Introduction
Orthopyroxene-bearing felsic gneisses are described variously as charnockite or charnockite gneiss, metaluminous granulite and C-type granite mainly on the basis of the lithological association (Rajesh and Santosh, Reference Rajesh and Santosh2004). In a comprehensive study of the world-wide distribution, Frost and Frost (Reference Frost and Frost2008) brought all such rocks under the umbrella of charnockite. Grantham et al. (Reference Grantham, Mendonidis, Thomas and Satish-Kumar2012) summarised six categories of these rocks that form by magmatic, metamorphic, or metasomatic processes. Magmatic charnockites are widespread in many regional granulite terranes including the Mawson charnockite of East Antarctica (Young et al., Reference Young, Zhao, Ellis and McCulloch1997). Metamorphic charnockites are usually limited in extent and formed by CO2-rich fluid invasion of amphibolite-facies biotite and/or hornblende-bearing rocks (Newton et al., Reference Newton, Smith and Windley1980a; Janardhan et al., Reference Janardhan, Newton and Hansen1982). As the source and supply of CO2 in lower and mid-crustal rocks is limited with the latter also providing little scope for fluid permeability, the metamorphic charnockites are reported along veins and patches with limited geographic extent (Rajesh et al., Reference Rajesh, Belyanin, Safonov, Kovaleva, Golunova and Van Reenen2013). However, there have been reports of metamorphic charnockites forming larger massifs (Santosh and Omori, Reference Santosh and Omori2008). Ambiguities in identifying metamorphic and igneous charnockites result from the general absence of temperature difference between charnockite magma and surrounding granulitic crust (Touret and Huizenga, Reference Touret and Huizenga2012).
Apart from these two categories, there are many examples of orthopyroxene-bearing felsic gneisses whose status has not been clearly defined. These rocks show neither clear-cut evidence for magmatic crystallisation nor do they occur along the fractures or fluid pathways. Most of them occur as thick-to-thin bands associated with migmatitic pelitic and basic granulites (cf. Nicoli et al., Reference Nicoli, Stevens, Moyen and Frei2015). This particular variety is highly deformed, commonly mylonitic, and associated with aluminous granulites that record ultrahigh temperature (UHT) metamorphism. As the origin of the granulitic rocks in such associations is linked invariably to the partial melting process, we explore the possibility of such a process to explain the origin of the orthopyroxene-bearing felsic gneiss. It is important that partial melting of the lower crust produces a variety of melt-restite combinations and that their eventual segregation is responsible for differentiation of continental crust (reviewed in Clemens and Stevens, Reference Clemens and Stevens2016). Our endeavour is to link the genesis of orthopyroxene-bearing felsic gneisses with the partial melting process through a multipronged approach.
Background geology
Regional geology
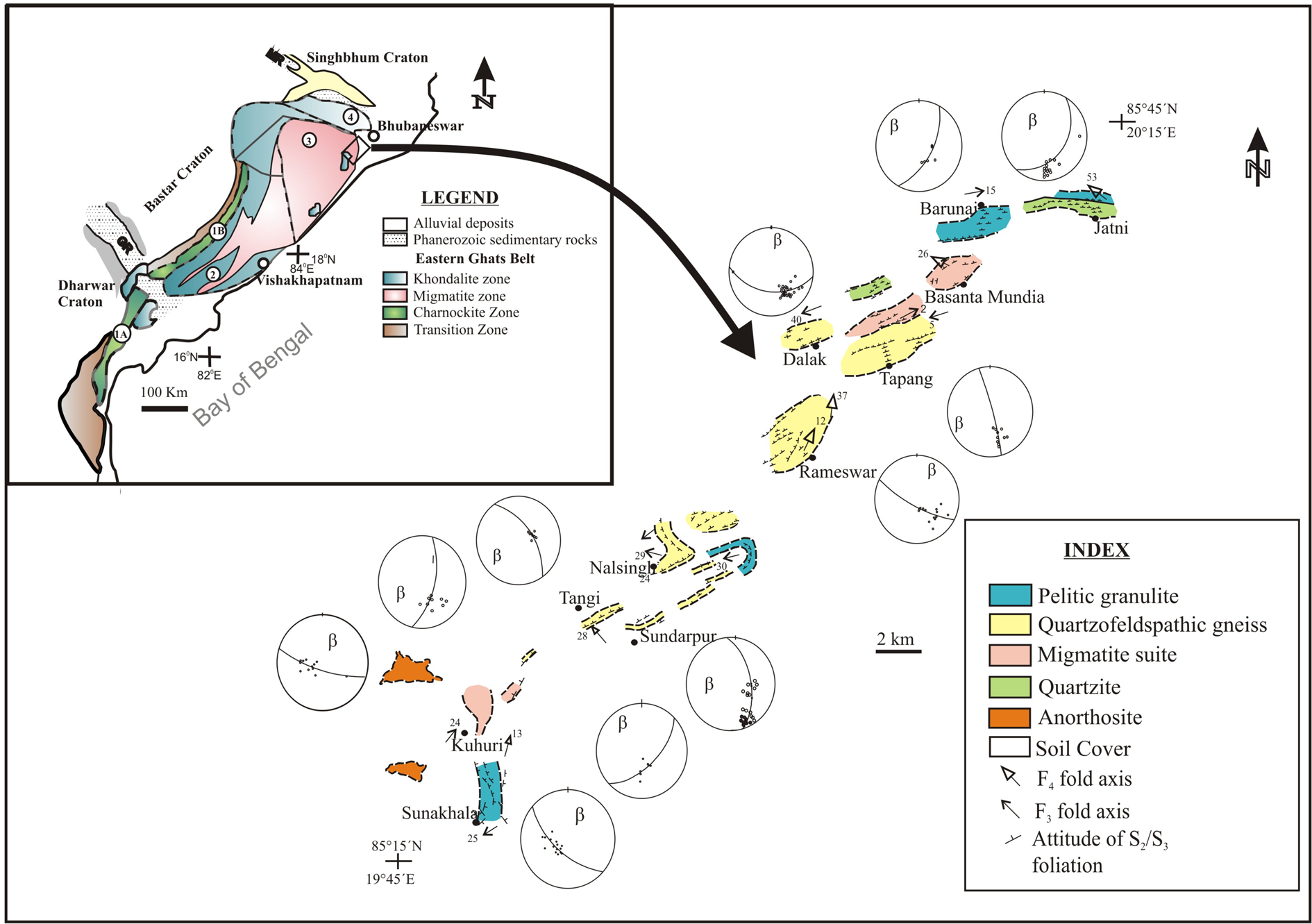
The Proterozoic Eastern Ghats Belt played a crucial role in connecting the cratonic blocks of India with East Antarctica (Dasgupta et al., Reference Dasgupta, Bose and Das2013, 2017; Saha et al., Reference Saha, Bhowmik, Bose and Sajeev2016; Bose and Dasgupta, Reference Bose and Dasgupta2018; Dasgupta, Reference Dasgupta2020; Bose, Reference Bose, Banerjee, Jain, Dasgupta and Bajpai2020 and references therein). This regionally extensive geologically complex orogenic belt exposes UHT metamorphosed lower crust that evolved during supercontinent cycles involving Columbia and Rodinia. The entire belt is thought to represent a collage of several crustal provinces and isotopic domains, out of which the centrally occurring Eastern Ghats Province evolved together with the Rayner Complex of East Antarctica as a single orogenic belt (the R-EG belt by Morissey et al., Reference Morissey, Hand and Kelsey2015) through subduction–accretion processes during the time frame c. 1.13−0.90 Ga (Mezger and Cosca, Reference Mezger and Cosca1999; Upadhyay et al., Reference Upadhyay, Gerdes and Raith2009; Bose et al., Reference Bose, Dunkley, Dasgupta, Das and Arima2011a; Das et al., Reference Das, Bose, Karmakar, Dunkley and Dasgupta2011; Korhonen et al., Reference Korhonen, Clarke, Brown, Bhattacharya and Taylor2013; Dasgupta et al., Reference Dasgupta, Bose, Bhowmik, Sengupta, Pant and Dasgupta2017; Bose and Dasgupta, Reference Bose and Dasgupta2018). Several studies from this belt reveal that the pressure–temperature–fluid history of the lower crust underwent multiple stages of metamorphism (Mohan et al., Reference Mohan, Sachan and Singh2003; Sarkar et al., Reference Sarkar, Santosh, Dasgupta and Fukuoka2003; Gupta et al., Reference Gupta, Panigrahi and Sarkar2005; Bose et al., Reference Bose, Das, Ohnishi, Torimoto, Karmakar, Shinoda and Dasgupta2009, Reference Bose, Das, Torimoto, Arima and Dunkley2016a; Das et al., Reference Das, Tomioka, Bose, Ando and Ohnishi2017; Ganguly et al., Reference Ganguly, Bose, Das, Torimoto and Ghosh2017). The Chilka Lake granulite complex occurs at the north-eastern corner of the Eastern Ghats Province (Fig. 1 inset) although it was assigned the status of a separate crustal domain. However, recent studies put question marks on this domain-based classification (Ganguly et al., Reference Ganguly, Das, Bose, Ghosh, Hayasaka and Hidaka2018; Ganguly and Chatterjee, Reference Ganguly and Chatterjee2020).
Fig. 1. Map of the Chilka Lake granulite complex (modified after Bose et al., Reference Bose, Das, Torimoto, Arima and Dunkley2016a). Inset shows the broad lithologic map of the Eastern Ghats Belt (after Ramakrishnan et al., Reference Ramakrishnan, Nanda, Augustin, Ramakrishnan, Paul and Mishra1998).
Chilka Lake granulite complex
The Chilka Lake granulite complex consists of granulite-facies migmatitic ortho- and paragneisses surrounding an anorthosite massif (Fig. 1). Gneissic rocks show evidence of multiple phases of deformation and metamorphism (Bhattacharya et al., Reference Bhattacharya, Sen and Acharyya1994; Sen et al., Reference Sen, Bhattacharya and Acharyya1995; Dobmeier and Raith, Reference Dobmeier and Raith2000; Dobmeier and Simmat, Reference Dobmeier and Simmat2002; Das et al., Reference Das, Bose, Karmakar and Chakraborty2012; Bose et al., Reference Bose, Das, Torimoto, Arima and Dunkley2016a). Diatexite migmatites in the Chilka Lake granulite complex show alternate layers of aluminous granulite (spinel–sapphirine–cordierite–garnet–orthopyroxene–quartz–sillimanite–K-feldspar), orthopyroxene-bearing felsic gneiss, garnet-bearing felsic gneiss (locally known as leptynite), and mafic granulite forming a large part of the highland situated NE of the anorthosite massif. The petrological history of these rocks is complex and was described initially as an example of high-P, high-T granulite metamorphism (Sen et al., Reference Sen, Bhattacharya and Acharyya1995). Sen et al. (Reference Sen, Bhattacharya and Acharyya1995) argued for a stepwise decompression-cooling retrograde P–T path based on their petrological data for aluminous granulites and orthopyroxene-bearing felsic gneiss (described as charnockite). A comprehensive P–T–t–fluid history has been proposed recently based on data obtained from two varieties of aluminous granulite (Bose et al., Reference Bose, Das, Torimoto, Arima and Dunkley2016a). This latter investigation suggested that the earliest metamorphism ensued through biotite-dehydration melting reactions along a possible clockwise P–T path. The peak metamorphic condition was reached at 8.5−9.0 kbar, 900−950°C at 988 ± 23 Ma (monazite U–Th-total Pb data). After an episode of cooling, the deep crust was exhumed up to mid-crustal level, which is texturally manifested by the breakdown of porphyroblastic garnet into myriad types of intergrowth. Decompression and final crystallisation of peraluminous granitic melt occurred at c. 781 ± 9 Ma (zircon U–Pb SHRIMP data, monazite U–Th-total Pb data). Renewed heating of the cooled crust at c. 550−500 Ma caused incipient melting of the biotite-bearing assemblage. In addition to this regional metamorphic scenario, thermal metamorphism at UHT conditions (>1000°C) has been characterised from Fe–Al rich granulites and calc-silicate granulites along the contact of the anorthosite massif (Raith et al., Reference Raith, Dobmeier and Mouri2007; Sengupta et al., Reference Sengupta, Dasgupta, Dutta and Raith2008), but its age is yet to be deciphered.
