Introduction
Merehead quarry (now called, Torr Works quarry by its current owners) is well known for its suite of rare Pb minerals found within so called ‘manganese pods’ (Turner and Rumsey, Reference Turner and Rumsey2010). It is the type locality for six Pb-oxychlorides and the neotype locality for the redefined lead basic carbonate, plumbonacrite (Rumsey et al., Reference Rumsey, Krivovichev, Siidra, Kirk, Stanley and Spratt2012).
The new Pb basic carbonate mineral somersetite was discovered in a sample that was collected during a Russell Society field trip to Merehead in summer 2006, but has since been visually identified on specimens collected in the 1980s. Somersetite (Cyrillic: сомерсетит) is named for the locality in the county of Somerset in South West England. The mineral and mineral name have been approved by the International Mineralogical Association Commission on New Minerals and Mineral Names (IMA2017-024). Type material is deposited in the collections of the Department of Mineralogy, Saint-Petersburg State University, Saint-Petersburg, Russia, under the catalogue number 1/19661. A number of other visually identical samples from the 2006 find and earlier collecting trips are in housed in the extensive collection of Merehead quarry specimens at the Natural History Museum in London.
Occurrence and association
Torr Works quarry is located near the town of Cranmore in Somerset, England, in the Mendip Hills. Worked for aggregate, it is well known for its suite of rare Pb minerals (Turner and Rumsey, Reference Turner and Rumsey2010). The quarry works Carboniferous limestone strata, which at a simplified regional level form a plunging anticline, having a roughly E–W axis and plunging towards the East. Folding, regional uplift, and subsidence caused the limestone to fracture, and therefore there are now numerous joints, as well as sub-parallel fractures and faults of all sizes, all roughly aligned with the axis of the anticline.
The regional uplift, surface erosion and subsequent subsidence and marine transgression has led to the Carboniferous strata being overlain disconformably by Jurassic Upper Oolites. The disconformity represents some 170 M.y. of erosion, and apart from rare and localized preserved wash features preserved in the palaeosurface, the entire Permo–Triassic succession has been removed by erosion. The basement consists of Silurian volcanics, mostly andesites, which are exposed locally at Moons Hill quarry, for example.
Across the Mendip Hills, this structural geology has controlled mineralization, including the emplacement of Mississippian Pb ore veins bearing galena, fluorite, calcite and baryte into the Carboniferous limestone, resulting in a large area being irregularly mineralized with sub-parallel ore veins. After their deposition, regional uplift, erosion and subsequent marine transgression allowed the ingress of seawater into many of these Pb veins, where auto-catalysis then caused deposition of manganate minerals (Turner, Reference Turner2006). Much later, multiple hydrothermal events occurred, and hot hydrothermal fluids followed the same structural features. Hot enough to silicify the limestone wallrocks in places, they caused irregular thermal alteration of existing Pb veins, eventually leading to formation of the ‘manganese pods’ for which Merehead is famous. These hydrothermal events also deposited quartz and other minerals, and must have led to the remobilization of Pb into solution as hydrocerussite is (locally) common in these hydrothermal veins, even where there are no other Pb minerals present. Crude crystals of hydrocerussite up to 50 mm in size and aggregates to 150 mm have been found in this environment. The new mineral somersetite was found in one such vein. Associated with symesite, calcite, aragonite and quartz (in well-formed crystals up to ~50 mm – exceptionally large for the Mendips) were masses of what appeared to be hydrocerussite of an unusual pale green colour. Up to ~25 mm across, these crystals have proved to contain somersetite and plumbonacrite. Further somersetite samples at the Natural History Museum from earlier finds have a similar mineralogical assemblage and are associated largely with calcite within manganese oxide, however, a few have been identified that show other minerals in close association; directly adjacent to orange-yellow mereheadite, thin coatings of cerussite and tiny flecks of blue diaboleite, and in proximity to pale-yellow mimetite and in one example, well-formed large crystals of baryte.
Physical properties and optical data
In the samples studied, somersetite forms plates and subhedral grains (Fig. 1a) up to 5 mm across and up to 2 mm thick. In bi-coloured crystals (Fig. 1b) it forms intergrowths with plumbonacrite (Fig. 2). Note that in the samples investigated, hydrocerussite does not associate directly with somersetite, which may indicate different stability fields of these minerals. Somersetite is green or white (most commonly, its visually similar to hydrocerussite-like minerals but with a mint-green tint), with white streak and adamantine lustre. It is brittle with a perfect cleavage on (001). Parting was not observed, and its fracture is uneven across the cleavage. Density could not be measured due to a lack of material and the absence of heavy liquids with densities higher than 7 g cm–3. Density calculated using the empirical formula is equal to 7.01 g cm–3. Somersetite has a Vickers Hardness Number (VHN20) of 140.4 kg/mm2 (n = 3, range 111–167 kg/mm2), which corresponds to a Mohs hardness of ~3.

