Introduction
Numerous guano deposits occur in the Atacama Desert, Chile, in a narrow band (69°30’ to 70°10'W by 19°30’ to 26°S) attached to the Coastal Range composed of Late Paleozoic and Mesozoic igneous rocks and stretching along the northern coast of Chile. Most of these deposits are situated on low-lying hills that were formerly islands. Some details of the geological setting and the mining history of guano deposits in the Tarapacá region are published elsewhere (Ericksen, Reference Ericksen1981; Pankhurst and Herve, Reference Pankhurst, Herve, Moreno and Gibbons2007; Appelton and Nothold, Reference Appleton and Nothold2002; Bojar et al., Reference Bojar, Walter, Baumgartner and Färber2010).
The guano deposit located on Pabellón de Pica Mountain, 1.5 km south of Chanabaya village, Iquique Province, Tarapacá Region, Chile (20°55'S, 70°08'W) has become known in the last decade as an interesting, very unusual mineralogical object being the type locality of several nitrogen-bearing and organic minerals: ammineite, CuCl2(NH3)2 (Bojar et al., Reference Bojar, Walter, Baumgartner and Färber2010), joanneumite, Cu(C3N3O3H2)2(NH3)2 (Bojar and Walter, Reference Bojar and Walter2012), chanabayaite, Cu4(N3C2H2)4(NH3)4Cl2(Cl,OH)2·H2O (Chukanov et al., Reference Chukanov, Zubkova, Möhn, Pekov, Pushcharovsky and Zadov2015a), shilovite, Cu(NH3)4(NO3)2 (Chukanov et al., Reference Chukanov, Britvin, Möhn, Pekov, Zubkova, Nestola, Kasatkin and Dini2015b), antipinite, KNa3Cu2(C2O4)4 (Chukanov et al., Reference Chukanov, Aksenov, Rastsvetaeva, Lysenko, Belakovskiy, Färber, Möhn and Van2015c) and möhnite, (NH4)K2Na(SO4)2 (Chukanov et al., Reference Chukanov, Aksenov, Rastsvetaeva, Pekov, Belakovskiy and Britvin2015d).
Specimens with the new mineral triazolite, NaCu2(N3C2H2)2(NH3)2Cl3·4H2O, were collected at Pabellón de Pica in 2012 by one of the authors (G.M.). The mineral is named for the presence of 1,2,4-triazolate anion.
The new mineral and its name were approved by the International Mineralogical Association Commission on New Minerals, Nomenclature and Classification (IMA2017-025 Chukanov et al., Reference Chukanov, Zubkova, Möhn, Pekov, Belakovskiy, Van, Britvin and Pushcharovsky2017). The holotype specimen is deposited in the collection of the Fersman Mineralogical Museum of the Russian Academy of Sciences, Moscow, Russia, with the registration number 5037/1.
Occurrence, general appearance and physical properties
Triazolite occurs in the contact zone between altered guano and the host rock, a gabbro which consists of amphibole, plagioclase and minor clinochlore and containing accessory chalcopyrite. Well-shaped prismatic crystals of triazolite up to 0.1 mm × 0.15 mm × 0.75 mm elongated along b and their radial aggregates up to 1.5 mm in diameter (Fig. 1) occur in cavities in aggregates of salammoniac (with subordinate halite) near the contact of a guano deposit with the host rock, or grow over the surface of the host rock. Other associated minerals are dittmarite, joanneumite, chanabayaite, nitratine, natroxalate and möhnite. Nitratine is present as an interspersed trace admixture. Joanneumite is observed in association with triazolite only sporadically.

Fig. 1. Aggregates of triazolite crystals on salammoniac (field of view 2.2 mm). Photograph: M. Burkhardt.
The observed forms of triazolite crystals are: pinacoids {001}, {010} and {100} and prism {hk0} or {0kl}.
The colour of the new mineral is deep blue. The streak is blue. Crystals of triazolite are translucent, with a vitreous lustre. The new mineral is brittle, with a Mohs hardness of 2. Cleavage is perfect on (001) and imperfect on (100) and (010). No parting is observed. The fracture across the perfect cleavage plane is uneven. Triazolite is non-fluorescent in ultraviolet light.
All attempts to measure the density of triazolite by flotation in heavy liquids gave an unsatisfactory, definitely erroneously low value ~1.85 g cm–3. This could be explained by the fact that triazolite crystals, although appearing perfect (Fig. 1), contain many microcracks (Fig. 2). In addition, micro-inclusions of salammoniac are common in triazolite crystals that could also decrease the bulk measured density. The density calculated using the empirical formula is 2.028 g cm–3.
