Introduction
The still actively forming bat-guano assemblage in the Rowley mine in southwestern Arizona, USA, has proven to be a prolific source of new minerals. Including the new mineral described herein, relianceite-(K), eight new minerals have now been described from this assemblage. Relianceite-(K), K4Mg(V4+O)2(C2O4)(PO3OH)4(H2O)10, is one of only five minerals known to include both phosphate and oxalate group, the others being davidbrownite-(NH4), (NH4,K)5(V4+O)2(C2O4)[PO2.75(OH)1.25]4⋅3H2O (Kampf et al., Reference Kampf, Cooper, Rossman, Nash, Hawthorne and Marty2019a), dendoraite-(NH4), (NH4)2NaAl(C2O4)(PO3OH)2(H2O)2 (Kampf et al., Reference Kampf, Cooper, Celestian, Ma and Marty2022), phoxite, (NH4)2Mg2(C2O4)(PO3OH)2(H2O)4 (Kampf et al., Reference Kampf, Celestian, Nash and Marty2019b), and thebaite-(NH4), (NH4,K)3Al(C2O4)(PO3OH)2(H2O) (Kampf et al., Reference Kampf, Cooper, Celestian, Nash and Marty2021a); all of these, except phoxite, are known only from the Rowley mine bat guano assemblage. One of these, dendoraite-(NH4), is intimately associated with relianceite-(K) and is described in a companion paper (Kampf et al., Reference Kampf, Cooper, Celestian, Ma and Marty2022).
In 1922, the Rowley Copper Mining Company was reorganised as the Reliance Copper Company in an effort to raise funds through stock offerings. Although the effort was unsuccessful and the newly formed Rowley Mines, Inc. took control of the mine in 1927, the mine was often referred to as the Reliance mine in subsequent years. The mineral name ‘relianceite’ is based upon this alternate name for the mine. For naming and species definition, the total combined occupancy of the four large cation sites in the structure is employed; thereby, the ‘-(K)’ suffix in the name reflects the fact that K+ > NH4+. If an analogue with NH4+ > K+ were found, it would be named relianceite-(NH4).
The new mineral and name were approved by the Commission on New Minerals, Nomenclature and Classification of the International Mineralogical Association (IMA2020-102, Kampf et al., Reference Kampf, Cooper, Celestian, Ma and Marty2021b). The holotype specimen of relianceite-(K) is deposited in the collections of the Natural History Museum of Los Angeles County, Los Angeles, California, USA, catalogue number 75275. This is also the holotype for dendoraite-(NH4).
Occurrence
Relianceite-(K) was found on the 125-foot level of the Rowley mine, ~20 km NW of Theba (small settlement and railroad depot), Maricopa County, Arizona, USA (33°2′57″N 113°1′49.59″W). The Rowley mine is on the western slope of the Painted Rock Mountains (in the Painted Rock mining district) and overlooks the Dendora Valley, immediately to the west. It is a former Cu–Pb–Au–Ag–Mo–V–baryte–fluorspar mine that exploited veins presumed to be related to the intrusion of an andesite porphyry dyke into Tertiary volcanic rocks. Although the mine has not been operated for ore since 1923, collectors took notice of the mine as a source of fine wulfenite crystals around 1945. An up-to-date account of the history, geology, and mineralogy of the mine was published recently by Wilson (Reference Wilson2020).
The new mineral was found in a hot and humid area of the mine (see figure 26 in Wilson, Reference Wilson2020) in an unusual bat guano-related, post-mining assemblage of phases that include a variety of vanadates, phosphates, oxalates and chlorides, some containing NH4+. This secondary mineral assemblage is found growing on baryte–quartz-rich matrix and, besides relianceite-(K), includes allantoin (Kampf et al., Reference Kampf, Celestian, Nash and Marty2021c), ammineite, antipinite, aphthitalite, bassanite, biphosphammite, cerussite, davidbrownite-(NH4) (Kampf et al., Reference Kampf, Cooper, Rossman, Nash, Hawthorne and Marty2019a), dendoraite-(NH4) (Kampf et al., Reference Kampf, Cooper, Celestian, Ma and Marty2022), fluorite, halite, hydroglauberite, mimetite, mottramite, natrosulfatourea (Kampf et al., Reference Kampf, Celestian, Nash and Marty2021c), perite, phoxite (Kampf et al., Reference Kampf, Celestian, Nash and Marty2019b), rowleyite (Kampf et al., Reference Kampf, Cooper, Nash, Cerling, Marty, Hummer, Celestian, Rose and Trebisky2017), salammoniac, struvite, thebaite-(NH4) (Kampf et al., Reference Kampf, Cooper, Celestian, Nash and Marty2021a), thénardite, urea, vanadinite, weddellite, willemite, wulfenite, and several other potentially new minerals. Relianceite-(K) was found in intimate association with antipinite, dendoraite-(NH4), fluorite, mimetite, mottramite, rowleyite, salammoniac, struvite, vanadinite, willemite, wulfenite, and at least one other potentially new species.
