk. Cow’s milk also makes significant contributions to intakes of other major minerals(Reference Bates, Lennox and Prentice19). In this sense, cow’s milk makes a substantial contribution to, and is the main dietary source of, calcium (26 %), iodine (37 %), riboflavin (25 %), magnesium (10 %) and potassium (14 %) in the diets of primary-school-aged children(Reference Roberts, Steer and Maplethorpe20). While volume-specific data are limited, in the UK, the National Diet and Nutrition Survey (NDNS) provides a nationally representative assessment concerning dietary habits of individuals, aged 1.5 years and older, living in private households and remains the only surveillance programme to do so. Temporal and age-related trends of cow’s milk to average daily total energy and macro and micronutrient intake from 2009/10 through to 2015/16 are presented in Table 2 for children (4–10 years) and adolescents (11–18 years). Despite the clear value of cow’s milk in the everyday diet, it is clear that intakes steadily decline as children age. This trend is not only restricted to the UK; it is also true in the USA(Reference Cavadini, Siega-Riz and Popkin21,Reference Fiorito, Mitchell and Smiciklas-Wright22) , Australia(Reference Baird, Syrette and Hendrie23) and other European countries(Reference van Rossum, Fransen and Verkaik-Kloosterman24). This is of great concern among children, especially as dietary habits may track into adulthood, as milk avoidance may have detrimental implications over time, leaving populations vulnerable to micronutrient deficiencies (e.g. calcium, iodine and riboflavin deficiencies to name a few(Reference Black, Williams and Jones25)) and lasting nutrition- and health-related complications (e.g. cardiovascular disease(Reference Appel, Moore and Obarzanek26), metabolic syndrome(Reference Pereira, Jacobs, David and Van Horn27), hypertension(Reference Pereira, Jacobs, David and Van Horn27,Reference Elwood, Pickering and Fehily28) , poor weight management and bone health(Reference Black, Williams and Jones25,Reference Rockell, Williams and Taylor29) ).
Table 1. Nutritional composition of cow’s milk

Note: N/A = values not available for this food; Trace = nutrient is present in less than 0·1 g per 100 g. *Data taken from a sample of strawberry- and banana-flavoured sugar-sweetened milk. Adapted from the Dairy UK. Available at: http://www.milk.co.uk/publications/default.aspx
Table 2. Percentage contribution of cow’s milk to average daily total energy and macro- and micro-nutrient intake over time by sex and age

Note: Percentage contribution information taken from National Diet and Nutrition Survey (NDNS) rolling programme. Percentage contributions for vitamin B6, vitamin B12, vitamin C and phosphorus are not reported in the NDNS. *The NDNS rolling programme for 2015/16 (https://www.gov.uk/government/statistics/ndns-results-from-years-7-and-8-combined) stopped providing sex-specific intake data, so the data are presented as boys and girls combined.
Scope and Methodology of the Narrative Review
Evidence from adult populations suggests that adequate cow’s milk consumption is associated with a reduced risk of cardiovascular disease(Reference Soedamah-Muthu and de Goede30), metabolic syndrome(Reference Elwood, Pickering and Fehily28,Reference Crichton, Bryan and Buckley31) and obesity(Reference Abargouei, Janghorbani and Salehi-Marzijarani32–Reference Dougkas, Reynolds and Givens34). Emerging data also suggest that cow’s milk may have a role in overall dietary quality, appetite control, hydration and cognitive function. Although the evidence to date is limited compared with the adult literature, these benefits appear echoed in recent paediatric studies. There is a need to review the literature to assess whether there is sufficient evidence of a beneficial or detrimental effect of cow’s milk in the diet and health of children. The aim of this narrative review is therefore to summarise and appraise the scientific literature to form an accurate, evidence-based evaluation of the associated nutrition-, physical- and health-related benefits of cow’s milk consumption in primary-school-aged children (4–11 years). Based on recent publications, it is not the intention of this narrative review to focus on body weight and body composition, as it is known with some confidence that cow’s milk consumption is inversely (or not) associated with body weight and body composition in children and adolescents(Reference Dougkas, Barr and Reddy35). Where possible, this review focuses solely on literature in children aged 4–11 years; however, where there is no applicable literature, data from general child studies will be reviewed (and possibly some adult studies). This is particularly true for mechanistic considerations, as much of this evidence comes from adult studies; therefore, any adult-specific data should be interpreted with some caution as the observations cited may not always be replicated in children. The narrative review will also highlight current knowledge gaps in this field and suggest directions for future research.
To identify research articles to facilitate this narrative review, PubMed (US National Library of Medicine National Institutes of Health) and Web of Science (Thomson Reuters, UK), were searched using various combinations of keywords relevant to the scope of the review up to August 2020. Search terms included: humans, child, milk, flavoured milk, animal milk, cow milk, infant, adolescent, preschool, primary-school, paediatric, diet, energy intake, nutritional status, diet quality, obesity, school-age children, food habits, dairy products, milk beverages, height, stature, anthropometry, calcium, health association, health benefits, dental health, caries, decay, bone health, appetite, cognition, memory, mental performance, school milk, insulin, glucose, hydration, snack. Studies were considered eligible for inclusion in the review if (i) they were conducted in children 4–11 years; (ii) participants received cow’s milk, and not supplemental forms of dairy minerals (unless a milk group was included); and (iii) the study had been peer-reviewed. Results from studies that grouped data over a wider age range were also considered where this was deemed appropriate. This was especially true for prospective and intervention studies, yet the mean age of participants had to fall between the stipulated age range. Studies were excluded if (i) human participants were not used; (ii) participants lay directly outside of the stipulated age range; (iii) studies used supplements only; (iv) studies used different dairy foods (i.e. yogurt, cheese, dairy desserts); (v) the milk consumed included different sources of animal milk; or (vi) data reporting was poor and/or not published in English.
In this narrative review, we have summarised the evidence base, which we identified by nutrition-, physical- and health-related related benefits and provide a summary for the studies identified. As this is not a systematic review, we did not perform a quality assessment of each study (based on design and implementation) or conduct a meta-analysis by study design but summarise the findings for each study and sub-section in Tables 3 (nutrition-related benefits), 4 (physical-related benefits) and 5 (health-related benefits). Symbols ↑ and ↓ indicate a statistically significant positive and negative effect/relationship, respectively, between cow’s milk and related subsections, with ↔ indicating no statistically significant effect/relationship or a neutral effect. This approach has successfully been used in a recent narrative review in this journal(Reference Dougkas, Barr and Reddy35), and therefore readers should be directed to this paper for justifications and interpretations for this approach. In brief, however, a tabulated summary allowed us to draw overall conclusions for this narrative review, although we did not undertake a detailed assessment of quality of individual studies.
Table 3. Studies in primary-school-aged children that measured nutritional status and hydration with cow’s milk consumption

Abbreviations in order of appearance: FM = flavoured milk; NHANES = National Health and Nutrition Examination Survey; SSB = sugar-sweetened beverage; FWR = free water reserve; W = water; CES = carbohydrate/electrolyte solution; SM = skimmed milk; Lo-PRO = low protein; Hi-PRO = high protein.