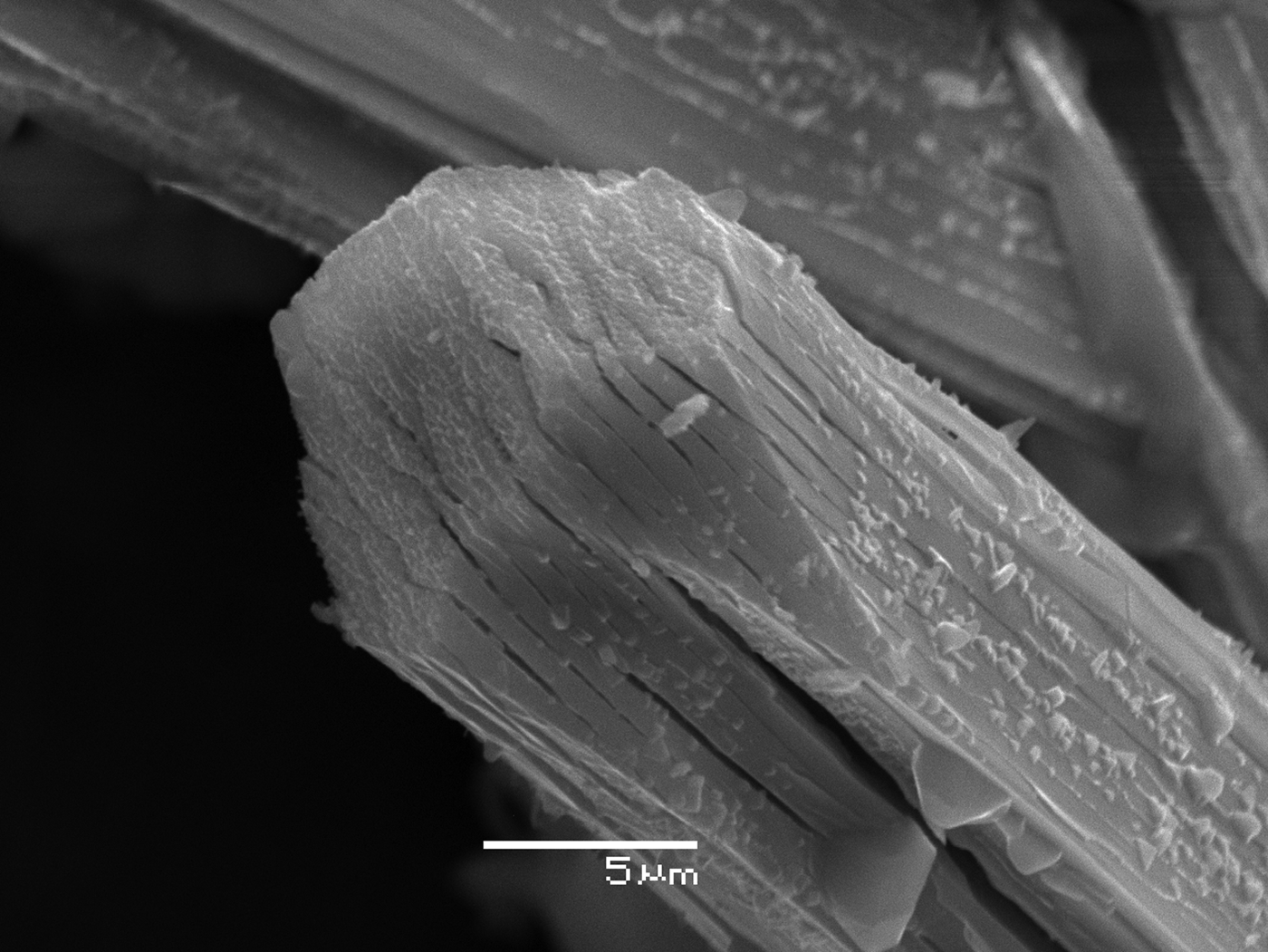
Fig. 2. Scanning electron microscopy image of a triazolite crystal.
Triazolite is optically biaxial (–), α = 1.582(4), β = 1.625(3), γ = 1.625(3), 2Vmeas = 5(3)° and 2Vcalc. ≈ 0° (λ = 589 nm). The dispersion of optical axes is not observed. The orientation is: X = b. Pleochroism is distinct: Y (violet) > X ≈ Z (greenish-blue).
