Introduction
Sulfate minerals are common products associated with volcanism and geothermal activity throughout the world and include hydrous sulfate minerals (e.g. gypsum and alunogen) and anhydrous sulfate minerals (e.g. anhydrite and baryte). Such minerals have been found in various terrestrial environments, including fumaroles (Africano and Bernard, Reference Africano and Bernard2000; Hynek et al., Reference Hynek, McCollom, Marcucci, Brugman and Rogers2013; Adams et al., Reference Adams, Lynch, Buckland, Johnson and Tratt2017), active crater lakes (Pierre and Alain, Reference Pierre and Alain1994; Rodríguez and van Bergen, Reference Rodríguez and van Bergen2017), hot springs (Bonny and Jones, Reference Bonny and Jones2003; Sakae et al., Reference Sakae, Okada, Okamura and Nakano2011; Tang et al., Reference Tang, Ehreiser and Li2014), caves in geothermal or volcanic settings (Rodgers et al., Reference Rodgers, Hamlin, Browne, Campbell and Martin2000; White, Reference White2010), and other environments with no volcanism or geothermal activities (Fernández-Remolar et al., Reference Fernández-Remolar, Morris, Gruener, Amils and Knoll2005; Stracher et al., Reference Stracher, Prakash, Schroeder, McCormack, Zhang, Van Dijk and Blake2005; Valente and Gomes, Reference Valente and Gomes2009). Apart from some sulfate minerals precipitated from aqueous solutions (superficial water bodies), most of these minerals in volcanic and/or geothermal environments are ordinarily present as fumarolic encrustations, volcanic sublimates and efflorescences (Stoiber and Rose, Reference Stoiber and Rose1974; Zhu et al., Reference Zhu, Tong and You1980; Ciesielczuk et al., Reference Ciesielczuk, Zaba, Bzowska, Gaidzik and Glogowska2013; Piochi et al., Reference Piochi, Mormone, Balassone, Strauss, Troise and De Natale2015; Rodríguez and van Bergen, Reference Rodríguez and van Bergen2016). Unlike sulfate minerals deposited in seas or lakes, the formation mechanisms of these sulfate minerals are not solely confined to the evaporation of water under ambient conditions. Alteration of rocks by gas condensate on volcanoes is considered to be the primary formation mechanism (Kodosky and Keskinen, Reference Kodosky and Keskinen1990; Africano and Bernard, Reference Africano and Bernard2000; McCollom et al., Reference McCollom, Hynek, Rogers, Moskowitz and Berquo2013). In addition, several other less common formation mechanisms of volcanic/fumarolic sulfate minerals have also been proposed, such as capillary action with subsequent evaporation of pore water, deposition of vapours around volcanic vents, precipitation from thermal spring water, and acid-fog deposition around active volcanoes (Delines, Reference Delines1975; Martin et al., Reference Martin, Rodgers and Browne1999; Bonny and Jones, Reference Bonny and Jones2003; Schiffman et al., Reference Schiffman, Zierenberg, Marks, Bishop and Dyar2006).
At the Zhenzhu Spring (also known as Pearl Spring or Zhenzhuquan), located in Tengchong, Yunnan Province, southwestern China (Fig. 1), abundant sulfate deposits have formed around the spring (Fig. 1d), which are located in a subaerial, acid and humid environment. The acid aerosols (fog/steam), derived from pool water, are copious and appear to be related to the formation of the sulfate minerals. However, the mineralogical and chemical features, and the genesis of these minerals, remain unclear. Previous investigations of the Zhenzhu Spring have only focused on the spring water and gas geochemistry (e.g. Shangguan and Huo, Reference Shangguan and Huo2002; Zhang et al., Reference Zhang, Liu, Liu, Jin and Han2008; Guo et al., Reference Guo, Liu, Li, Zhang and Wang2014), and microbiology (e.g. Hou et al., Reference Hou, Wang, Dong, Jiang, Briggs, Peacock, Huang, Huang, Wu, Zhi, Li, Dodsworth, Hedlund, Zhang, Hartnett, Dijkstra and Hungate2013; Briggs et al., Reference Briggs, Brodie, Tom, Dong, Jiang, Huang, Wang, Hou, Wu, Huang, Hedlund, Zhang, Dijkstra and Hungate2014; Liu et al., Reference Liu, Salam, Jiao, Jiang, Zhou, Yin, Ming and Li2016). This investigation mainly focuses on the sulfate minerals in contact areas of acid aerosol and rock. Using samples collected from the Zhenzhu Spring, this research: (1) investigates the spring water hydrochemistry; (2) describes the distribution, mineralogical composition, crystal morphology, major-element geochemistry and sulfur-oxygen isotope signatures of subaerial minerals; and (3) compares the sulfur-oxygen composition of sulfate minerals from the Zhenzhu Spring with those from the Rehai geothermal field and other modern steam-heated environments. By combining this information, this investigation provides valuable insights into the formation mechanism of sulfate minerals at the Zhenzhu Spring and the sulfur-oxygen isotope geochemistry of the Rehai hydrothermal system.
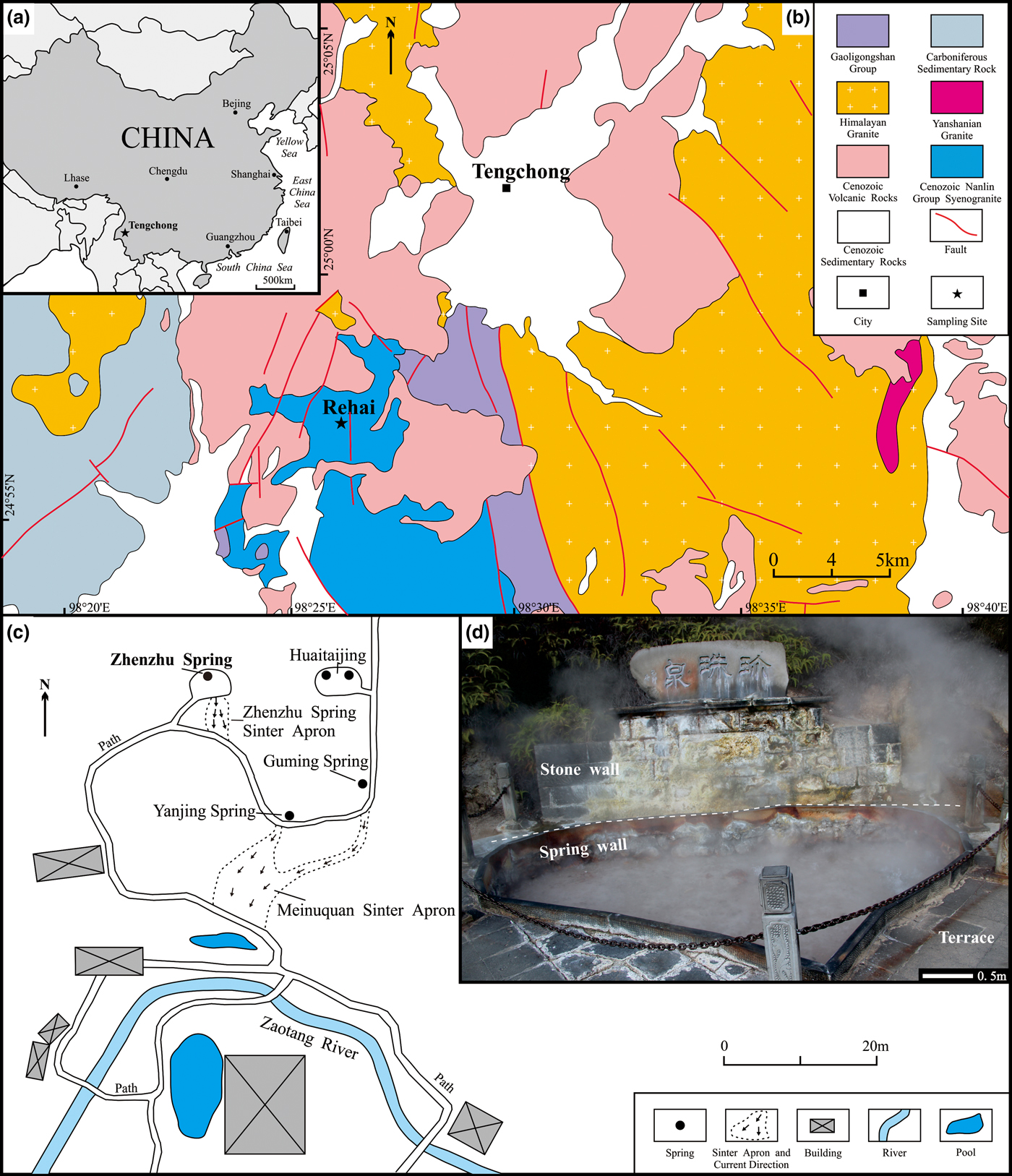
Fig. 1. Location of the Zhenzhu Spring. (a) Location of Tengchong in western Yunnan, southwest China. (b) Geological map of the area around the Rehai geothermal field where the Zhenzhu Spring is located, showing the distribution of granitic rocks in the Rehai geothermal field. (c) Map of the Zhenzhu Spring in the Rehai geothermal field, showing the locations of the Zhenzhu Spring and adjacent springs and sinter deposits. (d) General view of the heart-shaped Zhenzhu Spring showing the spring wall that surrounds the vent pool and the stone wall near the pool.