Nutrition-Related Benefits of Cow’s Milk
Nutritional Status
The importance of regular cow’s milk consumption in children has been recognised for over 40 years(Reference Cook, Irwig and Chinn36). As described, cow’s milk is a naturally nutrient-dense foodstuff, providing a rich source of many essential nutrients. While the potential of cow’s milk to improve nutritional status may be unsurprising, the effect of this in primary-school-aged children has only been investigated by numerous observational studies(Reference Campmans-Kuijpers, Singh-Povel and Steijns37–Reference Wang, Shang and Johnson-Down42) and three intervention-based studies(Reference Kuriyan, Thankachan and Selvam43–Reference Lien, Nhung and Khan45) (Table 3). All reported improved nutritional status with increased cow’s milk consumption, yet, because of the study designs utilised, findings must be interpreted with caution. In the few intervention-based studies(Reference Kuriyan, Thankachan and Selvam43–Reference Lien, Nhung and Khan45), which ranged from 16 weeks to 6 months, primary school children were provided with flavoured milk or no drink (control)(Reference Albala, Ebbeling and Cifuentes44), or 200 ml(Reference Kuriyan, Thankachan and Selvam43) and 250 ml(Reference Lien, Nhung and Khan45) of multi-micronutrient-fortified milk or unfortified milk, to establish whether unfortified and fortified cow’s milk influences micronutrient status in primary-school-aged children. In those consuming fortified versus unfortified cow’s milk, significant increases in blood riboflavin and vitamin B12 were observed, with all other analytes (selenium, ferritin and vitamin D) comparable between drinks(Reference Kuriyan, Thankachan and Selvam43). Additionally, significant increases in serum ferritin and zinc have been observed after 6 months with both fortified versus unfortified cow’s milk compared with a control drink(Reference Lien, Nhung and Khan45). With respect to nutritional status, dietary patterns characterised by high cow’s milk consumption (both plain and flavoured milk) resulted in greater intakes of energy, protein, phosphorus, magnesium, calcium, potassium, vitamin A, zinc, riboflavin (vitamin B2) and niacin compared with children who seldom consumed cow’s milk. Notably, diets high in cow’s milk may also limit intakes of foods and beverages high in fat and sugar. In studies of Dutch(Reference Campmans-Kuijpers, Singh-Povel and Steijns37) and American primary-school-aged children(Reference Fiorito, Marini and Mitchell46), for example, cow’s milk intake was inversely related with the intake of sugar-sweetened beverages. In these studies, children with low cow’s milk intakes had lower protein, fibre, calcium, magnesium, potassium and phosphorus intakes. Low cow’s milk consumption has significant implications for intakes of several key nutrients that are of importance in childhood. Based on the nutritional contribution to dietary intakes, it is important to note that children who drink cow’s milk regularly are more likely to meet dietary recommendations for many nutrients, and thus have a better nutritional status(Reference Rozenberg, Body and Bruyère13). It could therefore be argued that milk intake might be a marker for healthier eating habits(Reference Campmans-Kuijpers, Singh-Povel and Steijns37).
With the above in mind, in the UK, it should be noted that dietary intakes of vitamin D among primary-school-aged children are low. Considering UK cows’ milk is generally not a good source of vitamin D because it is not fortified, as it is in other countries, this narrative review may provide justification for UK policy makers to reconsider widespread fortification. There is evidence (albeit from a theoretical modelling perspective) from Northern Ireland that cows’ milk can be used as a successful vehicle for vitamin D fortification(Reference Weir, Johnston and Lowis47). Fortification of cows’ milks with 1 μg, 1·5 μg and 2·0 μg/100 g theoretically increased median vitamin D intakes from 2·0 μg/d to 4·2 μg/d, 5·1 μg/d and 5·9 μg/d, respectively and may therefore provide strong evidence for the efficacy of widespread fortification. This modelling appears to translate to human studies. In support of this, a recent review comprising 20 studies showed positive associations between the consumption of vitamin D fortified milk and 25(OH)D status in different population groups(Reference Itkonen, Erkkola and Lamberg-Allardt48). Furthermore, in Finland, Canada and the USA, who exercise a national vitamin D fortification policy (covering various fluid milk products), milk products contributed 28–63 % to vitamin D intake, while in countries without a fortification policy, or where the fortification covered only some dairy products (Sweden, Norway), the contribution was much lower or negligible(Reference Itkonen, Erkkola and Lamberg-Allardt48). Based on the above, widespread fortification of milk seems to help bolster vitamin D intakes and could be adopted by the UK to increase vitamin D intakes in primary-school children.
Concerns about added sugars in foods and beverages have increased recently. Accordingly, many schools have limited access to foods and beverages high in added sugars, including flavoured cow’s milk. In some studies, removal of flavoured cow’s milk from the school environment reduces energy and sugar intake, but negatively impacts essential nutrient intake and even reduced the overall intake of non-flavoured cow’s milk(Reference Hanks, Just and Wansink49,Reference Henry, Whiting and Phillips50) . In this sense, total milk consumption (both plain and flavoured) decreased by 12·3 %(Reference Henry, Whiting and Phillips50). Contrariwise, children who drink more flavoured cow’s milk generally have higher non-flavoured milk intakes and display nutritional intakes similar to children who only consume non-flavoured milk(Reference Fayet-Moore51). While greater cow’s milk consumption (both plain and flavoured) is generally associated with higher daily energy intake, we know with some confidence that this does not impact body mass or composition(Reference Dougkas, Barr and Reddy35). If daily energy intake is of concern for children predisposed to overweight and obesity, results of a recent study suggest that replacement of whole cow’s milk or semi-skimmed cow’s milk with skimmed cow’s milk may help reduce total energy intake, without impacting nutrient provision(Reference Rehm, Drewnowski and Monsivais52).
When taken together, these results suggest that cow’s milk consumption (both plain and flavoured) might serve as a useful strategy to boost nutritional status in primary-school-age children and may act as a surrogate marker of diet quality. There may be a need for widespread vitamin D fortification of cow’s milk, however, and based on the above seems justified and should be reviewed by UK policy makers. Cow’s milk is a readily available, accessible and affordable means of providing valuable essential nutrients to the diets of primary-school children. The nutritional implications of cow’s milk provision and/or removal in the school environment must be considered and is especially relevant in children suffering from nutritional inadequacies. The data are, however, primarily limited to observational investigations and require verification in more controlled intervention-based studies covering differing populations.