Previous studies mostly used monazite petrochronological data from pelitic granulites (locally known as khondalites) and aluminous granulites to determine the times of metamorphism (Dobmeier and Raith, Reference Dobmeier and Raith2000; Dobmeier and Simmat, Reference Dobmeier and Simmat2002; Simmat and Raith, Reference Simmat and Raith2008). These studies estimated different age clusters from a large number of monazite datasets. As petrological constraints were not imposed, some of the age interpretations remain problematic. For example, the continuous spectrum of ages between c. 800 Ma and 500 Ma was grouped into several clusters without any petrological context. Bose et al. (Reference Bose, Das, Torimoto, Arima and Dunkley2016a) argued that such ages could be artefacts and possibly result from fluid-induced changes in monazite and zircon. The age of emplacement of anorthosite is similarly a debated issue as the reported data are quite contrasting, e.g. 792 ± 2 Ma (Krause et al., Reference Krause, Dobmeier, Raith and Mezger2001), 983 ± 2.5 Ma (Chatterjee et al., Reference Chatterjee, Crowley, Mukherjee and Das2008) and 855 ± 31 Ma (Chakrabarti et al., Reference Chakrabarti, Basu, Bandyopadhyay, Zou, Ray, Sen and Ghosh2011). If all these data are assumed to be correct, a repetitive emplacement history of the anorthosite magma is indicated. Apart from contact metamorphism along its peripheries, the regional effect of anorthosite magmatism on the granulites is yet to be ascertained.
Mafic granulites and orthopyroxene-bearing felsic gneiss (charnockite, as mentioned in earlier studies) constitute integral components of the migmatite granulite suite (Bhattacharya et al., Reference Bhattacharya, Sen and Acharyya1994). While the status of the charnockite is controversial, mafic granulites are identified as deformed and metamorphosed mafic dykes emplaced at > c. 800 Ma (Bose et al., Reference Bose, Das, Chakraborty, Miura and Srivastava2011b). Some of the orthopyroxene-bearing felsic gneisses were identified previously as either larger charnockite bodies or as smaller charnockite patches (Bhattacharya et al., Reference Bhattacharya, Sen and Acharyya1994). The latter variety is hosted in felsic gneiss and interpreted to have formed by migmatisation of the earlier variety (Sen et al., Reference Sen, Bhattacharya and Acharyya1995). The same elliptical charnockite patches were interpreted to form by an in situ fluid-induced process during a later stage of the deformation history in a subsequent study (Dobmeier and Raith, Reference Dobmeier and Raith2000).
Methodology
Mineral compositional data were obtained from a CAMECA SX-100 electron probe microanalyser at the Department of Geology and Geophysics, Indian Institute of Technology, Kharagpur, India. Operating conditions of the instrument were 15 kV accelerating voltage, 15 nA beam current and 1–2 μm beam diameter. Natural silicate and oxide standards were used for calibration and the raw data were corrected using the ZAF program. Representative mineral compositions are presented in Tables 1 and 2. The complete dataset is provided in Supplementary data (Table S1, see link below).
Table 1. Representative mineral compositional data from the orthopyroxene-bearing felsic gneiss samples of the Chilka Lake area for othropyroxene (Opx), garnet (Grt), ilmenite (Ilm) and cordierite (Crd).

‘–’ = below detection limit
X Mg = Mg/(Mg+Fe2+); X Prp = Mg/(Mg+Fe2++Mn+Ca); X Alm = Fe2+/(Mg+Fe2++Mn+Ca); X Grs = Ca/(Mg+Fe2++Mn+Ca); X Sps = Mn/(Mg+Fe2++Mn+Ca); X Hem = Fe3+/(Fe2++Fe3++Mg); X Ilm = Fe2+/(Fe3++Fe2++Mg); X Gk = Mg/(Fe3++Fe2++Mg)
Table 2. Representative mineral compositional data from the orthopyroxene-bearing felsic gneiss samples of the Chilka Lake area for plagioclase (Pl), K-feldspar (Kfs) and biotite (Bt).

‘–’ = below detection limit
X Mg = Mg/(Mg+Fe2+); X Ab = Na/(Na+Ca+K); X An = Ca/(Na+Ca+K); X Or = K/(Na+Ca+K)
Fluid inclusions were investigated in doubly polished thin wafers (~150−200 μm in thickness) prepared from representative samples. The nature of occurrence of inclusions, their distribution patterns, shapes, sizes, and phase categories were studied carefully and documented under the petrological microscope at varying magnifications after the techniques outlined by Touret (Reference Touret2001) and van den Kerkhof and Hein (Reference Van den Kerkhof and Hein2001). Microthermometric measurements were performed with a Linkam heating/freezing system at Hokkaido University, Japan. Calibrations were undertaken with synthetic standard materials supplied by Fluid Inc., Denver, USA. The calibrations were performed at 0°C (triple point of H2O), −5.5°C (triple point of n-tridecane), −9.6°C (triple point of n-dodecane), −45.6°C (triple point of chlorobenzene), −63.4°C (triple point of chloroform), 82°C (melting point of dodecamethylene glycol) and 306.8°C (melting point of sodium nitrate). Heating rates of the samples were 1°C/min for melting and 5°C/min for homogenisation temperatures. Repeated microthermometric measurements indicated that the precision of microthermometric results reported in this study was within +0.1 and +0.2°C for melting and homogenisation temperatures, respectively. The results of microthermometric measurements are summarised in Table 3 and the Supplementary data are given in Table S2. In all experimental cases, CO2 was homogenised into the liquid phase. Fluid densities and isochores were calculated using the FLINCOR software (Brown, Reference Brown1989), which is based on the equation and thermodynamic data of Brown and Lamb (Reference Brown and Lamb1989). For isochore calculations, all analysed carbonic fluid inclusions were regarded as pure CO2.
Table 3. Fluid-inclusion analysis data from the orthopyroxene-bearing felsic gneiss samples of the Chilka Lake area.

Laser Raman spectroscopy was carried out at the National Institute of Polar Research, Japan using a JASCO-NRS 1000 Raman spectrometer. The analytical accuracy of wavenumber measurements of the Raman spectrometer was 1 wavenumber (cm-1). Raman spectroscopic analyses were undertaken on relatively large inclusions (>10 μm) to obtain good quality Raman signals. Raman data were acquired at room temperature for single-phase inclusions. Acquired data were then compared with the Raman spectral data listed by Burke (Reference Burke2001) for fluids and solid crystals found in the inclusions to identify each species. The Raman bands for the host phases were compared with the software Crystal sleuth (Laetsch and Downs, Reference Laetsch and Downs2006) to correct identification. Raman bands for the host crystals and fluids (+ solids, if any) in individual inclusions were then compared to identify different species (CO2, H2O, CH4, N2 among others).
For the geochemical investigation, representative samples were analysed at Actlabs, Canada for major, trace, and rare earth elements (REE) and the results are presented in Table 4. The whole-rock analysis was obtained by X-ray Fluorescence (XRF) on fused beads and the analytical detection limit for the major elements was 0.01%, except for Ti (0.001%). Trace and rare earth elements were analysed by inductively coupled plasma mass spectrometry. The analytical detection limits were in the 2−5 ppm range for the trace elements and 0.1 ppm for the REE (except Pr and Eu where detection limit was 0.05 ppm). Data processing was done using the software GCDkit (Janoušek et al., Reference Janoušek, Farrow and Erban2006).
Table 4. Whole-rock major, trace and REE data of the orthopyroxene-bearing felsic gneiss samples of the Chilka Lake area. Calculated norms are also shown.

For the geochronological investigation, all the samples were initially crushed, and zircon grains were concentrated using water table and magnetic separation at the Yokohama National University, Japan. Individual crystals were selected and handpicked using optical microscopy for mounting. Zircon crystals for SHRIMP U–Th–Pb analysis from all the samples were prepared in a 1” diameter, Au-coated, polished grain mount. Spots within the zircon crystals were selected using optical, back-scattered electron (BSE) spectroscopy, and cathodoluminescence (CL) imaging and were analysed on the SHRIMP II at the National Institute of Polar Research, Japan using a 30 μm diameter, 3.5 nA, 10 kV and negative O2 primary ion beam. Positive secondary ions were extracted at 10 kV and analysed at mass resolution 5000 using a single ETP electron multiplier and magnetic field switching. Each analysis consisted of six scans through the isotopes of interest and took nearly 14 min. The Pb isotopic compositions were measured directly. Pb/U and Pb/Th ratios were determined relative to chips of zircon standards SL13 (U = 238 ppm) and FC1 (radiogenic 206Pb/238U = 0.1859; age 1099 Ma; Paces and Miller, Reference Paces and Miller1993) using a power-law relationship between Pb+/U+ and UO+/U+ (Claoué-Long et al., Reference Claoué-Long, Compston, Roberts, Fanning, Berggren, Kent, D.V., Aubrey and Hardenbol1995) and an exponent of 1.55. The standard also provided an estimate of Pb isotopic fractionation (2.5 + 2.0 /amu). Analytical data are presented in Table 5 and plotted in concordia diagrams. Uncertainties quoted in the table and the text for individual analyses (ratios and ages) are at 1σ level whereas those shown in error ellipses on concordia diagrams and weighted average ages are at a 95% confidence level including the decay-constant errors of the concordia curve. Plots and age calculations have been made using SQUID (version 1.12a, Ludwig, Reference Ludwig2001) and ISOPLOT/EX (version 3.6, Ludwig, Reference Ludwig2008).
Table 5. Zircon U–Pb SHRIMP data from the selected OFG samples of the Chilka Lake area.
*204Pb corrected
Description of the orthopyroxene-bearing felsic gneiss
Field description
Aluminous granulite, orthopyroxene-bearing felsic gneiss, mafic granulite and felsic gneiss constitute a lithological package of the migmatitic rock suite of the Chilka Lake granulite complex. Whereas most of the hillocks in the central part of the granulite complex are made up of this rock suite, the best sections are exposed in the numerous stone quarries situated around the Tapang-Dadhimachigadia area. The different lithological units occur as continuous-to-discontinuous bands of variable size. The thickness of orthopyroxene-bearing felsic gneiss bands varies from a few metres to a few centimetres. These are commonly interbanded with migmatitic aluminous granulite and felsic gneiss (Fig. 2a). Such bands run parallel to the composite S2/S3 foliation (Fig. 2b) and locally exhibit outcrop-scale F3 folding (Fig. 2c). The gneissic foliation present in the rock is conformable with that of the adjoining aluminous granulite. In some occurrences, the rock looks massive (Fig. 2d), but foliation is present on a finer scale. The mutual boundary between this rock and migmatitic aluminous granulite is direct and well-defined (Fig. 2d). A coarser variety of orthopyroxene-bearing felsic gneiss contains cordierite-rich bluish layers that run parallel to the S2/S3 foliation (Fig. 2e). Leucosome layers, lenses, and fragments are locally present within the rock (Fig. 2f) where the layers show rootless folds. When occurring adjacent to the massive-type aluminous granulite, similar rootless folds of leucosome layers are present in the latter.

Fig. 2. Photographs showing field features of the orthopyroxene-bearing felsic gneiss (OFG). (a) Orthopyroxene-bearing felsic gneiss layer interbanded with felsic gneiss. (b) Interbanded orthopyroxene-bearing felsic gneiss and felsic gneiss where the bands of the former occur parallel to the composite S2/S3 foliation. (c) The S2 fabric in the orthopyroxene-bearing felsic gneiss showing outcrop-scale F3 folding. (d) Massive variety of orthopyroxene-bearing felsic gneiss showing a sharp boundary with aluminous granulite. (e) Garnet–orthopyroxene–cordierite-rich layers in orthopyroxene-bearing felsic gneiss parallel to the S2/S3 foliation. (f) Rootless folding of leucosome layers in orthopyroxene-bearing felsic gneiss.