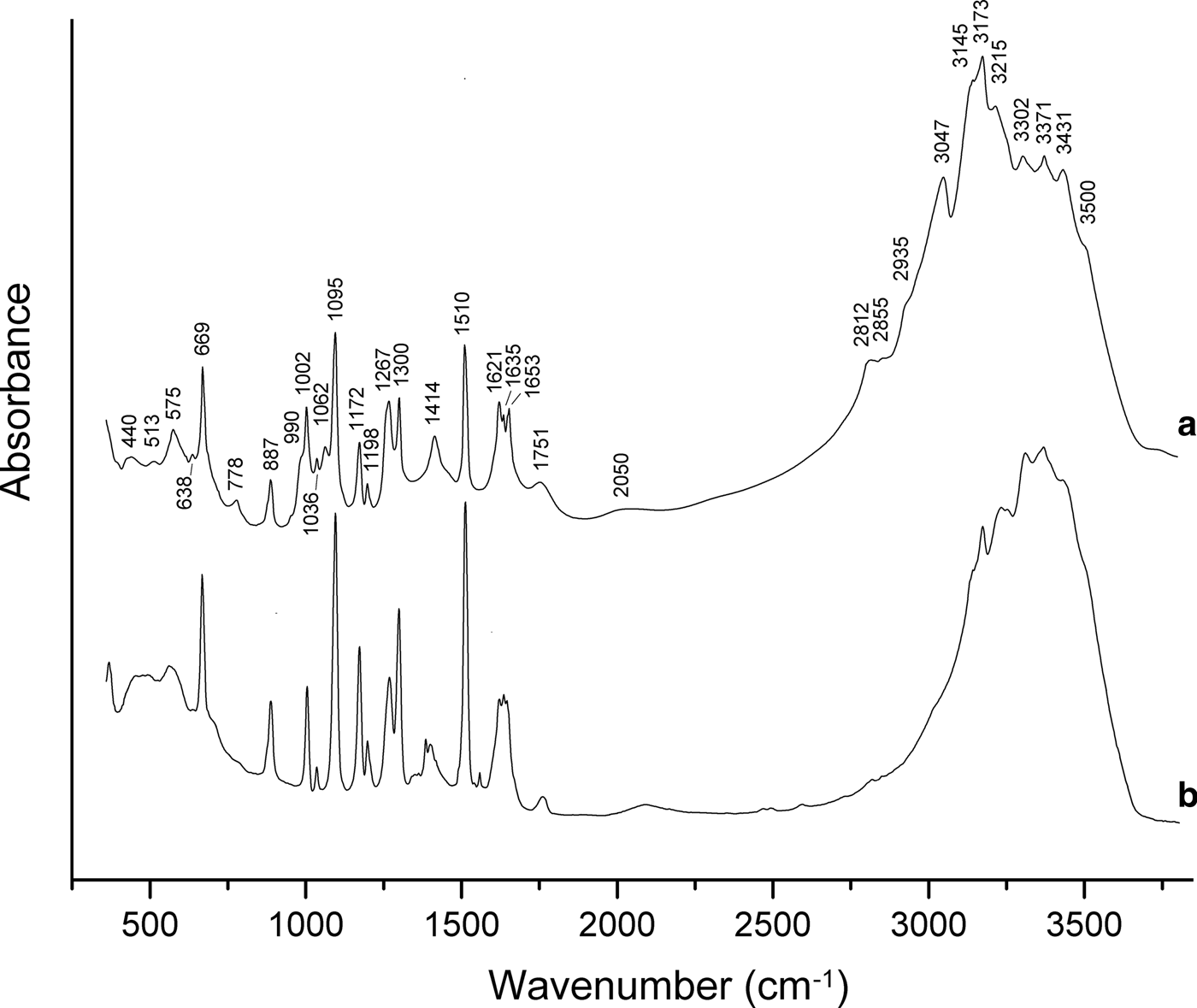
In order to obtain infrared (IR) absorption spectra (Fig. 3), powdered samples were mixed with dried KBr, pelletized and analysed using an ALPHA FTIR spectrometer (Bruker Optics) with a resolution of 4 cm–1. Sixteen scans were obtained. The IR spectrum of an analogous pellet of pure KBr was used as a reference.