Physical and optical properties
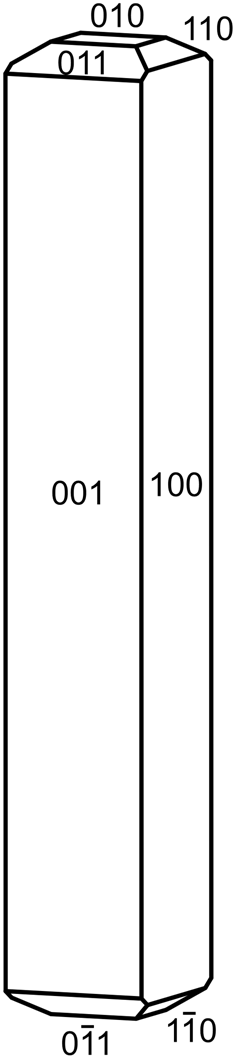
Crystals of relianceite-(K) are sky blue prisms, up to ~0.1 mm in length, often forming radiating sprays (Fig. 1). The blades are elongate on [010] with shallow pyramidal terminations; the observed crystal forms are {100}, {${\bar 1}$![]() 00}, {001} and {00${\bar 1}$
00}, {001} and {00${\bar 1}$![]() }; although terminal forms could not be measured, the appearance of crystals suggests some combination of the forms {010}, {110}, {${\bar 1}$
}; although terminal forms could not be measured, the appearance of crystals suggests some combination of the forms {010}, {110}, {${\bar 1}$![]() 10}, {011} and {01${\bar 1}$
10}, {011} and {01${\bar 1}$![]() } (Fig. 2). No twinning was observed, but inversion twinning is inferred from structure refinement. The streak is very pale blue, the lustre is vitreous, the Mohs hardness is ~2½, the tenacity is brittle and the fracture is splintery. No cleavage could be observed with certainty because of the small crystal size; however, the structure suggests two cleavages in the [010] zone, probably perfect on {100} and good on {001}. The tiny crystals are virtually invisible in density liquids making the measurement of their density impossible. The calculated density is 2.111 g⋅cm–3 using the empirical formula and 2.204 g⋅cm–3 using the ideal (K-end-member) formula. Relianceite-(K) is non-fluorescent in long- and short-wave ultraviolet light. The mineral is insoluble at room temperature in H2O, but easily soluble in dilute HCl.
} (Fig. 2). No twinning was observed, but inversion twinning is inferred from structure refinement. The streak is very pale blue, the lustre is vitreous, the Mohs hardness is ~2½, the tenacity is brittle and the fracture is splintery. No cleavage could be observed with certainty because of the small crystal size; however, the structure suggests two cleavages in the [010] zone, probably perfect on {100} and good on {001}. The tiny crystals are virtually invisible in density liquids making the measurement of their density impossible. The calculated density is 2.111 g⋅cm–3 using the empirical formula and 2.204 g⋅cm–3 using the ideal (K-end-member) formula. Relianceite-(K) is non-fluorescent in long- and short-wave ultraviolet light. The mineral is insoluble at room temperature in H2O, but easily soluble in dilute HCl.

Fig. 1. Spray of relianceite-(K) prisms on the holotype specimen (#75275); field of view 0.3 mm across.
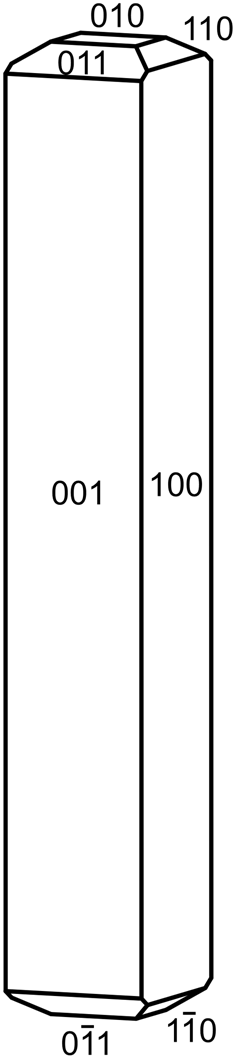
Fig. 2. Crystal drawing of relianceite-(K); clinographic projection in non-standard orientation, b vertical.
Relianceite-(K) is optically biaxial (+) with α = 1.528(2), β = 1.529(2) and γ = 1.562(2) determined in white light. The 2V measured using extinction data with EXCALIBR (Gunter et al., Reference Gunter, Weaver, Bandli, Bloss, Evans and Su2004) is 22(1)°; the calculated 2V is 20.1°. The partially determined optical orientation is Z = b (length slow). The mineral is pleochroic: X = colourless, Y = pale blue, Z = pale blue; and X < Y ≈ Z.
Raman spectroscopy
Raman spectroscopy was conducted on a Horiba XploRA PLUS spectrometer using a 532 nm diode laser, 100 μm slit and 1800 gr/mm diffraction grating and a 100× (0.9 NA) objective. Full pattern peak fitting was performed using the least-squares approach using Gaussian peak shapes to minimise the difference between measured and calculated profiles, and cubic-spline was used for base-line modelling. The spectrum from 3800 to 60 cm–1 is shown in Fig. 3 including labelled mode assignments based on several references: Frost (Reference Frost2004), Frost et al. (Reference Frost, Palmer and Pogson2011), Hardcastle and Wachs (Reference Hardcastle and Wachs1991), Kouvatas et al. (Reference Kouvatas, Alonzo, Bataille, Le Pollès, Roiland, Louarn and Le Fur2017), Ma and He (Reference Ma and He2012), Mohaček-Grošev et al., (Reference Mohaček-Grošev, Grdadolnik, Stare and Hadži2009), Rudolph and Irmer (Reference Rudolph and Irmer2007), Sergeeva et al. (Reference Sergeeva, Zhitova and Bocharov2019), Števko et al. (Reference Števko, Sejkora, Uher, Cámara, Škoda and Vaculovič2018) and Yakovenchuk et al. (Reference Yakovenchuk, Pakhomovsky, Konopleva, Panikorovskii, Bazai, Mikhailova, Bocharov, Ivanyuk and Krivovichev2018).
