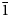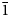Introduction
Minerals of the garnet supergroup are common rock-forming and accessory minerals of different metamorphic and metasomatic rocks, including skarns and skarn-like rocks associated with carbonatites in alkaline-ultrabasic complexes. In particular, Ti-rich andradite is a common mineral of skarn-like rocks of the Kovdor massif, Kola Peninsula, Russia (Ivanyuk et al., Reference Ivanyuk, Yakovenchuk and Pakhomovsky2002), where it forms well shaped rhombic dodecahedral crystals (up to 3 cm in diameter), spherical porphyroblasts (up to 5 cm in diameter) and coarse-grained monomineralic segregations (up to 20 cm in diameter).
The late stage of skarn formation may include low-temperature crystallisation of secondary garnet-group mineral phases with the ‘hydrogarnet-type’ substitution according to the scheme 4H+ + Z□ → □ + ZSi4+ (Lager et al., Reference Lager, Armbruster, Rotella and Rossman1989; Grew et al., Reference Grew, Locock, Mills, Galuskina, Galuskin and Hålenius2013). Such a phase was indeed found as thin crusts covering andradite crystals at Kovdor. Its detailed study led to its recognition as a new mineral species, nikmelnikovite, Ca12Fe2+Fe3+3Al3(SiO4)6(OH)20, which, in contrast to all known garnet-supergroup minerals, has a trigonal symmetry (Krivovichev et al., Reference Krivovichev, Yakovenchuk, Panikorovskii, Savchenko, Pakhomovsky, Mikhailova, Selivanova, Kadyrova and Ivanyuk2019). In the present contribution, we provide the results of its crystal-structure determination and describe its relationship to other minerals of the garnet supergroup. It is remarkable that nikmelnikovite was found in association with manaevite-(Ce), the first H2O-bearing member of the vesuvianite group, which also formed by the substitution of (SiO4)4– groups with [H4O4]4– (Moiseev et al., Reference Moiseev, Panikorovskii, Aksenov, Mazur, Mikhailova, Yakovenchuk, Bazai, Ivanyuk, Agakhanov, Shilovskikh, Pekov, Kasatkin, Rusakov, Yapaskurt, Karpenko and Krivovichev2020).
Materials and methods
Background information
Nikmelnikovite crystals studied in this work were sampled from the abandoned Mica open pit (kar'er ‘Slyuda’), also known as the Phlogopite open pit, in the Kovdor massif of ultrabasic, alkaline and carbonatitic igneous rocks and related metasomatites. The massif is situated in the southwestern part of the Murmansk Region, Russia (67°33′N, 30°31′E) (Fig. 1). The crystals were extracted from a coarse-grained spotted rock composed of white calcite, scolecite and natrolite–gonnardite (up to 5 cm in diameter), pale-blue pectolite (up to 15 cm long), colourless glagolevite (up to 3 cm in diameter), dark-brown andradite (up to 6 cm in diameter) with relict grains of magnetite, bright-green to dark-brown vesuvianite (up to 4 cm in diameter) and black magnesio-hastingsite (up to 1.5 cm long). Nikmelnikovite forms thin (up to 1 mm thick) epitaxial coatings on the octahedral faces of andradite crystals included in cavernous calcite (Fig. 2a,b,c). These crusts are in turn encrusted by crystals (up to 100 μm in diameter, Fig. 2d,e) and spherical globules (up to 5 μm in diameter, Fig. 2d) of H4O4-substituted mineral of the andradite–grossular series. In back-scattered electron images, the examined crystals of andradite comprise a sector-zoned core, where variations in average atomic number reflect different Al/Fe ratios, and a thin (up to 10 μm thick) marginal zone composed of an inner Fe-rich and outer Al-rich layers. Merohedral twinning with a twofold axis oriented parallel to [110] was observed (see below).

Fig. 1. Schematic geological map of the Kovdor massif (after Mikhailova et al., 2016), block-diagram of the Kovdor Phlogopite Deposit (after N.M. Manaev, unpublished map) and a photo of the Mica open pit (after Ivanyuk et al., Reference Ivanyuk, Yakovenchuk and Pakhomovsky2002), indicating the nikmelnikovite type locality.

