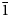Introduction
The Reaphook Hill Zn deposit is one of several non-sulfide zinc occurrences discovered in the northern Flinders Ranges of South Australia during the 1960s. Rock chip sampling near Reaphook Hill by the South Australian Department of Mines in 1963 and subsequent stream sediment sampling undertaken by Kennecott Explorations (Australia) Pty. Ltd. revealed zinc concentrations of up to 1170 ppm (Johns, Reference Johns1972). Scholzite was discovered as prismatic needles in three discrete near-surface mineralised zones associated with ferruginised and manganiferous limestone. Exploratory drilling failed to disclose sulfides or mineralisation below the base of oxidation and the prospect was considered to be incapable of sustaining a mining operation. The deposit was mined for specimens in the late 1960s and 1970s and many hundreds of specimens are now in collections worldwide (Johnson, Reference Johnson1978; Johnston and Hill, Reference Johnston and Hill1978). Investigations of collinsite, tarbuttite, parahopeite and scholzite from Reaphook Hill were undertaken by Hill and Milnes (Reference Hill and Milnes1974) and Hill (Reference Hill1975) using powder X-ray diffraction and electron-probe analyses. An X-ray single-crystal study of scholzite was also completed (Hill et al., Reference Hill, Johnson and Jones1973; Hill, Reference Hill1975). Parahopeite crystals were found to be compositionally zoned with their cores enriched in Fe and Mn and outer zones enriched in Mg, with MgO contents of up to 6.3 wt.%. Parahopeite at Reaphook Hill has several morphological varieties belonging to two generations. First-generation parahopeite occurs as translucent white to yellow–brown crystals with a prismatic habit and up to 1 cm in length. On the surface of some crystals, a second generation of parahopeite and reaphookhillite has formed as parallel growths. These second-generation crystals are colourless to white in colour and are bladed and thin tabular to equant in habit. In the present study, electron-probe analyses of 24 second generation parahopeite crystals found that the content of MgO is typically in the range 2.3 to 4.6 wt.%. Two of the analysed crystals had a MgO content in the range 5.5 to 9.7 wt.% and these represent the new mineral reaphookhillite, the Mg analogue of parahopeite. The new species (IMA 2018-128) and its name were approved by the Commission on New Minerals Nomenclature and Classification of the International Mineralogical Association (IMA2018-128, Elliott Reference Elliott2019). The holotype specimen, registration number G34798, is deposited in the mineral collections of the South Australian Museum, Adelaide, South Australia, Australia.
Occurrence
Reaphook Hill deposit comprises three discrete, near-surface mineralised zones, less than 3 m below the surface in unmetamorphosed sediments of the Lower Cambrian Parachilna Formation. These sediments comprise sandy, poorly sorted argillaceous siltstones with conglomerate lenses containing well-rounded quartz pebbles dispersed in a matrix of weakly consolidated sand and grit (Johns, Reference Johns1972). The Wilkawillina Limestone conformably overlies the Parachilna Formation and contains high Mn, Zn and P concentrations. Phosphate deposit occurrences in the Parachilna Formation have resulted from the near-surface enrichment by groundwater of zinc and phosphorus derived from originally low-grade mineralisation. The mineralised zones at Reaphook Hill have ferruginous and manganiferous cappings, which grade downwards into complexly fractured phosphatic pebble conglomerates, sandstones, and siltstones. They seem to have developed in fractured and faulted zones in the Parachilna Formation as a result of the action of groundwater causing near-surface enrichment of manganese, iron, zinc and phosphorus (Hill and Milnes, Reference Hill and Milnes1974).
Appearance, physical and optical properties
The holotype specimen of reaphookhillite comprises crystalline parahopeite. Parahopeite crystals line small cavities and reaphookhillite crystals have formed as an overgrowth on parahopeite crystals (Fig. 1). Crystals are bladed to thin tabular in habit and are up to 0.6 mm in width. Reaphookhillite is colourless and transparent. It has a vitreous lustre and shows a perfect cleavage on {010}. The mineral is brittle and shows an irregular fracture. By analogy with parahopeite, Mohs hardness is estimated at ~ 4. The calculated density is 3.09 g/cm3 from the empirical formula; 3.03 g/cm3 from the ideal formula. Optically, the mineral is biaxial (+) and is nonpleochroic. The indices of refraction are α = 1.583(3), β = 1.596(3), γ = 1.611(3) and 2Vcalc = 88.7°.