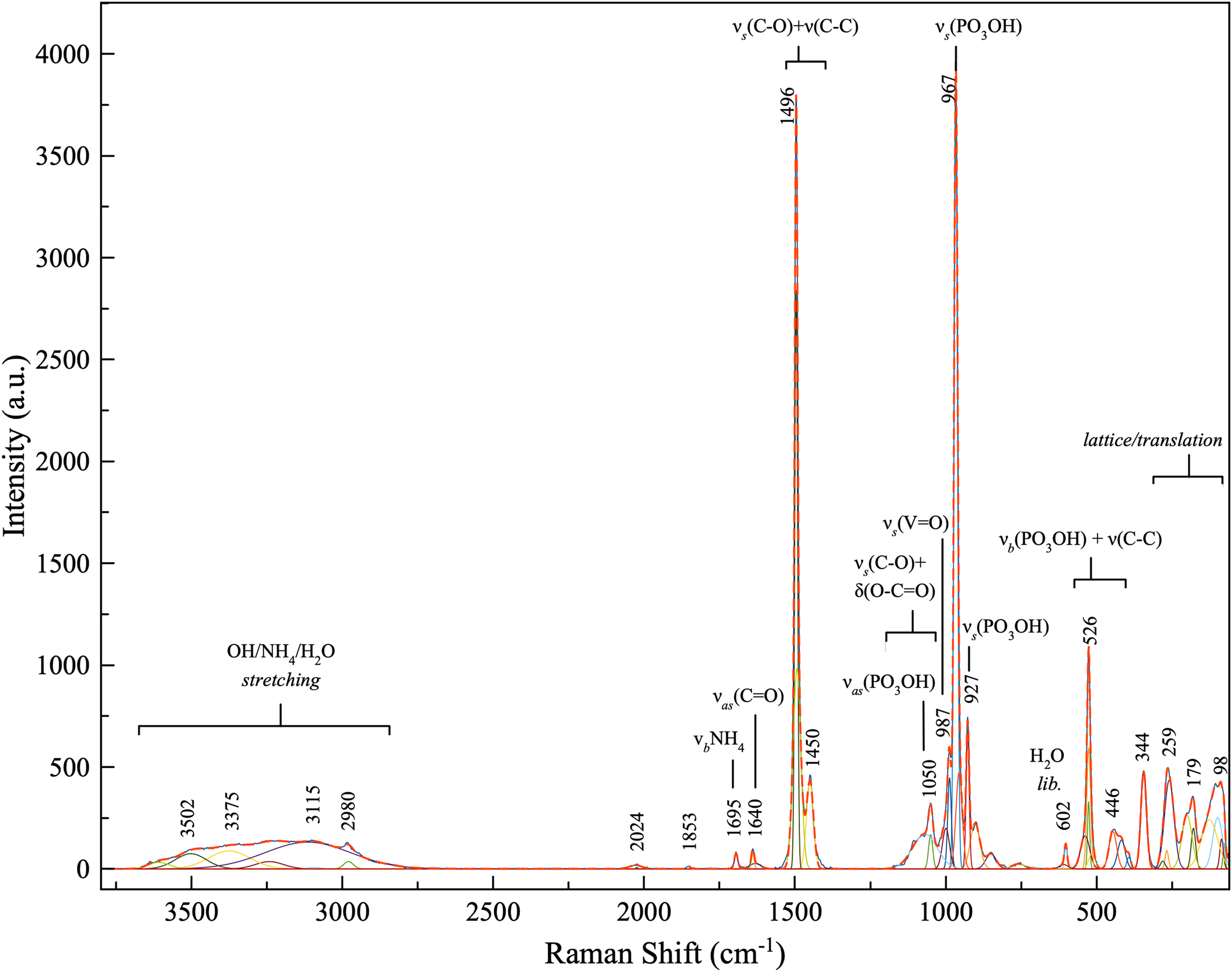
Fig. 3. Raman spectrum of relianceite-(K).
Chemical analysis
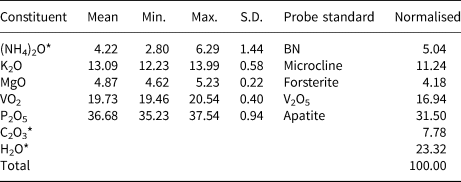
Analyses (6 points) were performed at Caltech on a JEOL 8200 electron microprobe in wavelength dispersive spectroscopy mode. Analytical conditions were 15 kV accelerating voltage, 5 nA beam current and 5 μm beam diameter. During vacuum deposition of the conductive carbon coat required for electron probe microanalysis (EPMA), relianceite-(K) clearly suffered loss of much of the weakly held H2O and probably a portion of its NH4. Relianceite-(K) was very sensitive to the electron beam and additional loss of these components probably occurred during the EPMA. The very large loss in H2O resulted in much higher concentrations for the remaining constituents than are to be expected for the fully hydrated phase; therefore, the other analysed constituents have been normalised to provide a total of 100% when combined with the calculated H2O content. To account for the loss of NH4, (NH4)2O was calculated so that K + NH4 = 4 atoms per formula unit (apfu) in accord with the structure. We attribute the large variation in analysed (NH4)2O content to analytical problems, rather than to actual compositional variation. Insufficient material is available for CHN analysis; however, the fully ordered structure and detailed bond-valence analysis unambiguously established the anion (O, OH, H2O and C2O4) identities and the corresponding quantitative contents of H2O and CO2. Analytical data are given in Table 1.
Table 1. Analytical data (wt.%) for relianceite-(K).
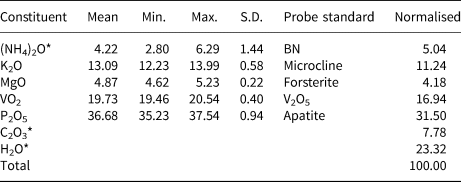
* (NH4)2O, C2O3 and H2O values in the Normalised column are based on the structure.
S.D. – standard deviation.
The empirical formula (based on P + V = 6 and O = 32 apfu) is [K2.21(NH4)1.79]Σ4.00Mg0.96(V4+0.95O)2(C2O4)[P1.03O3.03(OH)0.97]4(H2O)10. The simplified formula is (K,NH4)4Mg(V4+O)2(C2O4)(PO3OH)4(H2O)10 and the ideal (K-end-member) formula is K4Mg(V4+O)2(C2O4)(PO3OH)4(H2O)10, which requires K2O 19.49, MgO 4.17, VO2 17.16, P2O5 29.37, C2O3 7.45, H2O 22.36, total 100 wt.%. The Gladstone-Dale compatibility (Mandarino, Reference Mandarino2007) 1 – (K p/K c) is –0.001 in the range of superior compatibility for the empirical formula.
