Introduction
Nausea and vomiting of pregnancy (NVP) are normal, albeit unpleasant, symptoms of early pregnancy that appear on a spectrum of severity. At the extreme end of that spectrum is the complication hyperemesis gravidarum (HG) with symptoms so severe it can lead to weight loss, dehydration, poor quality of life and, without treatment, life-threatening complications(Reference Dean, Shemar, Ostrowski and Painter1). Indeed, HG was a common cause of maternal mortality until the 1950s when intravenous (IV) hydration was introduced(Reference Fejz, o, MacGibbon and Mullin2). Despite affecting 1–2 % of pregnancies globally(Reference Einarson, Piwko and Koren3) the aetiology remains unclear and is likely to be multifactorial. Recent research has implicated appetite genes GDF15 and IGFBP7 as likely culprits(Reference Fejzo, Sazonova, Sathirapongsasuti, Hallgrímsdóttir and Vacic4) and builds on previous research suggesting a predominantly genetic aetiology(Reference Zhang, Cantor and MacGibbon5). A personal or family history of HG is thought to be the strongest risk factor, and recurrence in subsequent pregnancies is common(Reference Dean, Bruin and O’Hara6). Carrying a female foetus or having a multiple pregnancy may also increase risk of HG(Reference Fiaschi, Nelson-Piercy and Tata7).
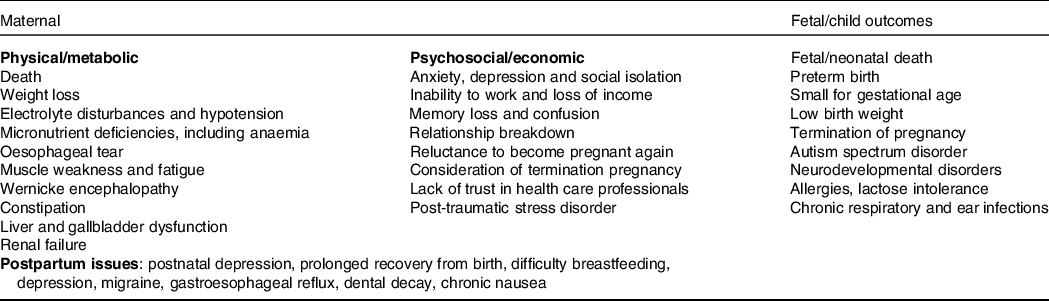
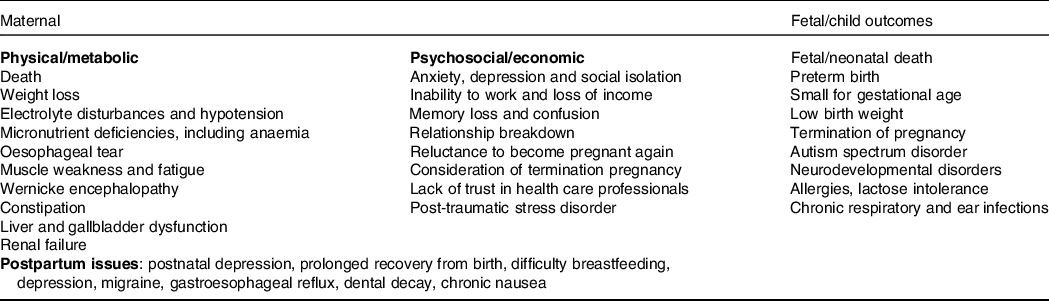
Although historical theories regarding psychosocial causes are still responsible for stigmas and mismanagement(Reference Dean8), they have now been widely debunked(Reference Fejzo and MacGibbon9), and it is well established from a systematic review of fifty-nine studies that depression and/or anxiety, while strongly associated with HG, is a consequence rather than a cause(Reference Mitchell-Jones, Gallos, Farren, Tobias, Bottomley and Bourne10). The historical belief that HG is self-limiting and does not have long-term consequences was incorrect(Reference Fejzo, Trovik and Grooten11). The wide-ranging physical and psychosocial adverse consequences of HG are summarised in Table 1. These include severe weight loss, which can be >15 % of pre-pregnancy weight(Reference Fejzo, Poursharif and Korst12), post-traumatic stress syndrome, relationship breakdown(Reference Christodoulou-Smith, Gold and Romero13), long periods off work(Reference Mitchell-Jones, Lawson and Bobdiwala14,Reference Trovik and Vikanes15) , elective termination of pregnancy(Reference Poursharif, Korst, Macgibbon, Fejzo, Romero and Goodwin16) and reduced willingness to become pregnant again in the future(Reference Heitmann, Nordeng, Havnen, Solheimsnes and Holst17).
Table 1. Summary of potential adverse outcomes of hyperemesis gravidarum
Hyperemesis gravidarum has typically been a condition that has been under-researched. By way of example, although it has a similar prevalence to type 1 diabetes in pregnancy, it receives significantly less research funding, despite recognition that it causes maternal malnutrition with direct effects on the unborn child. In recent years, there has been growing momentum for the need to prioritise nutrition research in the management of HG. A James Lind Alliance Priority Setting Partnership exercise published this year highlighted the importance of furthering our understanding of nutritional aspects of HG by clinicians, researchers and patients(Reference Dean, Bierma and Clarke18). Additionally, an international consensus document(Reference Jansen, Koot and Van’t Hooft19) emphasised the importance of consistent outcome reporting in HG, specifying that food and fluid intake, weight, maternal wellbeing and perinatal outcomes are to be included in future studies. The present review aims to comprehensively summarise and critically appraise the current literature regarding nutritional implications and management of HG in developed countries. The review will not include the topics of mild-to-moderate nausea and vomiting in pregnancy (NVP) or herbal/alternative therapies for the management of HG, which are considered outside the remit.