Fig. 1. (a) Somersetite crystal (green subhedral grain in the vug), field of view = 1 cm and (b) multi-coloured somersetite crystal (2 cm across) with inclusions of plumbonacrite and colourless transparent cerussite in the rim. The green zone is enriched in somersetite, but plumbonacrite was also determined from this area, whereas the white zone is enriched in plumbonacrite. All of these minerals can be identified reliably, by X-ray techniques.

Fig. 2. Scanning electron microscopy image showing mutual intergrowths of somersetite (dark grey) with plumbonacrite (lighter grey) and cerussite (very dark) in the rim.
Somersetite is optically negative. Despite the transparency of somersetite, its optical properties were investigated in reflected light because of the high values of refractive indices. A calculated mean refractive index is 2.00 and K C = 0.1451 was obtained from the chemical data in Table 1. Reflectance measurements were made using a SiC standard in air in the range 400–700 nm (Table 2). In reflected light, somersetite is grey. It is non-pleochroic, with white internal reflections and a very weak bireflectance. The measured anisotropism is ΔR589 = 1%; under the optical microscope, the relatively weak anisotropy is masked by the abundant internal reflections of white and blue with yellowish tints.
Table 1. Chemical composition of somersetite.

*Calculated values.
S.D. – Standard deviation.
Table 2. Reflectance values of an unoriented section free of internal reflections on a measured area of 20 µm × 20 µm (SiC standard, measured in air) for somersetite.

Note: The values required by the Commission on Ore Mineralogy are given in bold.
Chemical composition
Two samples of somersetite have been studied, the first is a 15 µm × 10 µm crystal, which was used for single-crystal analysis, and the second is a large sample (ca. 7 mm × 5 mm × 7 mm) consisting of somersetite, plumbonacrite, cerussite, symesite and an unidentified Pb-Cl-C(?) mineral (Fig. 2). The composition of somersetite (Table 1) was determined by wavelength-dispersive spectrometry (WDS) using a Cameca SX-100 electron microprobe (Natural History Museum, London, UK) with an acceleration voltage of 20 kV, beam current of 20 nA and a variable electron-beam diameter of 1, 5 and 10 µm. The mineral is stable under the electron beam and WDS produced consistent compositional data. Full spectrometer scans were obtained using the following diffracting crystals: LPC0 (d = 45 Å), TAP (d = 25.745 Å), LPET (8.742 Å) and LLIF (4.027 Å). Only peaks originating from Pb and O were observed in the scans. Spectrometer scans were not obtained from a larger ‘d’ spacing crystal as the sample was carbon coated. Vanadinite was used as the standard for the determination of Pb. Calcium, Fe, Mn, Co, Zn, V, Mo and As were included in the analytical protocol, but all determined values were below the detection limit. Fluorine, Cl and S were also found to be below the detection limit (530, 720 and 260 ppm respectively). Corrections for peak interferences (Pb/S, Pb/Si and Pb/Mo) were applied from empirical measurements taken from standards. These elements were also determined to be below the detection limit. CO2 was initially calculated by difference to the analytical total prior to PAP matrix correction (Pouchou and Pichoir, Reference Pouchou, Pichoir, Heinrich and Newbury1991). Stoichiometric CO2 and H2O were then derived by calculation from the crystal structure (5 (CO3)2– and 4 (OH)– groups).
The content of PbO within somersetite from two analysed crystals is slightly different (average values are 86.77 wt.% for the first sample and 88.12 for the second). The empirical formulas of the mineral (Table 1), calculated on the basis of 20 O atoms per formula unit, are identical Pb8.00C5.00H4.00O20. The simplified formula is Pb8O(OH)4(CO3)5, which requires: PbO = 87.46, CO2 = 10.78, H2O = 1.76, total 100.00 wt.%.
Infrared spectroscopy
In order to obtain infrared (IR) absorption spectra, powdered samples of crushed single crystals previously checked by single-crystal XRD were mixed with anhydrous KBr, pelletized, and analysed using an ALPHA FTIR spectrometer (Bruker Optics) with the resolution of 4 cm–1; 16 scans were obtained. The IR spectrum of analogous pellet of pure KBr was used as a reference.
The IR spectrum of somersetite is similar to that of plumbonacrite and, to a lesser degree, hydrocerussite (Fig. 3). The wavenumbers of absorption bands in the IR spectrum of somersetite and their assignments are (cm–1; s – strong band, w – weak band, sh – shoulder): 3549, 3280w (O–H-stretching vibrations); 2524w (combination mode), 1734w (overtone of in-plane bending vibrations of CO32− anions); 1403s (asymmetric C–O-stretching vibrations of CO32− anions); 1217 (possibly, combination mode involving Pb–O-stretching and O–C–O bending vibrations); 1046w (symmetric C–O-stretching vibrations of CO32− anions); 849 (out-of-plane bending vibrations of CO32− anions); 738w, 690sh, 683s (in-plane bending vibrations of CO32− anions); 615w (Pb···O–H bending vibrations), 507w, 391 (Pb–O-stretching vibrations). The absence of absorption bands in the range from 1550 to 1700 cm–1 indicates the absence of H2O molecules in somersetite.