X-ray crystallography and structure determination
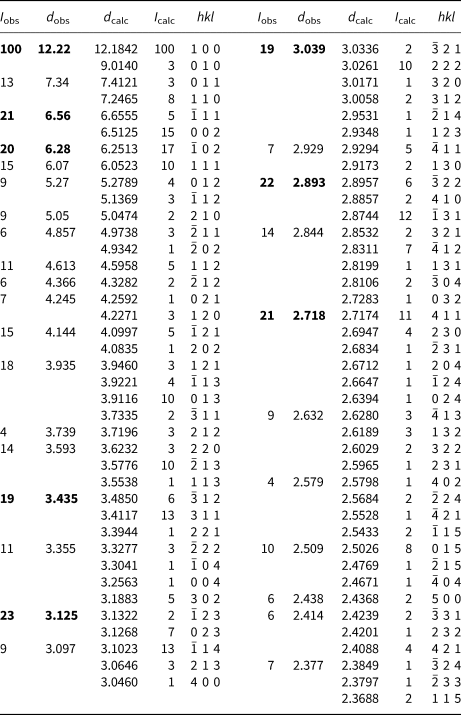
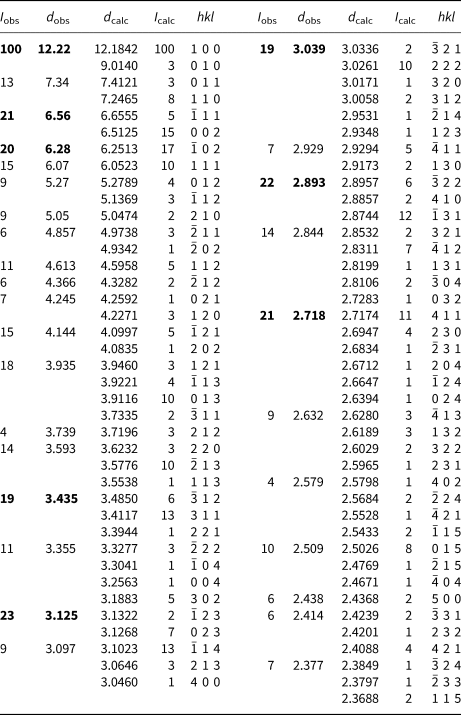
Powder X-ray studies were done using a Rigaku R-Axis Rapid II curved imaging plate microdiffractometer with monochromatized MoKα radiation. A Gandolfi-like motion on the φ and ω axes was used to randomise the sample. Observed d values and intensities were derived by profile fitting using JADE Pro software (Materials Data, Inc., USA). The powder data are presented in Table 2. Unit-cell parameters refined from the powder data using JADE Pro with whole pattern fitting are a = 12.424(10), b = 9.030(10), c = 13.273(10) Å, β = 100.75(2)°, and V = 1463(2) Å3.
Single-crystal X-ray studies were done using a Bruker D8 three-circle diffractometer equipped with a rotating anode generator (MoKα X-radiation), multilayer optics and an APEX-II CCD area detector. Crystals of relianceite-(K) generally exhibit subparallel composite growth. The best crystal found after extensive searching still provided split diffraction spots. Using 40 s frames with a 0.3° frame width, a total of 34,188 reflections were integrated. The unit-cell dimensions were obtained by least-squares refinement of 4016 reflections with I o > 10σI. Empirical absorption corrections (SADABS; Sheldrick, Reference Sheldrick2015) were applied and equivalent reflections were merged. Systematically absent reflections are consistent with the presence of a c-glide plane. The E statistics (|E 2 – 1| = 0.841) did not provide an unambiguous choice between centrosymmetric and noncentrosymmetric space groups. The structure was subsequently solved in space group Pc by direct methods using SHELXS-2013. The structure was refined using SHELXL-2016 (Sheldrick, Reference Sheldrick2015) and was modelled as an inversion twin. Four large-cation sites were refined with joint occupancies by K and N. Difference-Fourier syntheses failed to locate H atoms; however, detailed bond-valence assessment, including hydrogen-bond assignments, allowed O sites to be ascribed as O, OH or H2O. Data collection and refinement details are given in Table 3, atom coordinates and displacement parameters in Table 4, selected bond distances in Table 5 and a bond-valence analysis in Table 6. The crystallographic information files have been deposited with the Principal Editor of Mineralogical Magazine and are available as Supplementary material (see below).
Table 3. Data collection and structure refinement details for relianceite-(K).

*R int = Σ|F o2–F o2(mean)|/Σ[F o2]. GoF = S = {Σ[w(F o2–F c2)2]/(n–p)}½. R 1 = Σ||F o|–|F c||/Σ|F o|. wR 2 = {Σ[w(F o2–F c2)2]/Σ[w(F o2)2]}½; w = 1/[σ2(F o2) + (aP)2 + bP] where a is 0.0758, b is 0 and P is [2F c2 + Max(F o2,0)]/3.
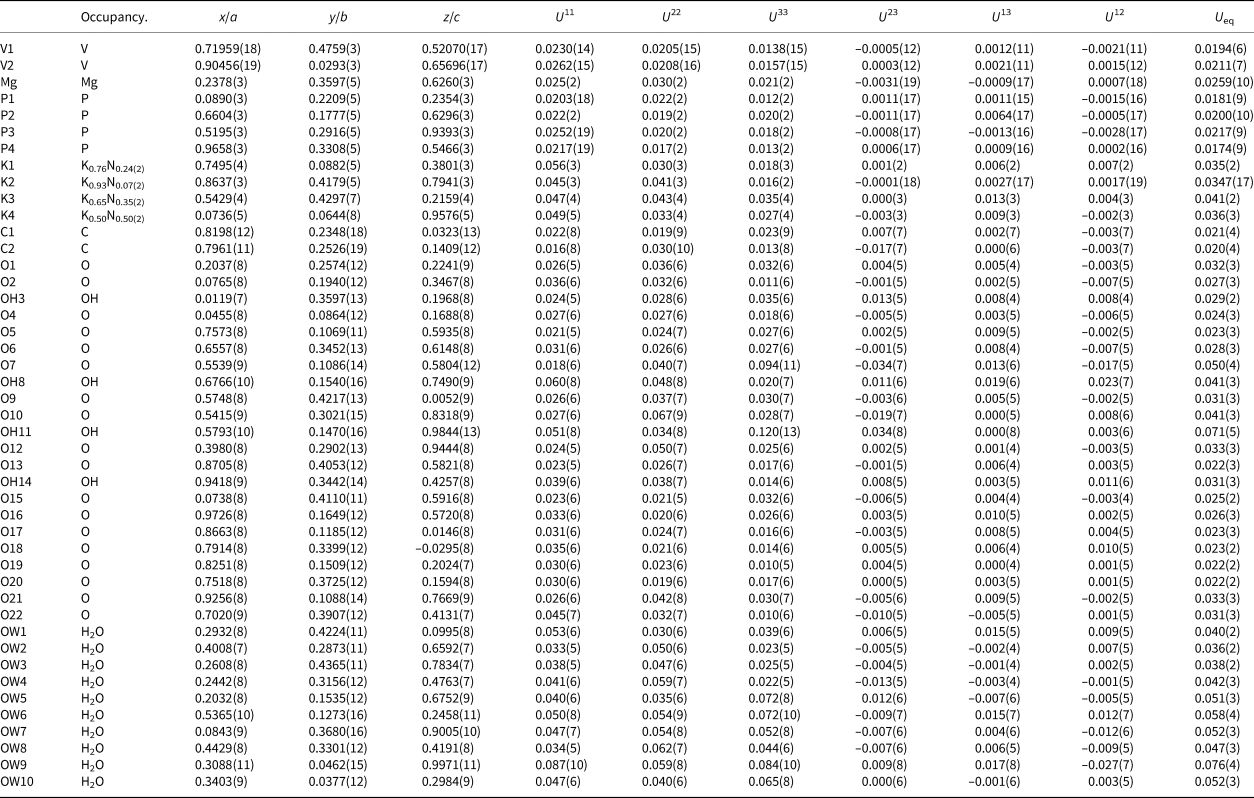
Table 4. Atom positions, occupancy and displacement parameters (Å)2 for relianceite-(K).