Fig. 1. Crystal of reaphookhillite, 0.3 mm in width, on parahopeite.
Chemical analysis
Chemical data for reaphookhillite (Table 1) were obtained using a CAMECA SX50 electron microprobe operating in wavelength dispersive mode. Operating conditions were: accelerating voltage 15 kV, beam current 20 nA and beam diameter 5 μm. The following standards were used: willemite (ZnKα), almandine–pyrope (MgKα), rhodonite (MnKα) and apatite (PKα). No other elements with Z > 8 were detected from analyses using energy dispersive spectroscopy mode. Raw X-ray intensities were corrected for matrix effects with a φ(ρz) algorithm (Pouchou and Pichoir, Reference Pouchou and Pichoir1985). The empirical formula (based on 12 O atoms per formula unit) is Mg0.83Zn2.16Mn2+0.02(PO4)2.01⋅3.97H2O. The ideal formula is MgZn2(PO4)2⋅4H2O which requires MgO 9.66, ZnO 39.02, P2O5 34.03, H2O 17.28, Total 100.00 wt.%.
Table 1. Analytical data for reaphookhillite.

* based on the structure refinement. S.D. – standard deviation
Infrared spectroscopy
The infrared absorption spectrum of reaphookhillite (Fig. 2) was obtained using a powdered sample using a Nicolet 5700 Fourier-transform infrared (FTIR) spectrometer (range 4000 to 650 cm–1, transmission mode) equipped with a Nicolet Continuum IR microscope and a diamond-anvil cell. A broad band is observed in the OH-stretching region with maxima at 3456, 3331 and 3174 cm–1 and a shoulder at 3068 cm–1. Calculated d(O⋅⋅⋅O) hydrogen donor–acceptor distances are in the range ~2.9–2.6 Å using the correlation function given by Libowitzky (Reference Libowitzky1999) which are in good agreement with the results of the single-crystal structure analysis. The band at 1690 cm–1 is attributable to H–O–H bending vibrations of water molecules. The bands at 1062 and 957 cm–1 are assigned to ν3 antisymmetric stretching vibrations of the PO4 tetrahedra ν1 and bands at 802 and 704 cm–1 to ν1 symmetric stretching vibrations of the PO4 tetrahedra.

Fig. 2. The FTIR spectrum of powdered reaphookhillite.
Powder X-ray diffraction
Powder X-ray diffraction data of reaphookhillite (Table 2) were collected using a Rigaku Hiflux Homelab diffractometer (CuKα X-radiation, λ = 1.541870 Å). The experimental powder pattern was indexed based on the calculated values of intensities obtained from the crystal-structure refinement, using the LAZY PULVERIX program (Yvon et al., Reference Yvon, Jeitschko and Parthé1977). The unit-cell parameters were derived from least-squares refinement using the program UNITCELL (Holland and Redfern, Reference Holland and Redfern1997): a = 5.7625(4), b = 7.5412(5), c = 5.2881(4) Å, α = 93.405(7), β = 91.135(7), γ = 91.317(5)° and V = 229.28(1) Å3.
Table 2. Calculated* and observed powder X-ray diffraction data for reaphookhillite.