Diagnosis and screening
There is no distinct point at which NVP becomes HG. A lack of diagnostic criteria has led to challenges within research as well as in the management of HG and access to treatment(Reference Grooten, Roseboom and Painter20). Clinical guidance documents from the United Kingdom(21) and the United States(22) both include ‘persistent vomiting in pregnancy in the absence of other causes’ as required criteria, with additional criteria of ‘>5 % weight loss and electrolyte imbalance’. A systematic review of definitions of HG used in trials identified eleven different definition items(Reference Koot, Boelig and Van’t Hooft23), with vomiting, nausea and gestational age at onset of symptoms being the most common. Symptom severity, ketonuria and need for hospital treatment were also commonly used.
Patient history to rule out other potential causes, and assessment of clinical presentation to look for signs of dehydration and/or malnutrition, is required. Assessment of the impact symptoms are having on quality of life and mental health should be assessed(Reference Dean, Shemar, Ostrowski and Painter1). The Pregnancy Unique Quantification of Emesis (PUQE) score is a validated tool for assessing the severity of NVP(Reference Koren, Boskovic, Hard, Maltepe, Navioz and Einarson24). It includes questions on the duration of nausea, the number of vomiting episodes, occurrence of retching and overall quality of life. Symptoms during the past 24 h yield a summary score from 3 to 15; the higher the score the more severe the NVP symptoms. However, it has not been validated as a diagnostic tool for HG and does not consider aspects such as nutritional intake, medication or urination frequency. More recently, an HG-specific tool, the HyperEmesis Level Prediction score, was found to perform better than PUQE in identifying patients with severe symptoms requiring intervention(Reference MacGibbon, Kim, Mullin and Fejzo25).
Effects of HG on offspring
Direct short- and long-term effects of HG on the offspring have been widely reported in the literature and are summarised in Table 1. The largest cohort study to date, which analysed >8 million pregnancies over 15 years in England found that women with HG had a higher risk of preterm birth and babies born small for gestational age(Reference Fiaschi, Nelson-Piercy, Gibson, Szatkowski and Tata26), with a systematic review of twenty-four studies finding similar results(Reference Veenendaal, van Abeelen, Painter, van der Post and Roseboom27). A population-based study of 2·2 million births in Norway, of which 20 004 women were reported to have HG, found babies exposed to HG had reduced birth weight and gestational length(Reference Vandraas, Vikanes, Vangen, Magnus, Stoer and Grjibovski28). The study also found an association with perinatal death, when exploring data from 1967 to 2009; however, authors suggest interpreting this with caution, as the finding was not replicated when examining a subsample of infants born between 1999 and 2009, when different disease classification systems were used.
Recently published research reports that offspring born to women with HG are at increased risk of having developmental delay(Reference Fejzo, Magtira, Schoenberg, Macgibbon and Mullin29) and autism spectrum disorder(Reference Fejzo, Kam, Laguna, MacGibbon and Mullin30,Reference Getahun, Fassett and Jacobsen31) . Specifically, a retrospective longitudinal cohort study using medical records of pregnant women and their children (n = 469 789), found that children exposed to HG in utero had higher rates of physician-diagnosed autism spectrum disorder than unexposed children, which was not associated with medications and not explained by confounding variables(Reference Getahun, Fassett and Jacobsen31). Although causality cannot be proven based on this observational study, there is strong biological plausibility based on the effect of maternal malnutrition on the developing brain at critical time points(Reference Getahun, Fassett and Jacobsen31,Reference Koren, Ornoy and Berkovitch32) . A study of 312 children exposed in utero to HG found they have a 3·82-fold increase of being diagnosed with conditions including allergies, chronic constipation, growth restriction and chronic respiratory infections(Reference Fejzo, Schoenberg, Macgibbon, Magtira, Martin and Mullin33). The authors postulate that failure to gain enough weight during pregnancy puts the child at risk for intra-uterine growth restriction, which in turn could incur greater risk for other neurodevelopmental and physical problems. The study was limited by sample size, retrospective recall of symptoms and self-report of conditions.
Looking at the longer-term impact of HG on offspring into adolescence, there is less robust evidence, due to lack of long-term follow-up and prospective studies. There is emerging evidence of potential effects on the metabolic profile of offspring, similar to effects observed in those exposed to undernutrition in pregnancy famine studies(Reference Bellver and Mariani34,Reference Ayyavoo, Derraik and Hofman35) . However, evidence for this theory is equivocal, possibly due to heterogenous populations. A longitudinal analysis of a Finnish birth cohort of 8953 women with HG, did not find any evidence that prenatal exposure to HG has negative consequences on cardiometabolic health of the offspring at 16 years(Reference Koot, Grooten and Sebert36); however, this study did not have data on duration, severity or onset of symptoms. It is important to recognise that none of these studies was designed to measure dietary intake or its effects, as the majority of research on HG to date has focused on medical management, with little attention paid to the outcomes of poor nutrition(Reference Koren, Ornoy and Berkovitch32). Additionally, most studies have employed a retrospective rather than a prospective study design, meaning they are potentially subject to recall bias. It is thought that retrospective evaluation of NVP/HG can distort the perception of the effectiveness of antiemetics and associations with long-term outcomes(Reference Koren, Maltepe, Navioz and Wolpin37).