Hydration
Whole cow’s milk is approximately 87 % water(Reference Nagpal, Behare and Kumar1) and may therefore be a beneficial choice for hydration. For children, maintenance of euhydration is important for good health(Reference Popkin, D’Anci and Rosenberg53) but may also increase concentration and mental performance (cognitive function)(Reference D’Anci, Constant and Rosenberg54) while helping reduce instances of headaches, constipation and other disorders(Reference Popkin, D’Anci and Rosenberg53). This is important as children are at a greater risk of dehydration compared with adults, having relatively greater fluid losses at rest and during exercise and being less able to recognise thirst(Reference D’Anci, Constant and Rosenberg54). Given that water is continually being lost from the body water pool, it is important to constantly replenish fluid losses to prevent dehydration. Establishing beverages that encourage longer-term fluid retention and maintenance of euhydration has real clinical and practical implications(Reference Maughan, Watson and Cordery55). This is particularly true in situations where free access to fluids is limited or when frequent breaks for urination are not desirable(Reference Maughan, Watson and Cordery55), such as in in the school setting where many children arrive at school in an already hypo-hydrated state(Reference Bonnet, Lepicard and Cathrin56). There is little research concerning the impact of cow’s milk on hydration in primary-school-aged children (Table 3). To date, three studies have been conducted(Reference Montenegro-Bethancourt, Johner and Remer57–Reference Volterman, Obeid and Wilk59). Two of these were exercise-based intervention studies conducted by the same research group in Canada(Reference Volterman, Moore and Obeid58,Reference Volterman, Obeid and Wilk59) , and the remaining study was cross-sectional(Reference Montenegro-Bethancourt, Johner and Remer57). Nonetheless, all reported improved hydration status with cow’s milk in comparison with water or alternative beverages. While these findings show promise, additional controlled-intervention studies should be performed in different settings to examine repeatability of these effects.
In the two exercise-based intervention studies, researchers examined the influence of post-exercise cow’s milk consumption on rehydration in 7–11-year-old(Reference Volterman, Obeid and Wilk59) and 10–12-year-old children(Reference Volterman, Moore and Obeid58). The rehydration potential of cow’s milk (or milk protein) was compared with water or a carbohydrate–electrolyte drink or zero or low cow’s milk protein beverage, given at 100 % and 150 % of the children’s body mass losses following exercise, respectively. In both studies, children exercised in the heat (∼34·5°C) and consumed the test beverages immediately following exercise. The children were subsequently observed for a period of 2 h(Reference Volterman, Obeid and Wilk59) and 4 h(Reference Volterman, Moore and Obeid58), respectively, during which measures of hydration status were collected (urine output and fluid balance). Findings over 2 h showed cow’s milk was more effective than both water and the carbohydrate–electrolyte drink at replacing fluid loss during exercise(Reference Volterman, Obeid and Wilk59). This was similar over 4 h. The authors suggested that the protein in cow’s milk may be a factor responsible for its rehydrating properties(Reference Volterman, Moore and Obeid58).
Montenegro-Bethancourt and colleagues(Reference Montenegro-Bethancourt, Johner and Remer57) conducted a cross-sectional study designed initially to establish the contribution of fruit and vegetable intake on hydration status. Children (4–10 years old) recorded all food and beverages consumed over 3 d using a weighed food diary alongside 24 h urine samples. Regular intake of fruit and vegetables made a substantial contribution to hydration status, but notably, cow’s milk consumption was also a strong dietary predictor of hydration status. In particular, cow’s milk increased free water reserve by 25 ml in boys and 33 ml in girls per 100 g of intake. These findings corroborate studies in adult populations(Reference James60–Reference Shirreffs, Watson and Maughan62).
Based solely on adult studies, there are a number of potential mechanisms explaining the greater fluid retention (and thus hydration potential) with cow’s milk(Reference James, Stevenson and Rumbold63). Firstly, milk contains modest amounts of sodium (∼20 mmol/L, similar to most commercial sports drinks) and large amounts of potassium (∼40 mmol/L). Sodium, as the main cation in the extracellular fluid, plays a major role in fluid retention(Reference Maughan and Leiper64), whilst potassium may exert some beneficial effects(Reference Maughan, Owen and Shirreffs65), although this is not a consistent finding. Secondly, the protein content of cow’s milk may help facilitate greater fluid retention(Reference James, Clayton and Evans66,Reference James, Evans and Madin67) . While water and alternative beverages meet the basic intentions of rehydration, they do not offer the abundance of nutrients present in cow’s milk that will also aid in normal growth and maturation. Nevertheless, it should be considered that, while these findings suggest that cow’s milk helps improve the hydration status of children, there remains room for further studies to clarify the role of cow’s milk in hydration, especially in a free-living school setting. While the intervention-based studies show promise, many primary-school children do not exercise in such conditions.
Physical-Related Benefits of Cow’s Milk
Dental Health
Milk contains multiple nutrients that may offer anticariogenic properties, protecting against the development of dental caries, and thus supporting dental health in children(Reference Aimutis68,Reference Levine69) . The nutrients principally believed to play a role in dental health include calcium, phosphorus and protein(Reference Aimutis68–Reference Moynihan70). As reported earlier, cow’s milk intake has been shown to be inversely related with the intake of sugar-sweetened beverages(Reference Campmans-Kuijpers, Singh-Povel and Steijns37,Reference Fiorito, Marini and Mitchell46) . Although speculative, one could argue that increased cow’s milk intake might indirectly improve dental health. Calcium is required for bone and tooth formation, whereas phosphorus ions work alongside calcium to maintain tooth strength(Reference Moynihan70). Cow’s milk proteins (particularly αs1-, αs2- and ß-casein) may act to prevent tooth enamel erosion and demineralisation of the tooth surface by producing casein phosphopeptides(Reference Ferrazzano, Cantile and Quarto71). Based on the available evidence, inverse associations between cow’s milk intake and the incidence of tooth decay have frequently been reported(Reference Levine, Nugent and Rudolf72–Reference Curtis, VanBuren and Cavanaugh75) (Table 4). The evidence, however, has been derived from cross-sectional research, so causal conclusions cannot be justified and caution must be exercised.
Table 4. Studies in primary-school-aged children that measured dental health, bone health and physical stature with cow’s milk consumption

Abbreviations in order of appearance: DXA = dual energy X-ray absorptiometry; CaCO3 = calcium carbonate.