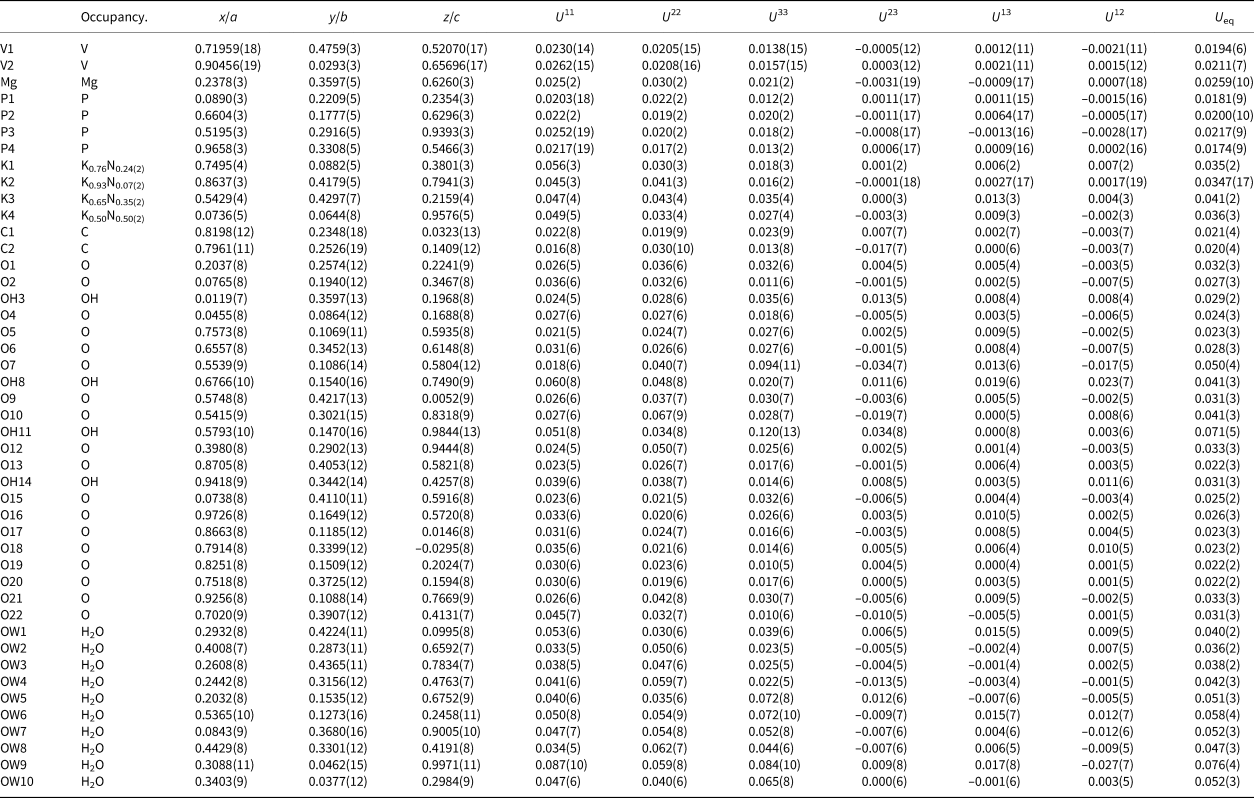
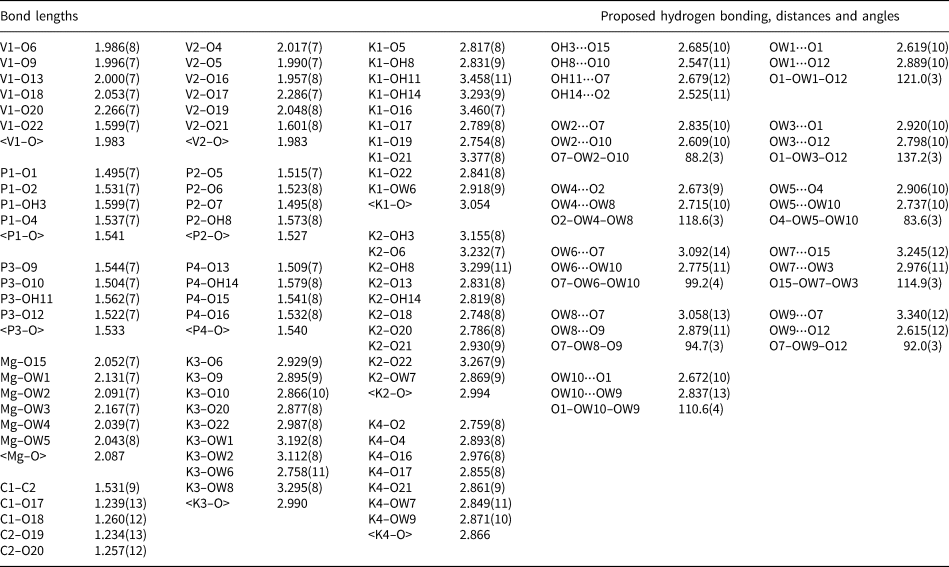
Table 5. Selected bond lengths (Å) and angles (°) for relianceite-(K).