Nutritional implications of HG
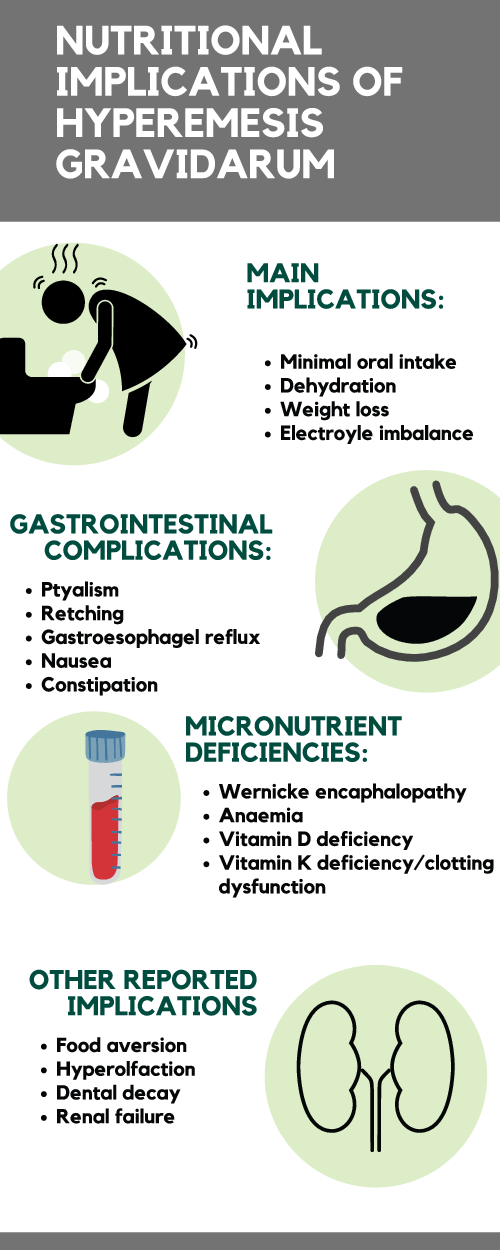
Nutritional implications of HG are shown in Fig. 1 and detailed below.
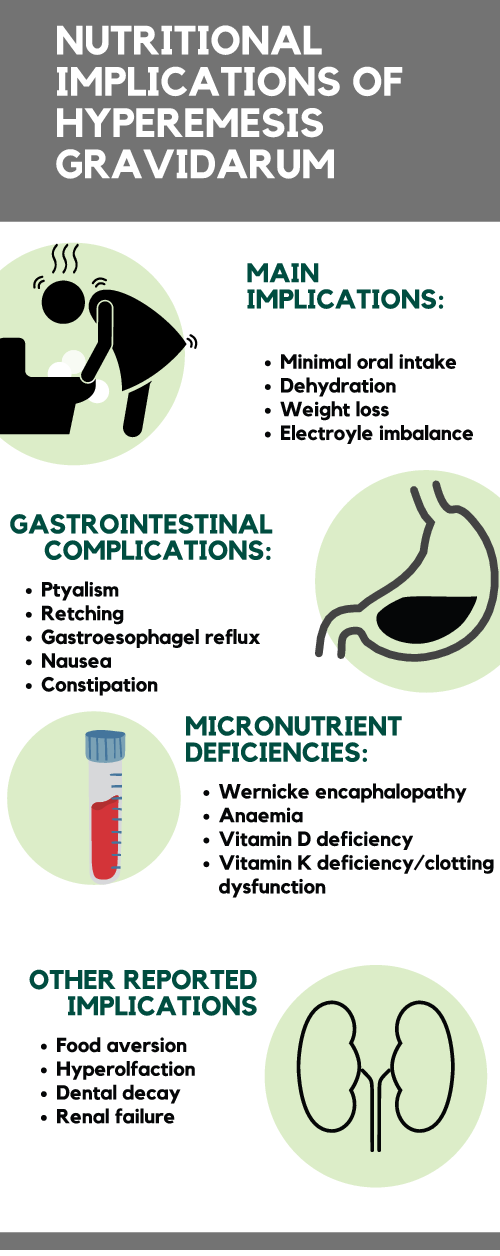
Fig. 1. Nutritional implications of hyperemesis gravidarum (see separate pdf)
Sensory issues relating to taste and olfaction
Although it is generally accepted that taste changes occur during pregnancy, specific scientific evidence is lacking(Reference Faas, Melgert and de Vos38). Similarly, although there is abundant anecdotal evidence for a heightened sense of smell during pregnancy, there is a lack of conclusive studies(Reference Cameron39). It is hypothesised that an evolutionary mechanism exists, whereby increased olfactory sensitivity protects the developing embryo by reducing the likelihood that the mother will ingest toxins(Reference Cameron39,Reference Nordin, Broman, Olofsson and Wulff40) . It is not known whether this applies to pregnancies affected by HG, as limited studies on this topic have had mixed results. Yasar et al.(Reference Yasar, Sagit, Zeki Uludag and Ozcan41) reported that odour and taste identification scores were different between pregnant women and non-pregnant women; however, there was no difference between women with HG and controls. A more recent study found the opposite. Tan et al.(Reference Tan, Kartik, Thanendran, Zakaria, Win and Omar42) reported a deficiency in taste and smell identification in women hospitalised for HG. Specifically, those with HG were hypersensitive to taste, with the exception of sweet taste, compared with gestation-matched controls. Sweet, crunchy and uncooked (fresh) food characteristics were preferred by women experiencing HG. Limitations exist with these sensory studies, namely small sample sizes and applicability to nutritional intake not having been demonstrated.
Nutritional intake
Although poor intake is a key feature of HG, there is a distinct lack of research about this topic(Reference Koren, Ornoy and Berkovitch32,Reference Grooten, Roseboom and Painter43,Reference Dean, Shemar, Ostrowski and Painter44) . A systematic literature search conducted in April 2020(Reference Maslin, Shaw, Dean, Brown and Shawe45) has identified only four previous research studies which assess nutritional intake in women with HG(Reference van Stuijvenberg, Schabort, Labadarios and Nel46–Reference Crozier, Inskip, Godfrey, Cooper and Robinson49). Studies by van Stuijvenberg(Reference van Stuijvenberg, Schabort, Labadarios and Nel46) and Birkeland(Reference Birkeland, Stokke and Tangvik47), which both assessed women admitted to hospital, reported that many with HG had energy intakes <50 % of recommended levels and were significantly deficient compared with control participants. Median energy intakes of 443(Reference van Stuijvenberg, Schabort, Labadarios and Nel46) and 990 kcal(Reference Birkeland, Stokke and Tangvik47) (1854 and 4141 kj), respectively, over a 24-h period were recorded, compared with recommendations of 2500 and 2285 kcal (10460 and 9560 kj), respectively. Actual intake is likely to have been even lower, due to amounts lost through vomiting. With such low energy intakes, the intake of the majority of macro- and micronutrients were also significantly lacking. Of note, increasing frequency and severity of symptoms was inversely related to nutritional intake. A Turkish study(Reference Fatma, Irfan, Umur and Yusuf48) examined antioxidant intake, finding that vitamin E, E equivalent, vitamin C, carotene and vitamin A levels were significantly lower in women with HG, compared with control participants. Each of these studies examined nutritional intake at one time point only, with relatively small sample sizes. None of the studies assessed fluid intake.