Fig. 2. Paragenesis and morphology of nikmelnikovite and associated minerals. (a,b) Crusts of nikmelnikovite (1) on andradite (2) associated with glagolevite (3), tobermorite (4) and natrolite (5). (c) Back-scattered electron image of cross-section of andradite with epitaxial nikmelnikovite crust, natrolite and later strontianite bunches (6). (d) Secondary-electron images of nikmelnikovite crust with spherulites of H4O4-substituted grossular (7). (e) Octahedral aggregates of complex hydrogarnet-related phases associated with glagolevite and tobermorite.
The chemical composition of the mineral corresponds to the empirical formula Ca11.81(Fe2+0.74Mn0.12Mg0.16)Σ1.02(Al3.95Fe3+1.98)Σ5.93[Si6.14O24](OH)20⋅1.18H2O (Krivovichev et al., Reference Krivovichev, Yakovenchuk, Panikorovskii, Savchenko, Pakhomovsky, Mikhailova, Selivanova, Kadyrova and Ivanyuk2019); its idealised formula can be written as Ca12Fe2+Al4Fe3+2[SiO4]6(OH)20.
Single-crystal X-ray diffraction
The single-crystal X-ray diffraction study of nikmelnikovite was done using an Agilent Technologies Xcalibur Eos diffractometer equipped with a CCD area detector and operated at 50 kV and 40 mA. A hemisphere of three-dimensional data was collected at room temperature using monochromatic MoKα X-radiation (λ = 0.71069 Å) with frame widths of 1° and 150 s count for each frame. The intensity data were reduced and corrected for Lorentz, polarisation and background effects. Empirical absorption correction was applied in the CrysAlisPro (Agilent Technologies, 2014) program complex using spherical harmonics, implemented in the SCALE3 ABSPACK scaling algorithm. The crystal structure was solved by direct methods with the ShelX program package (Sheldrick, Reference Sheldrick2008) and refined to R 1 = 0.046 for 1184 independent reflections with F o > 4σ(F o). Crystal data, data collection information and structure refinement details are given in Table 1, final atom positions, displacement parameters and site occupancies are listed in Table 2. Selected interatomic distances are reported in Table 3. The crystal structure of nikmelnikovite was solved in the space group R $\bar{3}$![]() using a merohedral twin model (2-fold axis along [110]) with a twin ratio of 0.134/0.866. The low-occupancy O9, Mn and Si2 sites were refined in an isotropic approximation, because the attempts to refine them anisotropically resulted in physically unrealistic displacement parameters. The occupancies of the O7, O9, Mn and Si2 sites were fixed in order to generate a crystal-chemically reasonable model. Anisotropic displacement parameters and other details of the crystal-structure refinement are in the crystallographic information file, which has been deposited with the Principal Editor of Mineralogical Magazine and is available as Supplementary material.
using a merohedral twin model (2-fold axis along [110]) with a twin ratio of 0.134/0.866. The low-occupancy O9, Mn and Si2 sites were refined in an isotropic approximation, because the attempts to refine them anisotropically resulted in physically unrealistic displacement parameters. The occupancies of the O7, O9, Mn and Si2 sites were fixed in order to generate a crystal-chemically reasonable model. Anisotropic displacement parameters and other details of the crystal-structure refinement are in the crystallographic information file, which has been deposited with the Principal Editor of Mineralogical Magazine and is available as Supplementary material.
Table 1. Crystal data, data collection and structure refinement parameters for nikmelnikovite.

Table 2. Atom coordinates, displacement parameters (Å2) and site occupancies (s.o.f.; apfu = atoms per formula unit) in the structure of nikmelnikovite.

*fixed during refinement.
Table 3. Selected bond distances (Å) in the crystal structure of nikmelnikovite.