Fig. 3. IR spectra of (a) somersetite; (b) plumbonacrite from Långban, Sweden and (c) hydrocerussite from Merehead quarry. England.
Powder XRD data
Powder XRD data (Britvin et al. Reference Britvin, Dolivo-Dobrovolsky and Krzhizhanovskaya2017) were obtained after single-crystal XRD from crushed fragments of 1/19661 using a Rigaku RAXIS Rapid II single-crystal diffractometer (CoKα) equipped with a cylindrical image plate detector using Debye-Scherrer geometry (with d = 127.4 mm). Data are given in Table 3. The unit-cell parameters were refined for a hexagonal unit cell, space group P63/mmc, a = 5.249(1), c = 40.679(2) Å, V = 970.89(55) Å3 and Z = 2.
Table 3. Powder XRD data for somersetite*.

*The eight strongest lines are shown in bold.
Crystal structure
Experiment
A crystal of somersetite from sample 1/19661 was mounted on a thin glass fibre and mounted on a Bruker DUO APEX II CCD four-circle diffractometer with a Mo-IμS micro-focus tube at 50 kV and 40 mA. More than a hemisphere of XRD data was collected with frame widths of 0.5° in ω, and with 90 s spent counting for each frame. The data were integrated and corrected for absorption using an empirical ellipsoidal model using the APEX and XPREP Bruker programs. The observed systematic absences for somersetite were consistent with the space group P3̅1c. In this space group, light atoms (C, O) could not be refined anisotropically. The obtained structure model was transformed to the space group P63/mmc using the ADDSYM algorithm incorporated in the PLATON program package (Le Page, Reference le Page1987; Spek, Reference Spek2003). The structure was refined successfully with the use of SHELX (Sheldrick, Reference Sheldrick2015). Structure refinement resulted in the crystallographic agreement index R 1 = 0.031 (Table 4). The final atomic coordinates and anisotropic displacement parameters are given in Table 5 and selected interatomic distances in Table 6. Hydrogen atom positions were not localized. The structural model involves disorder of several intrablock Pb positions. Attempts to eliminate the disorder by using different space groups were not successful, indicating that disorder is characteristic of the structure. This feature is typical for the other Pb basic carbonate minerals structurally related to hydrocerussite (Krivovichev and Burns, Reference Krivovichev and Burns2000; Martinetto et al., Reference Martinetto, Anne, Dooryhée, Walter and Tsoucaris2002; Siidra et al., Reference Siidra, Nekrasova, Depmeier, Chukanov, Zaitsev and Turner2018a,Reference Siidra, Nekrasova, Depmeier, Chukanov, Zaitsev and Turnerb,Reference Siidra, Nekrasova, Depmeier, Chukanov, Zaitsev and Turnerc). The crystallographic information files have been deposited with the Principal Editor of Mineralogical Magazine and are available as Supplementary material (see below).
Table 4. Crystallographic data and structure refinement details for somersetite.

Table 5. Atomic coordinates and displacement parameters (Å2) for somersetite.

*Site occupancy factor (SOF) = ⅙; ** SOF =½; *** SOF = ⅓. † BVS – bond-valence sums calculated using bond-valence parameters from Krivovichev and Brown (Reference Krivovichev and Brown2001) for the Pb2+–O bonds and from Brese and O'Keeffe (Reference Brese and O'Keeffe1991) for the C–O bonds.
Table 6. Selected interatomic distances in the crystal structure of somersetite.

Single-crystal XRD is a reliable method for the determination of Pb basic carbonate minerals related to hydrocerussite. However, in some cases confusion may occur during the preliminary check of the unit-cell parameters. Somersetite and plumbonacrite form very fine mutual intergrowths (Fig. 2) in the aggregates from the Torr Works quarry. These intergrowths may mimic a good quality ‘single crystal’. The latter results in the wrong determination of the unit-cell parameters as a = 9.0929(5) Å and c = 40.660(6) Å. The a parameter is identical with plumbonacrite, whereas the c value is the same as in somersetite (Table 7). ‘Crystals’ with this pseudo unit-cell were checked by powder XRD and expectedly provided patterns of the somersetite with plumbonacrite mixture. We suggest that some of the ‘hydrocerussite’-like phases from Torr Works quarry, studied previously at the Natural History Museum, London and described in Turner and Rumsey (Reference Turner and Rumsey2010) also correspond to the mixtures of layered Pb basic carbonate minerals. As different stackings of the electroneutral sheets described below may result in the other unusual minerals, the structural architectures related to hydrocerussite and all hydrocerussite occurrences should be evaluated carefully.
Table 7. Comparative data of somersetite, an unnamed slag phase, hydrocerussite and plumbonacrite.