Table 5. Studies in primary-school-aged children that measured appetite and cognitive function with cow’s milk consumption
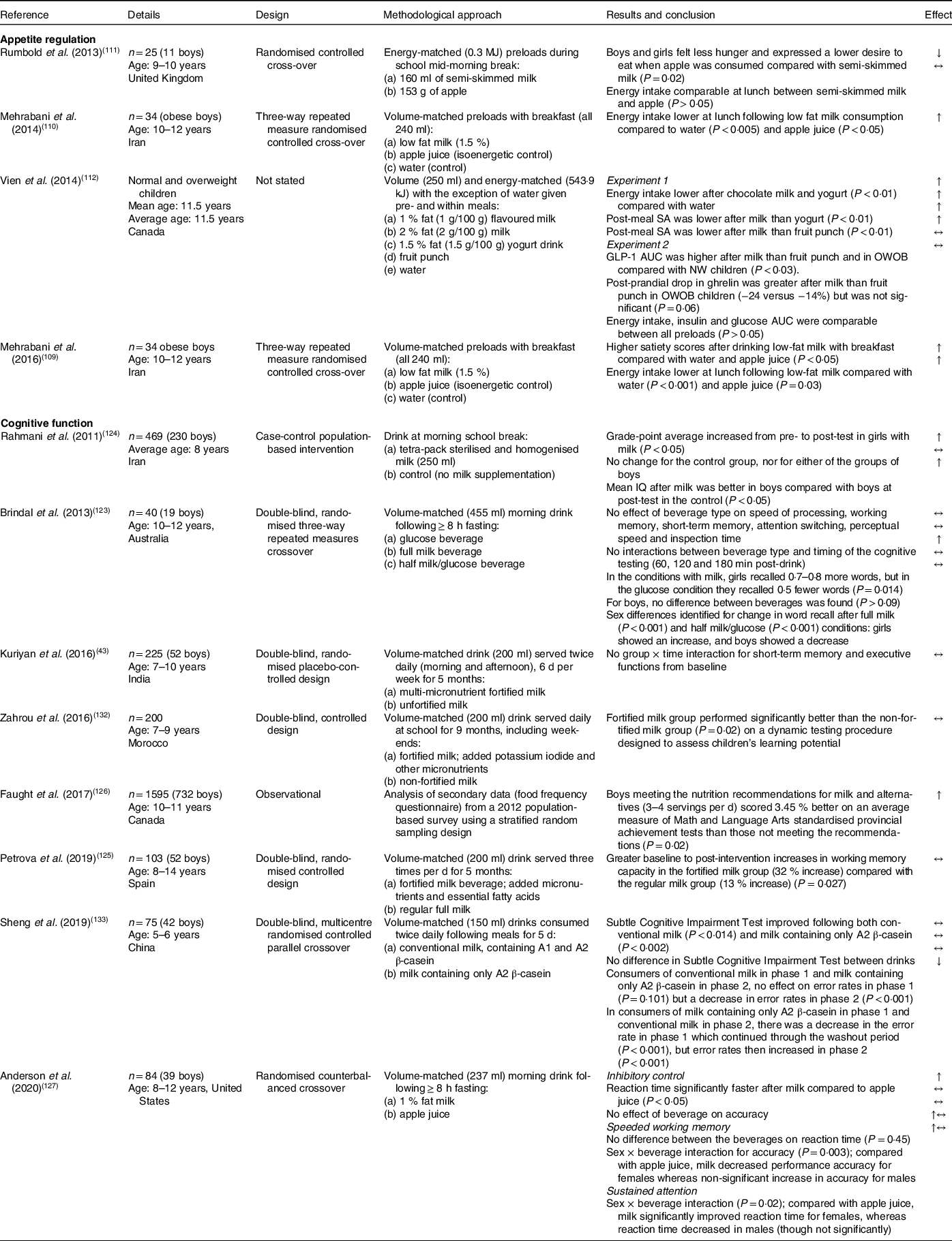
Abbreviations in order of appearance: SA = subjective appetite; GLP-1 = glucagon like peptide-1; OWOB = overweight and obese; NW = normal weight; AUC = area under the curve; IQ = intelligence quotient.
The findings of the cited studies were generated from recall methods which have obvious shortcomings. Nevertheless, all studies (following visual examinations accompanied with food frequency questionnaires) reported inverse associations with cow’s milk and the development of dental caries. Interestingly, though sugar is suggested to possess acidogenic and cariogenic potential(Reference Giacaman, Campos and Muñoz-Sandoval76), one study(Reference Petti, Simonetti and D’Arca74) reported that the association between cow’s milk consumption and protection against of dental caries was stronger for children with diets high in sucrose. This might suggest that cow’s milk offers protection against the harmful effects of sugar, though this is speculative. To this end, cow’s milk intake during primary-school years has been reported as a predictor of incidences of caries later in childhood(Reference Levine, Nugent and Rudolf72). In this sense, greater cow’s milk intake is inversely associated with indices of dental caries. Collectively, these studies support the suggestion that dietary habits established and maintained during primary-school years could have longer-term effects on health outcomes.
Although no controlled trials have been conducted in primary-school children, the available literature appears to suggest that cow’s milk could help reduce the incidence of dental caries and contribute to dental health in children. To reduce the occurrence of tooth decay, it is recommended that primary-school children limit their consumption of sugary beverages (especially when not consumed with a meal) and increase consumption of cow’s milk. The exact mechanism by which cow’s milk reduces the incidence of dental caries remains uncertain, though calcium, phosphate and casein phosphopeptides may all play a role(Reference Aimutis68–Reference Moynihan70). Casein phosphopeptides are phosphorylated casein-derived peptides produced by tryptic digestion of casein in the duodenum(Reference Ferrazzano, Cantile and Quarto71). The anticariogenic activity of casein phosphopeptides is due to their ability to stabilise high levels of amorphous calcium phosphate on tooth surface, preventing demineralisation and enhancing remineralisation of enamel caries(Reference Cross, Huq and Palamara77). In addition, milk fat could be adsorbed onto the enamel surface and may have a protective role. Thirdly, milk enzymes may have a role in reducing the growth of acidogenic plaque bacteria(Reference McDougall78). Where prior observational research provides a solid foundation, any future work should seek to implement robust randomised clinical trials (RCT) to confirm any causal relationships between cow’s milk consumption and dental health in primary-school-aged children.
Bone Health and Physical Stature
Childhood is a critical time for bone growth and lasting bone health(Reference Weaver79,Reference Wiley80) , and the nutritional composition of cow’s milk has evolved to stimulate and support this(Reference Ebringer, Ferenčík and Krajčovič2,Reference Rezaei, Wu and Hou5) . The scientific opinion that regular cow’s milk consumption is associated with greater physical stature has a long history, dating back to the 1920s(Reference Leighton and Clark81,Reference Orr82) . Cow’s milk contains multiple nutrients that may support childhood growth(Reference Wiley80) and lasting bone health(Reference Weaver79). The beneficial effects of cow’s milk consumption on bone health in children may include increased bone mineral content (BMC) and bone mineral density (BMD), characteristics necessary for the prevention of bone-related diseases later in life (osteoporosis). Evidence of these benefits, however, is equivocal, showing a beneficial-to-neutral effect of cow’s milk on these constructs(Reference Black, Williams and Jones25,Reference Rockell, Williams and Taylor29,Reference Bonjour, Carrie and Ferrari83–Reference Zhou, Hu and Ma88) . In this sense, of the cited studies, two reported greater total body BMC(Reference Du, Zhu and Trube85,Reference Zhou, Hu and Ma88) , while one reported no effect. One study reported no effect on total body BMD(Reference Iuliano-Burns, Wang and Evans87), yet three studies reported increased regional BMC(Reference Rockell, Williams and Taylor29,Reference Bonjour, Carrie and Ferrari83,Reference Goulding, Rockell and Black86) , and another two studies reported increased regional BMD(Reference Black, Williams and Jones25,Reference Chan, Hoffman and McMurry84) (Table 4). The mixed findings reported throughout these studies may be due to a lack of consistency in methodological approaches, length of study, location and measures of bone health, all of which confound comparisons and prevent a clear conclusion. Furthermore, age and sex differences must be considered, especially as puberty and bone mineralisation typically occurs earlier in girls compared with boys(Reference Feskanich, Bischoff-Ferrari and Frazier89).