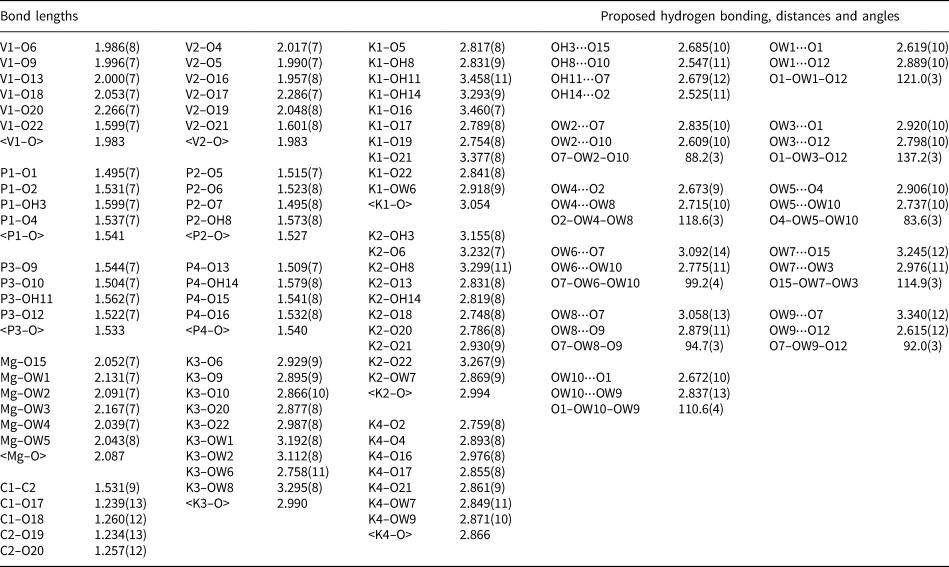
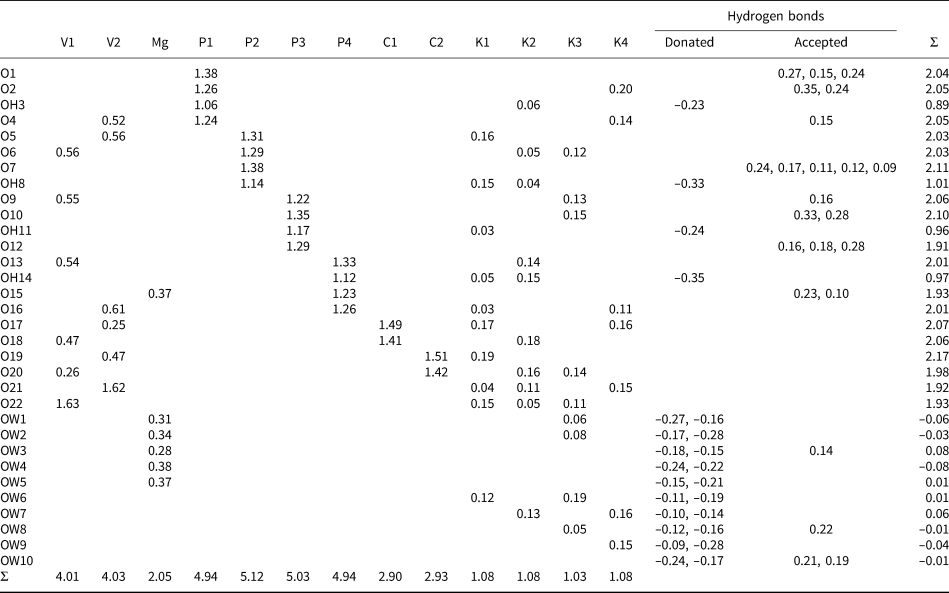
Table 6. Bond-valence analysis for relianceite-(K). Values are in valence units (vu).
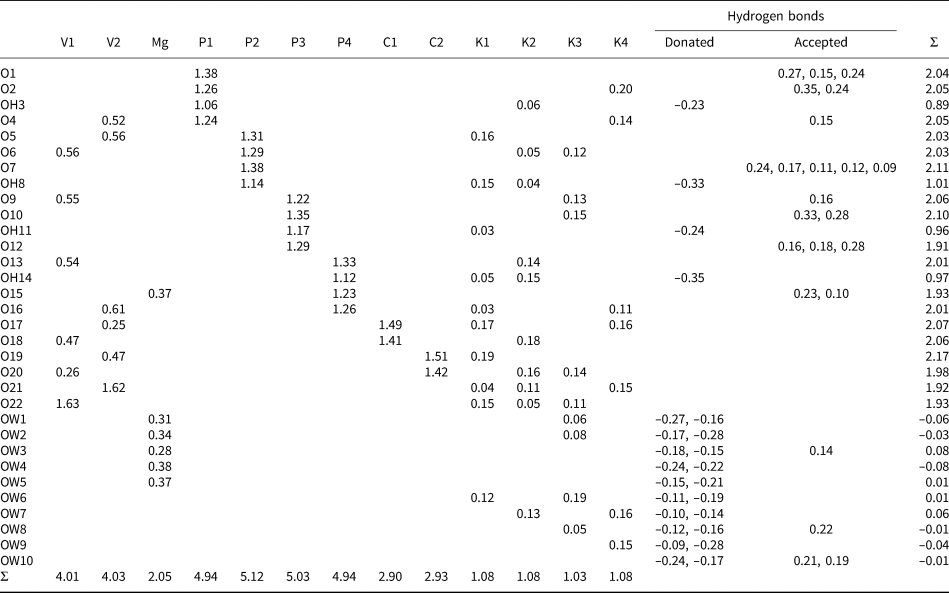
Bond-valence parameters for NH4+–O are from Garcia-Rodriguez et al. (Reference García-Rodríguez, Rute-Pérez, Piñero and González-Silgo2000); all others are from Gagné and Hawthorne (Reference Gagné and Hawthorne2015). The K/N sites were modelled using refined occupancies. Hydrogen-bond strengths are based on O–O distances according to the relation of Ferraris and Ivaldi (Reference Ferraris and Ivaldi1988).