*Palache et al. (Reference Palache, Berman and Frondel1951) and Anthony et al. (Reference Anthony, Bideaux, Bladh and Nichols2003) do not provide structural data.
Cation and anion coordination
The structure of somersetite contains five symmetrically unique Pb positions (Table 5). Coordination spheres of the Pb2+ cations are different due to the different degree of the stereochemical activity of 6s 2 lone electron pairs. In one coordination hemisphere, Pb1 and Pb3 atoms are coordinated by seven oxygen atoms at rather short Pb–O distances <3.0 Å. The resulting PbO7/PbO6OH [1 + 6] configuration is complemented by three additional long Pb–O bonds in another coordination hemisphere. The half occupied split Pb4 site is coordinated rather symmetrically by six Pb4–O2 bonds of 2.6688(11) Å each in plane and two additional apical Pb4–O4 bonds of 2.18(3) Å each. Coordination spheres of the split Pb2 site (SOF = ⅙) and Pb5 site (SOF = ⅓), despite the strong disorder, also demonstrate a rather uniform distribution of short and long bonds, which suggests that these atoms are not as stereochemically active as the other Pb sites. Very similar disorder for the Pb2 site was observed in the structure of the synthetic analogue of hydrocerussite: Pb3(OH)2(CO3)2 (Martinetto et al., Reference Martinetto, Anne, Dooryhée, Walter and Tsoucaris2002), determined by synchrotron XRD and hydrocerussite from Merehead quarry, England (Siidra et al., Reference Siidra, Nekrasova, Depmeier, Chukanov, Zaitsev and Turner2018a).
The carbonate triangles are very similar and show typical bond lengths, with a <C–O> of 1.294(8), 1.289(8) and 1.278(11) Å for C1-, C2- and C3-centred triangles, respectively.
The OH1 site attributed to hydroxyl group forms one short OH1–Pb1 bond (0.61 valence units) and three weaker OH1–Pb2 bonds. Coordination of the OH1 site is identical to ones in hydrocerussite from Merehead quarry (Siidra et al., Reference Siidra, Nekrasova, Depmeier, Chukanov, Zaitsev and Turner2018a) and synthetic powder material (Martinetto et al., Reference Martinetto, Anne, Dooryhée, Walter and Tsoucaris2002). In the synthetic analogue of hydrocerussite, Pb3(OH)2(CO3)2, the OH1 site demonstrates additional static disorder, whereas this is absent in somersetite and hydrocerussite (Siidra et al., Reference Siidra, Nekrasova, Depmeier, Chukanov, Zaitsev and Turner2018a), as well as in NaPb5(CO3)4(OH)3 (Siidra et al., Reference Siidra, Nekrasova, Chukanov, Pekov, Yapaskurt, Katerinopoulos, Voudouris, Magganas and Zaitsev2018b). The OH2 site is coordinated similarly by one Pb3 atom at 2.23(1) Å and three Pb5 atoms at 2.760(3) Å each. The O4 atom is coordinated tetrahedrally by four Pb atoms, which results in the formation of oxocentred OPb4 tetrahedra (Krivovichev et al., Reference Krivovichev, Mentré, Siidra, Colmont and Filatov2013; Siidra et al., Reference Siidra, Krivovichev and Filatov2008a) in somersetite. The disorder of the O4 atom (SOF = ½) correlates with the disorder of the Pb4 (SOF = ½) site.
Bond-valence sums for the cations and anions were calculated using the structure are listed in Table 5. They are in general agreement with the expected oxidation states, taking into account the strong disorder of several Pb sites.
Structure description
Polyhedra PbOn (with Pb–O bonds <3.1 Å) share common oxygen atoms with CO3 triangles thus forming two types of the two-dimensional (2D) blocks shown in Fig. 4. The first block is formed by Pb1, Pb2, C1, O1 and OH1 atoms, whereas Pb3, Pb4, Pb5, C2, C3, O2, O3, O4 and OH2 atoms build the second block. Each of the blocks can be split into separate sheets (Fig. 5). The outer ordered sheets in each block are topologically identical and correspond to the [PbCO3]0 composition. Each [PbCO3]0 sheet consists of Pb2+ cations, coordinated by three CO3 triangles. The [PbCO3]0 cerussite-type (denoted as C) sheets can be cut from the framework structure of cerussite PbCO3. The stereochemically active lone electron pairs on Pb2+ cations in the Pb1 and Pb3 sites are accommodated between the layers and point to each other (Fig. 4). The [Pb(OH)2]0 lead hydroxide (denoted as LHO) sheet is sandwiched between the two [PbCO3]0 sheets, shifted one relative to the other and denoted as C1 and C1’ in Figs 4 and 5. The resulting [Pb3(OH)2(CO3)2]0 block with a 2:1 (2[PbCO3] : 1[Pb(OH)2]) sheet structure and the disorder of Pb atoms in LHO sheet is very similar to that in the structure of hydrocerussite Pb3(OH)2(CO3)2 (Fig. 6) (Siidra et al., Reference Siidra, Nekrasova, Depmeier, Chukanov, Zaitsev and Turner2018a; Martinetto et al., Reference Martinetto, Anne, Dooryhée, Walter and Tsoucaris2002) and the hydrocerussite-type block in the NaPb5(CO3)4(OH)3 slag phase (Siidra et al., Reference Siidra, Nekrasova, Chukanov, Pekov, Yapaskurt, Katerinopoulos, Voudouris, Magganas and Zaitsev2018b).