In two recent studies(Reference Du, Zhu and Trube85,Reference Zhou, Hu and Ma88) , school milk interventions increased total body and regional (forearm) BMC and BMD compared with children who seldom drank cow’s milk. In these studies, the beneficial effects of cow’s milk on bone health were observed in n = 435–757 children (mean age 11 years) following daily school milk intake for 1–2 years.
On a comparative basis with other animal sources, whole cow’s milk is the richest source of calcium and represents the biggest contributor of dietary calcium during childhood(Reference Sunyecz90). In addition, considering beef and eggs for example, cow’s milk is the cheapest source of protein, calcium, phosphorus and vitamin D(Reference Gaucheron91). During childhood, the beneficial effects of cow’s milk on bone health in children are commonly attributed to calcium(Reference Caroli, Poli and Ricotta92). However, many other nutrients, including phosphorus and protein, are needed for normal growth and lasting bone health(Reference Rizzoli93). In 5–11-year-old children (n = 99), for example, calcium supplementation for 10 months did not influence total body or regional BMC(Reference Iuliano-Burns, Wang and Evans87). In contrast, in an earlier study where cow’s milk (and dairy) was supplemented daily for 1 year (distributed to deliver 1200 mg calcium daily), lumbar (lower back) BMD increased compared with children who maintained their usual eating habits(Reference Chan, Hoffman and McMurry84). This may illustrate that calcium and other nutrients work together for bone growth and, thus, lasting bone health(Reference Rizzoli93). It is important to note, however, that children in the calcium supplementation study were already consuming near daily recommended amounts, which may illustrate that intakes exceeding calcium recommendations (from either supplemental calcium or cow’s milk) offer no further benefit to bone health in children.
In several studies, it has been observed that children who avoid consuming cow’s milk characteristically exhibit low calcium intakes, short statures, increased fatness and lower BMC (and thus exhibit reduced bone health) compared with their cow’s milk-drinking counterparts(Reference Black, Williams and Jones25,Reference Goulding, Rockell and Black86) . In children (mean age 8 years) who previously avoided cow’s milk, the introduction of cow’s milk to the diet increased not only habitual cow’s milk consumption but also increased total body BMC(Reference Rockell, Williams and Taylor29). This may suggest it is never too late for children to introduce cow’s milk into their diet for bone health benefits.
From a stature perspective, available data appear to suggest that cow’s milk consumption almost certainly has a positive effect on growth in children. To date, 14 studies in primary-school-aged children have evaluated this aspect of cow’s milk consumption(Reference Black, Williams and Jones25,Reference Rockell, Williams and Taylor29,Reference Cook, Irwig and Chinn36,Reference Albala, Ebbeling and Cifuentes44,Reference Leighton and Clark81–Reference Bonjour, Carrie and Ferrari83,Reference Du, Zhu and Trube85,Reference Baker, Elwood and Hughes94–Reference Wiley99) . Seven were intervention-based, four were observational and the remaining three were prospective designs. Based on these studies, the evidence suggests that cow’s milk intake positively influences physical stature in primary-school-aged children. All six intervention studies showed increased stature with increased cow’s milk (one study included milk calcium). In addition, all prospective studies reported increased stature with increased cow’s milk. With regard to the cross-sectional studies, two illustrated that cow’s milk avoiders displayed stunted growth compared with cow’s milk drinkers, while the remaining study showed increased adult stature with increased cow’s milk consumption in childhood. Although trends are consistent across studies, there remains a need for evidence from robust controlled-intervention trials in primary-school-aged children to verify causality.
Beneficial effects on physical stature were observed with both whole- and reduced-fat cow’s milk, distributed at a range of 190–568 ml daily. In several of these studies, it was reported that childhood cow’s milk intake was associated with higher skeletal development (BMD of the hip and the forearm), bone growth and periosteal bone expansion. These were likely established earlier during growth periods and maintained into late adolescence and young adulthood(Reference Matkovic, Landoll and Badenhop-Stevens96–Reference Wiley99), supporting the notion that dietary habits established and maintained during the primary- and secondary-school years may not only induce short-term effects but offer lasting benefits. Indeed, in children with prolonged cow’s milk avoidance, stunted growth and physical stature is observed compared with children who habitually consume cow’s milk, and this is maintained into adulthood(Reference Black, Williams and Jones25,Reference Rockell, Williams and Taylor29,Reference Goulding, Rockell and Black86) . During a pubertal growth spurt, about 37 % of the entire skeletal mass is accumulated(Reference Matkovic, Jelic and Wardlaw100). Therefore, inadequate calcium intake during this period may compromise volumetric bone density and overall stature attained. Notably, in a study that explored both cow’s milk and supplemental calcium, those children in the cow’s milk (and dairy) group were 3 cm taller (166 cm) compared with the supplemental calcium (163 cm) and placebo (163 cm) group(Reference Matkovic, Landoll and Badenhop-Stevens96). This may indicate that cow’s milk has more of a beneficial effect on physical stature and growth than single cow’s milk constituents (i.e. calcium).
The precise mechanisms or nutrients in cow’s milk responsible for stimulating growth and lasting bone health are not yet clear. Evidence suggests that maintaining adequate calcium intake during childhood might be advantageous for the attainment of peak bone mass, which may be crucial in reducing the risk of bone-related diseases later in life(Reference Luiza Loro, Sayre and Roe101). Interestingly, it appears that whole foods may offer greater benefits than the equivalent amount of calcium in supplemental form. It has also been suggested that the growth-stimulating effect of cow’s milk is likely attributed to hormonal effects that can be influenced by ingested cow’s milk proteins (predominantly whey protein and the release of amino acids during digestion but also casein), micronutrients and also energy(Reference Hoppe, Mølgaard and Vaag102–Reference Rogers, Emmett and Gunnell104). Observations from child studies show that these nutrients stimulate the secretion of insulin-like growth factor-1 and insulin, both of which are anabolic hormones that play an essential role in the regulation of growth and accrual of bone mass during childhood(Reference Hoppe, Mølgaard and Vaag102–Reference Rogers, Emmett and Gunnell104), though there is some controversy that cow’s milk intake upregulates insulin and insulin-like growth factor-1 signalling and thus promotes chronic diseases such as cancer (prostate, breast and colorectal) and cardiovascular disease(Reference Melnik9), though these perceptions are hypothetical at present and not supported by evidence.
Nonetheless, when taken together, it appears that cow’s milk consumption promotes health and may increase physical stature in primary-school-aged children. This suggests that cow’s milk consumption during childhood might be important to ensure full growth potential is achieved. While there is some suggestion about the improved bone health with increased cow’s milk consumption and the mechanisms responsible for the growth-stimulating properties of cow’s milk, more intervention studies are needed to elucidate the components responsible for these effects and to prove and/or disprove the chronic disease hypotheses. This is especially prudent when considering bone health, as the methodological approaches previously employed have been diverse in nature.