Aluminous granulites occur within the quartzofeldspathic gneiss as layers and lenses bearing the penetrative regional planar gneissic fabric. In most of the quarry surfaces, aluminous granulites and orthopyroxene-bearing felsic gneiss show intimate associations as migmatite complexes. Migmatites are mostly stromatic wherein individual centimetre-thick layers show tight folding.
Petrography and phase chemistry
Orthopyroxene-bearing felsic gneiss shows broadly similar textures in all the samples, yet some tangible mineralogical variations exist. The gneissic foliation is marked by alternate thin (up to 1 mm) orthopyroxene-rich layer and thick (2−5 mm) quartzofeldspathic layers. Orthopyroxene grains are mostly small, equant, and recrystallised, although flattened orthopyroxene grains are present in strongly deformed rocks. In a highly deformed variety orthopyroxene shows extreme flattening, pinch-and-swell structures, and grain refinement. Equant polygonal grains of orthopyroxene and plagioclase in most of the samples suggest static recrystallisation as the main deformation mechanism. Based on the mineralogical variation, we categorised the rock in two broad associations. ‘Association-1’ is composed of orthopyroxene + plagioclase + quartz + ilmenite + garnet with a subordinate amount of cordierite, biotite and K-feldspar. ‘Association-2’ is composed of orthopyroxene + K-feldspar + quartz with a subordinate amount of plagioclase and biotite.
In Association-1, recrystallised orthopyroxene, quartz, and plagioclase constitute the mineralogy of most of the samples. Porphyroblastic orthopyroxene occasionally shows an elliptical shape with rounded biotite and quartz inclusions (Fig. 3a). Some of the porphyroblastic orthopyroxene grains show flattening parallel to the foliation. Garnet porphyroblasts are present in some samples and these grains contain inclusions of plagioclase, biotite and quartz (Fig. 3b). Ilmenite is present as a smaller matrix phase. In cordierite-bearing samples, recrystallised grains of cordierite form a stable association with garnet and orthopyroxene (Fig. 3c). Orthopyroxene grains show flattening in highly deformed rocks. Quartz grains in such rocks show ribbon structures. Grain-scale deformation is marked by flattening and development of dislocation in orthopyroxene and bending of twin lamellae in plagioclase. Biotite flakes are present on garnet and orthopyroxene grains. K-feldspar is rare in most samples, but present as microperthite grains in a few samples. Locally, veins of pyrite and chalcopyrite are present along the foliation. In the felsic layers, quartz grains show cuspate grain outlines (Fig. 3d). Microperthite grains contain rounded quartz inclusions (Fig. 3e). A thin film of K-feldspar occurs surrounding elongated quartz inclusions in plagioclase (Fig. 3f). A similar K-feldspar layer also develops surrounding plagioclase that occurs within garnet grains in the vicinity (Fig. 3b). These textures are indicative of the former presence of a melt phase (Sawyer, Reference Sawyer2001). Quartz, garnet and sometimes plagioclase contain fluid-inclusion trails. The composition of orthopyroxene shows minor variation in terms of Al (Al2O3 = 2.69−3.34 wt.%) and Fe–Mg distribution (X Mg = 0.47−0.51). Garnet consists of almandine (68−71 mol.%) and pyrope (22−28 mol.%) with low grossular (4−7 mol.%) and spessartine (0−2 mol.%) components. The biotite composition is slightly variable in terms of Ti content and Fe–Mg distribution. Those occurring as inclusions in garnet show very high Ti (up to 7.5 wt.% TiO2) and are more Mg rich (X Mg = 0.71) compared to the matrix phases (TiO2 = 5.2−6.1 wt.%; X Mg = 0.61−0.65). Plagioclase shows 51−54 mol.% albite and 44−46 mol.% anorthite with low orthoclase (2−3 mol.%) components (Fig. 4a). Ilmenite contains a small but variable amount of hematite component (6.9−13.0 mol.%). The geikielite component is low (3.4−5.3 mol.%), and the pyrophanite component is negligible (<1 mol.%). In cordierite-bearing samples, garnet is slightly Mg rich (35–38 mol.% pyrope). Orthopyroxene is also Mg rich (X Mg = 0.66) and contains slightly higher Al-content (up to 5.6 wt.% Al2O3). Cordierite is Mg rich (X Mg = 0.81), but the oxide total is low (~96 wt.%) which indicates the possible presence of volatiles in its structure.
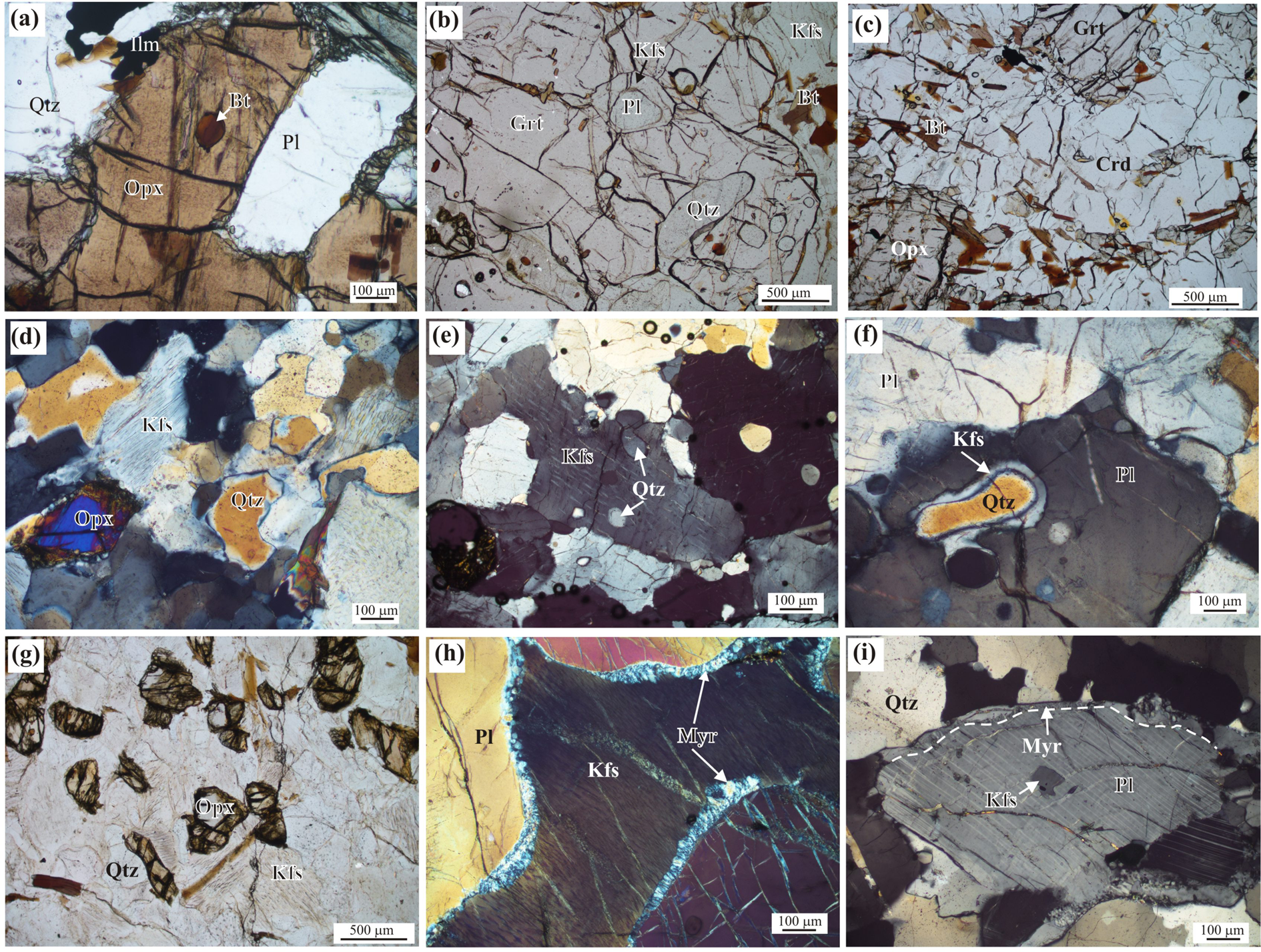
Fig. 3. Photomicrographs showing textural features of orthopyroxene-bearing felsic gneiss. (a) Orthopyroxene porphyroblast containing a rounded biotite inclusion in association of quartz and plagioclase. (b) Garnet porphyroblasts containing plagioclase, biotite and quartz inclusions. Note the occurrence of thin K-feldspar layer surrounding plagioclase enclosed within garnet. (c) Cordierite showing stable association with garnet and orthopyroxene. (d) Cuspate quartz grains in the leucocratic layers. (e) Rounded quartz inclusions within microperthitic K-feldspar. (f) Thin film of K-feldspar surrounding quartz inclusions within plagioclase. (g) Random orientation of orthopyroxene, quartz and K-feldspar grains. (h) Myrmekitic intergrowth at the margin of coarse microperthitic K-feldspar. (i) Thin K-feldspar layer surrounding plagioclase developing myrmekite. Abbreviations: Bt – biotite; Crd – Cordierite; Grt – Garnet; Kfs – K-feldspar; Myr – myrmekite; Opx – Orthopyroxene; Pl – plagioclase; Qtz – quartz.

Fig. 4. (a) Plot of feldspar compositions in the ternary system albite–anorthite–K-feldspar plane. (b) Plot of total Al+Fe3+ vs. Si+Mg+Fe2+ per formula unit of orthopyroxene.
In Association-2, orthopyroxene shows granoblastic fabric with K-feldspar and quartz. In places, the texture of the rock is massive with a random orientation of orthopyroxene and K-feldspar grains (Fig. 3g). In the foliated rocks, the foliation is marked by alternate orthopyroxene-bearing melanocratic and K-feldspar + quartz-bearing leucocratic layers. Plagioclase is rarely present in the leucocratic layers. Orthopyroxene is usually coarse-grained and elliptical with biotite inclusions, but stretched grains are present in deformed rocks. K-feldspar is mesoperthite in most samples (Fig. 3h). Evidence of deformation is present at the grain-scale. Biotite is present along the fractures of orthopyroxene. Quartz grains in the leucocratic layers show cuspate grain outlines, while smaller rounded quartz grains occur as inclusions within larger K-feldspar grains. These textures again indicate melt-induced crystallisation. Myrmekitic intergrowth is present at the margin of K-feldspar grains and such intergrowth is thicker in places (Fig. 3h). Thin K-feldspar layers surrounding plagioclase also shows the formation of myrmekite (Fig. 3i). Quartz grains contain fluid inclusions. The orthopyroxene composition shows a wide variation in different samples. The Al2O3 content varies from 0.96 to 5.75 wt.%, while X Mg varies within the range of 0.32−0.80. A plot of total Al+Fe3+ vs. Si+Mg+Fe2+ (Fig. 4b) suggests that the Tschermak substitution is the main cause of compositional variation in orthopyroxene.