Fig. 4. General projections of the crystal structures of somersetite and plumbonacrite (only strong Pb–O bonds <3.0 Å are shown). Identical types of sheets are marked by the same colours. The crystal structure of somersetite is based on 2D blocks of two types: (i) [Pb3(OH)2(CO3)2]0 formed by three sheets and identical in topology and composition to those in hydrocerussite and (ii) [Pb5O(OH)2(CO3)3]0 similar to those in plumbonacrite. OPb4 oxocentred tetrahedra are ordered in [Pb5O(OH)2(CO3)3]0 blocks in the structure of plumbonacrite. Lone pairs on Pb2+ cations between the blocks in the structure of somersetite and plumbonacrite are symbolized by ‘e’. OPb4 tetrahedra are marked by red. See text for details.
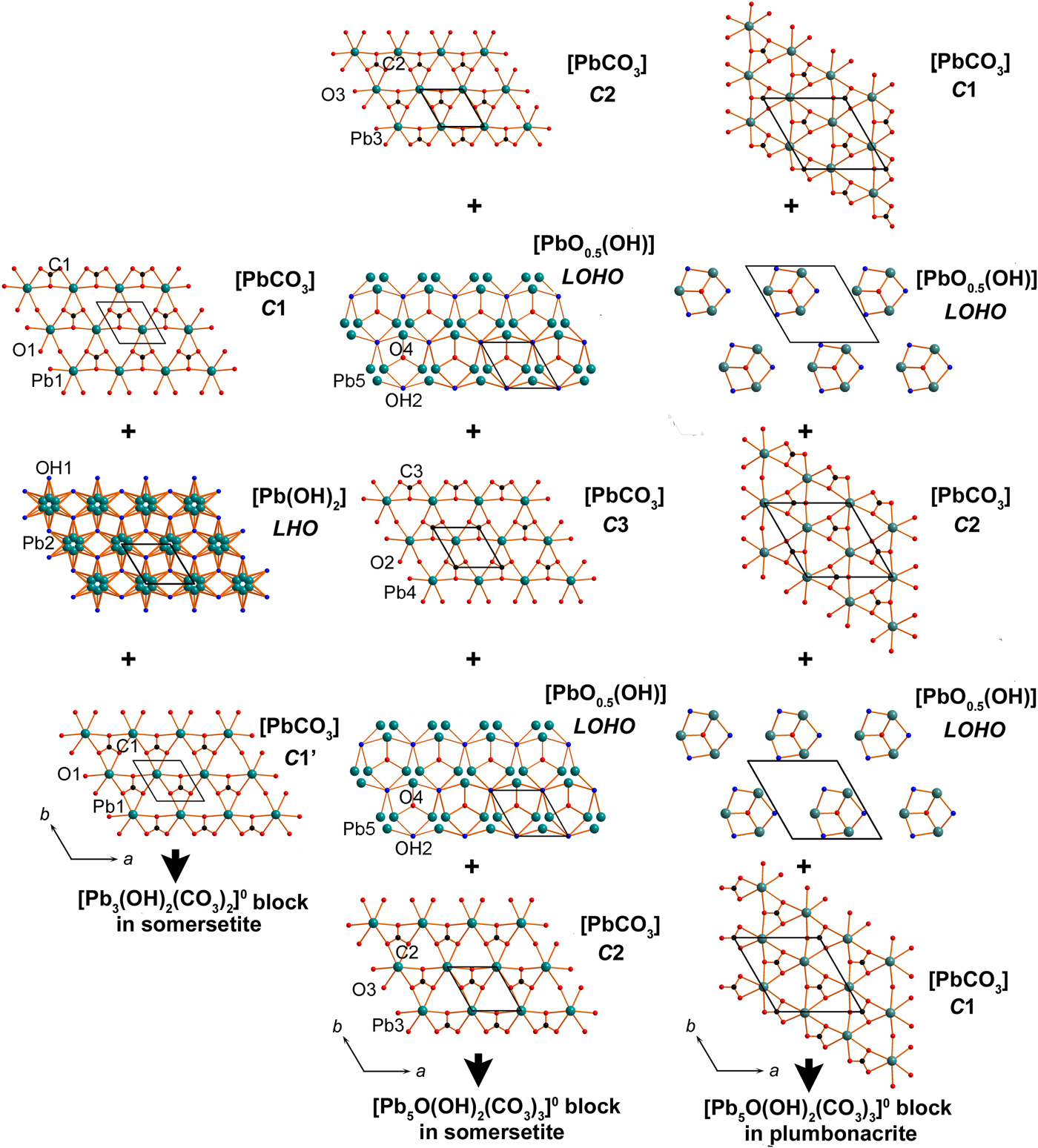
Fig. 5. Stackings of sheets in 2D blocks in the structures of somersetite and plumbonacrite. See text for details.