Health-Related Benefits of Cow’s Milk
Appetite Regulation
Appetite comprises numerous regulatory processes associated with the initiation and termination of eating and the selection and amount of food consumed. The regulation of appetite and eating behaviour depend on the detection and integration of signals relaying nutritional status, and their interaction with signals associated with food palatability and gastrointestinal handling, in addition to circadian, social, emotional, habitual and other situational influences(Reference Woods, Lutz and Geary105). Consequently, appetite and the regulation of eating behaviour are complex processes, regulated by homeostatic and non-homeostatic influences(Reference Berthoud106). Cow’s milk contains a host of nutrients that might exert a favourable effect on appetite and eating behaviour(Reference Aziz, Anderson and Saarela107), yet in primary-school children there is limited evidence concerning the short-term effect of cow’s milk on these measures. There are also no data on the moderate- and longer-term effects of daily cow’s milk consumption on these outcomes in primary-school children.
From the available studies(Reference Kavezade, Mozaffari-Khosravi and Aflatoonian108–Reference Vien, Patel and Panahi112), cow’s milk has principally been given as a mid-morning snack or alongside breakfast, with the volume of cow’s milk offered to children ranging from 160 ml to 250 ml. Based on these studies, the evidence concerning cow’s milk and appetite regulation is inconclusive. Three of these studies found a decrease in energy intake after cow’s milk consumption, yet three reported no effect. Two studies reported that cow’s milk consumption reduced subjective appetite, and one reported increased subjective appetite compared with a fruit-based snack, while two studies did not measure subjective appetite. Only one study measured hormonal indicators of appetite and reported that cow’s milk consumption stimulated the secretion of glucagon-like peptide-1.
In two studies of 34 overweight and obese boys (mean age 11 years)(Reference Mehrabani, Safavi and Mehrabani109,Reference Mehrabani, Salehi-Abargouei and Asemi110) , when compared with volume- and energy-matched servings of water or fruit juice, 240 ml of low-fat cow’s milk with breakfast reduced energy intake at an ad libitum lunchtime meal. In another study comprising 48 obese children (mean age 11 years), girls reported higher satiety scores 4 h after drinking whole cow’s milk compared with skimmed milk, and low-fat cow’s milk significantly reduced appetite compared with apple juice 2 h after consumption. These differences, however, did not translate to changes in energy intake at an ad libitum lunchtime meal across all conditions in girls, boys and the group as a whole(Reference Kavezade, Mozaffari-Khosravi and Aflatoonian108). As mentioned, only one investigation (comprising two experiments) has sought to establish the effect of cow’s milk (and other dairy products) on appetite and feeding behaviour in normal weight and overweight/obese children (mean age 11.5 years), where subjective appetite and appetite-related analytes were measured(Reference Vien, Patel and Panahi112). In both experiments, preloads (experiment 1: 1 % fat (1 g/100 g) chocolate cow’s milk, 2 % (2 g/100 g) fat cow’s milk, 1.5 % (1.5 g/100 g) fat yogurt drink, fruit punch or a water drink; experiment 2: 2 % (2 g/100 g) fat cow’s milk or a fruit punch) were provided 60 min preceding and during an ad libitum pizza meal. All preloads were matched for volume (250 ml) and energy content (543·9 kJ; except water in experiment 1). The first experiment comprised measures of subjective appetite, whereas the second experiment included measures of subjective appetite together with appetite-related analytes (serum glucose, insulin and plasma GLP-1 and peptide YY). Reduced energy intake was observed following chocolate cow’s milk and yogurt consumption compared with a water drink in the first experiment. Consistent with a reduction in energy intake, subjective appetite (combined appetite score) was significantly lower following 2 % (2 g/100 g) fat cow’s milk compared with the yogurt drink only. No additional effects were observed concerning energy intake following the consumption of 2 % (2 g/100 g) fat cow’s milk and fruit punch or on subjective measures of appetite after 1 % fat chocolate cow’s milk, 1.5 % (1.5 g/100 g) fat yogurt drink, fruit punch or water. Compared with the fruit punch preload, cow’s milk consumption resulted in a significantly greater GLP-1 area under the curve. Nonetheless, ad libitum energy intake, insulin and glucose AUC were comparable between trials. Considering all aforementioned studies, it is important to consider that, in these studies, energy intake was principally assessed via ad libitum assessments which may not be reflective of free-living eating behaviour.
The mechanism(s) by which cow’s milk consumption might influence eating behaviour are not fully understood, but there are several plausible suggestions from the adult literature and constituents of cow’s milk that may act synergistically to explain possible actions. In the studies where cow’s milk reduced appetite and subsequent eating behaviour, it is probably unsurprising that cow’s milk consumption suppressed energy intake at ad libitum assessment meals, considering the macronutrient composition of cow’s milk compared with fruit-juice drinks and water. Cow’s milk contains considerably more protein than fruit-juice drinks and water. Although it is not a universal finding, it is widely recognised that dietary proteins are more satiating than energetic equivalents of carbohydrate and fat under most conditions, commonly suppressing eating behaviour at the next meal(Reference Anderson and Moore113–Reference Rolls, Hetherington and Burley115). Consequently, cow’s milk proteins (whey and casein, and their products of digestion) may act to potentiate peptides of gastrointestinal, pancreatic and adipose tissue origin, increasing perceptions of satiety and acutely reducing energy intake (anorexigenic behaviours)(Reference Anderson and Moore113). Moreover, medium-chain triglycerides, conjugated linoleic acid (CLA) and lactose may also be implicated in the reduction of energy intake after cow’s milk intake(Reference Aziz, Anderson and Saarela107). Medium-chain triglycerides are absorbed directly into the portal circulation and transported to the liver for rapid oxidation. A combined action of increased energy expenditure, decreased fat deposition and increased satiety may reduce of energy intake via pre-absorptive signals, post-absorptive changes in metabolites and appetite-related analytes(Reference Aziz, Anderson and Saarela107), and similar appetite-related analyte responses have been observed with lactose(Reference Bowen, Noakes and Trenerry116). When considering CLA intake and its potential implications in appetite regulation, cow’s milk (and dairy) and cattle meat (cows and lamb) represent the almost exclusive dietary sources(Reference Terpstra117) where the predominant isomer of CLA (accounting for more than 90 % of the total CLA intake) is cis-9,trans-11-CLA. It is, however, strongly proposed that other isomers, such as trans-10, cis-12-CLA, may influence body-weight and fat changes(Reference Li, Huang and Xie118). In agreement with an earlier narrative review(Reference Dougkas, Reynolds and Givens34), it remains unknown whether cow’s milk (and dairy), when providing physiological doses of CLA, can elicit any meaningful impact on appetite and body-weight regulation in humans. This is especially prudent when considering experimental design, age, sex, energy intake and CLA metabolism of the participants, and the dose and chemical form of the CLA isomer administered, as differences may arise solely from these methodological differences(Reference Plourde, Jew and Cunnane119). From a child perspective, potential underlying mechanisms of CLA on appetite regulation are poorly understood, though evidence from adult studies suggests that CLA can inhibit fatty acid synthase and stearoyl-CoA desaturase-1(Reference Li, Huang and Xie118), enhance fat oxidation and thermogenesis and reduce lipogenesis and preadipocyte differentiation and proliferation(Reference Wang and Jones120).