Results
Geometrical relationship to the ideal cubic garnet unit cell
Most of the garnet-supergroup minerals crystallise in the cubic space group Ia $\bar{3}$![]() d. Their unit cell parameters depend upon the chemical composition; e.g. for the minerals chemically related to nikmelnikovite, grossular, Ca3Al2(SiO4)3, andradite, Ca3Fe2(SiO4)3, and katoite, Ca3Al2(OH)12, a is equal to 11.847, 12.063 and 12.38 Å, respectively (Geiger and Armbruster, Reference Geiger and Armbruster1997; Armbruster and Geiger, Reference Armbruster and Geiger1993; Sacerdoti and Passaglia, Reference Sacerdoti and Passaglia1985). Nikmelnikovite is the first mineral species in the garnet supergroup that has a trigonal (rhombohedral) symmetry and the third IMA-recognised mineral species in the supergroup that has a non-cubic symmetry (the two others are tetragonal henritermierite and holtstamite). Nikmelnikovite crystallises in the rhombohedral space group R $\bar{3}$
d. Their unit cell parameters depend upon the chemical composition; e.g. for the minerals chemically related to nikmelnikovite, grossular, Ca3Al2(SiO4)3, andradite, Ca3Fe2(SiO4)3, and katoite, Ca3Al2(OH)12, a is equal to 11.847, 12.063 and 12.38 Å, respectively (Geiger and Armbruster, Reference Geiger and Armbruster1997; Armbruster and Geiger, Reference Armbruster and Geiger1993; Sacerdoti and Passaglia, Reference Sacerdoti and Passaglia1985). Nikmelnikovite is the first mineral species in the garnet supergroup that has a trigonal (rhombohedral) symmetry and the third IMA-recognised mineral species in the supergroup that has a non-cubic symmetry (the two others are tetragonal henritermierite and holtstamite). Nikmelnikovite crystallises in the rhombohedral space group R $\bar{3}$![]() with the unit cell parameters in the hexagonal setting given in Table 1. The relationship between its unit cell parameters (a h, b h, c h) and those of the pseudocubic cell (a c, b c, c c) are shown in Fig. 3 and can be described by the following equations:
with the unit cell parameters in the hexagonal setting given in Table 1. The relationship between its unit cell parameters (a h, b h, c h) and those of the pseudocubic cell (a c, b c, c c) are shown in Fig. 3 and can be described by the following equations:

Fig. 3. The relationship between the unit cell of nikmelnikovite (blue) and the ideal cubic unit cell of garnet (red).
The corresponding transformation matrix Tc→h = [1$\bar{1}$![]() 0 | 01$\bar{1}$
0 | 01$\bar{1}$![]() | ½½½] has its determinant det(T) = 3/2 that corresponds to the transition between the unit cell volumes of the real (rhombohedral in hexagonal axes) unit cell (V h) and the pseudocubic unit cell analogous to ‘common’ garnet-supergroup minerals (V pc). The latter value is equal to 1806.7 Å, which is comparable to the cubic unit cell volumes of grossular, andradite and katoite (1662.7, 1755.4 and 1897.4 Å3, respectively). The pseudocubic unit cell is a rhombohedron with the parameters a c = 12.180 Å and α = 89.89° (i.e. the rhombohedral distortion is within 0.12°).
| ½½½] has its determinant det(T) = 3/2 that corresponds to the transition between the unit cell volumes of the real (rhombohedral in hexagonal axes) unit cell (V h) and the pseudocubic unit cell analogous to ‘common’ garnet-supergroup minerals (V pc). The latter value is equal to 1806.7 Å, which is comparable to the cubic unit cell volumes of grossular, andradite and katoite (1662.7, 1755.4 and 1897.4 Å3, respectively). The pseudocubic unit cell is a rhombohedron with the parameters a c = 12.180 Å and α = 89.89° (i.e. the rhombohedral distortion is within 0.12°).
Cation coordination and cation arrays
The idealised crystal-chemical formula of nikmelnikovite, Ca12Fe2+Al4Fe3+2[SiO4]6(OH)20, can be rewritten as {Ca12}[Fe2+Al4Fe3+2](Si6)O24(OH)20, in order to conform with the general formula of garnet-supergroup mineral species, {X 3}[Y 2](Z 3)φ12, where X, Y and Z are cations in eightfold, octahedral and tetrahedral coordinations, respectively; φ = O, OH (Grew et al., Reference Grew, Locock, Mills, Galuskina, Galuskin and Hålenius2013). In contrast to the ideal garnet structure with the space group Ia $\bar{3}$![]() d, the nikmelnikovite structure is strongly modified through the formation of vacancies, cation ordering and substitution mechanisms. In this section, we briefly outline the modifications of cation arrays in the structure of nikmelnikovite in comparison to the ideal patterns observed in the Ia $\bar{3}$
d, the nikmelnikovite structure is strongly modified through the formation of vacancies, cation ordering and substitution mechanisms. In this section, we briefly outline the modifications of cation arrays in the structure of nikmelnikovite in comparison to the ideal patterns observed in the Ia $\bar{3}$![]() d structure type (archetype).
d structure type (archetype).
The crystal structure of nikmelnikovite contains two eightfold-coordinated X sites, Ca1 and Ca2, that are fully occupied by Ca. Cation arrays in the archetype and nikmelnikovite structures are shown in Fig. 4a and b, respectively. Except for slight geometrical distortions, the Ca arrays are identical and therefore are not involved in the transition between the two structure types.