Dehydration, ketonuria and electrolyte disturbances
Dehydration is a widespread consequence of HG that can lead to severe electrolyte imbalances, the most frequently reported being hypokalaemia(Reference Fejzo, Trovik and Grooten11). Dehydration can be assessed using a combination of patient-reported fluid intake and output, reduced or concentrated urine output, skin turgor, dry mucous membranes, reduced blood pressure and/or tachycardia(Reference Armstrong, Kavouras, Walsh and Roberts50). IV hydration should be used for those who cannot tolerate oral liquids for a prolonged period or if clinical signs of dehydration are present(22). Normal saline with additional potassium chloride is recommended and should be guided by daily electrolyte monitoring(21). Thiamine (IV or orally) should also be given to those admitted with prolonged vomiting who require rehydration(21,22) (see late paragraph on thiamine deficiency). Overall, IV hydration has been found to be highly effective in symptom relief, compared with other treatment modalities. In a survey of 765 women from twenty-six countries, IV hydration and antihistamines were the most commonly used treatment modalities, with 83 % reporting IV hydration as ‘effective or maybe effective’(Reference Goodwin, Poursharif, Korst, MacGibbon, Romero and Fejzo51).
Ketonuria is a condition in which ketone bodies are present in the urine. It is often listed as a diagnostic criterion with dehydration(Reference Koot, Boelig and Van’t Hooft23) and can be an indication that the body is using fat as an alternative source of energy to glucose, as occurs in starvation. It is commonly used in the assessment of HG and often as a decision-making criterion for treatment, particularly IV hydration(Reference Koot, Boelig and Van’t Hooft23). However, ketones do not indicate dehydration and may in fact provide misleading information about the severity of the condition, either underestimating how unwell a patient is or indeed preventing discharge from hospital where they are still present(Reference Koot, Grooten and Post52). The risks associated with misleading results outweigh the potential benefit of identifying malnutrition, which could be more accurately assessed in other ways(Reference Dean, Shemar, Ostrowski and Painter1,Reference Niemeijer, Grooten and Vos53) . It is therefore now recommended that ketonuria is not used to identify or assess HG(Reference Koot, Grooten and Post52).
Malnutrition and weight loss
‘Malnutrition’, a term often used interchangeably with the term ‘undernutrition’, is widely described as a state of nutritional imbalance in calories, macronutrients, vitamins and/or minerals. However, specific definitions for malnutrition during pregnancy or ‘gestational malnutrition’ are lacking from current international guidelines(Reference Cederholm, Barazzoni and Austin54,Reference White, Guenter, Jensen, Malone and Schofield55) . Nutritional screening is a rapid, simple and general procedure used by nursing, medical or other staff, often at first contact with the patient, to detect those with significant risk of nutritional problems, so that clear guidelines for action can be implemented(Reference Elia56). It is not known to what extent formal nutritional screening takes place in patients with HG admitted to hospital, and variation in practice may exist(Reference Maslin, Billson, Dean and Abayomi57). The combination of physiological changes in lean mass, fat mass, weight and blood volume during pregnancy means that standard nutrition screening tools and biochemical reference ranges used in the adult non-pregnant population are not appropriate. As such, an evidence review conducted in 2018 concluded that more research is needed to examine the validity and reliability of screening/assessment tools in identifying malnutrition in pregnancy, which would help to standardise care pathways and treatment goals(58).
The gastrointestinal symptoms of HG (including nausea, vomiting and retching, but hyperolfaction, ptyalism, abdominal pain, gastroesophageal reflux) profoundly affect the ability to eat, digest and absorb nutrients and maintain caloric balance. These symptoms have the potential to persist throughout the entire pregnancy(Reference Mullin, Ching and Schoenberg59), causing malnutrition, dehydration and extreme weight loss(Reference Fejzo, Poursharif and Korst12). As such, HG has been likened to a form of prolonged starvation(Reference van Stuijvenberg, Schabort, Labadarios and Nel46,Reference Birkeland, Stokke and Tangvik47) , and unintended weight loss or inability to achieve gestational weight gain guidance is common. It is difficult to quantify average weight loss/gain in those with HG for a number of reasons. Studies on gestational weight gain/loss often rely on recall of self-reported preconception weight, which may be subject to error. Weight change during pregnancy may be expressed in a number of different ways, using a number of different timeframes (e.g. percentage weight loss, absolute weight loss, change in BMI, or categories of suboptimal/expected/excessive gestational weight gain). Two small cross-sectional hospital-based studies reported a measured mean weight loss of ∼3 kg from preconception to admission(Reference van Stuijvenberg, Schabort, Labadarios and Nel46,Reference Birkeland, Stokke and Tangvik47) , whereas a longitudinal study of hospital admissions for HG reported a mean weight loss of 4·4 kg at 9 weeks gestation (n = 892)(Reference Meinich and Trovik60). A recent international survey study of 445 people with HG reported a self-reported average weight loss of 12·5 % of pre-pregnancy weight(Reference MacGibbon, Kim, Mullin and Fejzo25). However, it has been suggested that, due to women’s tendency generally to underreport their weight, there is potential for an underestimation of initial weight loss in HG(58). Although a threshold of >5 % weight loss is commonly used as a clinical diagnostic criterium for HG, ‘extreme weight loss’, being defined as >15 % of preconception weight has been estimated to affect 25 %(Reference Fejzo, Trovik and Grooten11). However, it has been suggested that, due to women’s tendency generally to underreport their weight, there is potential for an underestimation of initial weight loss in HG(Reference Meinich and Trovik60). Although a threshold of >5 % weight loss is commonly used, a clinical diagnostic criterium for HG, ‘extreme weight loss’, being defined as >15 % of preconception weight has been estimated to affect 25 %(Reference Fejzo, Poursharif and Korst12). Those that experience this extreme weight loss were more likely to be hospitalised and have prolonged symptoms, gallbladder dysfunction, liver dysfunction and renal failure(Reference Fejzo, Poursharif and Korst12).