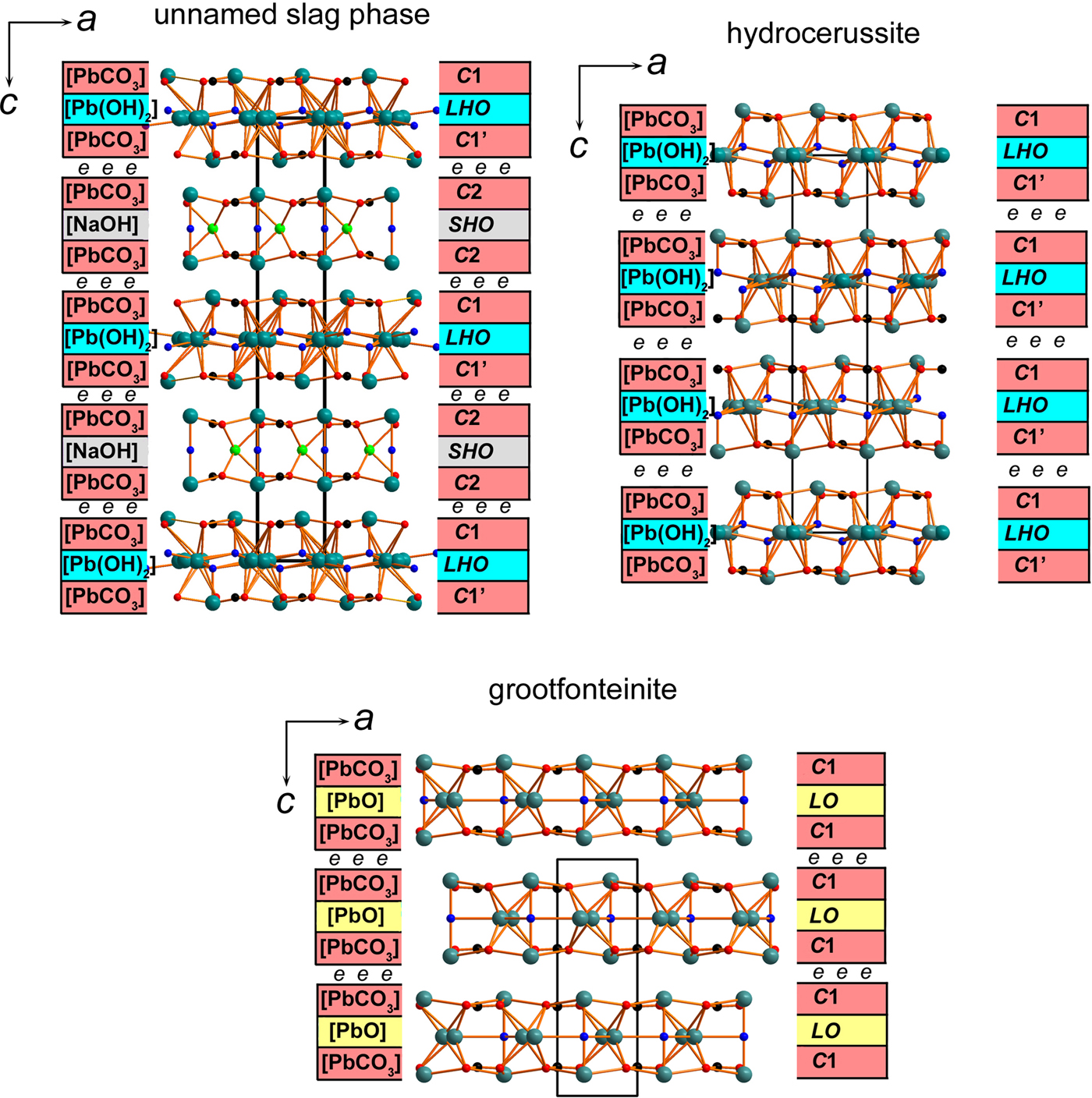
Fig. 6. General projections of the crystal structures of NaPb5(CO3)4(OH)3 unnamed phase from Lavrion (Siidra et al., Reference Siidra, Nekrasova, Depmeier, Chukanov, Zaitsev and Turner2018a); hydrocerussite (Siidra et al., Reference Siidra, Nekrasova, Chukanov, Pekov, Yapaskurt, Katerinopoulos, Voudouris, Magganas and Zaitsev2018b); and grootfonteinite (Siidra et al., Reference Siidra, Jonsson, Chukanov, Nekrasova, Pekov, Depmeier, Polekhovsky and Yapaskurt2018c). The structural architecture of NaPb5(CO3)4(OH)3 is similar to somersetite based on 2D blocks of two types: (i) [Pb3(OH)2(CO3)2]0 hydrocerussite type and (ii) [NaPb2(OH)(CO3)2]0 abellaite type. Lone pairs on Pb2+ cations are symbolized by ‘e’ between the blocks in the structures of Pb basic carbonates. See text for details.