General setting
The Tengchong volcanic and geothermal field lies to the southeast of the Tibetan Plateau, in the western Yunnan Province, China (Fig. 1a). Sixty eight volcanoes exist in Tengchong (Zou et al., Reference Zou, Fan, Schmitt and Sui2010). The volcanoes developed along the Gaoligong dextral strike-slip fault and near its intersection with the Ruili-Mandalay sinistral strike-slip fault (Huang et al., Reference Huang, Zhou, Wang, Robinson, Zhao and Qi2013). There are three famous volcanoes in Tengchong: Dayingshan; Maanshan; and Heikongshan. The volcanic zone is quite active; the latest eruption of Maanshan volcano, the youngest volcano, took place only ~3800 years ago (Jiang, Reference Jiang1998). Tengchong has a wide distribution of volcanic rocks with an outcrop of up to 1000 km2 (Fig. 1b), including basalt, basaltic andesite, dacite and andesite (Jiang et al., Reference Jiang, Li, Tu, Wei, Zhang, Wang and Dai2018). Tengchong has a highland subtropical climate and can be broadly divided into two seasons: the dry season from October to April and the wet season extending from May to September (Jones and Peng, Reference Jones and Peng2015). Temperature, rainfall and sunshine variations are apparent between the dry season (generally 8 to 18°C, 0 to 150 mm/month, 170 to 270 hours/month, respectively) and the wet season (generally 18 to 22, 140 to 340 mm/month, 70 to 180 hours/month, respectively) in Tengchong.
The Rehai geothermal field, located ~11 km southwest of Tengchong city (Fig. 1b), has an area of ~10 km2. A large number of springs, with water temperatures varying from 42 to 96°C (Guo, Reference Guo2012), occur at the Rehai geothermal field, distributed mainly in the valley of Zaotanghe River (Zhang et al., Reference Zhang, Liu, Liu, Jin and Han2008). Previous research (e.g. Hong et al., Reference Hong, Zhang, Jin, Liu, Han and Ling2009; Guo et al., Reference Guo, Liu, Li, Zhang and Wang2014) shows that most of the springs have near-neutral to alkaline waters. Only a few springs produce acidic water (pH < 4), including the Zhenzhu, Diretiyanqu and Laogunguo Springs. Furthermore, other evidence of geothermal activity has been found, including hydrothermal explosions, fumaroles, steaming ground, sinters, travertines and hydrothermal alteration. One magma chamber with a temperature of >400°C, is located below the Rehai geothermal field (Zhao et al., Reference Zhao, Ran and Chen2011). This magma chamber is proven by the existence of areas of low-velocity anomalies and high conductivity in deep seismic sounding profiles (e.g. Wang and Gang, Reference Wang and Gang2004; Xu et al., Reference Xu, Yang, Li and Liu2012; Yang et al., Reference Yang, Hu, Hu, Duan and Li2013). It is located 7 km below the surface and has a thickness up to 20 km (e.g. Bai et al., Reference Bai, Liao, Zhao and Wang1994; Bai et al., Reference Bai, Meju and Liao2001; Jiang et al., Reference Jiang, Tan, Zhang, Peng, Li, Zhang, Xu and Wang2012). Residual heat from the magma chamber heats the groundwater, creating a hydrothermal convective system which forms hot or warm springs on the surface. The temperatures of the geothermal reservoirs, which are composed of Proterozoic metamorphic rocks and Yanshanian granitic rocks (Guo and Wang, Reference Guo and Wang2012), vary between 60 and 450°C, but are mainly in the range 160 to 260°C (Liao et al., Reference Liao, Shen and Guo1991; Shangguan, Reference Shangguan2000; Du et al., Reference Du, Liu, Fu, Ninomiya, Zhang, Wang, Wang and Sun2005). The geothermal water from hot springs at the Rehai geothermal field is essentially derived from meteoric water (Du et al., Reference Du, Liu, Fu, Ninomiya, Zhang, Wang, Wang and Sun2005).
Methods
Detailed field observations, descriptions, photography and measurements of water parameters (T, pH) were taken in June 2016 and January 2017. Water samples were filtered through a 0.45 μm nylon membrane and collected in 500 ml polyethylene bottles which had been pre-washed with deionised water. A condenser was used to collect 50 ml of condensed steam. This apparatus has been employed by Hynek et al. (Reference Hynek, McCollom, Marcucci, Brugman and Rogers2013) to collect gas condensates from fumaroles. After sampling, the water samples were promptly sent for cation and anion analysis. A Thermo Corporation IRIS Intrepid II XSP ICP-OES was used to analyse major cations (Na, K, Ca, Mg, Fe and Al) and SiO2 concentrations. Carbonate and bicarbonate concentrations were determined by titration with HNO3 (0.1 M), described in Alcicek et al. (Reference Alcicek, Bulbul and Alcicek2016). The anions, including Cl− and SO42−, were measured using ion chromatography (Dionex Thermo Corporation ICS 1100). The precision of the analyses is < 5% (RSD) for cations and SiO2, and < 10% (RSD) for Cl− and SO42−.
Scanning electron microscope (SEM) analyses and energy-dispersive X-ray (EDX) analyses were used to identify micromorphology and elemental compositions, respectively. Small solid samples (≤ 1 cm) were extracted and mounted on SEM stubs, after being bonded with conductive glue and coated with a thin gold layer. Three-dimensional images were produced by a QUANTA 250 FEG-scanning electron microscope at 5 kV, while EDX analyses were done with an accelerating voltage of 20 kV using an Oxford INCAx-max20 X-ray spectrometer. The average EDX spot size used was ~1 μm. Numerous EDX analyses were carried out to distinguish the minerals and precisely verify their composition. These analyses were conducted at the State Key Laboratory of Oil and Gas Reservoir Geology and Exploitation, Chengdu University of Technology.