Recently published research from a Norwegian cohort has identified that not regaining pre-pregnancy weight specifically by week 13–18 is an independent risk factor for delivering a baby born small for gestational age, even when adjusted for total pregnancy weight gain, pre-pregnancy body mass index (BMI), parity, age and smoking status. The results from 892 women hospitalised for HG between 2002 and 2016 demonstrated on odds ratio of 2·66 for infants born small for gestational age, underlining the importance of medical and nutritional treatment for HG during early pregnancy, in order to reverse any first-trimester weight loss(Reference Meinich and Trovik60). The data also showed that weight gain patterns within pregnancy time intervals are different among different BMI categories. Recognition of weight loss (or poor weight gain) is particularly important in light of the fact that HG may be more common in women who are either under- or overweight(Reference Vikanes, Grjibovski, Vangen, Gunnes, Samuelsen and Magnus61). Indeed, with an increasing population of pregnant women entering pregnancy already living with overweight or obesity(Reference Devlieger, Benhalima and Damm62,Reference Poston, Caleyachetty and Cnattingius63) , there is a risk that weight loss due to persistent nausea and vomiting may be overlooked.
Thiamine deficiency
One of the most commonly occurring micronutrient deficiencies in HG is thiamine deficiency(Reference Fejzo, Trovik and Grooten11), a condition that can rapidly deteriorate into a medical emergency if not managed correctly(Reference Oudman, Wijnia, Oey, van Dam, Painter and Postma64). Thiamine is an essential nutrient for carbohydrate metabolism(Reference Wiley65). In pregnancy, thiamine requirements are estimated to increase by 45·5 %, based on an additional calorie needs(Reference Macgibbon66). Although thiamine is widespread in many foods and food groups, it is not included in sufficient levels in all preconception vitamin preparations. Levels therefore can rapidly deplete during pregnancy, especially in women with HG, who experience frequent vomiting, poor oral intake, and intolerance of oral vitamin preparations. To complicate matters, symptoms of thiamine deficiency include nausea, vomiting, anorexia and fatigue(Reference Wiley65), meaning it can mimic HG, making it difficult at times to determine when thiamine deficiency is present(Reference Fejz, o, MacGibbon and Mullin2).
When thiamine levels are rapidly depleted, Wernicke’s encephalopathy (WE) can occur. WE is an acute neuropsychiatric syndrome characterised by the classic triad of ataxia, eye movement disorders and mental status change(Reference Wiley65). It is most commonly observed in individuals with chronic alcoholism and accompanying malnutrition; however, numerous cases in women with HG have been reported. A systematic review published in 2019 examining cases of WE in HG identified 146 case studies reporting on 177 cases(Reference Oudman, Wijnia, Oey, van Dam, Painter and Postma64). Pregnant WE patients became thiamine depleted between 10 and 15 weeks of gestation, had been vomiting for a median of 7 weeks before WE was diagnosed and had lost an average of 12·1 kg. Commonly reported signs of WE across all cases were nausea and vomiting (100 %), double vision (37·4 %) and blurred vision (27·4 %). In half of the cases, spontaneous miscarriage occurred, and 5 % of the cases resulted in maternal fatality. In cases in which the offspring survived, patients had a shorter duration of excessive vomiting before the onset of WE, than in cases where the offspring did not survive (6·2 compared with 9 weeks)(Reference Oudman, Wijnia, Oey, van Dam, Painter and Postma64). This underlines the critical importance of obtaining medical help sooner rather than later when intractable vomiting of pregnancy occurs.
The systematic review found that thiamine supplementation was insufficient or absent from treatment plans, and in 14 % of cases it was explicitly reported that HG patients received intravenous glucose supplementation without thiamine, which exacerbates WE(Reference Oudman, Wijnia, Oey, van Dam, Painter and Postma64). This highlights the lack of awareness of the potential severity of the condition, which could have been prevented by giving prophylactic thiamine injections(Reference Oudman, Wijnia, Oey, van Dam, Painter and Postma64). Thiamine supplementation, either oral or intravenous, should be given to all women admitted with prolonged vomiting, especially before administration of dextrose or parenteral nutrition(21). The American College of Obstetricians and Gynecologists advise that 100 mg should be given, intravenously with the initial rehydration fluid and 100 mg daily for the next 2–3 d (followed by intravenous multivitamins), for women who require IV hydration and have vomited for more than 3 weeks(22). For those at home, it has been suggested that those with prolonged symptoms and/or weight loss who are unable to tolerate prenatal vitamins that include thiamine should have thiamine levels monitored regularly(Reference Macgibbon66). It is also recommended that basic screening for signs of WE should be shared with the patient and her family/caretakers, including signs of confusion, unsteady gait and oculomotor symptoms(Reference Macgibbon66).