In summary, evidence suggests that cow’s milk may have a unique potential to influence elements of energy balance. Macro- and micronutrients and other bioactive constituents might act individually, though probably synergistically, to impart beneficial effects on energy balance and body mass regulation through actions related to appetite, eating behaviour and metabolism. However, there is little mechanistic exploration of cow’s milk consumption and appetite regulation from a paediatric perspective. Understanding the relationship between cow’s milk consumption and appetite regulation could provide novel nutritional interventions to contribute toward the fight against childhood overweight and obesity(Reference Jo121), whilst bolstering nutritional status and improving elements of cognitive function and hydration. Controlled intervention studies are necessary to determine the best possible timings to administer cow’s milk and establish whether consumption delivers these benefits when administered during the school day.
Collectively, the effects of cow’s milk intake on appetite regulation in primary-school-aged children are unclear and could be dependent on BMI. The studies summarised suggest that mid-morning milk consumption influences eating behaviour at the next meal in overweight and obese children, showing that cow’s milk could be beneficial for reducing body mass. In lean children, the evidence suggests there is no effect of cow’s milk consumption on appetite and eating behaviour, but cow’s milk may boost the nutritional quality of the diet. There is much scope for further studies to clarify the role of cow’s milk on appetite and eating behaviour, especially in a free-living school environment where methodological approaches are more reflective of habitual eating behaviours. In addition, it will be important to fully distinguish the effects of cow’s milk on appetite- and on metabolism-related peptides and subsequent eating behaviour.
Cognitive Function
Compared with children with better dietary quality, those with nutritional inadequacies demonstrate decreased attention and academic performance(Reference Florence, Asbridge and Veugelers122). Aside from improving nutritional status and dietary quality, emerging evidence illustrates that cow’s milk may positively influence cognitive function in primary-school children(Reference Kuriyan, Thankachan and Selvam43,Reference Brindal, Baird and Slater123,Reference Rahmani, Djazayery and Habibi124) . Varying in duration from 2 h to 9 months, studies of an intervention-based nature generally report that cow’s milk consumption increases elements of cognitive function, though some of the specific outcome measures demonstrate no effect or, in some cases, a negative effect of cow’s milk. These studies highlight that consideration should be given to potential moderators such as sex and to the use of cow’s milk as a non-stigmatised method for providing nutrients through fortification(Reference Petrova, Bernabeu Litrán and García-Mármol125). One non-interventional study considered the adherence of n = 1595 children to Canadian nutrition recommendations in Grade 5 (10–11 years) and in relation to academic achievement in the provincial achievement tests taken approximately 1 year later(Reference Faught, Montemurro and Storey126). A positive association was identified for boys, with those who met the nutrition recommendations for milk and alternatives (at the time of the study: 3–4 servings/d) scoring 3.45 % better on an average measure of Math and Language Arts tests than those who did not meet the recommendations(Reference Faught, Montemurro and Storey126). An account of the intervention studies follows, but overall, the varying methodological approaches used suggest caution is needed in making firm conclusions about potential links between cow’s milk consumption and improved cognitive function.
In a study(Reference Rahmani, Djazayery and Habibi124) involving n = 469 boys and girls (mean age 8 years), evaluating the effects of daily mid-morning cow’s milk consumption on physical, mental and school performance, researchers found that a school feeding scheme focusing on daily cow’s milk intake had beneficial effects on school performance for girls. In this study, children received a serving of cow’s milk (250 ml) daily for 12 weeks. Assessments of physical, mental and school performance were conducted prior to and at the end of the 12-week supplementation period. Similarly, in a smaller study(Reference Brindal, Baird and Slater123) involving 40 children (mean age 11 years), the effects of a carbohydrate drink, a cow’s milk drink or a combination of both on subsequent cognitive function (processing speed, memory, attention and perceptual speed) were assessed over a 3 h period(Reference Brindal, Baird and Slater123). Findings showed cow’s milk consumption improved short-term memory. Children were able to recall 0·7–0·8 more words compared with 0·5 fewer words after the carbohydrate drink. However, this effect was only observed in girls and not boys(Reference Brindal, Baird and Slater123,Reference Rahmani, Djazayery and Habibi124) . There were slightly more mixed findings from a crossover study in which 84 children (mean age 10 years) were given 237 ml of cow’s milk or apple juice(Reference Anderson, Gunstad and Updegraff127). While the beverages were not standardised for temperature, participants were asked to complete computerised tasks of inhibitory control, speeded working memory and sustained attention at baseline and 30 min, 90 min and 120 min following beverage consumption. Although the significant results following cow’s milk compared with juice consumption were, again, only apparent in girls, there was a negative effect (decreased working memory accuracy) alongside the positive one (improved reaction time on the sustained attention task). There were non-significant trends in the opposite direction for boys. No significant effects of the beverages were observed for on-task behaviour during the testing.
The mechanisms responsible for the beneficial effects of cow’s milk on improved cognitive function are unclear. One suggestion is a sustained blood glucose response following consumption(Reference Amiel, Pottinger and Archibald128,Reference Gold, MacLeod and Thomson129) . Findings from Anderson et al. (Reference Anderson, Gunstad and Updegraff127), an adult-based study, suggest that glucoregulation may play a role, as participants with higher fasting glucose levels demonstrated faster reaction times on an inhibition task following cow’s milk compared with juice. There were, however, no sex differences in initial glucose elevation or change in glucose levels over time to explain the apparent sex differences in cognition. Such findings suggest a likelihood that factors other than glucoregulation explain why cow’s milk differentially affects the cognition and behaviour of boys and girls. The micronutrient content of cow’s milk is another potential mechanism for the observed effects(Reference Kuriyan, Thankachan and Selvam43). Low intakes of vitamin B1, folate and vitamin B12 affect short-term memory and impair learning, causing low cognitive scores and development in primary-school-age children(Reference Vaz, Pauline and Unni130). In addition, low iron and riboflavin intake may adversely affect motor skill development and psychomotor performance(Reference Swaminathan, Edward and Kurpad131). All of these micronutrients are heavily present in cow’s milk. In one of the longer intervention-based studies available (5 months), Kuriyan and colleagues(Reference Kuriyan, Thankachan and Selvam43) attempted to establish if fortification of cow’s milk with multiple micronutrients influenced the mental and physical performance of children (7–10 years) compared with an unfortified cow’s milk drink. The children were randomised into groups and were provided with cow’s milk (2 × 200 ml) 6 d per week for 5 months, with assessments of attention and executive function conducted at baseline and 5 months. The findings showed improved cognitive and physical performance in both groups, illustrating that further fortification of cow’s milk provided no additional benefits to cognitive and physical performance but did improve some elements of nutritional status(Reference LaRowe, Moeller and Adams39). Finally, in a double-blind RCT (9 months), the learning potential of 7–9-year-olds (31.9 % with moderate or severe iodine deficiency at baseline) improved to a greater degree following 200 ml daily of fortified cow’s milk [fortified with 45 μg iodine (given as potassium iodide) and other micronutrients] than following non-fortified milk (20·8 μg iodine)(Reference Zahrou, Azlaf and El Menchawy132).