P–T conditions of metamorphism
Pressure and temperature of metamorphism were calculated using conventional geothermobarometric methods. We used the garnet–orthopyroxene–plagioclase–quartz assemblage (Association-1) to calculate both the pressure and the temperature of equilibration of the assemblage. Using the garnet–orthopyroxene thermometer of Lee and Ganguly (Reference Lee and Ganguly1988), we calculated the temperature in the range of 664–803°C. The garnet–orthopyroxene–plagioclase–quartz barometer yields pressure in the range 5.6–6.6 kbar for both Fe and Mg end-member equilibria of Bhattacharya et al. (Reference Bhattacharya, Krishna Kumar, Raith and Sen1991). We also calculated pressure and temperature simultaneously from the same assemblage using the method outlined by Pattison et al. (Reference Pattison, Chacko, Farquhar and McFarlane2003) which takes into account the late-stage Fe+2–Mg resetting. For cordierite-free assemblages, the calculated values vary from 760°C, 6.97 kbar to 672°C, 6.36 kbar for core and rim compositions of the phases, respectively. For the cordierite-bearing assemblages, the estimated values range between 855°C, 7.06 kbar, and 838°C, 6.92 kbar for two different X Al models of orthopyroxene. The garnet–ilmenite thermometer after Bishop (Reference Bishop1980) yields a range of temperatures from 561°C to 745°C. The Ti-in-biotite model (Henry et al., Reference Henry, Guidotti and Thomson2005) yields temperatures in the range 790−843°C. The maximum temperature is calculated from the biotite included within garnet, i.e. the prograde biotite. The garnet–biotite thermometer after the model of Ganguly et al. (Reference Ganguly, Cheng and Tirone1996) yields 672−770°C at an assumed pressure of 8 kbar and ideal Fe–Mg mixing in biotite. Conventional pressure estimation was done using both the garnet–cordierite and orthopyroxene–cordierite barometers (Harris and Holland, Reference Harris and Holland1984). The calculated results are 4.6 kbar and 5.4 kbar respectively assuming the peak temperature of 850°C. For Association-2, we have only used the two feldspar thermometer of Whitney and Stormer (Reference Whitney and Stormer1977); using compositions of plagioclase lamellae and K-feldspar hosted in mesoperthite. The results show temperature values in the range of 740−870°C.
From the different conventional geothermometers used, the maximum temperature estimated was ~850°C at 6−8 kbar (Fig. 5). Although some of the mineral pairs such as garnet and biotite did not coexist at the peak stage, others (garnet and orthopyroxene) actually stabilised at the peak. Note that the peak temperatures show a wide range from ~850°C down to ~660°C. This is possibly caused by down-temperature resetting as observed from many high-temperature terranes.
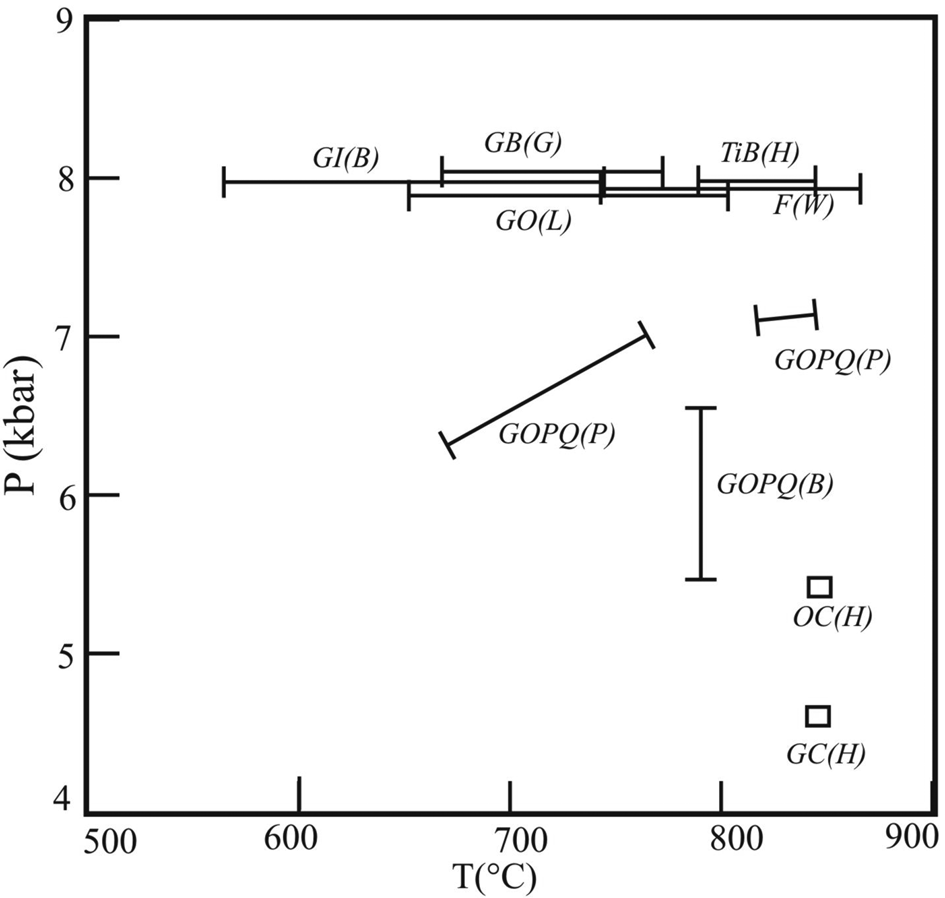
Fig. 5. Summary of results obtained from geothermobarometric calculations of the samples investigated in a P-T frame. Abbreviations used are as follows. GI(B): Garnet–ilmenite thermometer of Bishop (Reference Bishop1980); GB(G): Garnet–biotite thermometer of Ganguly et al. (Reference Ganguly, Cheng and Tirone1996); TiB(H): Ti-in-biotite thermometer of Henry et al. (Reference Henry, Guidotti and Thomson2005); GO(L): Garnet–orthopyroxene thermometer of Lee and Ganguly (Reference Lee and Ganguly1988); GOPQ(P): Garnet–orthopyroxene–plagioclase-quartz thermobarometers of Pattison et al. (Reference Pattison, Chacko, Farquhar and McFarlane2003); GOPQ(B): Garnet–orthopyroxene–plagioclase–quartz barometers of Bhattacharya et al. (Reference Bhattacharya, Krishna Kumar, Raith and Sen1991); F(W): Feldspar thermometer of Whitney and Stormer (Reference Whitney and Stormer1977); OC(H): Orthopyroxene–cordierite barometer of Harris and Holland (Reference Harris and Holland1984); GC(H): Garnet=cordierite barometer of Harris and Holland (Reference Harris and Holland1984).
For further constraining the stability fields of the two peak metamorphic assemblages of Association-1, we carried out phase equilibria modelling in the P–T range of 6–12 kbar, 600–1000°C using the programme Perple_X (Connolly, Reference Connolly2005). Two representative bulk compositions were taken from the experimental model greywacke compositions of Stevens et al. (Reference Stevens, Clemens and Droop1997). We added 1 wt.% CO2 in the two bulk compositions to check any variation of stability fields of the assemblages. The results show that for composition A (Mg#49), melting begins at ~750–800°C at 4–9 kbar and the assemblage garnet + orthopyroxene + plagioclase + K-feldspar + quartz coexists with the melt at ~7 kbar >840°C (Fig. 6a). This closely matches the P–T window for the cordierite-free assemblages in Association-1. For more magnesian bulk B (Mg#62), cordierite stabilises in the assemblage of garnet + orthopyroxene + plagioclase + K-feldspar + quartz + melt at ~7 bar >850°C (Fig. 6b). This also matches with the P–T results obtained from the cordierite-bearing assemblages of Association-1.

Fig. 6. Calculated phase diagram in the system Na2O–CaO–K2O–FeO–MgO–Al2O3–SiO2–H2O (NCKFMASH) for bulk compositions of Stevens et al. (Reference Stevens, Clemens and Droop1997). 1 wt.% CO2 was additionally considered in each case. (a) Composition A (Mg# = 49) and (b) composition B (Mg# = 62) of Stevens et al. (Reference Stevens, Clemens and Droop1997). The diagrams were calculated using the program Perple_X (Connolly, Reference Connolly2005). Solution models used are: garnet (Holland and Powell, Reference Holland and Powell1998); orthopyroxene (Holland and Powell, Reference Holland and Powell1998); cordierite (Holland and Powell, Reference Holland and Powell1998);melt (Holland and Powell, Reference Holland and Powell1998); biotite (Holland and Powell, Reference Holland and Powell1998); and plagioclase (Newton et al., Reference Newton, Charlu and Kleppa1980b). Note the assemblages garnet + orthopyroxene + quartz + cordierite + melt appears at 7−9 kbar at temperatures >850°C in association 1. At similar P–T conditions the stable assemblage in association 2 is orthopyroxene + quartz + K-feldspar + plagioclase + melt.
Fluid-inclusion study
We analysed three samples of the orthopyroxene-bearing felsic gneiss. A summary of the results is presented in Table 3. Descriptions of results for individual samples are presented below.
Fluid inclusions in sample Ch-BT7 occur within quartz and K-feldspar. Inclusions in quartz are both primary and pseudo-secondary varieties. Inclusions are of two types. Type 1 inclusions are primary, 5−10 μm in size and oval in shape (Fig. 7a). Type 2 inclusions are secondary and very small (2−5 μm). Vapour bubbles are occasionally present in larger primary inclusions. Pseudo-secondary inclusions are also small and flat in shape. Inclusions in K-feldspar are 5−10 μm in size, irregular in shape, and of type 2 variety. Type 1 inclusions in quartz show melting temperatures (T m) of –57.2 to –57.0°C (Fig. 7b) and homogenisation temperature (T h) of 26−28°C (Fig. 7c), which are slightly different from the type 2 inclusions (–57.2 to –56.6°C and 26−32°C, respectively). Pseudo-secondary inclusions in quartz show a similar melting temperature (–57.2 to –57.0°C), but lower homogenisation temperatures (10−14°C). Type 2 inclusions in K-feldspar show melting temperatures of –57.2 to –57.0°C and homogenisation temperatures of 14−18°C (Figs 7b, c). Raman spectra show that the trapped fluid is rich in CO2 (Fig. 7d) with daughter crystals of dolomite (Raman bands as inset in Fig. 7d). The calculated densities of type 1 fluids in quartz range 0.67 to 0.68 g/cm3, whereas the type 2 fluids show a much wider range (0.58−0.69 g/cm3). The pseudo-secondary fluids are denser (0.84−0.85 g/cm3). Type 2 fluids in K-feldspar show a density range similar to the pseudo-secondary inclusions (0.81−0.83 g/cm3).

Fig. 7. Results of fluid inclusion studies. (a) Primary type I fluid inclusions in quartz in sample Ch-BT7. Histograms plotting (b) temperature of melting T m and (c) temperature of homogenisation (T h) in sample Ch-BT7. (d) Raman spectroscopic data showing Raman band characteristics of CO2 in sample Ch-BT7. Inset shows possible Raman band of dolomite. (e) Primary type I fluid inclusions in quartz in sample Ch-46E. Histograms plotting (f) temperature of melting T m and (g) temperature of homogenisation (Th) in sample Ch-46E. (h) Raman spectroscopic data showing strong Raman band characteristics of CO2 in sample Ch-46E. Data also show Raman band characteristics of calcite. (i) Primary type I fluid inclusions in garnet in sample Ch-BT12. Histograms plotting (j) temperature of melting T m and (k) temperature of homogenisation (T h) in sample Ch-BT12. (l) Raman spectroscopic data showing strong Raman band characteristics of CO2 in sample Ch-BT12. Inset shows weak Raman band of CH4.
In sample Ch-46E, fluid inclusions occur within quartz. Inclusions are secondary (type 2) variety, oval in shape and 5−10 μm in size (Fig. 7e). These inclusions yield melting temperatures of –59.0 to –58.6°C and are homogenised to a vapour phase yielding homogenisation temperatures of 18−24°C (Figs 7f, g). Raman data show that the trapped fluid is rich in CO2 (Fig. 7h). Daughter crystals of calcite were identified from their Raman bands (Fig. 7h). The calculated densities are extremely low (0.18−0.21 g/cm3).