Iron deficiency anaemia
Hospital admission data from >8 million pregnancies in the United Kingdom suggests that those with HG have higher rates of anaemia; however, the timing of onset relative to HG was difficult to confirm, and it is unclear whether it is related to iron intake(Reference Fiaschi, Nelson-Piercy and Tata7). Data from small-scale studies of hospitalised patients with HG suggest iron intake is <50 % that of control participants(Reference van Stuijvenberg, Schabort, Labadarios and Nel46,Reference Birkeland, Stokke and Tangvik47) and that intakes are significantly lower in those with the most vomiting episodes per day(Reference van Stuijvenberg, Schabort, Labadarios and Nel46). Anecdotally, supplements containing iron can worsen nausea(Reference King and Murphy67), and those with HG may be advised to omit them, replacing with a separate folic acid supplement instead(21,22,68) . In the pregnant population generally, a review of eleven European studies showed that the prevalence of iron deficiency and iron deficiency anaemia was 28–85 % and 21–35 % at 32 and 39 weeks gestation, respectively, in those who did not take iron supplements(Reference Milman, Taylor, Merkel and Brannon69). It is therefore highly likely that those with HG are at risk of iron deficiency anaemia, and therefore, their offspring may be more susceptible to the potential negative consequences it can cause, including cognitive and neurodevelopmental disorders(Reference Pivina, Semenova, Doşa, Dauletyarova and Bjørklund70,Reference Young, Oaks, Tandon, Martorell, Dewey and Wendt71) .
Other micronutrient deficiencies
A range of different micronutrient deficiencies have been reported; however, it is impossible to compare rates because of the varying study designs used. In a South African study of women hospitalised with HG (n = 20)(Reference van Stuijvenberg, Schabort, Labadarios and Nel46), more than 60 % had suboptimal biochemical status of thiamine, riboflavin, vitamin B6 and vitamin A. Results need to be interpreted with caution owing to the dilutional affect that occurs with expansion of blood volume during pregnancy. A study examining factors associated with bone resorption indices found that serum 25OHD3 levels were significantly lower in women with HG compared with the control group(Reference Sahin, Madendag and Eraslan Sahin72). It was not clear, however, whether this was attributable to poor sun exposure or dietary intake, or a combination of factors. The authors hypothesised that women with HG may have an increased risk for lower bone mass in offspring due to maternal dietary deficiency and increased maternal bone mobilisation. Finally, a number of cases of maternal and neonatal vitamin K deficiency secondary to HG have also been reported in the literature(Reference Shigemi, Nakanishi, Miyazaki, Shibata and Suzuki73), as has biotin deficiency(Reference Onder, Guven, Demir, Mentese and Guvendag Guven74).
Clinical and nutritional management of HG, evidence for effectiveness and current guidelines
Overview of the clinical management of HG
In the absence of a definitive cause, the management of HG focuses on symptom relief and prevention of serious morbidity(Reference O’Donnell, McParlin and Robson75). First line advice is that women who have vomiting, but are not dehydrated, can be managed in the community with antiemetics, support, reassurance, oral hydration and dietary advice. Screening for thyroid dysfunction is recommended(21,22) . Medical management consists of anti-emetic medication applied in a stepwise approach(76); however, treatment can be challenging as some women simply do not respond to any anti-emetic treatment sufficiently(Reference O’Donnell, McParlin and Robson75,Reference Boelig, Barton, Saccone, Kelly, Edwards and Berghella77) . Medications with the most evidence of safety are generally used first. In addition to anti-emetic medications, anti-reflux medications may offer benefit. The proton pump inhibitor omeprazole is licensed for use in pregnancy as an antacid and may offer some benefit to women with HG where acid is painful or exacerbating symptoms(Reference Dean, Shemar, Ostrowski and Painter1). Laxatives also play a role in HG management, particularly for those prescribed ondansetron for which constipation is a significant side effect.
Hospital admission
In the United Kingdom, the Royal College of Obstetricians and Gynaecologists recommends that inpatient management should be considered if there is at least one of the following:
-
– continued nausea and vomiting and inability to keep down oral antiemetics,
-
– continued nausea and vomiting associated with ketonuria and/or weight loss (greater than 5 %) despite oral antiemetics
-
– and/or confirmed or suspected comorbidity(21). Additionally, the National Institute for Health and Care Excellence(78) recommends there should be:
-
– a lower threshold for admitting to hospital or seeking specialist advice if the woman has a co-existing condition (e.g. diabetes) which may be adversely affected by nausea and vomiting. Similar recommendations are in place internationally(22,68,79) .
Typically, secondary care involves admission to either an antenatal or a gynaecology ward for treatment with IV fluids, antiemetics and vitamin supplements. It is anticipated that oral intake would gradually be resumed followed by discharge back into the community. Resumption of symptoms would result in readmission and a repeat of previous care, possibly trying different antiemetics or a combination thereof(Reference O’Donnell, McParlin and Robson75). An analysis of HG hospital statistics from England between 1998 and 2011(Reference Fiaschi, Nelson-Piercy, Gibson, Szatkowski and Tata26) found the readmission rate to be 28 %, with 11 % having three or more admissions. Only 10 % of pregnancies with admissions for HG were managed as day cases, whereas 33 % had more than 4 d of inpatient hospital stay during the pregnancy. Developments in clinical practice research suggest, however, that ambulatory day case management is an effective direct alternative to inpatient management of severe NVP, proving more cost-effective(Reference Ucyigit80–Reference McParlin, Carrick-Sen, Steen and Robson82).
Dietary and lifestyle management
As there is little high-quality and consistent evidence supporting any one intervention in the management of HG(Reference Boelig, Barton, Saccone, Kelly, Edwards and Berghella83), effective treatment requires a combination of medical interventions, lifestyle changes, dietary changes, supportive care and patient education(Reference Macgibbon66). Women with HG can experience severe food aversions and poor appetite, meaning a dietetic consultation may be helpful in expanding food choices, prescribing oral nutritional support(Reference Maslin, Billson, Dean and Abayomi57) and monitoring nutritional deficiencies(Reference Macgibbon66). Assessment by a dietitian is recommended on admission to hospital in some countries(68,79) , although there is a lack of consensus on referral criteria and management(Reference Maslin, Billson, Dean and Abayomi57). A Cochrane review was published in 2016, which focused solely on interventions for HG (rather than mild or moderate NVP)(Reference Boelig, Barton, Saccone, Kelly, Edwards and Berghella83). It identified only one nutrition-related study, whereby women taking vitamin B6 had a slightly longer hospital stay compared with placebo. There was no clear evidence of differences in other outcomes, including vomiting episodes, readmission rate or side effects(Reference Tan, Yow and Omar84). The mechanism of action of vitamin B6 is unknown, and the review did not identify any lifestyle or dietary interventions.