Discussion of the structure
The structure of relianceite-(K) includes four large cation sites coordinated by O (O, OH and/or H2O) sites: K1 (ten coordinated), K2 (ten coordinated), K3 (nine coordinated) and K4 (seven coordinated). One octahedrally coordinated Mg site is surrounded by one O site and five H2O sites. There are four P sites, P1, P2, P3 and P4, all tetrahedrally coordinated by three O and one OH. One oxalate (C2O4) group includes two independent C sites, C1 and C2, and four independent O sites. The two V4+ sites, V1 and V2, are both octahedrally coordinated by O sites. Each of the V4+ bonds to three O atoms shared by phosphate groups with V–O bond distances ranging from 1.957 to 2.017 Å, two O atoms shared with the oxalate C2O4 group with longer V–O bonds from 2.048 to 2.286 Å, and one O atom that forms a short vanadyl V = O bond of 1.599 and 1.601 Å. The longest V–O bonds in each octahedron is trans to the short vanadyl bond, giving typical [1+4+1]-coordinations (Schindler et al., Reference Schindler, Hawthorne and Baur2000).
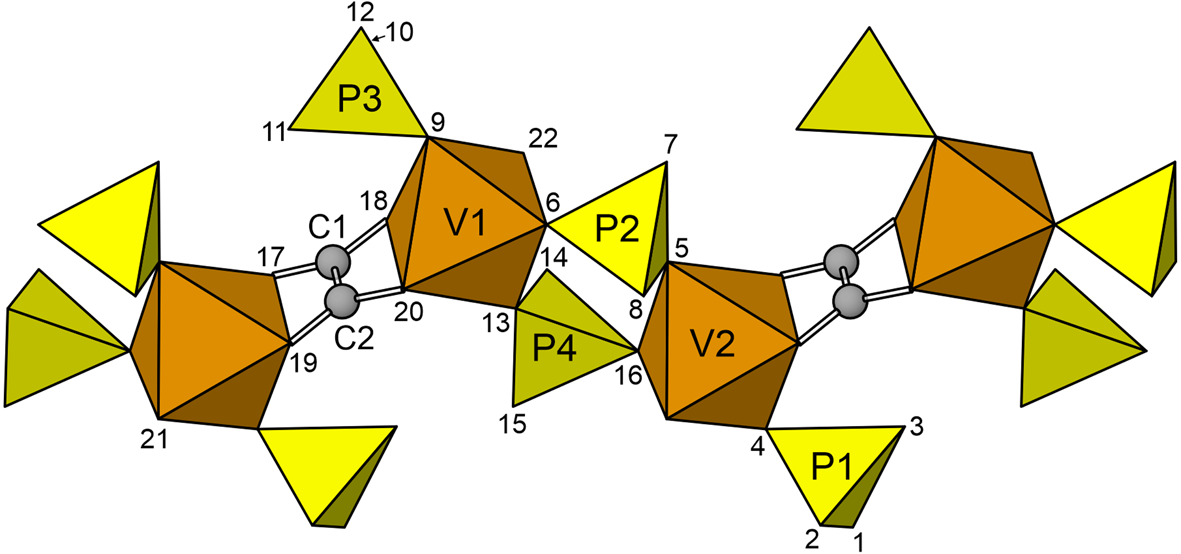
The structural unit is a chain along b constructed of VO6-octahedra, PO3OH tetrahedra and C2O4 oxalate groups. In this chain, pairs of VO6 octahedra, V1O6 and V2O6, are linked by the bridging oxalate group, forming [V2C2O12] dimers. Two PO3OH tetrahedra, P2O3OH and P4O3OH, form a double bridge between the VO6 octahedra, thereby linking the [V2C2O12] dimers into the chain. Additional PO3OH tetrahedra, P1O3OH and P3O3OH, decorate the chain. The decorated (V4+O)2(C2O4)(PO3OH)46- chain (Fig. 4) is identical to that in the structure of davidbrownite-(NH4), (NH4,K)5(V4+O)2(C2O4)[PO2.75(OH)1.25]4⋅3H2O (Kampf et al., Reference Kampf, Cooper, Rossman, Nash, Hawthorne and Marty2019a). The O site (O15) of the MgO(H2O)5 octahedron is the O site of the P4O3OH tetrahedron that does not link to V1 or V2. It can be considered a distant decoration on the (V4+O)2(C2O4)(PO3OH)4 chain. As seen in Fig. 5, the chains are linked to each other through an extensive system of K/NH4–O bonds and hydrogen bonds.