Fig. 4. The relationship between cation arrays in grossular (a, c, e) and nikmelnikovite (b, d, f): the arrays of octahedral cations (a, b), Ca (c, d) and Si sites (e, f).
The array of octahedrally coordinated Y cations in the archetype structure (Fig. 4a) is a cubic body-centred lattice with the a Y parameter being half that of the ideal Ia $\bar{3}$![]() d unit cell. There are four octahedrally coordinated cation sites in nikmelnikovite, Al, Fe1, Fe2 and Mn, all together forming the same Y-cation array as in the archetype arrangement (Fig. 4b). However, in contrast to the latter, the array is split into four distinct sites with different site occupancies. The Al site is predominantly occupied by Al with the <Al–O> bond length of 1.922 Å. The Fe1 site has the <Fe1–O> bond length equal to 2.137 Å. Its site occupancy is consistent with Fe as the major component with a small admixture of Al. The <Fe1–O> bond length corresponds to the occupation of this site by Fe2+; this is most probably also the site that accommodates Mg2+. The average <Fe2–O> bond length of 1.993 Å and the refined site occupancy agrees well with its population by Fe3+ and Al. Finally, the Mn site is predominantly vacant. The assignment of Mn to this site is rather tentative, as this site may be occupied by Fe as well. The overall ratio of the octahedral sites is Al:Fe1:Fe2:Mn = 3:1:3:1, which provides eight cations in total, in agreement with the general formula of the garnet-supergroup mineral {X 3}[Y 2](Z 3)φ12 multiplied by four, {X 12}[Y 8](Z 12)φ48.
d unit cell. There are four octahedrally coordinated cation sites in nikmelnikovite, Al, Fe1, Fe2 and Mn, all together forming the same Y-cation array as in the archetype arrangement (Fig. 4b). However, in contrast to the latter, the array is split into four distinct sites with different site occupancies. The Al site is predominantly occupied by Al with the <Al–O> bond length of 1.922 Å. The Fe1 site has the <Fe1–O> bond length equal to 2.137 Å. Its site occupancy is consistent with Fe as the major component with a small admixture of Al. The <Fe1–O> bond length corresponds to the occupation of this site by Fe2+; this is most probably also the site that accommodates Mg2+. The average <Fe2–O> bond length of 1.993 Å and the refined site occupancy agrees well with its population by Fe3+ and Al. Finally, the Mn site is predominantly vacant. The assignment of Mn to this site is rather tentative, as this site may be occupied by Fe as well. The overall ratio of the octahedral sites is Al:Fe1:Fe2:Mn = 3:1:3:1, which provides eight cations in total, in agreement with the general formula of the garnet-supergroup mineral {X 3}[Y 2](Z 3)φ12 multiplied by four, {X 12}[Y 8](Z 12)φ48.
The Ia $\bar{3}$![]() d → R $\bar{3}$
d → R $\bar{3}$![]() symmetry transition is associated with the splitting of the single Si site in the archetype structure into two symmetrically non-equivalent sites in the derivative, Si1 and Si2. The archetypal and derivative Si arrangements are shown in Fig. 4e and f, respectively. The Si1 site in nikmelnikovite is fully occupied by Si with the <Si1–O> bond length of 1.650 Å, whereas the Si2 site has a site-occupancy factor (s.o.f.) of 0.10 with the <Si2–O> bond length equal to 1.87 Å. The configuration around Si2 is remarkably similar to that observed in in the Ia $\bar{3}$