Anecdotally, advice concerning avoidance of fatty and odorous foods, eating small amounts of liquid or food at frequent intervals, avoiding an empty stomach, eating dry crackers and/or eating a high-protein snack before bed has been recommended(Reference King and Murphy67,Reference Ebrahimi, Maltepe and Einarson85) . However, there has been no evidence-based research on the effectiveness of these approaches. Although they may provide some symptomatic relief in women with mild or moderate NVP(Reference Chandra, Magee, Einarson and Koren86), an international survey of 765 women from twenty-six countries found only 22 % reported dietary interventions to be either ‘maybe effective’ or ‘effective’(Reference Goodwin, Poursharif, Korst, MacGibbon, Romero and Fejzo51). Overall research suggests that, for the severe symptoms of HG, lifestyle and dietary changes alone are insufficient(Reference Fejzo, Trovik and Grooten11). This does not necessarily reflect health care professional practices. An Australasian study of doctors(Reference Raymond87) investigating prescription practices in HG found the first choice of treatment was dietary advice, followed by metoclopramide and ondansetron. Of note, dietary advice was rated the most effective for HG by clinicians; however, there were no patient-reported data collected to support this finding.
Similarly, although ginger may be more effective than placebo in alleviating symptoms in some women with mild NVP, there is no evidence of its effect in women with severe symptoms. A survey conducted by the Pregnancy Sickness Support charity of >500 women with HG found that, although 88 % of respondents had tried ginger, 87·6 % of them found it not at all helpful, with more than half experiencing negative side effects including acid reflux(Reference Dean and O’Hara88). Some 60 % of respondents had been recommended to try ginger more than twenty times, by multiple different healthcare professionals, family members and strangers. Of note, 79 % of women who had ginger suggested by a healthcare professional reported that it eroded their trust and confidence in the healthcare professional. Separately, other qualitative research suggests that women with HG find some health care professionals to be unsympathetic, not fully appreciating the extent of their symptoms and the impact on their quality of life(Reference Havnen, Truong, Do, Heitmann, Holst and Nordeng89).
Enteral nutrition support (tube feeding)
Optimising medical therapy to relieve symptoms and enable sufficient oral intake is the goal in HG; however, that may not be achievable in all patients(Reference Macgibbon66). In situations where antiemetic medication and IV fluids are not sufficient to reduce the nausea and/or vomiting, ketonuria persists and the patient is unable to improve oral nutritional intake, additional nutritional therapy should be considered. A threshold of 8–10 % weight loss has been suggested(Reference Macgibbon66); however, this needs to be assessed on an individual basis, considering pre-pregnancy weight, comorbidities and clinical status. Modes for enteral nutrition delivery in HG include nasogastric tubes (NGT), nasojejunal tube (NJT), endoscopic gastrostomy and gastrojejunostomy. However, percutaneously inserted tubes in pregnancy are very rare and carry additional risks attributable to changing anatomy(Reference Saha, Loranger, Pricolo and Degli-Esposti90). Due to their recent absence of nutritional intake, weight loss and electrolyte imbalance, women with HG are at high risk of refeeding syndrome(Reference Poston, Caleyachetty and Cnattingius63). Clinical consensus guidelines recommend that those at risk of refeeding syndrome should be managed by starting with minimal nutritional support (i.e. 10–20 kcal/kg/d) and advancing feeding slowly to meet full needs by 4–7 d or by 33 % of goal every 1–2 d(91,Reference da Silva, Seres and Sabino92) . Oral or IV thiamine, plus potassium, phosphate and magnesium, may also be recommended, with close monitoring of fluid and electrolyte balance required. Dietetic advice can be very helpful to treat or avoid potentially serious complications of HG(Reference Dean, Shemar, Ostrowski and Painter44), and a dietitian should be consulted when tube feeding is being considered(21,79) .
A case series of data collected over 10 years of women hospitalised with HG in Norway (n = 558) found that, compared with IV fluid or peripheral parenteral nutrition regimens, NJT feeding (n = 107) was associated with adequate maternal weight gain and favourable pregnancy outcomes(Reference Stokke, Gjelsvik, Flaatten, Birkeland, Flaatten and Trovik93). Although inadvertent tube expulsions occurred in 54 % of patients, the majority (79 %) accepted a new tube placement. Those who were tube fed had significantly longer hospital stay, receiving tube feeding for a median of 5 d. Overall, the study concluded that enteral nutrition for HG might be both feasible and beneficial in reversing hyperemesis-induced weight loss, although patient acceptability was not assessed due to the retrospective study design. It also recommended that prospective studies are needed to investigate the optimal time point for initiating enteral nutrition and to evaluate the best tube placement (NGT/NJT). In a smaller case series of eleven women hospitalised with HG in Israel, Vaisman et al.(Reference Vaisman, Kaidar, Levin and Lessing94) concluded that NJT feeding could be an effective option in those with persisting symptoms despite IV fluids and antiemetic drugs. A clear reduction in vomiting was apparent within the first 48 h after tube insertion, with vomiting ceasing completely after a mean of 5 ± 4 d. The patients were encouraged to drink and eat along with tube feeding from day 3 onwards and were discharged 1–3 d after tube removal when no symptoms recurred. They noted that that post-pyloric feeding might be advantageous due to its effect on intestinal dysmotility, however acknowledged the aesthetic and discomfort issues that patients may feel with tube feeding.