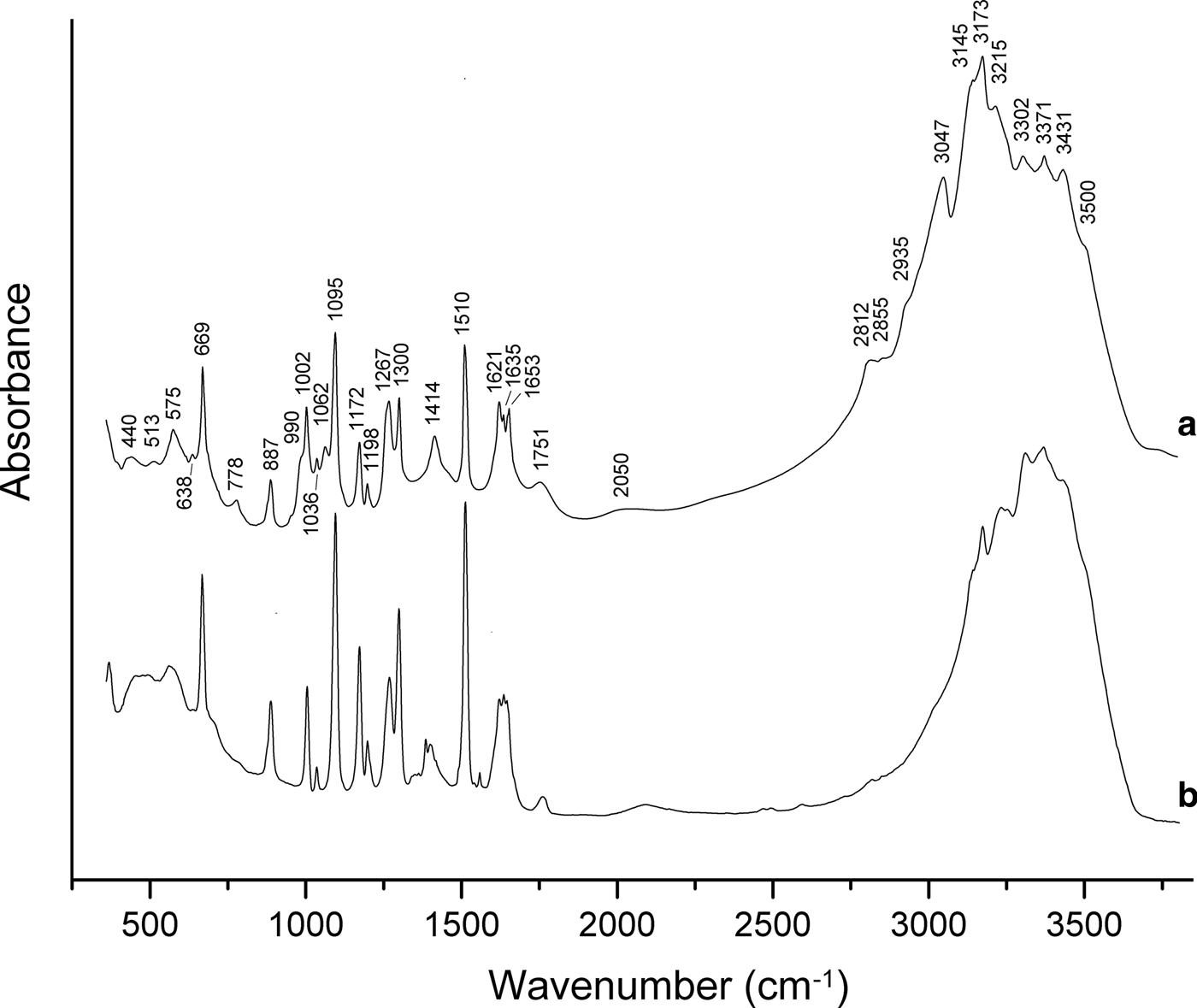
Fig. 3. Powder IR absorption spectra of (a) triazolite and (b) chanabayaite.
The assignment of absorption bands made in accordance with Grinshtein et al. (Reference Grinshtein, Strazdin and Grinvalde1970), Nakamoto (Reference Nakamoto2008, Reference Nakamoto2009), Chukanov et al. (Reference Chukanov, Zubkova, Möhn, Pekov, Pushcharovsky and Zadov2015a) and Chukanov and Chervonnyi (Reference Chukanov and Chervonnyi2016) is as follows. Strong bands in the range 3100–3500 cm–1 correspond to N–H and O–H stretching vibrations. The bands in the range 2800–3100 cm–1 correspond to C–H stretching vibrations of the 1,2,4–triazolate anion, possibly combined with N–H stretching vibrations. Bands in the range 1620–1660 cm–1 are due to degenerate bending vibrations of NH3 and bending vibrations of H2O molecules. Strong bands in the range 1095–1510 cm–1 correspond to in-plane stretching and mixed vibrations of the 1,2,4-triazolate ring and to symmetrical bending vibrations of NH3 molecules. The bands in the range 990–1062 cm–1 are assigned to in-plane bending vibrations of C–H bonds. The band at 887 cm–1 corresponds to in-plane bending vibrations of the 1,2,4-triazolate ring. Bands below 800 cm–1 are due to out-of-plane vibrations of the 1,2,4-triazolate anion, rocking vibrations of NH3 and librational vibrations of H2O molecules.
The broad bands at 1751 and 2050 cm–1 correspond to acid groups which may arise e.g. as a result of partial protonation of triazolate anions in accordance with the dynamic equilibrium C2N3![]() ${\rm H}_{\rm 2}^\ndash $ + H2O ↔ C2N3H3 + OH–.
${\rm H}_{\rm 2}^\ndash $ + H2O ↔ C2N3H3 + OH–.