X-ray diffraction (XRD) was used as the main method for mineral identification. Fourteen samples were chosen for XRD analysis utilising CuKα radiation with a step size of 0.02° and 2θ range from 5 to 60° (DX-2700, Dandong Haoyuan), and was conducted at the College of Materials and Chemistry & Chemical Engineering, Chengdu University of Technology. Eleven samples were chipped and powdered into near 200 mesh size for major-element analysis. The powdered samples were then sent to the Institute of Multipurpose Utilization of Mineral Resources, Chinese Academy of Geological Sciences, and analysed using an X-ray fluorescence spectrometer (XRF, Axios max, Panalytical).
The δ34S and δ18O values were determined at the Beijing Createch Testing Technology Co., Ltd. Prior to sulfur and oxygen isotope analysis, the water samples were acidified to a pH of 3, heated to 80°C, mixed with 10% barium chloride solution to precipitate BaSO4, washed repeatedly with distilled-deionised water and dried at 110°C. A dried BaSO4 sample was obtained from sulfate minerals by the following protocols from Szynkiewicz et al. (Reference Szynkiewicz, Modelska, Buczyński, Borrok and Merrison2013): The solid samples (~5 g) were dried and ground using an agate mortar and pestle. The contribution of elemental S and sulfide was negligible because neither were observed in the XRD analyses. We directly treated the powder sample with 6 N HCl to dissolve the sulfate minerals and exclude the acid non-soluble substances (e.g. quartz, amorphous silica). NaOH was used to adjust the pH to 9–10, in order to remove dissolved iron by precipitation of iron oxides. Lastly, the same procedure for the extraction of dissolved SO42− in the water samples described above (acidifying, adding 10% barium chloride solution, and drying) was used to obtain BaSO4. After the solid BaSO4 was converted to SO2 and CO, its δ34S and δ18O were measured with an Elemental Analyzer (EA) coupled with a Finnigan MAT 253 Plus (Thermo Fisher Scientific) ratio mass spectrometer. The analytical precision is estimated to be better than ± 0.2‰ (δ34S) and ± 0.5‰ (δ18O). The δ34S and δ18O were reported relative to the Vienna-Canyon Diablo Troilite (V-CDT) standard and the Vienna Standard Mean Ocean Water (V-SMOW), respectively.
Results
General description
Unlike most of the hot springs at the Rehai geothermal field, the Zhenzhu Spring is an acid sulfate spring with Na–SO4–Cl-type waters formed by condensation and oxidation of H2S-rich vapours in the shallow groundwater or surface water (Jiang et al., Reference Jiang, Li, Tu, Wei, Zhang, Wang and Dai2018). The Zhenzhu Spring has been artificially modified into a heart-shaped pool (Fig. 1d) with a depth of ~0.45 m and a maximum diameter of ~4.4 m. Characterised by a stable water depth thanks to a discharge channel, the Zhenzhu Spring has a water depth of ~8 cm. Numerous small vents are developed at the bottom. These vents release a large quantity of CO2 gas (CO2 = 94.5%) with very limited amounts of N2, O2, Ar, H2, CH4, He and Stotal (Stotal = 0.115%, including 0.101% H2S and 0.005% SO2) (Shangguan et al., Reference Shangguan, Bai and Sun2000). The intense activity of the spring water results in the occurrence of aerosols above the Zhenzhu Spring. Colourful sulfate minerals cover the stone wall and the spring wall (Fig. 1d). During field observations, it was also noticed that Fe-staining (reddened rocks) occurs on the spring wall (Fig. 1d).
Chemistry of spring water and condensed steam
The temperature, pH and major composition of fluids at the Zhenzhu Spring is given in Table 1. The spring waters are characterised by temperatures from 84.6 to 96°C and pH generally between 3.24 and 4.5. The waters predominately contain SiO2, SO42−, Na, Cl, K and HCO3 (in decreasing order) and minor Ca, Mg, Fe and Al. Although the acidic condensed steam (pH = 3.22) mainly formed by aerosol precipitation, and vapour condensation from the Zhenzhu Spring shows lower ionic concentrations than the spring waters, the Ca concentration of this sample (>5 mg/L) is approximately three times the concentration of the spring waters (Table 1). The most abundant cation and anion in the condensed steam are SO42− and Ca, respectively. The remaining ions are present with <1 mg/L in the condensed steam. A significant difference in charge balance error values was found in different studies (Table 1). Although the reasons for this variation are not clear, it might be caused by laboratory analytical errors, different sampling procedures, unfiltered samples, etc.
Table 1. Composition (mg/L) of spring water and condensed steam from the Zhenzhu Spring.
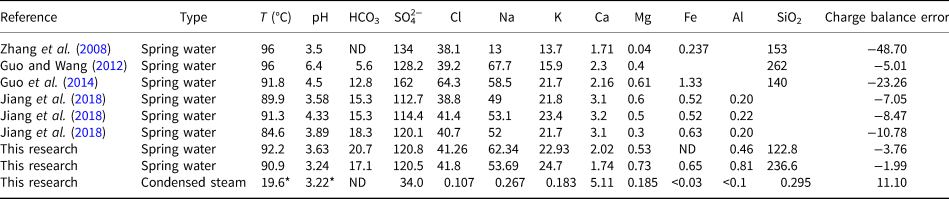
ND = not detected, blank = no data. * = measured in the lab.