The number of sheets in the second block in somersetite is five, identical to that in plumbonacrite (Figs 4 and 5). C2 sheets (in somersetite) and C1 sheets (in plumbonacrite) are not shifted relative to each other. Also, similarly to plumbonacrite, an additional [PbCO3]0 cerussite-type C3 sheet with the disordered Pb4 site is inserted in the middle of the [Pb5O(OH)2(CO3)3]0 block in somersetite. The composition of the [PbO0.5(OH)]0 lead oxide hydroxide (denoted as LOHO) sheets in somersetite is identical to that in plumbonacrite. However, LOHO sheets in somersetite demonstrate strong disorder not observed in plumbonacrite (Fig. 7). Taking into account ½ occupancy of the central O4 atoms in OPb4 tetrahedra and disorder of Pb5 in somersetite, the arrangement of isolated oxocentred tetrahedra is very similar to those in plumbonacrite.
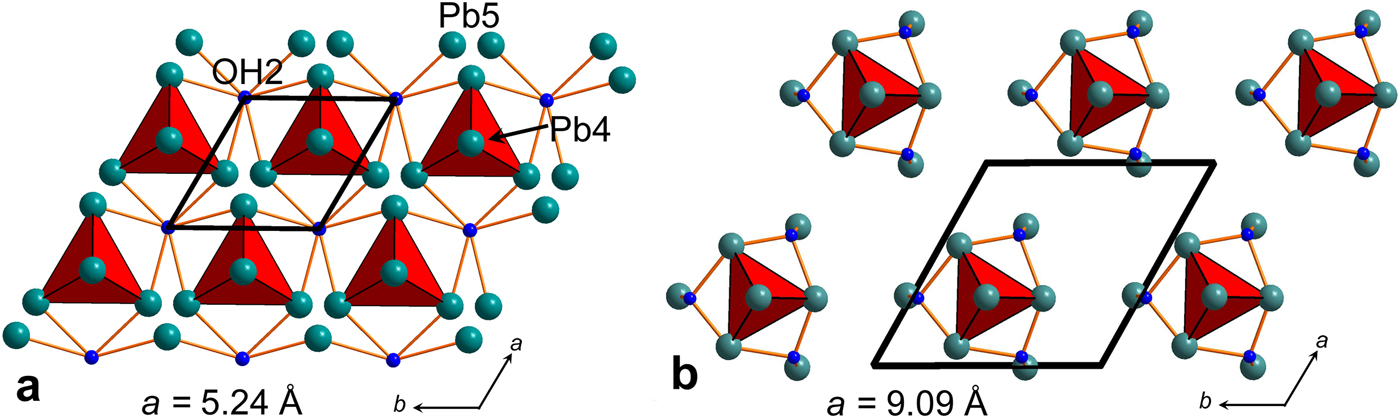
Fig. 7. Arrangement of O4-centred OPb4 oxocentred tetrahedra in the structure of somersetite (a). Site occupation factors of atoms are listed in Table 6. OPb4 oxocentred tetrahedra are ordered in plumbonacrite (b).
The structure of somersetite consists of the alternation of the [Pb5O(OH)2(CO3)3]0 and [Pb3 (OH)2(CO3)2]0 blocks separated by stereochemically active lone electron pairs on Pb2+. There are two electroneutral blocks of each type per unit cell, which corresponds to the formula [Pb5O(OH)2 (CO3)3][Pb3(OH)2(CO3)2] or Pb8O(OH)4(CO3)5 in a simplified representation.