Chemical data
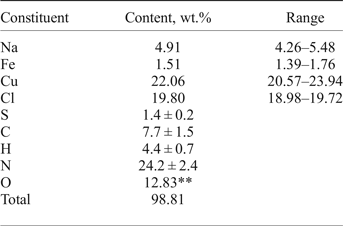
Chemical analyses (four for EMPA) were carried out using an electron microprobe (EDS mode, 20 kV, 600 pA, beam rastered on an area 10 µm × 10 µm in order to minimize unstable sample damage) for Na, Cu, Fe and Cl. The standards used are: albite for Na, metallic iron for Fe, CuFeS2 for Cu and NaCl for Cl. Attempts to use the wavelength dispersive mode, with higher beam current, were unsuccessful because of the instability of the mineral. H, N, C and S were analysed by gas chromatography of products of ignition in oxygen at 1200°C with a Vario Micro cubeanalyser (Elementar GmbH, Germany). Structural data and chemical tests show the absence of ![]() ${\rm CO}_{\rm 3}^{2\ndash} $ anions. All carbon belongs to the triazolate anion. Analytical data are given in Table 1.
${\rm CO}_{\rm 3}^{2\ndash} $ anions. All carbon belongs to the triazolate anion. Analytical data are given in Table 1.
Table 1. Chemical composition of triazolite*.
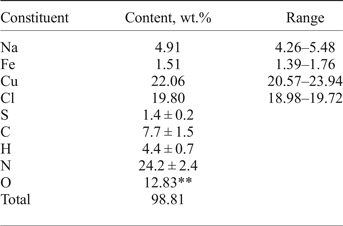
*Contents of other elements with an atomic number > 6 are below detection limits; **calculated from the idealized formula.
The empirical formula calculated on the basis of Cu + Fe = 2 apfu [because occupancy of the (Cu,Fe) sites is 100%] is Na1.14(Cu1.86Fe0.14)Σ2.0N9.23C3.43H23.34O4.29(Cl2.99S0.23)Σ3.22. Taking into account that the presence of organic minerals indicates mineral formation under reducing conditions, admixed sulfur is considered as S2–. The idealized formula is NaCu2(N3C2H2)2(NH3)2Cl3·4H2O (Z = 4; see description of the crystal structure), which requires Na 4.61, Cu 25.48, Cl 21.33, N 22.48, C 9.63, H 3.64, O 12.83, total 100.00 wt.%.
Reactions of triazolite solution in 20% HCl with potassium hexacyanoferrate(II) and potassium hexacyanoferrate(III) show that all the Cu in the mineral is bivalent. Triazolite dissolves in dilute hydrochloric and nitric acids without gas evolution. It is insoluble in water, but crystals of triazolite become dull after exposure to warm water. Heating in a closed tube gives a white sublimate of NH4Cl. Melting is accompanied by the release of water and an additional portion of sublimate. Stronger heating results in reduction to metallic Cu.
X-ray diffraction and crystal structure
Powder X-ray diffraction data (Table 2) were collected using a Stoe IPDS II image plate diffractometer (MoKα radiation, Gandolfi mode). Orthorhombic unit-cell parameters refined from the powder data are: a = 19.319(5), b = 7.149(2), c = 12.519(4) Å and V = 1729(1) Å3.
Table 2. Powder X-ray diffraction data of triazolite.
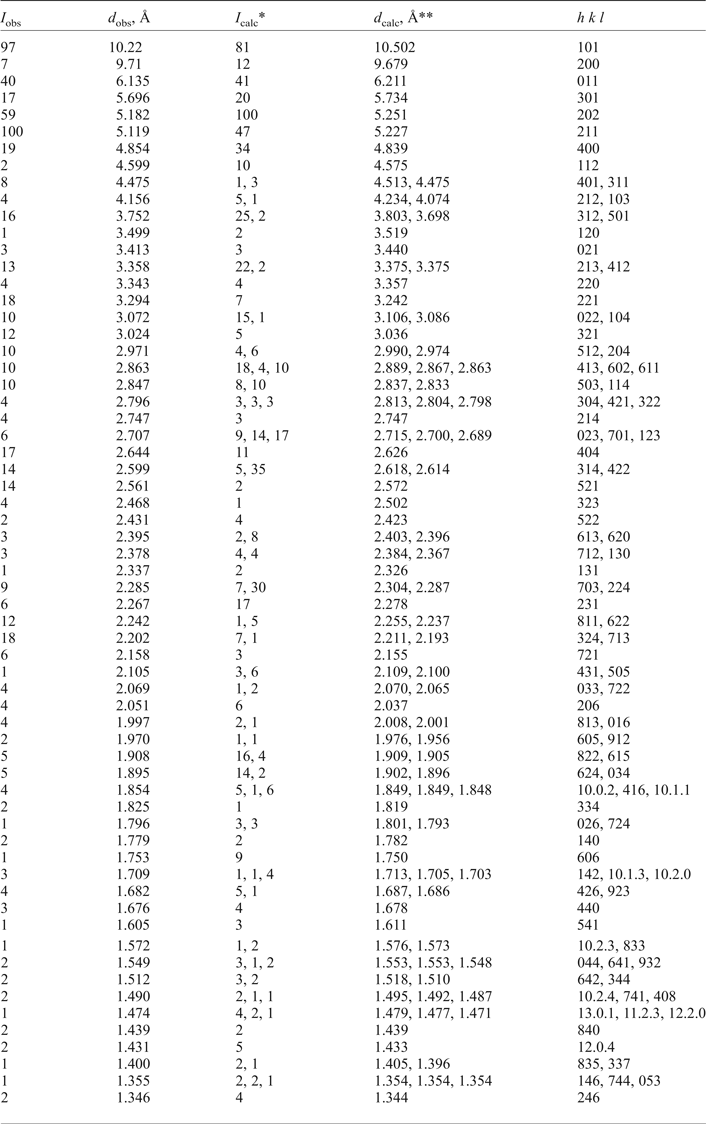
*Only reflections with I calc ≥ 1 are taken into account; **calculated with the unit-cell parameters obtained from single-crystal data.