Mineralogy
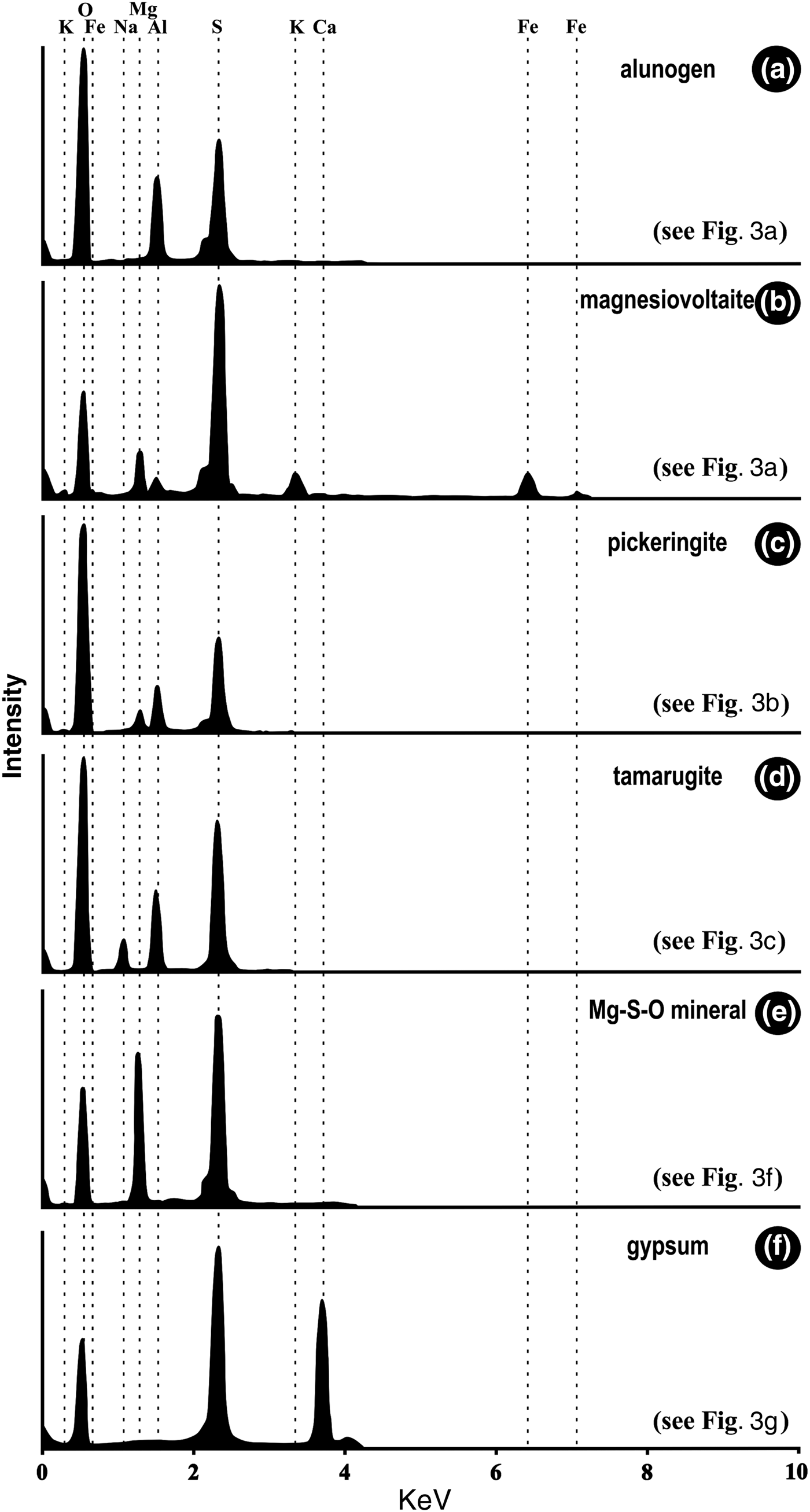
Detailed XRD analyses and SEM-EDX analyses can be seen in Figs 2 to 4 and Table 2. The minerals in the samples can be divided into three groups: (1) sulfate minerals (alunogen, pickeringite, tamarugite, magnesiovoltaite, gypsum and an Mg–S–O phase); (2) silica minerals (amorphous SiO2 and quartz); and (3) silicate minerals (K-feldspar and illite). Apart from the Mg–S–O phase, all the remaining minerals can be identified in XRD analyses (Fig. 2; Table 2). The quartz, K-feldspar and illite are derived mainly from the country rocks, which contain no metal-sulfide minerals, while the sulfate minerals and amorphous SiO2 are the secondary minerals. There is a high content of amorphous SiO2 in only one sample (Table 2). The amorphous SiO2 shows a broad ‘hump’ with no apparent peaks in the XRD spectra (Fig. 2b) and occurs mainly as tabular aggregates (Fig. 3h, i).
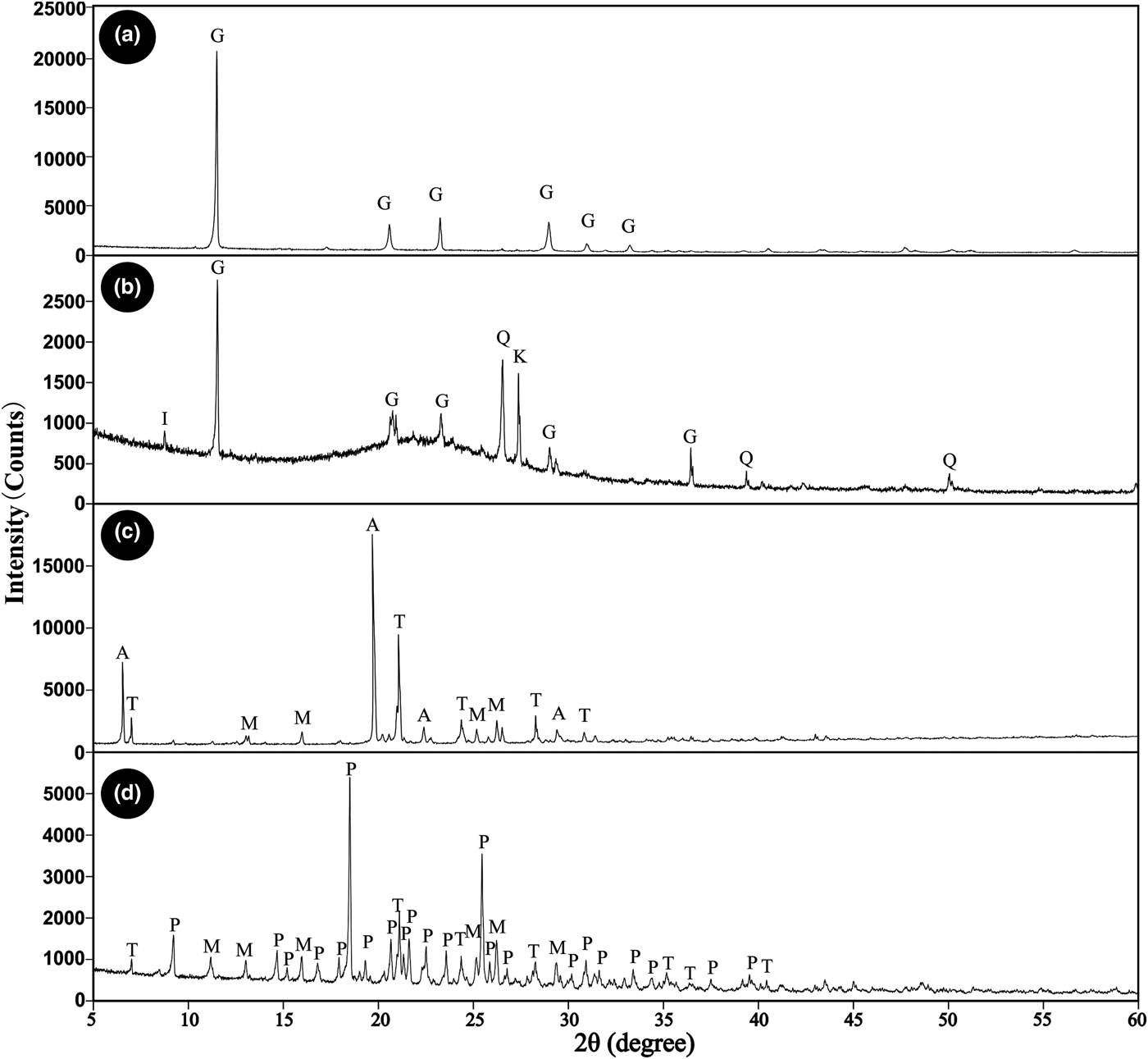
Fig. 2. XRD patterns for samples from the Zhenzhu Spring showing the presence of gypsum (G), amorphous SiO2, quartz (Q), K-feldspar (K), illite (I), alunogen (A), tamarugite (T), magnesiovoltaite (M) and pickeringite (P).