symmetry transition is associated with the splitting of the single Si site in the archetype structure into two symmetrically non-equivalent sites in the derivative, Si1 and Si2. The archetypal and derivative Si arrangements are shown in Fig. 4e and f, respectively. The Si1 site in nikmelnikovite is fully occupied by Si with the <Si1–O> bond length of 1.650 Å, whereas the Si2 site has a site-occupancy factor (s.o.f.) of 0.10 with the <Si2–O> bond length equal to 1.87 Å. The configuration around Si2 is remarkably similar to that observed in in the Ia $\bar{3}$![]() d structure of katoite (Sacerdoti and Passaglia Reference Sacerdoti and Passaglia1985), where the Si site has the occupancy of 0.214 and the <Si–O> bond length is equal to 1.892 Å.
d structure of katoite (Sacerdoti and Passaglia Reference Sacerdoti and Passaglia1985), where the Si site has the occupancy of 0.214 and the <Si–O> bond length is equal to 1.892 Å.
‘Hydrogarnet’ substitution and short-range order in nikmelnikovite
It is usually implied that the ‘hydrogarnet’ substitution in garnets involves the substitution scheme (SiO4)4- ↔ (O4H4)4-, where a silicate tetrahedron is replaced by a tetrahedral cluster of four (OH)– groups (e.g. Geiger and Rossman Reference Geiger and Rossman2018, Reference Geiger and Rossman2020a,Reference Geiger and Rossmanb and references therein). It is noteworthy that such a configuration-to-configuration scheme is favoured by the cubic symmetry of the Ia $\bar{3}$![]() d garnets, where all O atoms are symmetrically equivalent and Si is located in the 24d Wyckoff site with the point symmetry $\bar{4}$
d garnets, where all O atoms are symmetrically equivalent and Si is located in the 24d Wyckoff site with the point symmetry $\bar{4}$![]() . The situation in nikmelnikovite is different, as the Si2 site has a trivial symmetry and its coordination environment is highly asymmetrical (Fig. 5). The O1, O2 and O3 sites are fully occupied and the occupancy of each site can be formulated as (OH)0.882O0.118; i.e. if the Si2 site is vacant, these sites are populated by hydroxyl groups (in agreement with the ‘hydrogarnet’ substitution scheme). In contrast, the O9 site has a low occupancy and seems to be occupied only if the Si2 site is occupied by Si. This site has the neighbouring O7 site at 1.60 Å; i.e. the simultaneous occupation of the O9 and O7 sites by O is impossible. In contrast to the O9 site with trivial symmetry, the O7 site is located on the threefold axis and its assigned occupancy is (OH)0.882. The substitution scheme for the O9 and O7 sites can therefore be written as 3O9O2– ↔ O7(OH)–. The total ‘hydrogarnet’ substitution in nikmelnikovite corresponds to the scheme:
. The situation in nikmelnikovite is different, as the Si2 site has a trivial symmetry and its coordination environment is highly asymmetrical (Fig. 5). The O1, O2 and O3 sites are fully occupied and the occupancy of each site can be formulated as (OH)0.882O0.118; i.e. if the Si2 site is vacant, these sites are populated by hydroxyl groups (in agreement with the ‘hydrogarnet’ substitution scheme). In contrast, the O9 site has a low occupancy and seems to be occupied only if the Si2 site is occupied by Si. This site has the neighbouring O7 site at 1.60 Å; i.e. the simultaneous occupation of the O9 and O7 sites by O is impossible. In contrast to the O9 site with trivial symmetry, the O7 site is located on the threefold axis and its assigned occupancy is (OH)0.882. The substitution scheme for the O9 and O7 sites can therefore be written as 3O9O2– ↔ O7(OH)–. The total ‘hydrogarnet’ substitution in nikmelnikovite corresponds to the scheme:
which results in the essential anion deficiency compared to the ‘ideal’ cubic garnets. For one formula unit, the substitution scheme can be re-written as