Single-crystal X-ray studies were carried out using a Bruker Kappa X8 APEX CCD diffractometer (Institute of Mineralogy and Crystallography, University of Vienna), MoKα-radiation (λ = 0.71073 Å). A full sphere of three-dimensional data was collected at T = 200 K. Triazolite is orthorhombic, space group P212121, a = 19.3575(5), b = 7.15718(19), c = 12.5020(4) Å, V = 1732.09(8) Å3 and Z = 4. The crystal structure was solved by direct methods and refined with the use of SHELX software (Sheldrick, Reference Sheldriсk2008) to the final R = 0.0242 for 4210 unique reflections with I > 2σ(I).
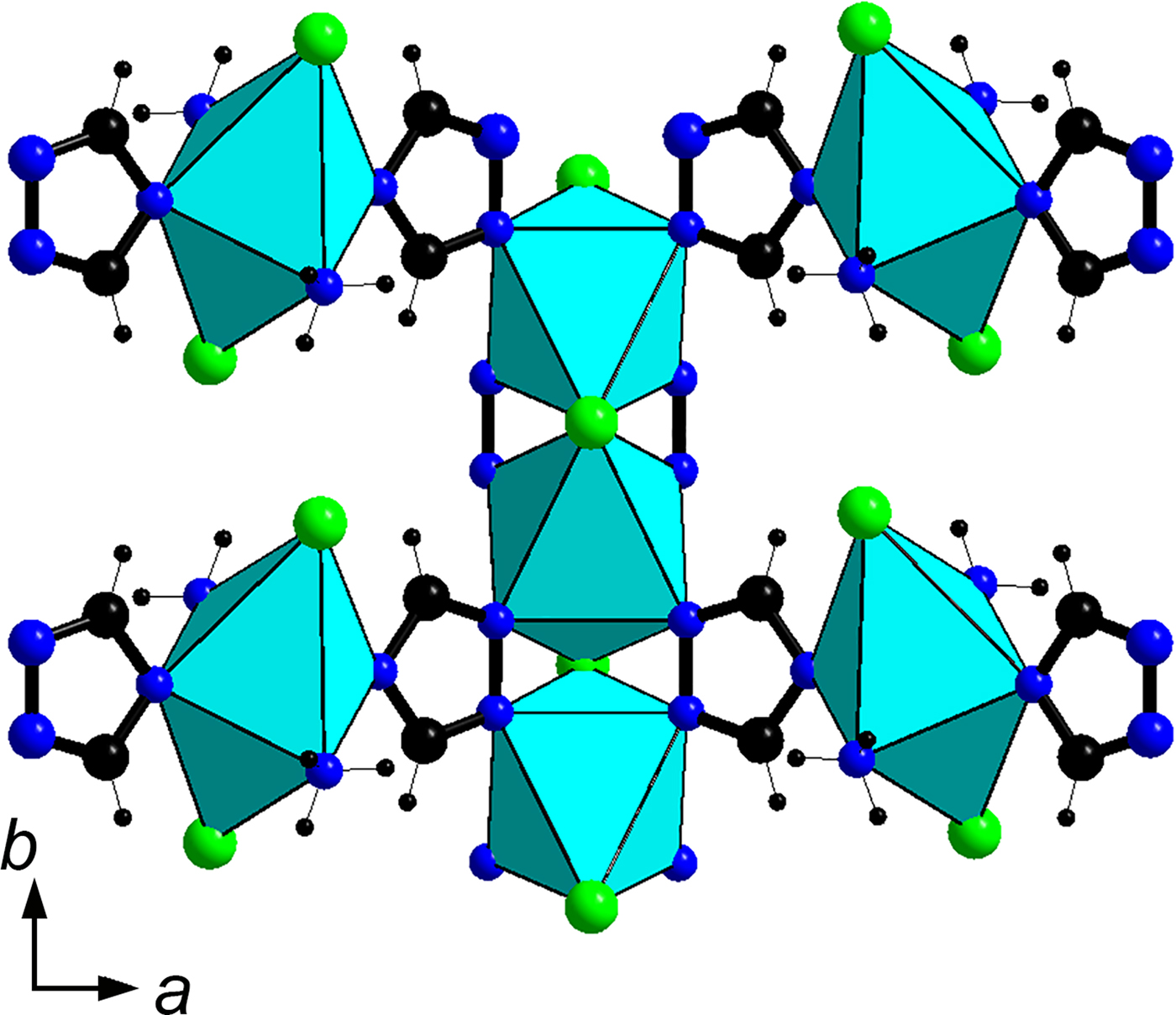
A detailed crystal-structure description is given by Zubkova et al. (Reference Zubkova, Chukanov, Pekov, Möhn, Giester, Yapaskurt, Lykova and Pushcharovsky2016). The crystal structure of triazolite (Fig. 4) contains zig-zag chains of the corner-sharing CuN4Cl2 octahedra (with N atoms belonging to four 1,2,4-triazole rings) running