* I calc calculated with the LAZY PULVERIX program (Yvon et al., Reference Yvon, Jeitschko and Parthé1977).
The strongest lines are given in bold.
Single-crystal X-ray diffraction
Structure determination
A crystal 125 μm × 30 μm × 15 μm in size was attached to a MiTeGen polymer loop and mounted on a an Oxford Diffraction Xcalibur E diffractometer equipped with an Eos CCD detector. An ω scan rotational method was used to collect the intensity data at room temperature using MoKα radiation (λ = 0.71073 Å). The unit cell was refined from 1697 reflections using the least-squares algorithm in the CrysalisPro software package (Rigaku Oxford Diffraction, 2015). CrysalisPro was used to process the structure data, including the application of an empirical absorption correction. The structure was solved by direct methods using SHELXT (Sheldrick, Reference Sheldrick2015a) and refined in space group P ${\bar 1}$![]() using the SHELXL–2018 program (Sheldrick, Reference Sheldrick2015b) implemented in the WinGX suite (Farrugia, Reference Farrugia1999). Following the solution of the structure, the atom coordinates were transformed to correspond to those in the structure of parahopeite (Chao, Reference Chao1969). The final R 1 is 2.91% for 889 observed reflections [F 0 > 4σ(F 0)]. Difference-Fourier syntheses located all H atom positions. The H sites were refined with soft restraints on the O–H distances of 0.90(3) Å, H–H restraints of 1.40 Å and temperature factors U iso(H) = 1.5U eq(O). Details of data collection and structure refinement are provided in Table 3. Fractional coordinates and atom displacement parameters are provided in Table 4, selected interatomic distances and hydrogen bonds in Table 5 and bond valences in Table 6. The crystallographic information files have been deposited with the Principal Editor of Mineralogical Magazine and are available as Supplementary material (see below).
using the SHELXL–2018 program (Sheldrick, Reference Sheldrick2015b) implemented in the WinGX suite (Farrugia, Reference Farrugia1999). Following the solution of the structure, the atom coordinates were transformed to correspond to those in the structure of parahopeite (Chao, Reference Chao1969). The final R 1 is 2.91% for 889 observed reflections [F 0 > 4σ(F 0)]. Difference-Fourier syntheses located all H atom positions. The H sites were refined with soft restraints on the O–H distances of 0.90(3) Å, H–H restraints of 1.40 Å and temperature factors U iso(H) = 1.5U eq(O). Details of data collection and structure refinement are provided in Table 3. Fractional coordinates and atom displacement parameters are provided in Table 4, selected interatomic distances and hydrogen bonds in Table 5 and bond valences in Table 6. The crystallographic information files have been deposited with the Principal Editor of Mineralogical Magazine and are available as Supplementary material (see below).
Table 3. Crystal data, data collection and refinement details for reaphookhillite.

* w = 1/[σ2(Fo2) + (0.0739 P)2 + 0.32 P]; P = (Max (F 02,0)] + 2Fc2)/ 3
Table 4. Fractional coordinates and displacement parameters (Å2) for atoms for reaphookhillite.

* Site occupancy: Mg0.735(6)Zn0.265(6)
Table 5. Selected interatomic distances and hydrogen bonds (d in Å, angles in °) for reaphookhillite.

Table 6. Bond-valence analysis for reaphookhillite*.

* Bond valence parameters are from Gagné and Hawthorne (Reference Gagné and Hawthorne2015). Hydrogen-bond strengths are based on O–O bond lengths from Ferraris and Ivaldi (Reference Ferraris and Ivaldi1988).
Description of the crystal structure
Reaphookhillite is isostructural to its Zn-analogue parahopeite, Zn3(PO4)2⋅4H2O, (Table 7) whose structural details were described by Chao (Reference Chao1969). In the crystal structure of parahopeite, there are two Zn sites with different coordination numbers: 6-fold coordination and 4-fold coordination. In reaphookhillite, the [6]-coordinated site is occupied by Mg. The refined site-scattering value at the site is 16.77 epfu (electrons per formula unit), which is slightly greater than the result from the electron-microprobe analysis of 15.26 epfu, but still consistent with strong dominance of Mg at the site. The average <M–O> distance is similar in both structures; 2.090 Å for reaphookhillite and 2.108 Å for parahopeite. Calculation of bond-length distortion (BLD) coefficients (Robinson et al., Reference Robinson, Gibbs and Ribbe1971) indicates that the site in reaphookhillite is more strongly distorted (BLD 5.10) than in parahopeite (BLD 2.00). The tetrahedrally coordinated Zn site in reaphookhillite has a <Zn–O> distance of 1.960 Å, similar to the same distance in parahopeite of 1.952 Å. Bond-length distortion coefficients are 1.19 and 1.13 respectively.
Table 7. Comparison of reaphookhillite and parahopeite.