Fig. 5. The arrangement of anions around the Si2 site in nikmelnikovite.
This latter formulation agrees well with the empirical and idealised formulae of nikmelnikovite (see above; Krivovichev et al., Reference Krivovichev, Yakovenchuk, Panikorovskii, Savchenko, Pakhomovsky, Mikhailova, Selivanova, Kadyrova and Ivanyuk2019). Note that both equations (2) and (3) are not charge-balanced. In the crystal of nikmelnikovite that we studied, the charge balance is achieved via two mechanisms: (1) incorporation of trivalent cations into the Mn site and (2) incorporation of Al3+ into the Fe1 site occupied primarily by Fe2+. Thus, the total substitution scheme can be written as follows:

The 3O9O2– ↔ O7(OH)– substitution mechanism affects the coordination environments of cations surrounding the respective configuration. In particular, the coordination of the Ca2 atom changes from eightfold (the O9 site is occupied and the O7 site vacant) to sevenfold (the O7 site is occupied and the O9 site vacant). The same applies to the coordination of the Mn site, which seems to be occupied only if the O9 site is non-vacant. The other sites in nikmelnikovite are not affected by the ‘hydrogarnet’ substitution.
Discussion
Taking into account the results of this study, the crystal-chemical relations between the garnet archetype structure and the derivative nikmelnikovite structure can be described by the following series of imaginary modifications:
(1) The symmetry is lowered according to the Ia $\bar{3}$
 d → R $\bar{3}$
d → R $\bar{3}$ group–subgroup transition arising from the unit cell transformations defined by equation (1) above;
group–subgroup transition arising from the unit cell transformations defined by equation (1) above;(2) As a result, the cation sites are split according to the following sequences: X → {X1, X2}; Y → {Y1, Y2, Y3, Y4}; Z → {Z1, Z2};
(3) the X sites remain occupied by Ca as, e.g. in grossular, andradite and katoite;
(4) each Y site is occupied predominantly by a distinct chemical species: Y1 → Al (Al site), Y2 → Fe2+ (Fe1 site), Y3 → Fe3+ (Fe2 site) and Y4 → vacancy (Mn site);
(5) one of the Z sites (Z1) remains occupied by Si, whereas the other site (Z2) is predominantly vacant; the modification of its coordination environment is described by equation (2) above.
The resulting structure of nikmelnikovite (Fig. 6) represents a porous three-dimensional octahedral–tetrahedral framework with cavities occupied by Ca2+ and H+ cations. We note that further structural variations are possible in the garnet supergroup with the formation of various derivative octahedral–tetrahedral framework topologies.

Fig. 6. The crystal structure of nikmelnikovite viewed along the c axis (only coordination polyhedra of cation sites with occupancies >50% are shown).
The complete transformation from the ideal garnet structure {X 3}[Y 2](Z 3)φ12 to that of nikmelnikovite can be described as

The latter formula takes into account the empirical Al:Fe ratio observed in nikmelnikovite. The more strict end-member formula (Bosi et al., Reference Bosi, Hatert, Hålenius, Pasero, Miyawaki and Mills2019) should be written as X{Ca12}Y[Fe2+Al3Fe3+3□]Z(Si6□6)O24(OH)20□4.
The crystal-chemical formula of nikmelnikovite refined from the crystal structure can be written as Ca12(Al2.79Fe3+0.21)(Fe2+0.89Al0.11)(Fe3+1.89Al1.11)(□0.882Mn0.118)(SiO4)6.708(OH)17.64 or Ca12Al4.01Fe3+2.10Fe2+0.89Mn0.118(SiO4)6.708(OH)17.64. This formula is in general good agreement with the empirical formula determined by the electron microprobe analysis reported by Krivovichev et al. (Reference Krivovichev, Yakovenchuk, Panikorovskii, Savchenko, Pakhomovsky, Mikhailova, Selivanova, Kadyrova and Ivanyuk2019) (see Introduction).
The low symmetry of nikmelnikovite that arises from complex substitutions and ordering phenomena is most likely the result of its low-temperature crystallisation. Nikmelnikovite was precipitated on the surface of primary andradite crystals, probably by heterogeneous nucleation through epitaxy, as noted previously for H4O4-substituted vesuvianite epitaxially grown on wiluite (Panikorovskii et al., Reference Panikorovskii, Krivovichev, Galuskin, Shilovskikh, Mazur and Bazai2016). It is noteworthy that the structural complexity of nikmelnikovite (4.529 bit/atom and 434.431 bit/cell, after H-correction: Krivovichev et al., Reference Krivovichev2013, Reference Krivovichev, Krivovichev and Hazen2018) is higher than those of andradite, grossular (1.595 bit/atom and 127.637 bit/cell) and katoite (1.658 bit/atom and 192.315 bit/cell), which is the result of its lower symmetry and the greater number of structure sites. Such an increase in structural complexity is typical for low-temperature minerals formed after primary minerals with simpler structures.
It should be noted that low-symmetry birefringent garnets have been observed and studied previously (Shtukenberg et al., Reference Shtukenberg, Popov and Punin2005; Frank-Kamenetskaya et al., Reference Frank-Kamenetskaya, Rozhdestvenskaya, Shtukenberg, Bannova and Skalkina2007). A comprehensive review of the relevant literature was provided recently by Tančić et al. (Reference Tančić, Kremenović and Vulić2020). However, nikmelnikovite is the first distinct garnet-supergroup member with a trigonal symmetry.
Acknowledgements
We are grateful to Evgeny Galuskin, Peter Leverett, and two anonymous referees for useful comments and Anton Chakhmouradian for correcting the English. This work was supported through the President of Russian Federation grant for young candidates of sciences (experimental work, grant MK-6240.2021.1.5 to T.L.P.) and Russian Science Foundation (theoretical analysis, grant 19-17-00038 to S.V.K.).
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1180/mgm.2021.55