Some of the components of cow’s milk may be detrimental for the cognitive performance of lactase-deficient children. In a study involving children of 5–6 years (85 % lactase deficient), information processing efficiency was assessed after 5 d consumption of 150 ml twice daily of conventional cow’s milk (containing A1 and A2 β-casein) or cow’s milk containing A2 β-casein only(Reference Sheng, Li and Ni133). While post-intervention response times were significantly improved from baseline for both cow’s milk products, error rates decreased in the A2 β-casein only condition. Furthermore, consuming conventional cow’s milk in the second phase of the crossover study appeared to undo the positive effects on error rate obtained from the A2 β-casein only milk in the first phase, which were maintained through the 9 d washout period.
It is prudent to highlight that none of the studies of cognition in primary-school children have compared cow’s milk with a control beverage. It is therefore difficult to ascertain whether these studies simply show less detrimental effects of cow’s milk than comparator beverages. Furthermore, where no comparators have been employed, this could reflect practice effects. Nonetheless, based on the published studies, and bearing in mind the varying methodological approaches employed, it is difficult to ascertain the role of cow’s milk in cognitive function for primary-school-aged children, but it may at the very least be a useful conduit for nutrient fortification. This could be particularly meaningful within the school environment. The intervention studies did not measure academic achievement directly, but their findings have relevant scholastic implications that warrant further investigation using RCTs to establish if increased milk consumption influences academic achievement in primary-school-aged children, especially given the identification of a positive association for boys between academic achievement and meeting the recommendations for consumption of milk and alternatives(Reference Faught, Montemurro and Storey126).
Future Directions and Conclusions
The aim of this narrative review was to evaluate evidence for the potential nutritional-, physical- and health-related benefits of cow’s milk consumption for primary-school-aged children (4–11 years). Cow’s milk consumption (both plain and flavoured) improves nutritional status without adversely impacting body mass and body composition(Reference Dougkas, Barr and Reddy35). With some confidence, cow’s milk also appears beneficial for hydration, dental and bone health and to have a beneficial-to-neutral effect on physical stature and appetite. Due to conflicting studies, reaching a conclusion has proven difficult concerning cow’s milk and cognitive function; therefore, a level of caution should be exercised when interpreting these results. All areas, however, would benefit from further robust investigation, especially in free-living school settings, to verify conclusions. Improving elements of cognitive function, hydration and appetite regulation could have important long-term health and scholastic implications that should certainly be explored further. Indeed, recent research involving adolescent populations illustrates that high intakes of cow’s milk are positively associated with academic performance(Reference Kim, Kim and Kang134) and increased motivation for learning(Reference Kim, Kim and Kang134) and impact favourably on eating behaviour following acute and chronic consumption(Reference Green, Stevenson and Rumbold135).
Despite a growing body of scientific literature exploring the potential benefits of cow’s milk consumption, there are several pertinent knowledge gaps that would benefit from further investigation. This is particularly true for cognitive function, hydration status and appetite regulation, where there is little research available, especially in free-living school settings. Further research, especially of a robust and methodologically sound nature (such as double-blind randomised controlled trials), should seek to explore the mechanisms responsible for any effects observed. At present, few studies on this population have attempted to establish mechanisms. Most purported mechanisms given throughout this narrative review come from adult-specific data and should therefore be interpreted with some caution, as the observations cited may not always be replicated in children. When working with child populations, there are various considerations that must be accounted for. The methodological approaches deemed most appropriate for the study of cognitive function, hydration status and appetite regulation in children will differ according to the objective of the study, type of data required and resources available(Reference Gibbons, Finlayson and Dalton136). In children, it is of great importance to adopt approaches that are non-invasive with a low level of participant stress. Current techniques available to assess cognitive function, hydration status and appetite are arguably invasive, elicit a moderate level of participant stress and have increased ethical risk. This might explain the current lack of studies and mechanistic insight from a child perspective. In recent years, however, research groups have been pursuing non-invasive techniques that offer an opportunity to conduct comprehensive mechanistic work in vulnerable populations. For example, developments in appetite and metabolism research have identified fingertip-capillary blood sampling as an efficacious, comparable and reproducible alternative to antecubital-venous blood sampling for the quantification of appetite-related peptides(Reference Allsop, Rumbold and Green137,Reference Green, Gonzalez and Thomas138) . These developments will certainly help provide a better understanding of mechanisms that influence appetite and eating behaviour in younger populations, where traditional methods of venous blood sampling might be contraindicated. Furthermore, fingertip-capillary blood sampling offers many advantages, including simplistic application, cost/time effectiveness and reduced volume of blood required for analysis(Reference Dayre McNally, Matheson and Sankaran139).
Considering the nutritional-, physical- and health-related impact of cow’s milk avoidance, the evidence begins to highlight the importance of increasing cow’s milk consumption. Cow’s milk is a naturally nutrient-dense foodstuff, providing a significant contribution of several essential nutrients and bioactive constituents that potentially impact human health favourably. Establishing and shaping healthy eating behaviours during the primary-school years is vital. Dietary behaviours shaped throughout the childhood years progress through to adolescence and adulthood(Reference Lake, Mathers and Rugg-Gunn140), making healthy eating environments crucial. For primary-school-aged children, the school setting may be an ideal environment to promote cow’s milk consumption, and school milk schemes are a great place to start developing healthy eating behaviours, given 35–40 % of children’s daily nutritional needs are met at school(Reference Gortmaker, Peterson and Wiecha141). Cow’s milk is a readily available, accessible and affordable means of providing valuable essential nutrients to the diets of primary-school children. Based on the evidence presented in this manuscript, there appears no reason for primary-school children to limit cow’s milk consumption, and there may, in fact, be many potential benefits to milk consumption.
Author Contributions
Benjamin Green and Penny Rumbold conceived and planned this narrative review. Penny Rumbold, Nicola McCullogh, Ruth Boldon and Benjamin Green sourced the relevant literature. All authors contributed to the writing and critically reviewed and approved the final manuscript.
Financial Support
This work was supported by funding from Cool Milk Ltd. The funding sponsors had no role in the design, collection, analysis or study interpretation, in the writing of the manuscript, and in the decision to publish the results.
Conflicts of Interest
Penny Rumbold has previously received funding from The Dairy Council (UK), Nourishmenow and Cool Milk Ltd. Benjamin Green has previously been supported by The Dairy Council (UK) in the award of a PhD studentship and has received funding from Cool Milk Ltd and is presently an employee of Danone Specialised Nutrition. Lewis James has previously received funding from Volac International Ltd. Emma Stevenson has previously received funding from The Dairy Council (UK) and Arla Food Ingredients. Collectively, the above (with the exception of Cool Milk Ltd) was not related in any way to the work presented in this article. Nicola McCullogh, Ruth Boldon and Crystal Haskell-Ramsay declare no conflicts of interest.