Garnet and quartz contain fluid inclusions in sample Ch-BT12 and all the inclusions are of both type 1 and type 2 varieties. Type 1 inclusions in garnet are large (10−20 μm) showing negative crystal shapes (Fig. 7i), while the type 2 varieties are finer (5−10 μm) although showing similar shapes. Inclusions in quartz grains are smaller (5−10 μm) and circular to oval in shape. Type 1 inclusions in garnet show melting and homogenisation temperatures of –57.4 to –57.2°C and 6−10°C respectively, while type 2 inclusions show wider homogenisation temperatures (2−20°C). Type 1 inclusions in quartz show melting and homogenisation temperatures of –57.2 to –57.0°C and 8−14°C respectively, while type 2 inclusions are homogenised to a vapour phase showing a narrower range of melting (–57.8 to –57.4°C) and higher homogenisation (24−28°C) temperatures (Figs 7j, k). Raman data show that the trapped fluid in garnet is rich in CO2 (Fig. 7l) while those in quartz additionally show weak CH4 Raman bands (inset in Fig. 7l). Daughter crystals of dolomite also show characteristic Raman bands in type 1 CO2 inclusions in garnet. The calculated densities of type 1 fluids in garnet range from 0.85 to 0.87 g/cm3, whereas the type 2 fluids show a wider range (0.80−0.91 g/cm3). Type 1 fluids in quartz show a density range of 0.85−0.87 g/cm3, which is slightly higher than those of type 2 fluids (0.69−0.71 g/cm3). Another set of type 2 inclusion in quartz shows a much lower density range (0.20−0.26 g/cm3).
Geochemical study
The whole-rock compositional data of representative samples are presented in Table 4. It is apparent from these data that considerable compositional heterogeneity exists between the samples. SiO2 varies from ~56 wt.% (Ch-DQ5) to ~70 wt.% (Ch-46E). Other oxides show a large compositional range. Alumina is usually low (<15 wt.%) except for samples Ch-41B (Al2O3 = 17.7 wt.%) and Ch-46E (Al2O3 = 16.49 wt.%). FeO(T) shows extreme inter-sample variation (1.92–15.65 wt.%), and similar variations are noted for MgO (1.23−6.62 wt.%), CaO (1.81−12.71 wt.%), Na2O (1.51−5.83 wt.%) and K2O (0.65−7.07 wt.%). TiO2 shows significant enrichment in sample Ch-39C (1.67 wt.%). LOI values were calculated to be <0.7 wt.%, indicating the anhydrous nature of the mineral assemblages. The compositions are metaluminous-to-weakly peraluminous with A/CNK ratios in the range of 0.56−1.18 (Table 4). Normative compositions show high orthoclase and albite with low corundum (Table 4). The wide compositional variation reflects variation in the modal mineralogy. Similar variations in trace-element compositions are noted with significant variation in Zr (19−1640 ppm), Ba (152−1478 ppm) and Y (8−125 ppm). Apart from Zr, concentrations of other high-field-strength elements are low. These wide variations are also reflected in trace-element distribution diagrams. (Fig. 8a) normalised to lower continental crust (Taylor and McLennan, Reference Taylor and McLennan1995). For the REEs, significant enrichments are noted in sample Ch-39C. ΣREE varies in the range 98−203 ppm, while that in sample Ch-39C is 404 ppm. This is reflected in the chondrite-normalised REE plot (Fig. 8b, after Boynton, Reference Boynton and Henderson1984). This plot shows an enriched LREE and flat HREE fractionation pattern including the sample Ch-39C although it shows an elevated REE pattern. The samples show both positive and negative Eu anomalies, implying a varied source. For comparison, we have also plotted the REE pattern for post-Archean shale (Taylor and McLennan, Reference Taylor and McLennan1985). Most of the samples plot within the range and follow a similar trend (Fig. 8b).

Fig. 8. Geochemical plots of the studied orthopyroxene-bearing felsic gneiss samples. (a) Trace elements of the samples normalised to lower continental crust (Taylor and McLennan, Reference Taylor and McLennan1995). (b) Chondrite-normalised REE plot after Boynton (Reference Boynton and Henderson1984). The shaded area represents the REE pattern of Post-Archean shale (after Taylor and McLennan, Reference Taylor and McLennan1985).
Geochronological study
The analytical results of three samples of the orthopyroxene-bearing felsic gneiss are presented in Table 5. Descriptions for the individual samples are presented below. Analyses of the zircon grains using BSE and CL reveal complex and multiple types of internal structure with characteristic CL responses. While neoblastic homogeneous to simple concentric zoned zircon shows no inheritance, other grains exhibit clear inherited core surrounded by single or multiple overgrowth(s). Extremely low-CL zircon grains with very high U content reveal no internal structures. Morphology, internal structure and chemistry of zircon grains vary widely for different samples, even among different grains in a single sample.
Sample Ch-41B
Zircon grains are elongated, anhedral, and oval (200−250 μm long and 100−150 μm wide). Some zircon shows simple zoning or unzoned characters at the interior part (Figs 9a–c), while others contain large high-CL inherited cores which commonly show oscillatory zoning and prismatic outlines (Figs 9d–f). The presence of biotite, quartz and apatite is conspicuous in such cores (Figs 9e, f). Most of the cores are highly fractured and truncated in nature and commonly embayed by the dark-CL resorption zone. The dark-CL resorption zone is thick (20−50 μm) and typically shows a convolute structure. The zone is mostly featureless, but traces of planar/oscillatory zoning are found in some grains. A few biotite, apatite and quartz inclusions are present in this zone too. The overgrowth domain is relatively thin (20−40 μm) and featureless. It forms discrete patches within the oscillatory-zoned core as well as a dark-CL resorption domain in some grains. Nineteen spots were analysed from 18 grains (Table 5). Most of the spots were analysed from the oscillatory zoned core domain (n = 11). Four spots each were analysed from the resorption and overgrowth domains. U (483−1889 ppm) and Th (144−198 ppm) contents in the overgrowth domain are highly variable with the Th/U ratio in the range of 0.08−0.42. Obtained spot ages from this domain are near-concordant in the range c. 740−520 Ma (207Pb/206Pb age). All the ages show minor reverse discordance (1−3%). Two spots from this domain show near-concordant ages in the span of c. 750−740 Ma, while the youngest age of c. 516 Ma is shown by grain 16.1. The near-concordant age of c. 616 Ma (spot 29.1) is a mixed one as the spot partially overlapped the core. Spot data from the resorption domain are discordant (4−17% discordance) with high U (3288−3946 ppm) and low Th (24−32 ppm) contents that result in a very low Th/U ratio (0.01). 207Pb/206Pb ages are spread in the range c. 1018−936 Ma, with more discordance towards the younger end. This range possibly represents an event of metamorphism with extensive melt/fluid/rock interaction. The inherited cores show variable composition in terms of U (131−1138 ppm) and Th (80−202 ppm), with a Th/U ratio of 0.14−1.29. With the exception of spot 18.2, all other spots have discordant ages in the range c. 2050−2450 Ma (207Pb/206Pb ages). In a Tera–Wasserburg plot, this dataset shows high scatter with no unique discordia line (Fig. 10a). The near-concordant date from spot 18.2 gives the minimum estimate for the crystallisation age of the core as c. 2445 Ma. Thus, the protolith of this rock has an Archean–Paleoproterozoic ancestry, with a possible metamorphic signature c. 1020−940 Ma, followed by re-metamorphism with growth and recrystallisation of zircon during c. 750−520 Ma. A probability density plot (Fig. 11) shows a hiatus in the period of c. 2000−1100 Ma, precluding any thermal activity during this period.

Fig. 9. CL images of representative zircon grains with spot 207Pb/206Pb data and spot Th/U ratios. (a–f) Zircon grains from sample Ch-41B. (g–k) Zircon grains from sample Ch-DQ5. (l–o) Zircon grains from sample Ch-KHQ4.

Fig. 10. Tera-Wasserburg plots of (a) sample Ch-41B, (b) sample Ch-DQ5 and (c) sample Ch-KHQ4.
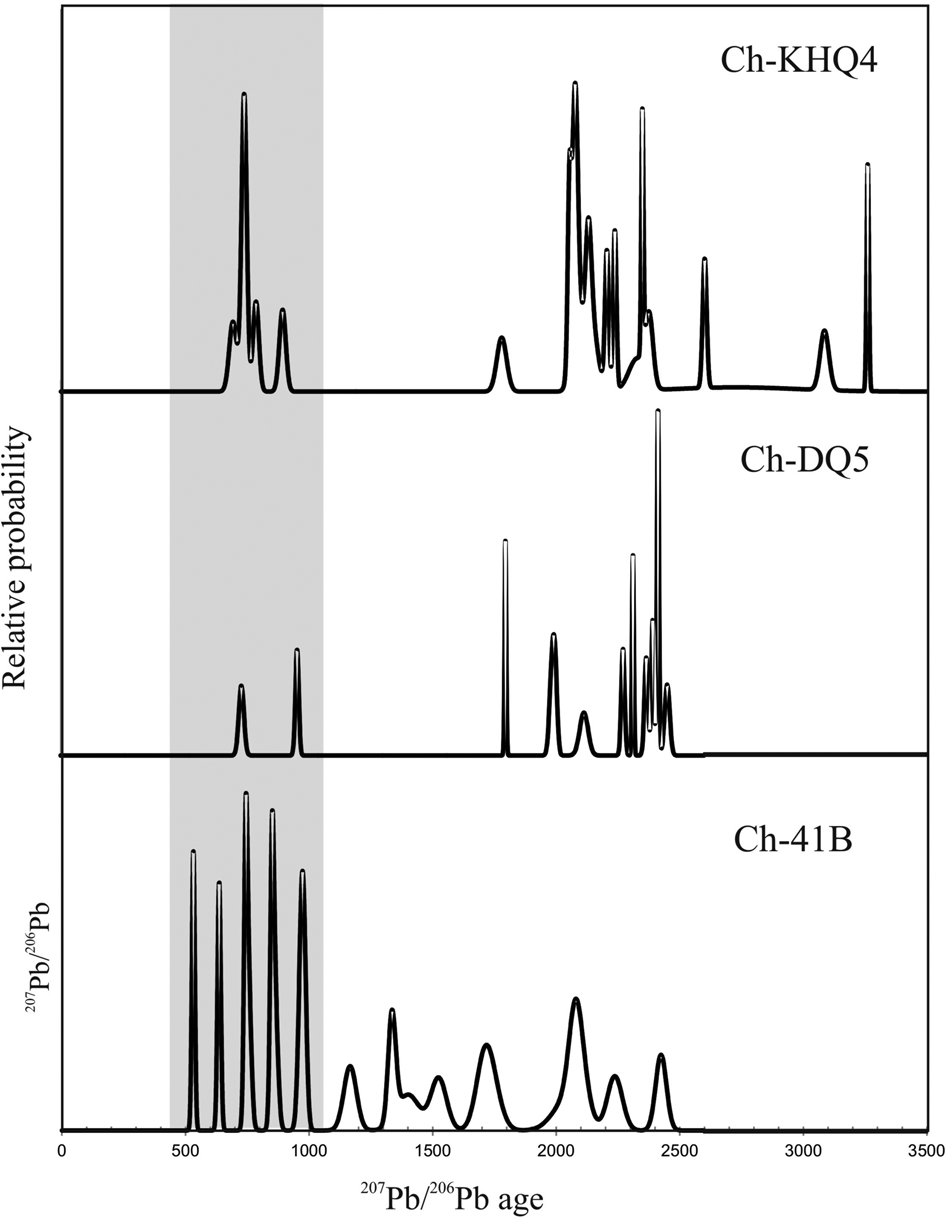
Fig. 11. Probability density plots of data points for three samples investigated.
Sample Ch-DQ5
In this sample, zircon grains are mostly oval-to-round in appearance with 150–200 μm in their long direction. Most of the grains contain an inherited core of variable sizes surrounded by variably thick dark-CL domains and moderate-CL overgrowth domains (Figs 9g–k). The outer overgrowth is narrow (<30 μm). A few neoblastic zircon grains are also present (Fig. 9i). Cores are oscillatory zoned with a bright-CL response, some of which show the presence of healed cracks. In some grains, cores have a euhedral grain outline, whereas, in others, these are abraded and truncated in appearance. Tiny rounded inclusions of quartz, apatite and K-feldspar are present in cores of some grains. The dark-CL domain is commonly featureless, although a few grains show traces of planar zoning. Occasionally, tiny alkali feldspar inclusions are present in this domain. Rarely, such a domain shows a patchy ghost structure with numerous inclusions of plagioclase and apatite grains. Thirteen spots were analysed from 12 grains (Table 5). One spot from the neoblastic grain domain (Th/U = 0.21) shows weak reverse discordance with the 207Pb/206Pb age of c. 730 Ma. One spot from the dark-CL zone (Th/U = 0.17) also shows reverse discordance with the 207Pb/206Pb date of c. 955 Ma. The rest of the data from the core domain shows high scatter in the Tera–Wasserburg diagram (Fig. 10b) with no unique discordia line and protolith ages span in the range c. 2450−1800 Ma (207Pb/206Pb age). The near-concordant date of c. 2450 Ma (207Pb/206Pb age) in grain 2.2 is the minimum age of crystallisation of the core domain. This is almost identical to the core age obtained from sample Ch-41B which shows similar petrological characteristics. These two rocks, therefore, inherited similar protolith signatures which are also evident from the probability density plot (Fig. 11).