* X-ray powder pattern from Anthony et al. (Reference Anthony, Bideaux, Bladh and Nichols2000).
Tetrahedra of ZnO4 and PO4 share common corners to form a sheet of 4-membered rings parallel to (001) (Fig. 3). Sheets are linked in the c direction by MgO2(H2O)4 octahedra which share O4 anions with P tetrahedra (Fig. 4). A system of hydrogen bonds (O⋅⋅⋅O range: 2.66–2.96 Å) further stabilises the structure (Table 5, Fig. 5). The structure contains two independent H2O molecules each belonging to the MgO2(H2O)4 octahedron. OW5 donates one hydrogen bond and OW6 donates two hydrogen bonds to neighbouring O atoms of the tetrahedral sheet. Both OW5 and OW6 donate hydrogen bonds to the other H2O group in the structure on an adjacent Mg octahedron.

Fig. 3. The sheet of corner-sharing ZnO4 and PO4 tetrahedra in the structure of reaphookhillite viewed along [010]. ZnO4 tetrahedra are orange; PO4 tetrahedra are green. The unit cell is outlined.

Fig. 4. The crystal structure of reaphookhillite viewed along [100]. MgO2(H2O)4 octahedra are blue; ZnO4 tetrahedra are orange; PO4 tetrahedra are green; hydrogen atoms are small white spheres. The unit cell is outlined.

Fig. 5. The hydrogen bonding in reaphookhillite.
Structural relations
Reaphookhillite is a rare example of a non-silicate oxysalt mineral in which Mg and Zn are ordered in separate sites. Other structures that contain [6]-coordinated Mg sites and [4]-coordinated Zn sites are falsterite (Kampf et al., Reference Kampf, Mills, Simmons, Nizamoff and Whitmore2012), ferraioloite (Mills et al., Reference Mills, Grey, Kampf, Macrae, Smith, Davidson and Glenn2016), rinmanite (Holtstam et al., Reference Holtstam, Gatedal, Söderberg and Norrestam2001) and zemannite (Cametti et al., Reference Cametti, Churakov and Armbruster2017; Missen et al., Reference Missen, Mills, Spratt, Birch and Brugger2019).
Reaphookhillite and parahopeite are structurally related to davidlloydite, (Zn,Cu)Zn2(AsO4)2⋅4H2O (Hawthorne et al., Reference Hawthorne, Cooper, Abdu, Ball, Back and Tait2012), which is also triclinic and contains topologically identical sheets of ZnO4 and XO4 tetrahedra that form 4-membered rings. However, the structures have different linkages between sheets. The structural relationship between parahopeite and davidlloydite as stacking variants was discussed by Hawthorne et al. (Reference Hawthorne, Cooper, Abdu, Ball, Back and Tait2012). Sheets of ZnO4 and XO4 tetrahedra, with X = P or As, of the form [ZnXO4] are found in the structures of phosphophyllite, FeZn2(PO4)2⋅4H2O (Hill, Reference Hill1977; Thomas and Weller, Reference Thomas and Weller1992), stergiouite, CaZn2(AsO4)2⋅4H2O (Rieck et al., Reference Rieck, Giester, Lengauer, Chanmuang and Topa2020) and in the orthorhombic members of the hopeite group; hopeite, Zn3(PO4)2⋅4H2O (Hill and Jones, Reference Hill and Jones1976), arsenohopeite, Zn3(AsO4)2⋅4H2O (Neuhold et al., Reference Neuhold, Kolitsch, Bernhardt and Lengauer2012) and nizamoffite, MnZn2(PO4)2⋅4H2O (Kampf et al., Reference Kampf, Falster, Simmons and Whitmore2013). In contrast to reaphookhillite, parahopeite and davidlloydite, the ZnXO4 sheets in phosphophyllite and related minerals are composed of tetrahedra that link to form 3- and 4-membered rings. Sheets link via MO2(H2O)4 octahedra, M = Mn, Fe, Zn.
Acknowledgements
Ben Wade of Adelaide Microscopy, The University of Adelaide is thanked for assistance with the microprobe analysis. The infrared spectrum was acquired with the assistance of the Forensic Science Centre, Adelaide. The single-crystal X-ray data set was collected at the Bragg Crystallography Facility, The University of Adelaide. Structures Editor Peter Leverett, Fernando Colombo and anonymous reviewer are thanked for constructive comments that improved the quality of the manuscript.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1180/mgm.2022.2