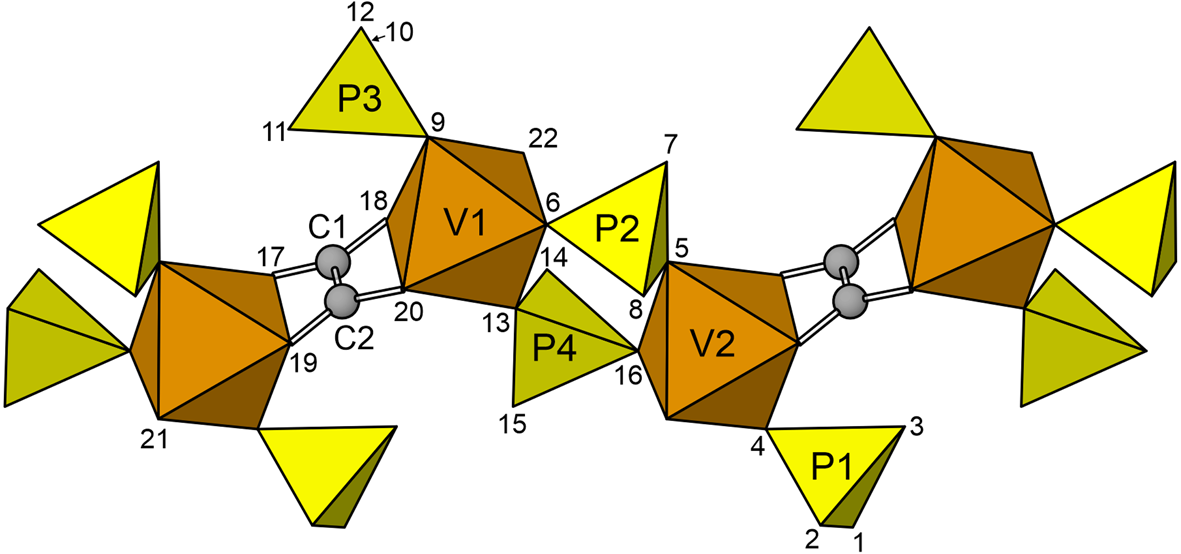
Fig. 4. The decorated chain in the relianceite-(K) structure. The O sites are numbered. The view is along [10${\bar 1}$![]() ] with [010] horizontal.
] with [010] horizontal.

Fig. 5. The relianceite-(K) structure viewed down [010]. The unit cell outline is shown with dashed lines.
Both relianceite-(K) and davidbrownite-(NH4) contain acid-phosphate groups that deliver strong intra- and inter-chain H-bonds (i.e. OH⋅⋅⋅OA distances 2.47–2.68 Å). Both structures contain two similar intra-chain H-bonds linking vertices of bridging and decorating P tetrahedra within the chain, and two inter-chain H-bonds that link decorating P tetrahedra of adjacent chains along [001] (Fig. 6). Davidbrownite-(NH4) contains additional OH bonded to its decorating P tetrahedra in the form of a single H atom bridging symmetrically equivalent OH8 anions on adjacent chains along [100]. In relianceite-(K), the neighbouring chains are more distant from each other in the [100] direction, and a Mg atom is located between chains in this region of the structure. The relative positioning of chains in the {010} plane differs between the two structures in relation to their different inter-chain connectivity, with relianceite-(K) having a greater β angle and a-translation.
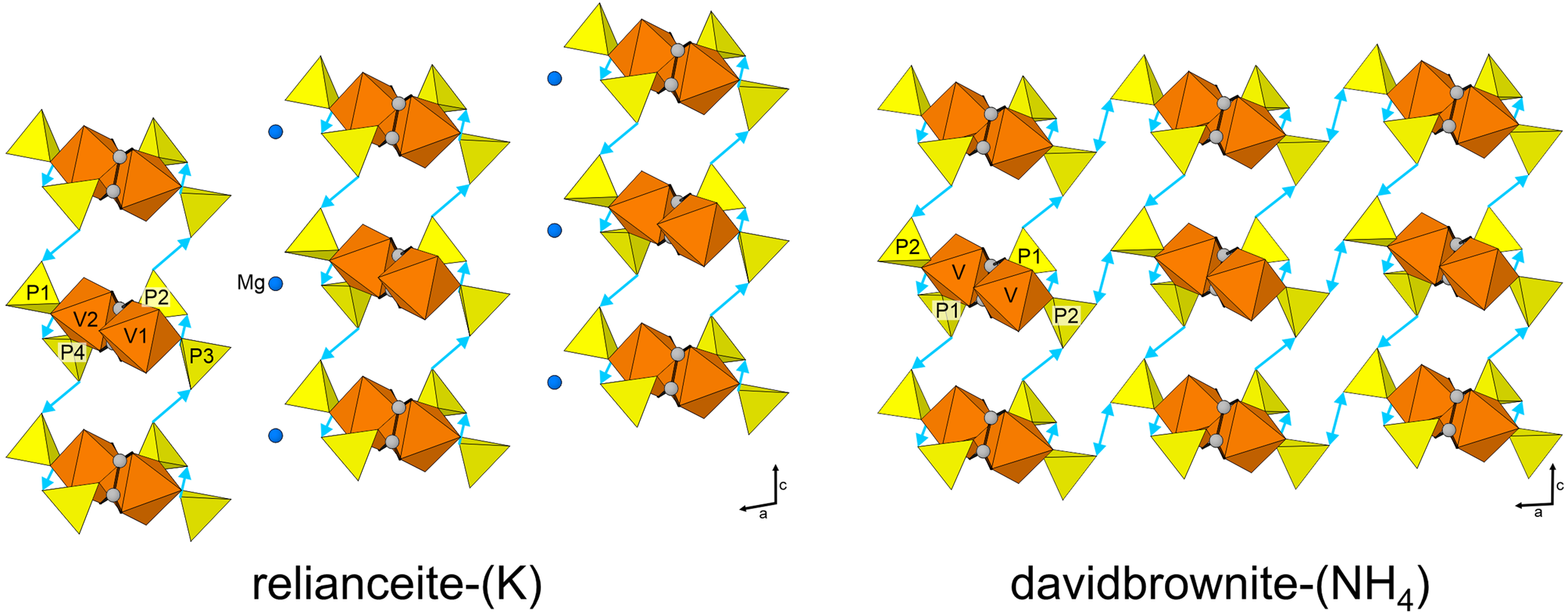
Fig. 6. Hydrogen-bond linkages between the chains in the structures of relianceite-(K) and davidbrownite-(NH4) viewed down [010]. The hydrogen bonds are shown as turquoise-coloured lines pointing from the donating OH group toward the receiving O atom. The double-ended arrow indicates the symmetrical H-bond between OH8 anions.
Acknowledgements
Peter Leverett and anonymous reviewers are thanked for constructive comments, which improved the manuscript. Keith Wentz, claim holder of the Rowley mine, is thanked for allowing underground access for the study of the occurrence and the collecting of specimens, along with Frank Hawthorne for providing access to the single-crystal instrument at the University of Manitoba. This study was funded, in part, by the John Jago Trelawney Endowment to the Mineral Sciences Department of the Natural History Museum of Los Angeles County.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1180/mgm.2021.99