In contrast, a trial investigating early NGT feeding in addition to standard care with intravenous rehydration and antiemetic treatment did not find an improvement in birth weight or secondary outcomes(Reference Grooten, Mol and van der Post95). The MOTHER (Maternal and Offspring outcomes after Treatment of HyperEmesis by Refeeding) trial (n = 116), based in hospitalised women in the Netherlands, also did not find beneficial effects of NGT on maternal weight gain, duration of stay, readmission rate, symptoms or quality of life. However, the study had a number of limitations. Firstly, more than half of the women who were eligible declined participation, and secondly, protocol completion was poor in the tube feeding group, both of which suggest low levels of acceptability of NGT feeding. A sensitivity analysis found that those with more marked weight loss were more likely to tolerate tube feeding, enabling them to complete the study protocol. The authors recommended that future trials are needed to study whether tube feeding is beneficial in women with severe HG that is complicated by marked weight loss and/or prolonged symptoms.
In a qualitative study of thirteen women from the Netherlands that investigated patient perspectives of HG management(Reference Havnen, Truong, Do, Heitmann, Holst and Nordeng89), eight had received NGT feeding and underlined the benefits of it. Women who did not have NGT said they wished they had, in order to prevent severe weight loss, dehydration and need for multiple hospital admissions. Other reasons cited for wanting to be fed via NGT were to provide sufficient nutritional intake for the baby and to reduce vomiting by preventing an empty stomach. The authors, however, acknowledged the limitations and lack of external generalisability of the study findings, highlighting differences in management strategies internationally. In some countries, enteral feeding is viewed as an effective but extreme method of supporting women suffering from very severe symptoms as a last resort(68,Reference Grooten, Mol and van der Post95) . The American College of Obstetricians and Gynecologists(22) recommends enteral tube feeding be initiated as the first-line treatment to provide nutritional support to those with HG who are not responsive to medical therapy and cannot maintain their weight. Other researchers note that the risks of enteral feeding may be less than those of chronic malnutrition and dehydration, especially in women with severe or prolonged symptoms(Reference Macgibbon66).
Total parenteral nutrition
In situations where conventional drug therapy has failed, total parenteral nutrition (TPN) may be an alternative to enteral feeding in women with a long course of HG accompanied by a significant weight loss(Reference Vaisman, Kaidar, Levin and Lessing94). TPN may be used in refractory cases to ensure sufficient calorie intake, but should be used only as a last resort in those where enteral feeding is not possible(21,22) , due to the associated risks and complications, including infection and thrombosis, as well as the high cost. Peripherally inserted central catheters (PICCs) are a popular alternative to other types of central venous access because of ease of insertion and perceived lower risk of complications(Reference Cape, Mogensen, Robinson and Carusi96); however, because use of PICCs may not meet the daily caloric needs of the pregnant woman, a prolonged period of IV administration will require central vein insertion(Reference Fejzo, Trovik and Grooten11).
A retrospective cohort study of women who had received TPN (n = 122) found that TPN support during early pregnancy is associated with a decreased risk for perinatal morbidity(Reference Peled, Melamed, Hiersch, Pardo, Wiznitzer and Yogev97). Specifically, compared with women with HG who did not receive TPN, administration of TPN during early pregnancy was associated with a lower rate of labour induction and preterm delivery. In addition, neonates in the subgroup of mothers who received TPN had a higher birth weight percentile, as well as a lower rate of composite morbidity and NICU admission. The authors highlighted that women who required TPN likely had a more severe presentation of HG, thereby further underlining the potential beneficial effect of TPN in the most critical cases.
In contrast, a retrospective study that compared TPN delivered via PICC line, compared with NGT/NJT or medication alone, reported serious complications of bacteraemia, sepsis and thrombosis observed in the majority (66·4 %) of the patients in the PICC line group(Reference Holmgren, Aagaard-Tillery, Silver, Porter and Varner98). Indeed, in several cases, the researcher noted the complications were severe enough to require admission to an intensive care unit. The authors recommended that, to avoid PICC, a more aggressive attempt at enteral feeding and hydration via insertion of NGT/NJT should be made. A recent systematic review on this topic yielded five eligible studies, concluding there are limited data regarding complication rates due to PICC use in pregnancy, with a high level of heterogeneity among existing studies stating a pooled rate of combined infectious and thromboembolic complications of 26 %(Reference Frolova, Shanahan, Tuuli, Simon and Young99).
Research gaps, priorities and recommendations for future research
As previously mentioned, results from a James Lind Alliance Priority Setting Partnership exercise were published this year, which underlined that clinicians, researchers and patients see nutrition as a priority in HG research(Reference Dean, Bierma and Clarke18). Three of the ten research priorities were specific to nutrition, namely:
-
- What are the immediate and long-term effects of HG (including malnutrition and dehydration) on the developing foetus?
-
- What are the immediate and long-term physical, mental and social consequences and complications of HG (including malnutrition and dehydration) on the pregnant person’s body?
-
- What are the nutritional requirements of the first, second and third trimesters, and how can people with HG achieve these goals?
In addition to these research questions, understanding the training needs of healthcare professionals in relation to nutrition to ensure consistent messaging is important. Dissemination and implementation of any new nutritional evidence into pragmatic clinical practice guidelines is also paramount. We acknowledge that this review has been narrative rather than systematic and has focused on research and healthcare in developed countries. Information on prevalence and management of HG in developing countries is limited, and future research should take a broader view, considering cultural, geographical and social issues known to impact maternal health(Reference Dean, Bierma and Clarke18).
Conclusion
Although poor nutritional intake and weight loss are both a characteristic and consequence of HG, very few studies have assessed the nutritional intake and extent of malnutrition in women with HG. The evidence base for dietary management of HG is poor, and it is not known what (if any) clinical nutrition advice is routinely provided, if it is acceptable and if it is effective. Although hospital admission and administration of IV fluids and vitamins is very common, enteral and parenteral nutrition are usually only used in exceptional circumstances, with a particularly limited evidence base around the use of NGT/NJT feeding. Better recognition and management of malnutrition in HG is required to prevent complications and optimise nutritional care. Future research should investigate the role and outcomes of targeted nutritional strategies in the management of HG, using agreed diagnostic criteria, guidelines and core outcomes.
Acknowledgements
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
K.M. planned and led the review. C.D. contributed to writing the review and approved the final version of the manuscript.
There are no conflicts of interest.