along the b axis and isolated Cu2+N4Cl2 octahedra (with two N atoms belonging to two 1,2,4-triazole rings and two N atoms of ammonia molecules NH3) connected with the chains via 1,2,4-triazolate anions C2N3![]() ${\rm H}_{\rm 2}^\ndash $. Na(H2O)6 octahedra share edges to form columns parallel to the b axis (Fig. 5). The system of hydrogen bonds formed by H2O and NH3 molecules connects isolated Cu-centred octahedra with each other and with the columns of Na(H2O)6 octahedra as well as the octahedral chains built by Cu and Na polyhedra.
${\rm H}_{\rm 2}^\ndash $. Na(H2O)6 octahedra share edges to form columns parallel to the b axis (Fig. 5). The system of hydrogen bonds formed by H2O and NH3 molecules connects isolated Cu-centred octahedra with each other and with the columns of Na(H2O)6 octahedra as well as the octahedral chains built by Cu and Na polyhedra.
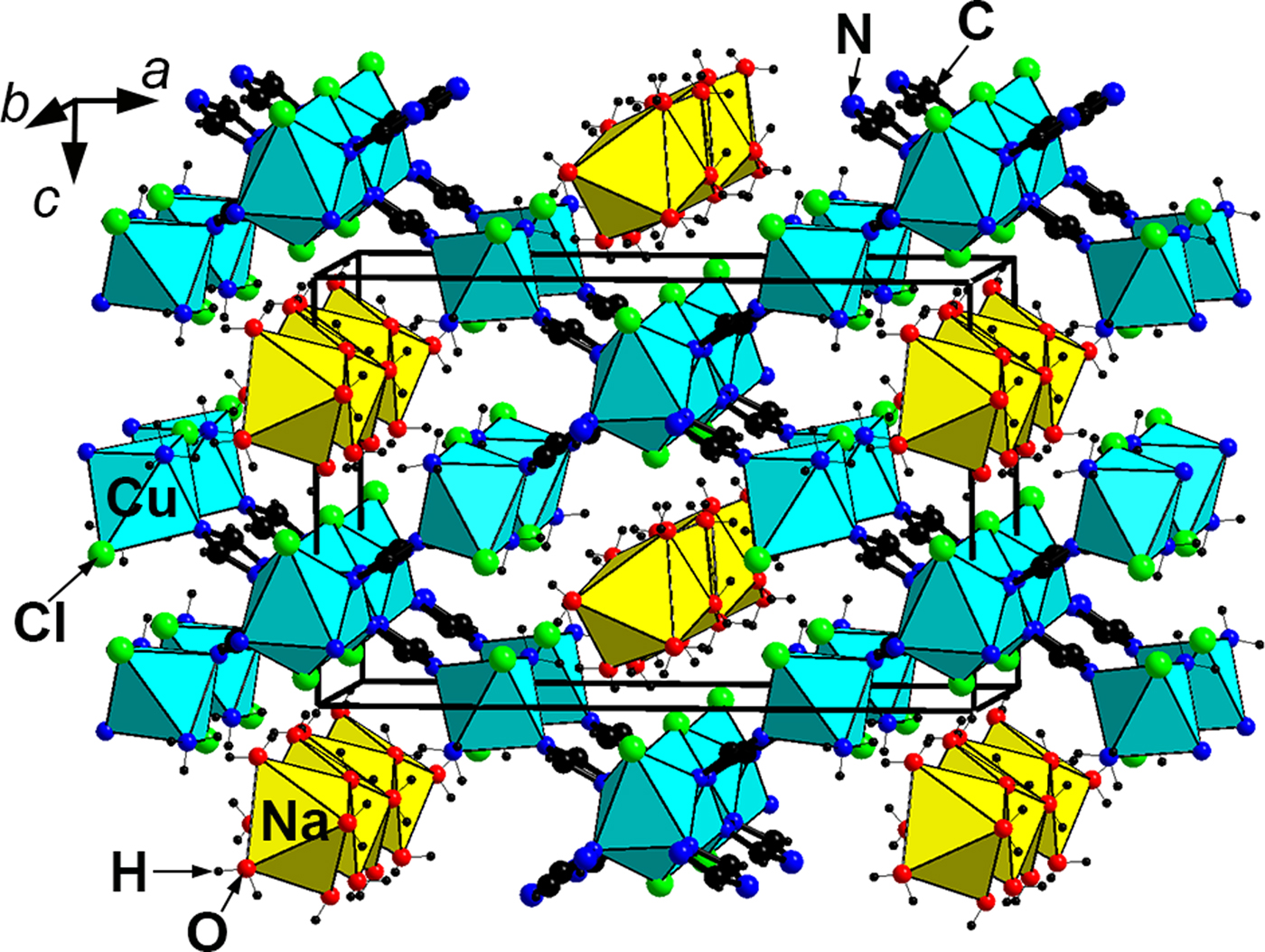
Fig. 4. The crystal structure of triazolite.