Sample Ch-KHQ4
This sample was collected from an outcrop close to the anorthosite massif. Zircon grains in the grain mount are mainly oval with some having irregular shapes. Most of the grains are 150−200 μm long and 100−150 μm wide. Except for a few, all the grains contain inherited core surrounded by a relatively thin (10–30 μm) overgrowth (Figs 9m–o). The inherited cores show oscillatory-to-planar zoning with dark-to-moderate-CL response (Figs 9m–o). A thin (<10 μm) bright-CL rim generally separates the core domain from the overgrowth. In the majority of the grains, the core domain shows patchy alteration with inclusions of biotite, alkali feldspar, plagioclase, monazite and quartz (Fig. 9o). Healed cracks are also present in many cores. The overgrowth domain is mostly featureless with moderate to low-CL response. In a few grains, however, such an overgrowth domain is characterised by a ‘soccer-ball’ texture. A few neoblastic grains also show soccer-ball textures with moderate-CL response (Fig. 9l). Twenty four spots were analysed from 22 grains, out of which six spots were analysed from the overgrowth and neoblastic grain domain (Table 5). Uranium content varies in the range 896−1592 ppm while Th content varies in the range 89−712 ppm in this domain. The Th/U ratio is thus quite variable (0.05−0.46). Data from this domain are near concordant. While five spots give near concordant (+3 to –7% discordance) ages in the range of c. 700−790 Ma (207Pb/206Pb date), the other spot (grain 20.2) yields a 207Pb/206Pb age of c. 900 Ma. Uranium and Th contents in the core domain are extremely variable (643−2248 ppm and 138−1574 ppm, respectively) giving a wide range of Th/U ratios (0.09−0.93). Obtained ages are all discordant (8−88%) in the range c. 3300−1800 Ma (207Pb/206Pb date). When plotted in the Tera–Wasserburg diagram (Fig. 9c), these show excess scatter without defining any unique mixing line. Except for one spot age of c. 900 Ma, the period of overgrowth formation can be interpreted as c. 790−700 Ma, which is comparable with data from the other two samples. The probability density plot (Fig. 11) mirrors the same. The possibility is that the Archean protolith was metamorphosed at c. 790 Ma and later modified by younger events, while the c. 900 Ma age represents a mixture between protolith and metamorphic ages (though without any apparent textural reason). Alternatively, the c. 900 Ma thermal event was present but any signatures were essentially erased by later events.
Data from the three analysed samples suggest that the major thermal event took place during the time frame of c. 800−520 Ma. Protoliths of all the rocks are Archean (c. 3300−2400 Ma). The major thermal event at c. 790−780 Ma (Tonian age) caused extensive metamorphism and partial melting of all the rock types. Zircon ages of c. 750−740 Ma, 720 Ma, and 690 Ma are near-concordant, but these possibly represent timing of fluid interaction as suggested by Bose et al. (Reference Bose, Das, Torimoto, Arima and Dunkley2016a) from similar age clusters in the aluminous granulite. New zircon growth at c. 520 Ma suggests thermal rejuvenation during the Pan-African age.
Discussion
Metamorphic history of the orthopyroxene-bearing felsic gneiss
We used field relations, textural characters, bulk chemical and phase chemical characters, fluid inclusion and zircon U–Pb geochronological data to understand the geological evolution of the orthopyroxene-bearing felsic gneiss of the Chilka Lake region of the Eastern Ghats Province. From the field relations, the rocks appear as a part of the migmatite complex wherein other members of the complex are represented by garnet-bearing felsic gneiss (leptynite) and spinel–sapphirine–cordierite–garnet-bearing aluminous granulite. Mafic granulite occurs as small layers, lenses, and detached boudins within the migmatite complex. The orthopyroxene-bearing felsic gneiss shows broadly two types of mineral associations: one garnet-bearing (with/without cordierite) and the other garnet-absent. In the former, the peak granulite-facies assemblage is represented by garnet + orthopyroxene + quartz + plagioclase + K-feldspar + ilmenite ± cordierite while apatite and zircon occur as accessory minerals. Biotite occurs in two contrasting textural modes: as inclusions within orthopyroxene and garnet (high Ti and X Mg), and as a retrograde phase over orthopyroxene, K-feldspar and/or garnet (low Ti and X Mg). Compositions of these two biotite phases are different. Textural data show cuspate grain outlines of quartz, small rounded quartz inclusions within K-feldspar and a thin film of K-feldspar over garnet and plagioclase. These are evidence of crystallisation in the presence of melt (Sawyer, Reference Sawyer2001; Sawyer et al., Reference Sawyer, Cesare and Brown2011). Thermobarometric estimations suggest a peak metamorphic condition of ~870°C at ~7 kbar. Combining field, textural and P–T data, we consider that the orthopyroxene-bearing felsic gneiss evolved due to partial melting of a metaluminous protolith under granulite-facies conditions followed by incomplete melt removal forming diatexite migmatite. Primary fluid inclusions in garnet and quartz indicate the fluid regime during the peak stage was dominated by dense CO2 (0.85 g/cm3) with the possible admixture of aqueous fluid. Whole-rock geochemical data of representative samples show wide compositional variation in terms of SiO2, Al2O3, FeOT, MgO, K2O and Na2O. A similar variation is observed in trace-element abundance and REE distribution. The chondrite-normalised REE pattern shows fractionated LREE and flat HREE patterns. Considering the protolith of the rocks as metaluminous sediments, the compositions show wide variation which implies inhomogeneity of the source (as discussed below).
Metamorphic evolution of the coexisting rocks
A major petrological examination of the Chilka Lake area was carried out by Sen et al. (Reference Sen, Bhattacharya and Acharyya1995) where aluminous granulite and charnockite were chosen as the study material. From the description of samples and their locations, it is clear that the charnockite investigated by Sen et al. (Reference Sen, Bhattacharya and Acharyya1995) is no different than the orthopyroxene-bearing felsic gneiss of this present study. From textural data and thermobarometric results, these authors showed a multistage decompression-cooling P–T history which was later revised (Bose et al., Reference Bose, Das, Torimoto, Arima and Dunkley2016a). Importantly, the peak metamorphic P–T data estimated from charnockite and aluminous granulite reported by Sen et al. (Reference Sen, Bhattacharya and Acharyya1995) are 8.3 kbar at 870°C, which is similar to the estimates of this study. In later work, Bose et al. (Reference Bose, Das, Torimoto, Arima and Dunkley2016a) reported five metamorphic cycles based on field, petrographic and geochronological investigations of the aluminous granulites of the same migmatitic complex of the Chilka lake area. Accordingly, the earliest metamorphic event (M1) produced an amphibolite-facies mineral assemblage which eventually produced the peak granulite (M2) assemblages at 900–950°C, 8.5–9.0 kbar at 988 ± 23 Ma. The third metamorphic event (M3) occurred along a decompressive P–T path down to 700–800°C, 6.0–6.5 kbar at 781 ± 9 Ma. This was followed by cooling (M4 at c. 730 Ma), and subsequent thermal overprinting (M5 at 550–500 Ma). The mafic granulite layers within the migmatite complex have been studied by Bose et al. (Reference Bose, Das, Chakraborty, Miura and Srivastava2011b) who showed that these layers are metamorphosed basic intrusives that underwent granulite-facies metamorphism and recrystallisation. As the intrusives invaded the c. 983 Ma anorthosite (Chatterjee et al., Reference Chatterjee, Crowley, Mukherjee and Das2008), Bose et al. (Reference Bose, Das, Chakraborty, Miura and Srivastava2011b) considered the age of emplacement younger than c. 983 Ma.
When correlated with the histories of the associated aluminous granulites, the orthopyroxene-bearing felsic gneiss shows a fragmentary history. The pre-granulite facies mineral assemblage (M1 assemblage of Bose et al., Reference Bose, Das, Torimoto, Arima and Dunkley2016a) is distinctly missing, except for the occurrence of prograde biotite and ilmenite. The peak granulite-facies assemblage in the rocks studied yields P–T estimates that are comparable to that of the M4 stage of Bose et al. (Reference Bose, Das, Torimoto, Arima and Dunkley2016a). This implies either that the orthopyroxene-bearing felsic gneiss failed to record the M2–M3 stages (high-temperature cooling and decompression), or that the imprints are lost from the rock record. From the field relations, it is clear that these rocks are associated intimately with the aluminous granulite, preserving similar structural features. Moreover, there are high Mg–Ti biotite and ilmenite inclusions present in some porphyroblastic phases indicating the existence of an earlier metamorphic, possibly a higher temperature, assemblage. We, therefore, consider the second possibility as the probable reason for this discrepancy. The preservation of M2 and M3 stages of metamorphism is exclusive to the aluminous granulites owing to the abundance of Al in the bulk-rock composition, which produced intricate mineral zoning and reaction textures. The orthopyroxene-bearing felsic gneiss is metaluminous and possibly failed to preserve the earlier histories as compared to its coexisting rock. Another reason could be relative melt removal from the source. While the aluminous granulites are grossly restitic with near-complete removal of melt, the orthopyroxene-bearing felsic gneiss is migmatitic with relatively higher melt retention. It is known that the attainment of high to ultrahigh temperature is possible only after significant removal of melt (White and Powell, Reference White and Powell2002). The rock also shows no evidence for late-stage (M5) heating which is preserved sporadically as local decomposition of biotite to orthopyroxene in the associated aluminous granulite (Bose et al., Reference Bose, Das, Torimoto, Arima and Dunkley2016a).
Zircon ages and possible source
Zircon U–Pb data from the three samples studied show a complex growth history. While the majority of the analysed grains show core-rim structure, a few neoblastic zircons were also analysed. The inherited cores are mostly detrital with traces of oscillatory zoning, whereas the overgrowth shows planar or no zoning. There is a dark U-rich patchy zone surrounding the cores and a luminescent outermost overgrowth. Considering the overall distribution of these zones, we have identified four distinct zircon domains in the samples. Data from the overgrowth domain and the neoblastic zircon show near-concordant ages in the range c. 780−730 Ma, but we could not calculate any group age due to lack of statistically viable data points. This arises as the overgrowth domain, in most circumstances, is narrower than 30 μm and could not be analysed by ion microprobe. This c. 780−730 Ma age suggests a prominent metamorphic event that is reflected clearly in the probability density plots of the samples (Fig. 11). This age can be correlated with the M3–M4 events of the coexisting aluminous granulite. The outermost overgrowth is also narrow in most of the grains, but spots from some grains indicate data in the range c. 520−530 Ma, which can be correlated with the Pan–African aged M5 event of the associated aluminous granulite. It is known that zircon growth was dominated by coupled dissolution and re-precipitation which is already recognised from other parts of the Eastern Ghats Province (Upadhayay et al., Reference Upadhyay, Gerdes and Raith2009; Bose et al., Reference Bose, Dunkley, Dasgupta, Das and Arima2011a, Reference Bose, Das, Torimoto, Arima and Dunkley2016a). A major problem lies with the dark U-enriched zircon mantle domain surrounding the cores. This implies large-scale destruction of the zircon structure (metamictisation) possibly augmented by fluid activities. The occurrence of high-density carbonic fluid inclusions is important in this context. It is well known that pure CO2 is incapable of large-scale redistribution of elements (including U) within the mineral structure (Manning, Reference Manning2018). A mixed fluid with CO2 and vapour is most likely, although the traces of the latter species are lost from the minerals. This can be explained by the higher mobility of H2O (narrower wetting angle) compared to CO2 (Watson and Brenan, Reference Watson and Brenan1987). Although this is speculative in the absence of direct evidence, it is apparent that zircon cores are largely affected by radiation damage and fluid activity. It is not clear whether any new tectonic and/or thermal imprint was imposed on zircon during such a fluid-induced alteration process. Spot ages from this domain are discordant in the time frame c. 1000−900 Ma. Incidentally, this time frame matches with the timing of M2 metamorphism (c. 988 Ma) of the Chilka lake region. It is, therefore, possible that the orthopyroxene-bearing felsic gneiss underwent the M1 stage which left cryptic signatures in zircon.