Discussion
Our study demonstrated the ‘green hydrocerussite’ from the Mendip Hills to be a new mineral formed by both the hydrocerussite-type [Pb3(OH)2(CO3)2]0 and plumbonacrite-type [Pb5O(OH)2(CO3)3]0 blocks. Somersetite is related directly to hydrocerussite and plumbonacrite and its structure type is new for both minerals and inorganic compounds. In its crystal structure (Fig. 4), stereochemically active 6s 2 lone electron pairs on Pb2+ cations act as ‘chemical scissors’ and are responsible for the formation of well-defined electroneutral structural blocks, similar to those in other hydrocerussite-related mineral species (Fig. 6). The block size in these structures can vary and may consist of either three or five sheets. The thickness of the [Pb5O(OH)2 (CO3)3]0 blocks in somersetite and plumbonacrite is nearly identical and corresponds to 10.18 Å and 10.14 Å, respectively. Hydrocerussite-type blocks in somersetite are half the size (5.08 Å) of the plumbonacrite type and are very similar to abellaite-type and grootfonteinite-type blocks. Directional lone electron pairs of the Pb2+ cations fill the volume between the blocks, which effectively reduces the dimensionality of the system from 3D in cerussite PbCO3 to 2D in hydrocerussite-related minerals. The 2D blocks are held together only by very weak Pb–O bonds and weak interactions between lone pairs (Pyykö, Reference Pyykö1997). This feature is characteristic for the other Pb basic carbonate minerals including: hydrocerussite Pb3(OH)2(CO3)2 (Siidra et al., Reference Siidra, Nekrasova, Depmeier, Chukanov, Zaitsev and Turner2018a; Martinetto et al., Reference Martinetto, Anne, Dooryhée, Walter and Tsoucaris2002), plumbonacrite Pb5O(OH)2(CO3)3 (Krivovichev and Burns, Reference Krivovichev and Burns2000; Rumsey et al., Reference Rumsey, Krivovichev, Siidra, Kirk, Stanley and Spratt2012), abellaite NaPb2(OH)(CO3)2 (Ibáñez-Insa et al., Reference Ibáñez-Insa, Elvira, Llovet, Pérez-Cano, Oriols, Busquets-Masó and Hernández2017), unnamed slag phase NaPb5(CO3)4(OH)3 (Siidra et al., Reference Siidra, Nekrasova, Chukanov, Pekov, Yapaskurt, Katerinopoulos, Voudouris, Magganas and Zaitsev2018b), grootfonteinite Pb3O(CO3)2 (Siidra et al., Reference Siidra, Jonsson, Chukanov, Nekrasova, Pekov, Depmeier, Polekhovsky and Yapaskurt2018c) and leadhillite polymorphs Pb4(SO4)(CO3)2(OH)2 (Giuseppetti et al., Reference Giuseppetti, Mazzi and Tadini1990; Steele et al., Reference Steele, Pluth and Livingstone1998; Steele et al., Reference Steele, Pluth and Livingstone1999). Electroneutral 2D blocks of only one topology per structure are observed in all of these minerals except for the slag phase NaPb5(CO3)4(OH)3. The structural architecture in somersetite is the same as in NaPb5(CO3)4(OH)3, where hydrocerussite-type [Pb3(OH)2(CO3)2]0 blocks alternate with abellaite-type [NaPb2(OH)(CO3)2]0 (Fig. 6). It is also similar in this sense to the layered crystal structure of synthetic [Tl+5(SiO4)(OH)]2[Tl+6(SO4)(OH)4] = Tl+16(SiO4)2(SO4)(OH)6 (Siidra et al., Reference Siidra, Britvin, Krivovichev, Klimov and Depmeier2014).
The presence of [Pb5O(OH)2(CO3)3]0 blocks in somersetite reveals structural relationships with the closely associated plumbonacrite. OPb4 tetrahedra are a typical feature of the various Pb oxychloride minerals found in association with hydrocerussite-type minerals at Torr Works: mereheadite Pb47O24(OH)13Cl25(BO3)2(CO3) (Krivovichev et al., Reference Krivovichev, Turner, Rumsey, Siidra and Kirk2009); chloroxiphite Pb3CuO2 (OH)2Cl2 (Siidra et al., Reference Siidra, Krivovichev, Turner and Rumsey2008b); Pb2O(OH)Cl yeomanite (Turner et al., Reference Turner, Siidra, Rumsey, Polekhovsky, Kretser, Krivovichev and Spratt2015); rickturnerite Pb7O4[Mg(OH)4](OH)Cl3 (Rumsey et al., Reference Rumsey, Krivovichev, Siidra, Kirk, Stanley and Spratt2012); rumseyite [Pb2OF]Cl (Turner et al., Reference Turner, Siidra, Krivovichev, Stanley and Spratt2012); symesite Pb10O7SO4Cl4(H2O) (Welch et al., Reference Welch, Cooper, Hawthorne and Criddle2000); parkinsonite (Pb,Mo,◻)8O8Cl2 (Symes et al., Reference Symes, Cressey, Criddle, Stanley, Francis and Jones1994); and mendipite Pb3O2Cl2 (Turner and Rumsey, Reference Turner and Rumsey2010).
Acknowledgements
We thank Mark Cooper for many comments and remarks that greatly helped to improve the manuscript and led to the changes in the formula of somersetite. Editorial handling by Peter Leverett, Juraj Majzlan and Principal Editor Peter Williams are highly appreciated. This work was supported by St. Petersburg State University through the internal grant 3.38.238.2015 and Russian Science Foundation, grant no. 14-17-00048 (IR spectroscopy studies, C.N.V.). Technical support by the X-Ray Diffraction and Geomodel Resource Centres of Saint-Petersburg State University is gratefully acknowledged.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1180/minmag.2017.081.087
