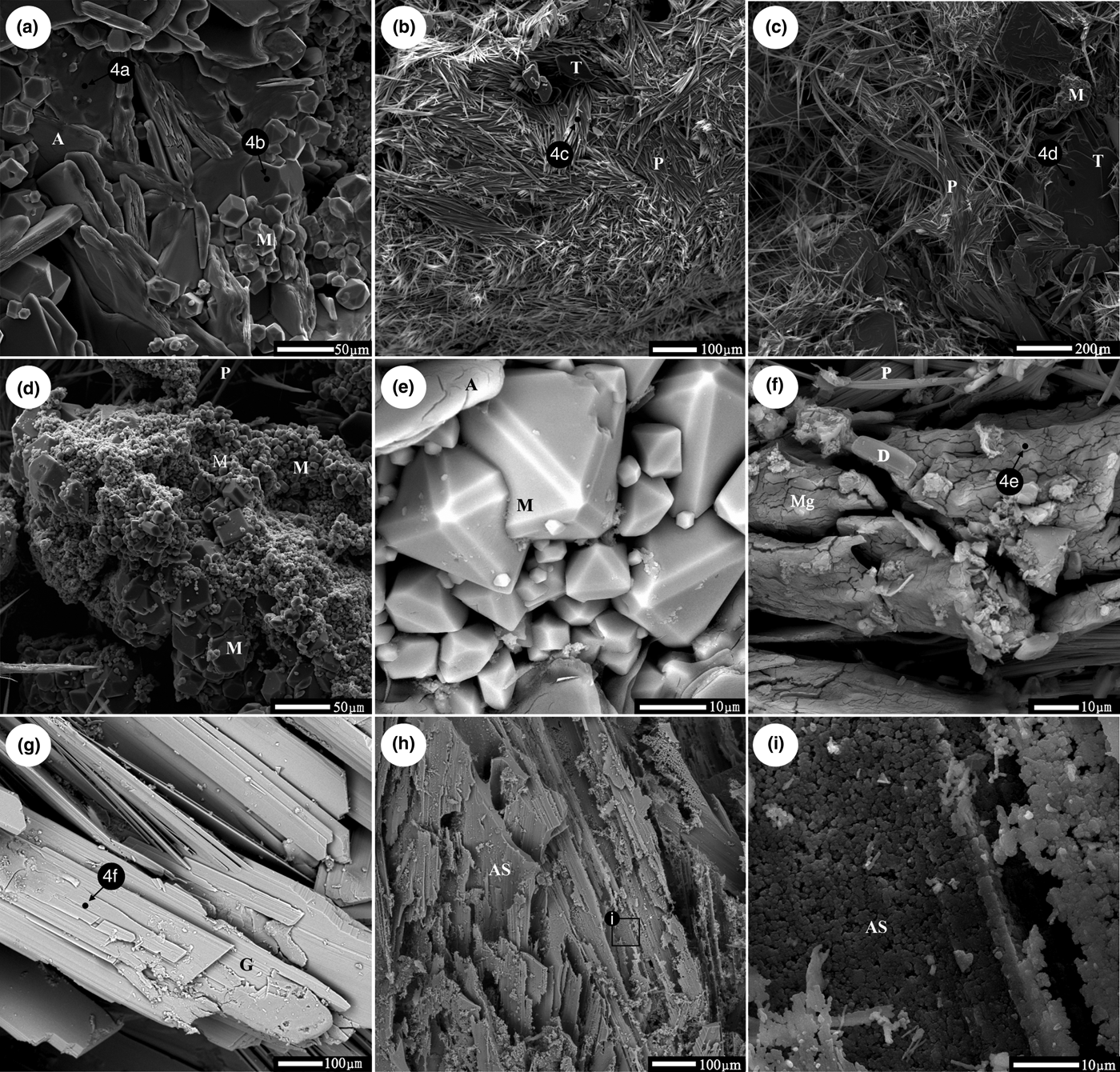
Fig. 3. Secondary electron (SE) and back-scattered electron (BSE) photographs of sulfate minerals (a–g) and amorphous SiO2 (h, i). A = alunogen, M = magnesiovoltaite, T = tamarugite, P = pickeringite, Mg = Mg–S–O phase, G = gypsum, AS = amorphous SiO2. Spot EDX analyses are in indicated in Fig. 4. (a) Tabular alunogen crystals and granular magnesiovoltaite crystals, SE. (b) Matted aggregate of pickeringite crystals with some tabular tamarugite crystals, SE. (c) Hair-like pickeringite crystals, tabular tamarugite crystals and aggregate of magnesiovoltaite crystals, SE. (d) Aggregates of magnesiovoltaite crystals of different sizes, SE. (e) Well-shaped magnesiovoltaite crystals, BSE. (f) Mg–S-O phase without distinct crystalline shapes and diatom (d), BSE. (g) Large thin tabular gypsum crystals which possess the same growing direction, BSE. (h) Tabular amorphous SiO2 aggregates, BSE. Black box indicates the position of (i). (i) Tabular amorphous SiO2 aggregates consisting of small amorphous SiO2 particles, SE.
Table 2. Minerals identified using X-ray diffraction.
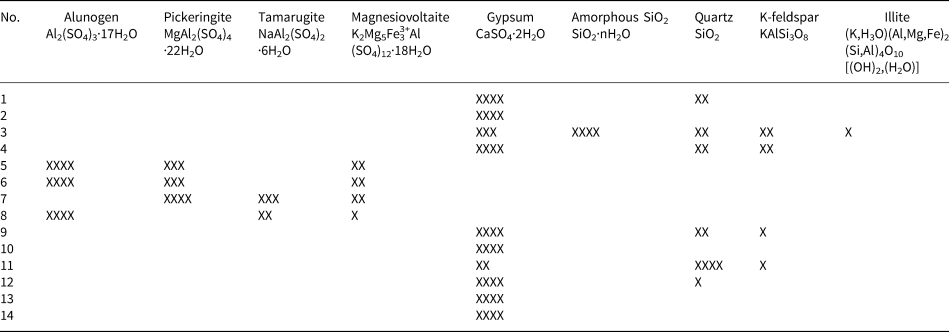
XXXX: content > 50%, XXX: 50% ≥content > 20%, XX: 20% ≥content > 5%, X: content ≤5%, Blank = no data
Sulfate minerals were observed in all samples from the Zhenzhu Spring and covered a range of compositions (Table 2). Gypsum is abundant in most of the samples from the spring wall and generally appears with silicate minerals or amorphous SiO2. SEM images show that the tabular gypsum crystals are common (Fig. 3g). Alunogen is also a major mineral, which has a tabular shape (Fig. 3a), and produces high O, S and Al X-ray peaks (Fig. 4a). Compared with the alunogen crystals, tamarugite, which occurs in lower concentrations, shows a smoother crystal surface and it is relatively thin in the microphotographs (Fig. 3c). Pickeringite, if present, is mainly developed as matted aggregates or hair-like crystals (Fig. 3b, 3c). Some of the samples contain an unusual mineral (magnesiovoltaite), which displays high Fe and K peaks in EDX analyses, in addition to O, Mg, Al and S X-ray peaks (Fig. 4b). This mineral generally exhibits a regular habit and appears as a mineral aggregate (Fig. 3d, e). Unlike other sulfate minerals from the samples, the Mg–S–O phase can only be found by SEM-EDX analyses and lacks normal geometric forms (Figs 3f, 4e). These Al-containing sulfate minerals (alunogen, tamarugite, etc.) were abundant in the samples from the stone wall (Fig. 1d).
Geochemistry
Table 3 lists the major element compositions of sulfate samples from the Zhenzhu Spring. SO3 contents are high and vary from 29.1 to 42.6 wt.%. Other components, ignoring SO3 however, are variable and can be divided into two groups (Table 2). SiO2 and CaO are the abundant components in Group 1, reflecting the enrichment of gypsum and amorphous SiO2, while Na2O, MgO, and Al2O3 are depleted. In contrast, Na2O, MgO, Al2O3 and Fe2O3 (total contents varying from 18.0 to 21.0 wt.%) are enriched in Group 2 because of the presence of many hydrated Al-containing sulfate minerals. Also, many of the precipitates have high H2O contents, as partly indicated by high loss on ignition (LOI) values (up to 42.2 wt.%), which might reflect the presence of hydrated phases (hydrated sulfate minerals and/or amorphous SiO2).