To explore the protolith sources from the detrital core ages of zircon (although this study is not focussed on the detrital zircon signature), we undertook a broad outline of the sediment provenance based on our limited SHRIMP data. Zircon cores for all the three samples show 207Pb/206Pb data >c. 1796 Ma. The probability density plots of 207Pb/206Pb data in all the samples show distinct peaks at c. 1796 Ma, c. 2081 Ma, c. 2356 Ma, c. 2412 Ma and c. 3267 Ma (Fig. 11). If we consider the source of zircon to be felsic igneous rocks (owing to the oscillatory zoned nature of the cores), the source felsic magmas must have intruded the adjacent cratonic blocks during these time frames. The presently adjacent Singhbhum Craton has a record of multiple felsic magmatism since the Mesoarchean time (Upadhyay et al., Reference Upadhyay, Chattopadhyay and Mezger2019; Olierook et al., Reference Olierook, Clark, Reddy, Mazumder, Jourdan and Evans2019 and references therein). When we compare these possible protolith ages with the felsic magmatic events in the Singhbhum Craton, we find no correlation except the c. 3267 Ma age, which can be correlated with the Singhbhum granite phase II (references as above). The terrane separating the Eastern Ghats Province and the Singhbhum Craton is represented by the Rengali Province where major magmatic and metamorphic events occurred during c. 3100−2800 Ma (Bose et al., Reference Bose, Das, Kimura, Hidaka, Dasgupta, Ghosh and Mukhopadhyay2016b). In contrast, we explored the possibility of finding a zircon source from East Antarctica which is considered to be contiguous to the Singhbhum Craton during the major part of the Precambrian time (Rogers and Santosh, Reference Rogers and Santosh2004; Harley, Reference Harley, Yoshida, Windley and Dasgupta2003). In the proposed Indo–Antarctic fit, the position of the Eastern Ghats Province was adjacent to the Rayner Province of East Antarctica (Harley, Reference Harley, Yoshida, Windley and Dasgupta2003; Dasgupta and Sengupta, Reference Dasgupta, Sengupta, Yoshida, Windley and Dasgupta2003; Bose et al., Reference Bose, Dunkley, Dasgupta, Das and Arima2011a) and the Chilka Lake area was positioned close to the Prydz Bay area of the Rayner Province. This geographical fit was later geologically vindicated in recent studies (Bose et al., Reference Bose, Das, Torimoto, Arima and Dunkley2016a, Sawant et al., Reference Sawant, Gupta, Clark, Misra, Pant and Dasgupta2017). On the basis of metamorphic characters, geochronological constraints and published Pb-isotopic signatures (Flowerdew et al., Reference Flowerdew, Tyrrell, Boger, Fitzsimons, Harley, Mikhalsky, Vaughan, Harley, Fitzsimons and Zhao2013), Bose et al. (Reference Bose, Das, Torimoto, Arima and Dunkley2016a) argued that the Chilka Lake area was a part of the Prydz Bay before it joined to the rest of the Eastern Ghats Province during c. 550−500 Ma. In the Prydz Bay area, geochronological records show multiple events of felsic magmatism (Mikhalsky et al., Reference Mikhalsky, Sheraton, Kudriavtsev, Sergeev, Kovach, Kamenev, Laiba, Harley, Fitzsimons and Zhao2013). In the Lambert Terrane of the northern Prince Charles Mountain – Prydz Bay area, felsic magmatism occurred at c. 2490−2420 Ma and c. 2120−2080 Ma (Boger et al., Reference Boger, Wilson and Fanning2001 Reference Boger, Maas and Fanning2008; Mikhalsky et al., Reference Mikhalsky, Beliatsky, Sheraton and Roland2006; Corvino et al., Reference Corvino, Boger, Henjes-Kunst, Wilson and Fitzsimons2008). In the Rauer Group area, felsic magmatism occurred at c. 3470−3270 Ma (Kinny et al., Reference Kinny, Black and Sheraton1993; Harley et al., Reference Harley, Snape and Black1998; Kelsey et al., Reference Kelsey, White, Powell, Wilson and Quinn2003, Reference Kelsey, Hand, Clark and Wilson2007, Reference Kelsey, Wade, Collins, Hand, Sealing and Netting2008). These felsic rocks can be considered as sources for the c. 2081 Ma, c. 2412 Ma and c. 3267 Ma zircon cores of the present study. The source of the c. 1796 Ma zircon core is problematic as we found no comparable felsic magmatic rock in the Prydz Bay area. However, the Fisher Terrane and Beaver Terrane of the Prydz Bay area show detrital zircon signatures of c. 2800–2500 Ma, 2200–2100 Ma and 1900–1700 Ma (Mikhalsky et al., Reference Mikhalsky, Henjes-Kunst, Belyatsky, Roland and Sergeev2010). It is possible that the detritus of the latter age group was also received in the Chilka lake area. This possibility needs to be verified with a more focussed detrital zircon study. Our preliminary zircon provenance data thus suggest similarities between a part of the Eastern Ghats Province and the Rayner Province in Antarctica over a possibly extended time period (c. 3300−500 Ma).
Partial melting of continental crust and its bearing on the Chilka Lake migmatite complex
Field and textural features encourage us to explore the nature of melting reactions that are responsible for the stability of the two mineral associations at granulite-facies conditions. Textural data suggest that a biotite-bearing precursor was stable at the pre-granulite condition and the peak granulite assemblages preserve evidence of melt-related textures. These features clearly suggest that the partial melting of a ‘suitable’ protolith was responsible for the formation of the orthopyroxene-bearing felsic gneiss.
Partial melting is considered as a major process of lower crustal evolution and a large number of granulite terranes of the world bear testimony to this. A review of literature shows that numerous natural occurrences, results of melting experiments, and theoretical models put tight constraints on the various melting processes (reviewed in Clemens, Reference Clemens, Brown and Rushmer2006). Partial melting experiments have been carried out on different bulk compositions to explain the diversity of melt compositions as well as the residual solids, commonly known as the restites. The majority of the partial melting reactions involved hydrous phases such as muscovite, biotite and hornblende as reactant phases together with quartz, plagioclase, K-feldspar and sillimanite depending on the composition of the protolith. Most of the experiments were carried out on pelitic protoliths where muscovite and biotite constitute the hydrous phases. Pelitic rocks are saturated with Al and sillimanite is taken as a reactant phase in most of the melting experiments (Waters and Whales, Reference Waters and Whales1984; Clemens and Vielzeuf, Reference Clemens and Vielzeuf1987; Le Breton and Thompson, Reference Le Breton and Thompson1988; Conrad et al., Reference Conrad, Nicholls and Wall1988; Vielzeuf and Holloway, Reference Vielzeuf and Holloway1988; Peterson and Newton, Reference Peterson and Newton1989; Patiño Douce and Johnston, Reference Patiño Douce and Johnston1991; Skjerlie and Johnston, Reference Skjerlie and Johnston1993; Skjerlie et al., Reference Skjerlie, Patiño Douce and Johnston1993; Carrington and Harley, Reference Carrington and Harley1995; Pickering and Johnston, Reference Pickering and Johnston1998; Das et al., Reference Das, Dasgupta and Miura2003). Partial melting of pelitic bulk compositions produces anhydrous assemblages of garnet + K-feldspar + quartz ± cordierite depending on the proportion of muscovite and biotite in the starting material and pressure–temperature–fluid conditions of metamorphism. The melting reactions include
The melt generated is usually S-type granite in composition, but its composition varies depending on the factors mentioned above. The first appearance of melt from biotite breakdown in these experiments is variable (over 100°C) together with the melt productivity. These variations are related to bulk rock TiO2, Na2O, H2O, X Mg, and α![]() $_{{\rm Si}{\rm O}_ 2}$ (Clemens, Reference Clemens, Brown and Rushmer2006). As sillimanite has never been stabilised in the rock and the bulk composition is mostly metaluminous, we rule out the pelitic sediment as the ‘suitable’ bulk composition in our study.
$_{{\rm Si}{\rm O}_ 2}$ (Clemens, Reference Clemens, Brown and Rushmer2006). As sillimanite has never been stabilised in the rock and the bulk composition is mostly metaluminous, we rule out the pelitic sediment as the ‘suitable’ bulk composition in our study.
Experiments with other protolith compositions have also produced interesting results. As a protolith, greywacke has been considered as a suitable material for partial melting. Partial melting experiments with metaluminous greywacke produced anhydrous minerals and granitic melts similar to pelitic protoliths (Vielzeuf and Montel, Reference Vielzeuf and Montel1994; Montel and Vielzeuf, Reference Montel and Vielzeuf1997; Stevens et al., Reference Stevens, Clemens and Droop1995, Reference Stevens, Clemens and Droop1997; Nair and Chacko, Reference Nair and Chacko2002). In such a case, the reactants include plagioclase instead of sillimanite and the reaction is
This reaction (3) explains the occurrence of orthopyroxene as a peritectic phase plus garnet and cordierite for slightly magnesian bulk (Association-1). This agrees with the data of Stevens et al. (Reference Stevens, Clemens and Droop1997) who showed experimentally that for a greywacke protolith, the garnet + orthopyroxene + cordierite assemblage forms as solid residue at 850°C and 5 kbar for more magnesian bulk (composition B of Stevens et al., Reference Stevens, Clemens and Droop1997). Our phase equilibria modelling using these bulk compositions suggest that both the cordierite-free and cordierite-bearing assemblages can stabilise at >840°C, 7 kbar (Figs 6a,b).
For the stability of orthopyroxene + K-feldspar (Association-2), the experimental data of Stevens et al. (Reference Stevens, Clemens and Droop1997) indicate a temperature above 900°C. Orthopyroxene was also produced as the only Fe–Mg phase in the experimental study of Patiño Douce and Beard (Reference Patiño Douce and Beard1995) where a similar starting material with intermediate Mg# was used. However, K-feldspar was not produced as a peritectic phase in the above experiment; instead, ilmenite was the product phase. It is therefore indicated that bulk compositions could play a major role in bringing out variation in the mineral associations.
Clemens (Reference Clemens, Brown and Rushmer2006) pointed out that greywacke has extreme variability in bulk composition in terms of SiO2 which varies from ~58 wt.% (quartz-poor volcaniclastic type) to ~79 wt.% (quartz-rich type). The former variety is metaluminous and enriched in FeO, MgO, CaO, Na2O, and TiO2, compositionally similar to andesites and dacites. Most of the experiments were carried out for Ca-poor bulk compositions (Vielzeuf and Montel, Reference Vielzeuf and Montel1994; Montel and Vielzeuf, Reference Montel and Vielzeuf1997; Stevens et al., Reference Stevens, Clemens and Droop1997), but average quartz-intermediate greywackes have more Ca (Dickinson, Reference Dickinson1982).