Fig. 5. A fragment of the triazolite structure showing mutual alignment of the zig-zag chain of Cu-centred octahedra and isolated Cu-centred octahedra connected via 1,2,4-triazolate anions. For better clarity, only the connection on the upper side of the chain is shown.
Discussion
Usually nitrogen is present in minerals as a ![]() ${\rm NO}_{\rm 3}^\ndash $ anion or N
${\rm NO}_{\rm 3}^\ndash $ anion or N![]() ${\rm H}_{\rm 4}^ + $ cation. There are only five minerals containing the neutral ammonia molecule
${\rm H}_{\rm 4}^ + $ cation. There are only five minerals containing the neutral ammonia molecule ![]() ${\rm NH}_{\rm 3}^0 $ as a species-defining component: ammineite, joanneumite, shilovite, chanabayaite and triazolite. All these species have been discovered at Pabellón de Pica, and in all these minerals ammonia molecules coordinate Cu2+. Note also that chanabayaite and triazolite are currently the only known minerals containing N–N bonds, even though the chemical properties and crystal chemistry of numerous synthetic triazole and triazolate complexes have been investigated in detail.
${\rm NH}_{\rm 3}^0 $ as a species-defining component: ammineite, joanneumite, shilovite, chanabayaite and triazolite. All these species have been discovered at Pabellón de Pica, and in all these minerals ammonia molecules coordinate Cu2+. Note also that chanabayaite and triazolite are currently the only known minerals containing N–N bonds, even though the chemical properties and crystal chemistry of numerous synthetic triazole and triazolate complexes have been investigated in detail.
A review of metal coordination compounds with 1,2,4-triazole derivatives as ligands is given by Haasnoot (Reference Haasnoot2000). In the 5-membered 1,2,4-triazole ring all C–N and N–N bonds are conjugated and have fractional bond orders between 1 and 2. As a result, C–N and N–N bond lengths are rather short (typically, between 1.30 and 1.38 Å). Deprotonated 1,2,4-triazole (= triazolate anion) is known as an active acyl transfer catalyst suitable for the aminolysis and transesterification of esters (Yang and Birman, Reference Yang and Birman2009). Some transition elements (copper, zinc, iron, etc.) show high affinity for the triazolate ligand (Hasnoot, Reference Haasnoot2000). Usually triazolate complexes contain additional ligands that stabilize their crystal structures. In triazolite such ligands are ![]() ${\rm NH}_{\rm 3}^0 $ and Cl–.
${\rm NH}_{\rm 3}^0 $ and Cl–.
Triazolite is a supergene mineral formed in the contact zone between an altered guano deposit and chalcopyrite-bearing gabbro. Guano was the source of organic matter and nitrogen. Oxidized chalcopyrite was the source of Cu. The high affinity of Cu for N as a ligand, including N in the 1,2,4-triazolate anion and NH3, promoted the formation of triazolite.
Triazolite is related structurally to chanabayaite (in part of the topology of the main structural unit containing zig-zag chains of the corner-sharing Cu-centred octahedra connected with isolated Cu-centred octahedra via 1,2,4-triazolate anions), which is a product of triazolite alteration as the result of dehydration and leaching of Na and a part of Cl (Zubkova et al., Reference Zubkova, Chukanov, Pekov, Möhn, Giester, Yapaskurt, Lykova and Pushcharovsky2016). Comparative data for triazolite and chanabayaite are given in Table 3.
Table 3. Comparative data for triazolite and chanabayaite.

Acknowledgements
The authors acknowledge Prof. Gerald Giester for his help in obtaining single-crystal X-ray diffraction data and Peter Leverett and Ian Grey for reviewing the paper, helpful comments and corrections. The authors thank the X-ray Diffraction Centre of Saint-Petersburg State University for instrumental and computational resources. This study was supported by the Russian Scientific Foundation, grant no. 14-17-00048.