Table 3. Major element (wt.%) compositions as determined by X-ray fluorescence from the Zhenzhu Spring samples.
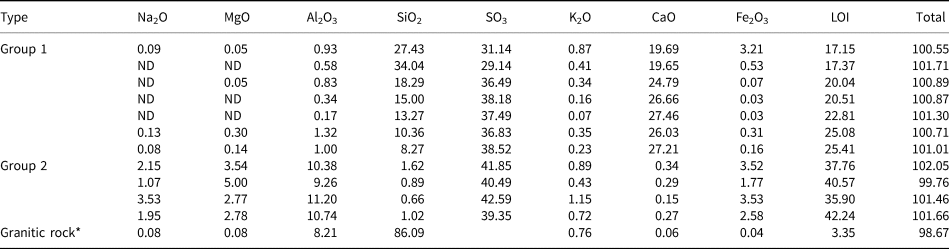
Blank = no data, * = average major element content of granitic rocks at the Rehai geothermal field (Lin et al., Reference Lin, Peng, Qiao and Li2014).
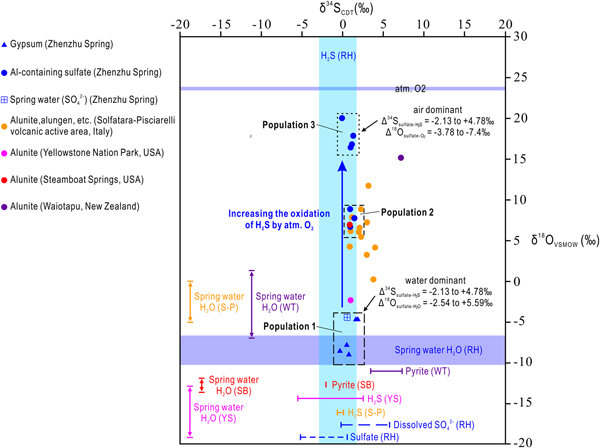
The results of the sulfur and oxygen isotope analyses of sulfate minerals, dissolved SO42−, H2S, sulfide and O2 at the Zhenzhu Spring and other springs at the Rehai geothermal field, and in some typical steam-heated environments, are present in Table 4 and Fig. 5. Samples from the Zhenzhu Spring show a narrow range of δ34S values (–0.33 to 1.8‰), which are close to the δ34S values of H2S (–2.9 to 1.8‰) and dissolved SO42− (–0.2 to 5.8‰) at the Rehai geothermal field. Additionally, a large δ18O range (–8.94 to 20.1‰) is observed, falling between the δ18O values of dissolved SO42− at the Rehai geothermal field (–10.19 to –6.7‰) and those of atmospheric O2 (~23.88‰ ± 0.02‰). The δ34S range of the Al-containing sulfate minerals (–0.05 to 1.47‰) from the Zhenzhu Spring is similar to the range of the gypsum (–0.33 to 1.88‰). However, compared with the negative δ18O values of the gypsum-containing samples (Population 1: –8.94 to –4.62‰) mainly occurring on the spring wall (approximating the spring water, Fig. 1d), the Al-containing minerals from the stone wall (Fig. 1d) have higher δ18O values (Population 2: 6.71 to 8.93‰, Population 3: 16.48 to 20.1‰). The spring water from the Zhenzhu Spring contains sulfur and oxygen isotope compositions that are consistent with the solid samples enriched in gypsum.
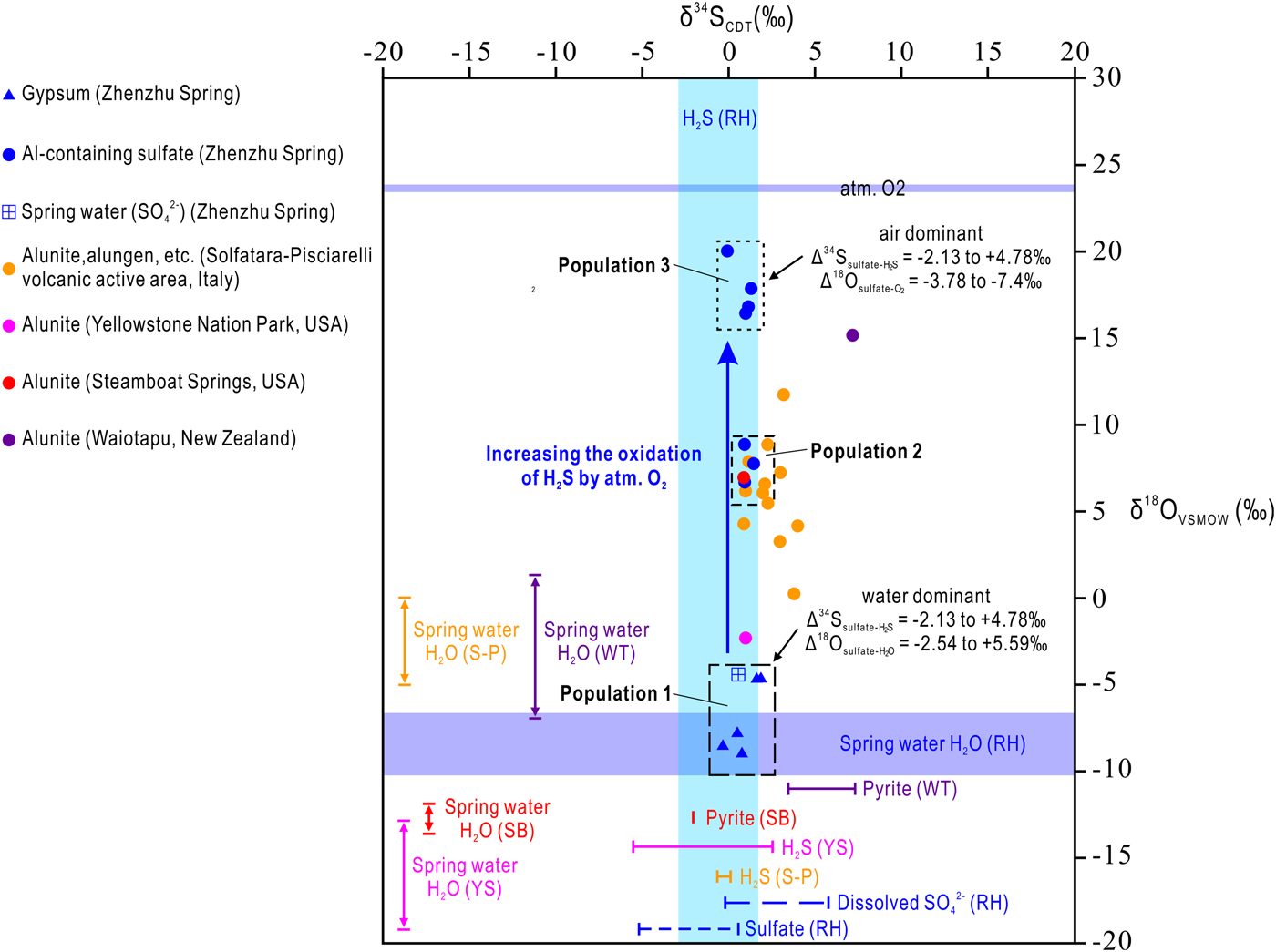
Fig. 5. Sulfur (δ34SCDT) and oxygen (δ18OVSMOW) isotope composition for sulfate minerals, H2S gases and spring water from the Rehai geothermal field, in comparison to potential sources and sulfur-containing minerals (alunite, pyrite and H2S gas) from other steam-heated environments. RH = Rehai geothermal Field, S-P = Solfatara-Pisciarelli volcanic active area (Italy), YS = Yellowstone National Park (USA), SB = Steamboat Springs (USA), WT = Waiotapu geothermal field (New Zealand) .