Experimental data of Vielzeuf and Montel (Reference Vielzeuf and Montel1994) suggest that melting began at ~900°C and at ~10 kbar. Patiño Douce and Beard (Reference Patiño Douce and Beard1995) used Mg-rich metagreywacke as the starting material and their data suggest that melting began at ~930°C and at ~15 kbar. A similar synthetic magnesian metagreywacke was used by Stevens et al. (Reference Stevens, Clemens and Droop1997). These latter studies explored the roles of low ![]() $f_{{\rm O}_ 2}$, Ti in biotite and magnesium number (Mg#) on melting. In all the cases, the melt compositions appeared to be strongly peraluminous. Patiño Douce and Beard (Reference Patiño Douce and Beard1996) used a low-Mg, low-Ca bulk composition which appears to be less fertile in nature owing to low SiO2. It is interesting to note that orthopyroxene is a ubiquitous product phase of the melting reaction. Nair and Chacko (Reference Nair and Chacko2002) showed experimentally that orthopyroxene, plus garnet and K-feldspar, formed from a biotite + plagioclase + quartz-bearing semipelitic protolith, stabilised in the system in the temperature range of 875−1025°C within 5−15 kbar pressure. The additional thermal stability of biotite in their experiment resulted from the Ti and F contents. Their study also implies that melting is insignificant up to 900°C for natural Ti- and F-bearing biotite, corroborating experimental data on synthetic systems (Forbes and Flower, Reference Forbes and Flower1974; Trønnes et al., Reference Trønnes, Edgar and Arima1985; Peterson et al., Reference Peterson, Chacko and Kuehner1991; Dooley and Patiño Douce, Reference Dooley and Patiño Douce1996).
$f_{{\rm O}_ 2}$, Ti in biotite and magnesium number (Mg#) on melting. In all the cases, the melt compositions appeared to be strongly peraluminous. Patiño Douce and Beard (Reference Patiño Douce and Beard1996) used a low-Mg, low-Ca bulk composition which appears to be less fertile in nature owing to low SiO2. It is interesting to note that orthopyroxene is a ubiquitous product phase of the melting reaction. Nair and Chacko (Reference Nair and Chacko2002) showed experimentally that orthopyroxene, plus garnet and K-feldspar, formed from a biotite + plagioclase + quartz-bearing semipelitic protolith, stabilised in the system in the temperature range of 875−1025°C within 5−15 kbar pressure. The additional thermal stability of biotite in their experiment resulted from the Ti and F contents. Their study also implies that melting is insignificant up to 900°C for natural Ti- and F-bearing biotite, corroborating experimental data on synthetic systems (Forbes and Flower, Reference Forbes and Flower1974; Trønnes et al., Reference Trønnes, Edgar and Arima1985; Peterson et al., Reference Peterson, Chacko and Kuehner1991; Dooley and Patiño Douce, Reference Dooley and Patiño Douce1996).
Fluid compositions of the lower crust have important controls over the formation of orthopyroxene-bearing assemblages from biotite-bearing ones. Experimental data show that a binary CO2–H2O fluid can influence the stability of biotite and orthopyroxene, but pure CO2 has no role except lowering the ![]() ${\rm \alpha }_{{\rm H}_2{\rm O}}^{} $ of the fluid and thereby raising the solidus temperature (Clemens, Reference Clemens1993; Clemens et al., Reference Clemens, Droop and Stevens1997; Graphchikov et al., Reference Graphchikov, Konilov and Clemens1999). In contrast, if halogen components are mixed with the fluid (H2O–CO2–KCl–NaCl), a biotite-bearing protolith can undergo melting at lower temperatures (750−800°C) and experimental data suggest that orthopyroxene is preferred as a solid residue if the fluid composition is salt-poor (Safonov et al., Reference Safonov, Elizaveta, Kosova, Rajesh, Belyanin, Golunova and Van Reenen2012). Although direct evidence of CO2-dominated fluid is found here, no indication of halogen species could be discerned from the fluid-inclusion study.
${\rm \alpha }_{{\rm H}_2{\rm O}}^{} $ of the fluid and thereby raising the solidus temperature (Clemens, Reference Clemens1993; Clemens et al., Reference Clemens, Droop and Stevens1997; Graphchikov et al., Reference Graphchikov, Konilov and Clemens1999). In contrast, if halogen components are mixed with the fluid (H2O–CO2–KCl–NaCl), a biotite-bearing protolith can undergo melting at lower temperatures (750−800°C) and experimental data suggest that orthopyroxene is preferred as a solid residue if the fluid composition is salt-poor (Safonov et al., Reference Safonov, Elizaveta, Kosova, Rajesh, Belyanin, Golunova and Van Reenen2012). Although direct evidence of CO2-dominated fluid is found here, no indication of halogen species could be discerned from the fluid-inclusion study.
Another important aspect is the fertility of protolith material. Experimental data show that greywackes can be more fertile than the pelites (Johannes and Holtz, Reference Johannes, Holtz, Ashworth and Brown1990; Clemens and Vielzeuf, Reference Clemens and Vielzeuf1987; Stevens et al., Reference Stevens, Clemens and Droop1995). Experimental data of Montel and Vielzeuf (Reference Montel and Vielzeuf1997) show that melt production from greywacke protolith decreases with higher pressure (> 8 kbar). This implies that the fertility of pelitic and greywacke changes at deep crustal conditions. Compared to these two common protoliths, other rocks are less fertile and require higher temperatures (>1000°C) for melting. The fertility of rocks depends directly on composition although rock association is another crucial factor. Patiño Douce and Johnston (Reference Patiño Douce and Johnston1991) showed experimentally that fertility increases in layered crust as a consequence of the exchange of melt-inducing components.
The migmatite complex and its probable protolith(s)
As the protolith of the orthopyroxene-bearing felsic gneiss investigated could be greywacke, we now discuss the tectonic implications of sedimentation in the context of the Eastern Ghats Province. It is argued that the Eastern Ghats Province evolved as an accretionary orogenic belt together with the Rayner Complex of East Antarctica during c. 1000–900 Ma (Dasgupta et al., Reference Dasgupta, Bose and Das2013, Reference Dasgupta, Bose, Bhowmik, Sengupta, Pant and Dasgupta2017; Bose and Dasgupta, Reference Bose and Dasgupta2018). Cawood et al. (Reference Cawood, Kröner, Collins, Kusky, Mooney, Windley, Cawood and Kröner2009) explained that the original geometry of the accretionary system could hardly be preserved due to the continuation of plate margin processes and subsequent deformation. They identified a significant number of large accretionary orogens dominated by deep-water turbidites which are tectonically interleaved with arc terranes and intruded by post-orogenic granites. The source could either be a part of the accretionary wedge or the backarc basin fill (Foster et al., Reference Foster, Gray, Spaggiari, Kamenov, Bierlein, Cawood and Kröner2009), but such deep water turbidites would show wide variation in composition. An important distinction could be made from the fact that accretionary prisms usually receive sediments from the adjacent arc and are dominated by lithic fragments (psammopelitic), while backarc basins would receive sediments from the continental interior and would be more arenaceous (psammitic) (Dickinson and Suczek, Reference Dickinson and Suczek1979; Dickinson and Valloni, Reference Dickinson and Valloni1980; Cawood, Reference Cawood1983, Reference Cawood1990; Dickinson, Reference Dickinson and Zuffa1985). This can be tested by the occurrence of younger-age zircon from arc-derived magmas in the turbidite sequence from an accretionary prism source, but older zircons from the distal continental source could also be possible (Parra et al., Reference Parra, Faugeres, Grousset and Pujol1997). In an unmetamorphosed sedimentary sequence, it is possible to decipher the provenance (magmatic arc, continental block and recycled orogen) on the basis of pristine sedimentary characters (Dickinson and Suczek, Reference Dickinson and Suczek1979; Chakraborty and Pal, Reference Chakraborty and Pal2001; McConnell et al., Reference McConnell, Rogers and Crowley2016) and geochemical proxies (Bhatia and Crook, Reference Bhatia and Crook1986; Wani and Mondal, Reference Wani and Mondal2016). These parameters have limited applications for a supposedly sedimentary sequence that has undergone high-temperature metamorphism, magmatism and deformation. Greywacke is a major component of the turbidite sequence, which in the classical case, is characterised by alternate sandy and muddy layers (Dżułiński and Walton, Reference Dżułiński and Walton1965; Walker, Reference Walker and Walker1984). It has been confirmed geochemically that a greywacke protolith can produce semipelitic schist upon metamorphism at low-temperature conditions (Winchester et al., Reference Winchester, Park and Holland1980). The occurrence of alternate pelitic and semipelitic migmatised layers in the lithopackage, thus can be a reflection of the turbidite sequence in a trench. In a classic example from the Nankai subduction zone, 3D modelling of the drill-core sites was carried out using core samples and geophysical data (Underwood and Moore, Reference Underwood, Moore, Busby and Azor2012). The reconstructed sections demonstrate the occurrence of turbidites (sandy and silty lithologies) and hemipelagic clay in all the sites. If such a sedimentary pile from a modern accretionary setting undergoes metamorphism, partial melting and deformation at lower crustal conditions (Fig. 12a), a lithological association such as that we described here could be possible. This is speculative at this stage, but a clearer picture might emerge with stable isotopic data and detailed detrital zircon analysis. A similar setting could have been developed during the subduction–accretion process involving the Indian and East Antarctic cratons (Fig. 12b) which produced the Rayer-Eastern Ghats (R-EG) orogenic belt during c. 1030−900 Ma and its subsequent disintegration at c. 780 Ma (Bose et al., Reference Bose, Das, Torimoto, Arima and Dunkley2016a).

Fig. 12. (a) Schematic diagram showing the possible tectonic setting for generation of the orthopyroxene-bearing felsic gneisses at the lower crust in a subduction zone setting. (b) Cartoon diagram showing subduction–accretion process involving Indian and East Antarctic cratons which formed the Rayner-Eastern Ghats (R-EG) orogeny during c. 1030–780 Ma (modified after Dasgupta et al., Reference Dasgupta, Bose, Bhowmik, Sengupta, Pant and Dasgupta2017). It is notable that the peak metamorphism in the Chilka Lake area occurred at c. 990 Ma (Bose et al., Reference Bose, Das, Torimoto, Arima and Dunkley2016a) through partial melting of the lower crust. The orthopyroxene-bearing felsic gneiss was also formed in this process and subsequently modified by later events (see text for details).
Existing models describing the origin of orthopyroxene-bearing felsic gneisses focus on charnockite. This is true for most of the cases as mentioned above, although our data suggest an alternative mechanism. Based on field occurrence, rock association, textural evidence and P–T–t–fluid history, we argue that partial melting of a greywacke-type protolith can produce restitic assemblages which closely resemble the orthopyroxene-bearing felsic gneiss. We also envisage a probable tectonic scenario where such a process can operate. On a larger scale, this warrants a revisit of the petrological history of similar rocks (often grouped under the charnockite umbrella) formed by subduction-related metamorphic processes in the deep crust.
Conclusion
The orthopyroxene-bearing felsic gneiss of the Chilka Lake granulite suite shows field and textural evidence of partial melting to produce two broad granulite-facies assemblages from slightly contrasting bulk compositions. Fluid-inclusion data suggest the presence of dense CO2-rich aqueous fluid during peak conditions while the later fluid was H2O rich with much lower density. Zircon grains from the orthopyroxene-bearing felsic gneiss have detrital cores giving Paleoproterozoic to Mesoarchean, whereas the overgrowth domains have Tonian to Pan African ages. We conclude that the orthopyroxene-bearing felsic gneiss has evolved from a sedimentary protolith similar to the adjacent aluminous granulites. A suitable precursor of the orthopyroxene-bearing felsic gneiss could be greywacke for which experimental data tally with our textural interpretation. Although no direct evidence is available, we propose a Proterozoic marine basin where arc-derived turbidity sequences produced the suitable protolith for the Chilka Lake granulite complex.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1180/mgm.2020.71
Acknowledgements
The authors thank Kazuyuki Shiraishi for his help during SHRIMP analysis. SB and KD received financial grants from the Department of Science and Technology (DST), Govt. of India during 2006–2010 when the samples were collected. Part of the SHRIMP analysis was done by SB under the JSPS post-doctoral fellowship (P07043). Editorial comments from Roger Mitchell and constructive criticisms by Jon Pownall and an anonymous reviewer have improved the quality of the paper. Utsa Bose helped us to improve the quality of English. The authors dedicate this work to Late Prof. Hiroharu Matsueda who introduced them to the world of fluid-inclusion study.