Table 4. Sulfur (δ34SCDT) and oxygen (δ18OVSMOW) isotopic analysis data for sulfate minerals, H2S gases (or pyrite) and spring waters (SO42−, H2O) from hot springs at the Rehai geothermal field and in some typical steam-heated environments and oxygen isotopic composition of atmospheric O2.
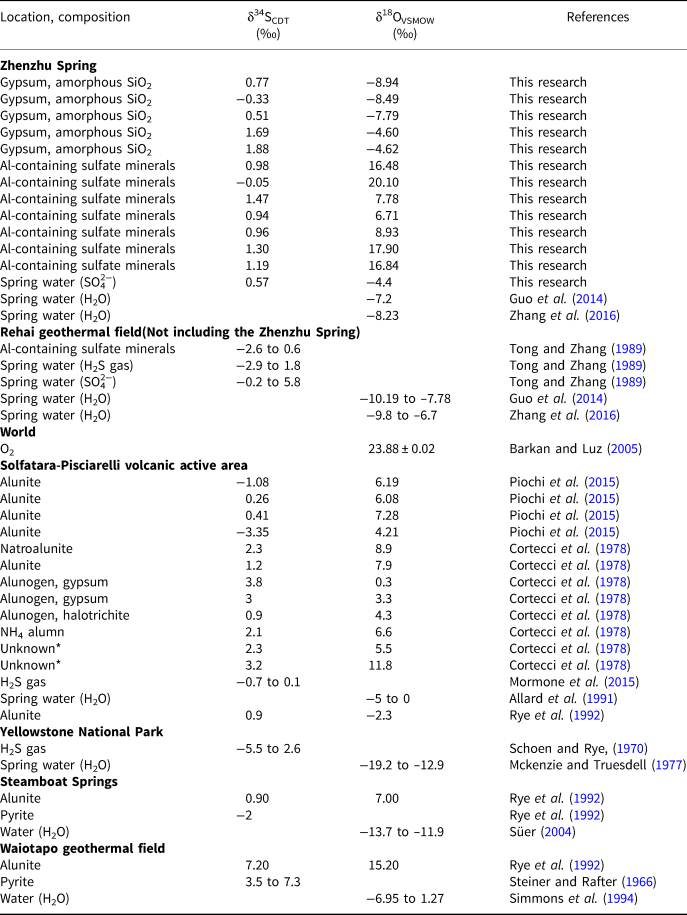
Blank = no data, Unknown* = samples without analysing their mineral composition.
Discussion
Genesis of sulfate minerals
Gas condensate alteration, which is usually considered to be a leading factor in the formation of sulfate minerals from fumaroles in volcanic and geothermal settings (Naughton et al., Reference Naughton, Greenberg and Goguel1976; Aguilera et al., Reference Aguilera, Layana, Rodríguezdíaz, González, Cortés and Inostroza2016), is discussed here in order to identify the genesis of the sulfate minerals at the Zhenzhu Spring. The main processes in gas condensate alteration include: (1) vapour condensation which causes the acidic gases (H2S and SO2) to dissolve in the condensate; (2) generation of H2SO4 in condensates (e.g. oxidation of H2S and SO2); and (3) interaction between gas condensates and rocks. A general characteristic of the alteration products is the co-occurrence of minerals, such as native sulfur (e.g. Mizutani and Sugiura, Reference Mizutani and Sugiura1966; Naughton et al., Reference Naughton, Greenberg and Goguel1976; Ikehata and Maruoka, Reference Ikehata and Maruoka2016) and silica minerals (e.g. cristobalite, tridymite and amorphous SiO2) (McHenry et al., Reference McHenry, Carson, Dixon and Vickery2017). The gas condensate alteration mechanism is considered as applicable to explain the formation of sulfate minerals around the Zhenzhu Spring.
The sulfur isotope composition is a robust indicator for recognising the origin of sulfate minerals in steam-heated environments because of their relatively small S isotope fractionation during abiotic oxidation of S-bearing minerals (generally Δ34S(sulfate–sulfide) = δ34Ssulfate – δ34Ssulfide < 3‰) (Fry et al., Reference Fry, Ruf, Gest and Hayes1988; Toran and Harris, Reference Toran and Harris1989; Balci et al., Reference Balci, Iii, Mayer and Mandernack2007) and sulfate precipitation from fluids (generally Δ34S(sulfate–dissolved SO42−) < 3‰) (Raab and Spiro, Reference Raab and Spiro1991). The δ34S of dissolved SO42− of the spring water and the sulfate samples from the Zhenzhu Spring vary in a way that is consistent with the variation in δ34S of the H2S gases, dissolved SO42− in other springs and sulfate minerals at the Rehai geothermal field (Fig. 5). This suggests that: (1) the dissolved SO42− in the spring waters from the Zhenzhu Spring and others springs at the Rehai geothermal field was contributed mainly by H2S oxidation; and (2) the sulfur in the sulfate minerals at the Zhenzhu Spring and the sulfate minerals from other areas at the Rehai geothermal field may originate from H2S oxidation and/or sulfate precipitation of spring water during an evaporation process. At the Zhenzhu Spring, the possibility of the H2S oxidation mechanism, instead of the sulfate precipitation from spring water, is probable because the spring water cannot inundate the areas where the sulfate minerals were formed.
Because oxygen in sulfate minerals is acquired during oxidation of H2S, the δ18O of sulfate minerals can provide useful information on their formation. Several factors proposed by Rye et al. (Reference Rye, Bethke and Wasserman1992) are known to be significant in controlling the δ18O of sulfate minerals in steam-heated environments, including: (1) the ratio of atmospheric oxygen to water oxygen involved in the oxidation of H2S; (2) the oxygen isotope composition of the fluid; and (3) the oxidation temperature and the degree of exchange of oxygen between aqueous sulfate and the fluid. Although low temperature can impede oxygen isotope exchange (Chiba and Sakai, Reference Chiba and Sakai1985; Holt and Kumar, Reference Holt, Kumar, Krouse and Grinenko1991), oxygen isotope exchange between dissolved SO42− and other fluids (e.g. meteoric water and magmatic water) at 50–100°C in low-pH fumarolic environments has been observed (Rye, Reference Rye2005). At the Rehai geothermal field, the low δ18O of dissolved SO42− in the spring water and the subaerial environment of sulfate minerals at the Zhenzhu Spring provide evidence for the weak influence of oxygen isotope exchange between sulfate minerals and spring water on the δ18O composition of sulfate minerals from the Zhenzhu Spring.
The δ18O of all sulfate samples from the Zhenzhu Spring falls between the two potential oxygen sources: H2O (either gas or liquid phase) and O2 (mainly gaseous O2 in atmosphere) (Fig. 5). The high δ18O of Population 3 shows the majority of sulfate oxygen is derived from atmospheric O2. These sulfate minerals have very high δ18O values (close to δ18O of atmospheric O2) and seem to be ‘primary sulfates’ as proposed by Holt and Kumar (Reference Holt, Kumar, Krouse and Grinenko1991). Primary sulfate has also been recorded in ‘steam caves’ (e.g. Adam and Aburi caves in in the Cerna Valley, Romania) and was considered to be formed during the oxidation process in which abundant atmospheric (and minor atmospheric gaseous H2O) 18O exchange occurred (Onac et al., Reference Onac, Wynn and Sumrall2011). Sulfate minerals of Population 2 suggest oxidation under less oxic conditions than those of Population 3, indicating that the sulfate oxygen of Population 2 had both atmospheric and H2O origins (steam, including gaseous and liquid H2O).
The low δ18O of gypsum from the Zhenzhu Spring may represent: (1) anoxic conditions in which 18O can only exchange with H2O; and (2) direct precipitation of gypsum from the fluid in oxic environments. The latter is more reasonable because the areas forming gypsum (i.e. the spring wall) are obviously oxic environments and the fluid precipitating gypsum is the condensed steam. Deposition of the aerosol/steam/fog (tiny water droplets and some vapour) produced by bubble bursting at the water surface forms condensed steam on the rock surface. This process leads the sulfate minerals of Population 3, which are precipitated from the condensed steam, to inherit the O and S isotope composition from spring waters (Fig. 5).
A main source of the metallic elements (Na, Mg, Al, K, Ca and Fe) contained in the sulfate minerals at the Zhenzhu Spring is probably rocks. Although Stoiber and Rose (Reference Stoiber and Rose1974) noted that vapours (gases) from high-temperature (above 330°C) fumarolic vents might contain small concentrations of voltaite cations (i.e. Na, K, Zn, Cu, V and Mo), metallic elements in the sulfate minerals at the Zhenzhu Spring are unlikely to be derived from the vapour because of the relatively low-temperature conditions. The high concentration of Ca in the condensed steam (Table 1) indicates Ca can be derived from the acid aerosols. However, only a few other cations can be transported by the acid aerosols from the Zhenzhu Spring. Under this scenario, rocks appear to be the main source of metallic elements in the formation of sulfate minerals at the Zhenzhu Spring. Acid condensed steams resulting from the precipitation of acid aerosol and condensation of vapour at the Zhenzhu Spring interact with rock debris (e.g. feldspar) and mobilised various elements (e.g. K, Na, Al and Ca) from rocks. Such an important cationic contribution of rocks to the formation of sulfate minerals has also been found in fumarolic environments (Hynek et al., Reference Hynek, McCollom, Marcucci, Brugman and Rogers2013; McCollom et al., Reference McCollom, Hynek, Rogers, Moskowitz and Berquo2013) and burning coal-waste heaps (Stracher et al., Reference Stracher, Prakash, Schroeder, McCormack, Zhang, Van Dijk and Blake2005; Masalehdani et al., Reference Masalehdani, Mees, Dubois, Coquinot, Potdevin, Fialin and Blanc-Valleron2009).
Collectively, the formation of all the sulfate minerals at the Zhenzhu Spring is controlled by acid aerosols. The following processes are responsible for sulfate mineral formation: (1) formation of underwater water bubbles resulting from intense activity of spring water around vents; (2) water bubble bursting when it arrives at the water surface and forming acid aerosols (composed of tiny water droplets with similar S–O isotope composition to the spring water, and vapour containing H2S); (3) oxidation of H2S to form SO42− with similar δ34S values to the spring water and different δ18O values depending on the ratio of air and water oxygen; (4) acid aerosols getting in contact with rocks and forming water droplets and/or films; and (5) fluid–rock interaction, releasing various elements and forming sulfate minerals.
Comparison with typical modern steam-heated environments
Rye et al. (Reference Rye, Bethke and Wasserman1992) indicated that most alunites in steam-heated environments (oxidation of H2S) have δ34S values the same as those of the precursor H2S (and as related sulfides, if present). This is demonstrated from the δ34S of alunite and its sulfur origins (pyrite and H2S) from Yellowstone National Park, Steamboat Springs, and Waiotapu geothermal field (Fig. 5). Although no alunite has been found at the Zhenzhu Spring, the δ34S of the sulfate minerals also falls within the δ34S range of H2S. This suggests that not only alunite, but also other sulfates (gypsum, alunogen, pickeringite, tamarugite, etc.) in modern steam-heated environments create negligible change in sulfur isotope compositions during their formation processes (oxidation of H2S and sulfide). Sulfur isotopic exchange between sulfate and H2S/sulfide might occur in steam-heated environments (e.g. Cunningham, Reference Cunningham1984; Ebert and Rye, Reference Ebert and Rye1997). However, this isotopic exchange process needs a considerable amount time to achieve equilibrium at low temperatures (4 × 105 years, if pH = 4 to 7, T = 100°C) (Rye, Reference Rye2005). In modern steam-heated environments (especially around fumaroles), sulfate minerals may have been formed for only a few years and have not been continuously in contact with the fluid. Thus, the sulfur isotope exchange in modern steam-heated environments is subtle and not significant.
The δ18O of sulfate minerals from all modern steam-heated environments (except those of Population 1) always reflects the incorporation of atmospheric O2 (Fig. 5). Δ18O(sulfate–H2O) values were found at Yellowstone National Park (10.7 to 16.9‰), Steamboat Springs (18.9 to 20.7.9‰), and Waiotapu (16.47 to 22.5‰), suggesting that the oxidation of H2S/sulfide occurred in a much more oxic environment and no or little H2O was involved in this process. The δ18O of sulfate minerals from the Solfatara-Pisciarelli volcanic area varies from 0.3 (close to the δ18O of the spring water, –5 to 0‰) to 11.8‰. The apparent variation has also been found in the sulfate minerals from the Zhenzhu Spring, and are largely formed by low ratios of atmospheric oxygen to water oxygen. In common with the sulfur isotope data, the δ18O of sulfate minerals in steam-heated environments can also be influenced by isotopic exchange (Rye et al., Reference Rye, Bethke and Wasserman1992; Rye, Reference Rye2005). However, the oxygen isotopic exchange in modern subaerial environments might not be common because the subaerial condition avoids the contact between sulfate or SO42− and fluids.
Conclusions
Detailed investigations of the samples from the Zhenzhu Spring show that these samples are mainly composed of sulfate minerals (gypsum, alunogen, pickeringite, tamarugite and magnesiovoltaite) and amorphous SiO2. The genesis of the sulfate minerals is related to acid aerosols of tiny water droplets and H2S vapour. The oxidation of H2S produces significant amounts of SO42− in the aerosol and deposition of this acid aerosol forms the acid condensate on the rocks. The condensate attacks the rocks to form the sulfate minerals with a similar δ34S value to that of the H2S and a greater δ18O value than the spring water. However, some subaerial sulfate minerals with the same δ18O values as the spring water have been also found and are considered to be the products of the evaporation of some condensed steam (mainly composed of water droplets formed by bubble bursting). This shows that although oxidation of H2S/sulfide plays a key role in the formation of sulfate minerals around acid hot springs, we cannot ignore the SO42− derived directly from the acid spring water by bubble bursting. The comparative studies of sulfur-oxygen isotope show that although the δ34S of the sulfate minerals in modern steam-heated environments displays the same δ34S values as those of the precursor H2S/sulfide, the δ18O values of these sulfate minerals are highly variable and influenced largely by the ratio of air and water oxygen. This investigation also suggests that the sulfur or oxygen isotopic exchange between the subaerial sulfate minerals and the fluid might be scarce in some modern steam-heated environments (at least at Zhenzhu Spring).
Acknowledgements
Financial support of this study was provided by the National Natural Science Foundation of China (grants 41572097 to Wen and 41472088 to Zheng). The authors wish to thank Yunnan Tengchong Rehai Tour Developing Co. Ltd. for providing permission to sample at Zhenzhu Spring. We also thank Dr. Alejandro Rodríguez and an anonymous reviewer for their constructive and thoughtful comments. The Principal and Production Editors are thanked for copy-editing this manuscript.